Abstract
Objective:
This study investigated the effects of an 8-week plyometric training (PT) session on countermovement jump (CMJ) height, take-off velocity, and Tensiomyography (TMG) derived contractile parameters in seniors.
Methods:
Twenty-three senior adults (age 66.7±5.2 years) were randomly divided into two groups: PLYO (n=11) and CTRL (n=12). Tensiomyography was measured in vastus lateralis (VL), biceps femoris (BF), tibialis anterior (TA), gastrocnemius medialis (GM), and lateralis (GL). Additionally, the electromechanical efficiency (EME) index was calculated in GM as a ratio between amplitudes of peak-to-peak M-wave and TMG (Dm) responses. Biochemical markers of muscle damage and inflammation were evaluated to provide indirect indices of exercise protocol safety.
Results:
The main effect of time (for take-off velocity p=.023; ɳ2= .236) and group x time interactions (for CMJ, Tc (BF, GM), Dm (BF) and EME p<.05; ɳ2= .136 - .236) were observed. Post hoc analysis showed a significant increase in CMJ height and take-off velocity, namely by 14.2% (p=.001) and 8.2% (p=.01) in PLYO, respectively. Contraction time (Tc) decreased in BF –5.7% (p=.001) and GM –9.6% (p=.001). Dm decreased only in BF –20.8% (p=.001), while the EME index of the GM improved by 22.9% (p=.002). There were no differences between groups or assessment time points for C-reactive protein (p=.122).
Conclusion:
The present study clearly supports the application of supervised PT exercise in seniors, since explosive power, muscle contractility, and EME of the lower limbs were markedly improved after training.
Keywords: Aging, Elderly, Countermovement Jump, Contractile Properties
Introduction
There is a large body of evidence suggesting that aging, in parallel with muscle disuse, negatively affects neuromuscular system functioning[1,2] and reduces work capabilities[3]. There is further evidence suggesting that aging is associated with the loss of muscle fibers and motor neurons, atrophy of muscle fibers, predominantly fast twitch, yielding a lower overall muscle mass, slower contraction time (Tc), and decreased muscle power[4-6]. However, those adaptations are not distributed symmetrically among muscle groups[5,6] It seems that aging, in combination with physical inactivity, predominantly affects (but not exclusively) fast twitch muscle fibers and at higher rates those located in postural compared to non-postural muscles[7-9]. Age-related increases in Tc were observed in older non-athletes and in master athletes, where power master athletes maintain shorter Tc with age[6], suggesting the importance of high-intensity exercise for maintaining shorter Tc.
Different resistance training (RT) strategies are commonly prescribed as a viable tool to counter age-related degenerative changes in muscle mass, function, and the ability to generate force[3,10,11]. RT protocols are generally implemented based on the recommendations prescribed by the American College of Sports Medicine[10]. The underlying, widely-accepted assumption is that traditional RT exercise would translate into greater muscle power output and consequently a lower frailty rate in the elderly[3,5]. However, there has been a growing debate in the literature regarding the efficiency of traditional RT exercise in preventing age-related atrophy and muscle fiber loss. For example, Aagaard et al.[3] reported that long term RT (from 3 to 5 months) can generate a 5-12% gain in muscle mass and volume in the elderly, while findings on the loss of muscle fiber count remain inconclusive. In a recent systematic review, Lopez et al.[11] suggested that even one well-structured and supervised weekly training session can assist in reducing the risk of falling and frailty in frail seniors due to increased muscle strength, muscle power, and functional capacities, while, conversely, two weekly sessions, held for 12 consecutive weeks, were not enough to induce these changes in non-frail seniors[12]. In a narrative literature review, Fischer et al.[13] concluded that >2 weekly RT sessions are needed to increase muscle mass, but fail to enhance muscle power development, when performing slow and controlled movements during RT exercise in seniors[14]. Furthermore, Steib et al.[15] provided evidence that high intensity RT exercise in seniors exhibits dose-dependent effects on muscle strength and power and functional outcomes, with high-intensity power trainings being more effective compared to moderate and low-intensity RT. Improvements after 10 weeks of high-velocity RT exercise were translated into greater improvements in functional and power tests (by 24-47%), but not in strength tests[16]. Importantly, improvements in power and functional status are far more beneficial for fall prevention[17]. Plyometric training (PT) is a type of high-velocity power training that has been extensively studied in athletes and younger populations. Marković and Mikulić[18] summarized the effects of PT in: (a) the positive adaptation of increased neural drive to the agonist muscles; (b) enhancement in muscle activation patterns; (c) adjustments in the mechanical characteristics of the muscle-tendon complex of plantar flexors; (d) changes in muscle size and structure; and (e) optimization in single-fiber kinetics. A recent meta-analysis showed moderate benefits of jump training (with overall effect size= 0.66 for >25 jumps per session) for to increase explosive power in seniors throughout all their muscle groups[19].
Likewise, Hoffrén-Mikkola et al.[20] established that hopping training in the elderly leads to an increase in the height of vertical jumps and capacity for rapid force production, primarily due to an increase in gastrocnemii muscle stiffness. However, there are no other studies reporting modifications of skeletal muscles’ contractile properties after PT in older populations. Recently, Zubac and Šimunič[21] demonstrated the use of Tensiomyography (TMG) for assessing the Tc of five different muscles after 8 weeks of PT in a young population and showed significant countermovement jump (CMJ) height improvements (∆12.2%; ɳ2=.498) whilst Tc, recorded in four (out of five) lower-limb muscles, significantly decreased (ranging from ∆8.7% to 32.9%). Importantly, the same authors demonstrated that pooled decrease in skeletal muscle Tc explained ~30% of CMJ height improvements. Since TMG Tc was found to be related to the proportion of myosin heavy chain type I (MHC-I) in vastus lateralis (VL)[22], they also reported an 8.2% decline in estimated MHC-I after 8 weeks of PT. Furthermore, due to a problematic and unreliable assessment of skeletal muscle electro-mechanical efficiency (EME)[23-25] the TMG amplitude (Dm) was recently integrated as a denominator to M-wave peak-to-peak amplitude (MPTP)[26]. However, this approach has never been applied in an experimental procedure thus far.
Therefore, this study aimed to examine the effects of 8 weeks of supervised PT on jumping performance, contractile capacity, and inflammatory response in seniors. Additionally, the applicability of the newly proposed EME index following supervised PT was explored[26]. We hypothesized that the PT would be instrumental to an increase in vertical jump ability and a decrease in muscle contractile properties (Tc, Dm) in PLYO compared to controls. More specifically, the EME index would be greater in PLYO compared to controls following the introduction of PT.
Materials and methods
Participants
Twenty-three participants completed all tests and study procedures. They were recruited from the Primorska region of Slovenia on a voluntary basis via presentations at Daily Activity Centers, on social media, and in local newspapers. Medical histories and a Physical Activity Readiness Questionnaire (PAR-Q) were administered prior to any data collection. The inclusion criteria were: age >65 years, body mass index <30 kg/m2, physical activity in supervised conditions 2-3 times per week, and having their doctor’s permission. The exclusion criteria were: any cardiovascular disease or electrocardiography (ECG) examination abnormalities, previous history of musculoskeletal and neurological disorder/injury, hormonal therapy that might interfere with training outcomes, regular alcohol consumption, and smoking. All participants were fully informed of the procedures and risks involved before written consent was obtained. The study protocol was reviewed and approved by the Republic of Slovenia National Medical Ethics Committee (approval number: 0120-447/2017/4). All procedures used conformed to the Declaration of Helsinki.
Study design
Participants were randomized into a plyometric group (PLYO, N=16) or control group (CTRL, N=15) via blinded lottery drawing. Six participants dropped from the study due to reasons unrelated to the experimental protocols; this included one participant who was afraid of injury, and another participant who was excluded due to methodological issues (loss of data). A general overview of the study procedures is displayed in [Flowchart 1] (Supplementary file). Data was collected on three separate occasions, including a familiarization trial, baseline, and final assessments. During familiarization, participants’ body height and mass were determined using a digital scale (Seca 769, Hamburg, Germany). Participants were then familiarized with laboratory equipment and testing procedures (i.e., vertical jump performance, TMG and EME measurements, warm-up and CMJ). All individuals participated in one session of jump familiarization a week before the baseline assessment in order to avoid any learning effects.
Flowchart 1.
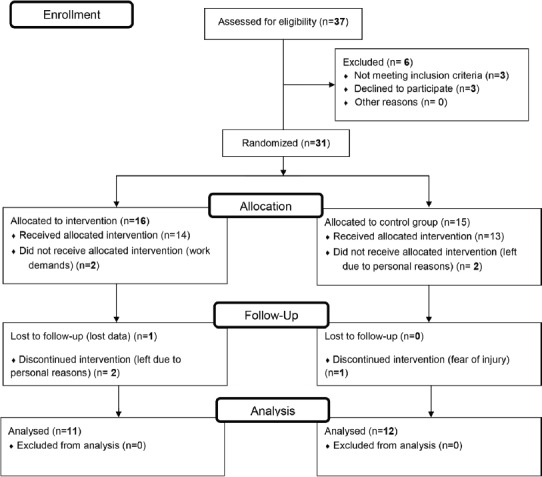
Study flow diagram.
Supplementary file.
During baseline and final data collection all participants reported to our laboratory at 08:00 a.m. for all experimental procedures following an overnight fast. All participants were asked to withdraw from vigorous physical activity two days prior to baseline assessment. Venous blood samples were taken after the participants were rested in a supine position for 15 minutes to examine their inflammatory response. After blood sampling, participants had a light breakfast and continued with other testing procedures (4 participants per hour). All assessments were performed in a closed and ventilated facility, with a temperature range of 20-22°C during the morning. Lastly, all testing procedures were conducted at the same facility, by the same researchers, using the same equipment at a similar time of the day (between 07:00-09:30 a.m.) throughout the investigation.
Plyometric training
All participants engaged in supervised physical training for 60 minutes, three times weekly. Plyometric training was introduced in a structured linear periodization model for nine consecutive weeks as originally proposed by Marković & Mikulić[18] (and later applied in a healthy younger population21). During the first 30 minutes all participants engaged in a 15-minute structured warm-up (jogging, dynamic stretching) followed by 15 minutes of various drills. Afterwards, PLYO continued with 5 minutes of warm-up jumps and 20-30 minutes of PT, while CTRL continued with 30 minutes of their habitual physical exercise, including non-weight bearing exercises (gymnastics, balance and coordination movements) and active stretching. PLYO were instructed and encouraged to jump (bounce, hop) vertically to a maximum jumping height and with minimum ground contact time. Between the two sets of exercises, subjects rested for 2-3 minutes. The number of vertical jumps was progressively increased. All subjects completed at least 80% of the planned sessions. All training sessions were supervised by an experienced kinesiologist and performed indoors, in a gym. PT included one-week rest (e.g., deloading week) after the fourth week of training (Table 1). Self-reported rate of perceived effort (RPE) scales were administered following each PT session.
Table 1.
Sets of x repetitions of plyometric exercise (bilateral consecutive vertical jumps) by week of study.
| Week 0: | Baseline assessment |
|---|---|
| Week 1: | 5x10 jumps |
| Week 2: | 5x10 jumps |
| Week 3: | 6x10 jumps |
| Week 4: | 6x10 jumps |
| Week 5: | Deloading |
| Week 6: | 7x10 jumps |
| Week 7: | 7x10 jumps |
| Week 8: | 8x10 jumps |
| Week 9: | 8x10 jumps |
| Week 10: | Final assessment |
Blood sampling and muscle inflammatory response
Blood samples of 3 ml were drawn from the antecubital vein before and after the 8 weeks of supervised PT. After collection, the samples were taken to the Izola General Hospital laboratory. Samples were centrifuged at room temperature at 3400 rpm for 10 minutes, and at 3880 rpm for 10 minutes. Biochemical markers of muscle damage (lactate dehydrogenases; LDH) and inflammation (C-reactive protein; CRP) were determined via previously standardized procedures established by the International Federation of Clinical Chemistry (IFCC) method (L→P) at 37°C and the immunoturbidimetric method (Beckman Coulter AU 680), respectively. Internal quality control of measurements showed very good reliability (CV=2.99% and 1.55% for serum LDH and CRP, respectively).
Tensiomyography
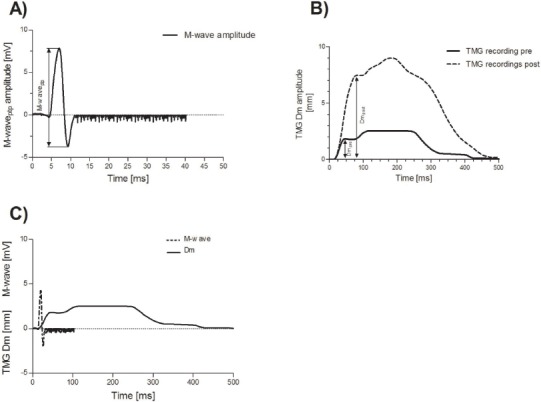
Tensiomyography was used to detect muscle belly enlargement in a transversal plane during an isometric twitch contraction[27] by using digital high-precision displacement sensor (digital–optical comparator, TMG-BMC Ltd, Slovenia) that was pressed by a spring (.2 N/cm2) on the muscle belly during the measurement to assure a high signal-to-noise ratio and high reliability[31]. The sensor was positioned perpendicular to the tangential plane on the skin above the muscle belly. The measuring point for muscle was determined on the basis of previous TMG studies[22,27-29] in VL, BF, GM, gastrocnemius lateralis (GL), and tibialis anterior (TA). All measurements were performed isometrically in relaxed pre-defined positions: for VL, in a supine position with the knee angle set at 30° flexion (where 0° represents the fully extended joint); for BF in a prone position with the knee angle set at 5° flexion; for TA supine with the ankle in a neutral position; and for GM and GL in a prone position with the ankle in a neutral position. Foam pads were used to support the joints. When necessary, the measuring point and electrode positions were adjusted to obtain maximal Dm of the muscle belly. Using an electrical stimulator (TMG-S2, TMG-BMC, Slovenia), a 1 ms rectangular (twitch) impulse was applied through stimulation electrodes that were positioned 5 cm distally (cathode) from and 5 cm proximally (anode) to the measuring point. Initially, the electrical current amplitude was set just above the threshold and was then gradually increased until the Dm readings stabilized. From two maximal twitch responses, the muscle contractile properties were calculated and an average was used for further analysis. All TMG-derived contractile parameters were extensively analyzed for their reliability. Indeed, Tc and Dm were found to be highly reliable with 95% ICC ranging >0.90, for all five lower-limb muscles.
From TMG response a MHC-1 proportion was estimated using a multiple linear regression model as proposed by Šimunič et al.[22] for VL muscle only:
Equation 1

where MHC-1 represents a myosin heavy-chain type I proportion, Td TMG derived delay time, Tc TMG derived contraction time, and Tr TMG derived half-relaxation time.
Electro-mechanical efficiency
This stimulation protocol was implemented in accordance with the recent investigation of Paravlić et al.[26], who suggested a high reproducibility for the MPTP and Dm following evoked twitch contractions. More precisely, the Dm and MPTP of GM were elicited using a maximal electrical impulse applied to the posterior tibial nerve through an electrical stimulator (TMG-S2, TMG-BMC, Slovenia). Accordingly, both the TMG and electromyography (EMG) sensors were positioned “one handbreadth below the popliteal crease on the medial mass of the calf.” The EMG recording electrodes (Ambu BlueSensor N, Ballerup, Denmark) were placed on the belly of the dominant leg GM muscle in a proximal-distal position towards the tip of the calcaneus bone based on previous recommendations of Delagi et al.[30], while the reference electrode was placed on the lateral malleolus of the ipsilateral leg. The maximal stimulation amplitude (monophasic pulse of 1 ms duration) through two square (50×50 mm) 2 mm thick reusable rubber-based self-adhesive stimulating electrodes (Dura-Stick Plus, Hanover, Germany) were positioned in the popliteal fossa, in a proximal-distal direction. The cathode was placed proximally while the anode was placed distally with ~4 mm of inter-electrode distance. Regular plantar flexion during stimulus was assessed visually so as to avoid peroneal nerve stimulation[31]. The maximal stimulation amplitude was achieved gradually by increasing the amplitude of electrical impulse from the threshold by ~30 mA to the maximal stimulator output (110 mA) just to be sure of achieving the highest amplitudes of both Dm and MPTP. Accordingly, the nerve was stimulated 2 times at a maximum intensity of 110 mA, where both the TMG and M-wave responses were recorded and saved for further analysis. Before the maximum stimulus was achieved, each subject received about the same number of stimuli. To minimize the effects of fatigue and potentiation, rest periods of a minimum of 10 seconds were given between each stimulus. Each measurement assessment lasted ~10 min. The EME of the GM was calculated as a ratio between Dm and MPTP (in mm/mV)[26]. A very high intra-assay reliability of the MPTP and Dm amplitude was confirmed, with ICC readings ranging from 0.88 to .92 for MPTP and Dm, respectively.
Vertical jump tests
CMJ performance was evaluated after the standardized warm-up involving a 5-minute step test, five minutes of whole-body dynamic stretching, and five minutes of jumping drills. CMJ performance was assessed using a force plate (HE600X600, AMTI, Watertown, MA, USA). Subjects performed 2-3 warm-up CMJs, followed by 3 maximal CMJs, where the CMJ with the highest jump was taken for further analysis. Between each trial there was a break of 60 seconds so as to avoid fatigue. Arms were kept akimbo. Jumping height was calculated from force impulse as previously reported in younger adults[27].
Statistics
Preliminary sample sizes were estimated using GPower software (version 3.1.5) and the following factors: for repeated measures within/between interactions ANOVA, effect size to be detected was .20 (calculated directly from pooled partial eta squared readings, previously documented in young adults[21). The alpha was set at .05, research power at .80; correlation among repeated measures .40. The resulting target sample size was 12 participants per group, initially to be recruited in the study. Unfortunately, in the PYLO group the target sample size was missed by one participant. Normality was confirmed using the Shapiro-Wilk test, with additional Q-Q plot visual inspection. Students’ independent t-test was applied to establish differences between participants in dependent variables of interest (i.e. age, body mass, body height, BMI). The main effects were analyzed using a mixed general linear model (GLM), taking into account the groups (CTRL, PLYO) and time (baseline and final assessment) as factors. After determining the interaction effect, secondary analysis was used to determine the time effect in both groups. Additionally, the degree of the effect was determined for dependent variables by using partial-eta squared (ɳ2) readings. Partial-eta squared readings of .02, .13, and .33 were rated as small, moderate and high differences, respectively[32]. The homogeneity of the variance was evaluated using Levene’s test, while Mauchly’s test was used to confirm compound symmetry. The analysis of covariance (ANCOVA) with baseline measurements entered as covariate was used for unmatched mean readings at the baseline. For non-parametric data, a Friedman-ANOVA test was applied, followed by a Sign-test separately for each scale. Linear regression analysis was performed after the calculation of the Pearson correlation and linearity test. SPSS 19.0 (IBM, Chicago, IL, USA) software was used for all calculations. All data are presented with mean ± standard deviation and 95% confidence intervals when appropriate. Statistical significance was accepted at p-values <.050 for the main effects and <.10 for the interaction effect.
Results
Distribution normality, variance homogeneity, and sphericity assumptions were not violated in any of the dependent variables (p>.050). The PAR-Q questionnaire outlined 3 participants with several positive answers who were subsequently removed from the study. Later, seven dropped out due to various personal reasons unrelated to the study design, while data were lost for one participant, leaving 23 participants who successfully completed all study procedures. Following randomization, the two groups did not differ significantly in any of the anthropometric measures at baseline assessment nor at final assessment (Table 2). Participants allocated into the PLYO group completed an average of 21±3 of a total of 24 PT sessions.
Table 2.
Anthropometric characteristics of the participants.
| All | Control group | Experimental group | Pgroup | |
|---|---|---|---|---|
| N (% men) | 23 (35%) | 12 (33%) | 11 (36%) | - |
| Age / years) | 66.8 ± 5.1 | 67.2 ± 5.8 | 66.3 ± 4.5 | .623 |
| Body height / m | 1.68 ± .08 | 1.67 ± .99 | 1.68 ± .06 | .861 |
| Body mass / kg | 74.3 ± 13.4 | 74.6 ± 13.8 | 73.8 ± 14.2 | .855 |
| Body mass index / kg/m2 | 26.3 ± 3.8 | 26.5 ± 3.6 | 25.9 ± 4.1 | .735 |
RPE scale
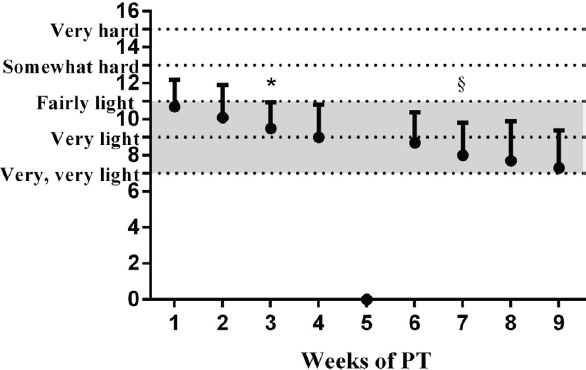
The PLYO group reported a decrease in RPE throughout the study, specifically after the third week of PT (Figure 1). The overall mean value of the RPE throughout the whole study duration was 9±2 (out of 19).
Figure 1.
Changes in the Borg’s self-perceived exertion scale throughout the 8-weeks of plyometric training (PT). * Significant time effect; indicates the first time-point which is different from the baseline (p<.05). § Significant time effect; indicates the first time-point different from the first (*) (p<.05).
CMJ performance
The main effect of time was found (p=.019, ɳ2=.233), while a significant interaction effect (time x group) was also observed for CMJ height (p=.057; ɳ2=.162). A Bonferroni post hoc revealed a 14.2% (.001) increment in CMJ for PLYO group exclusively. Likewise, a time effect was observed for Vv, which increased in PLYO by 8.2% (p=.023; ɳ2=.233). Unlike the CMJ height improvement, there was none for the relative CMJ power (time x group: p=.916) (Table 3).
Table 3.
Baseline and final data on countermovement jump (CMJ) performance, gastrocnemius (Ga.) medialis electro-mechanical efficiency (EME), tensiomyographic (TMG) assessment and inflammatory response of both groups.
| Control group | Plyometric group | p-level | ||||||
|---|---|---|---|---|---|---|---|---|
| pre | post | Pre | Post | time | group | interaction | ɳ2 | |
| CMJ | ||||||||
| Height (cm) | 10.2±2.4 | 10.2±3.2 | 10.5±4.5 | 11.7±4.5 | .019* | .524 | .057† | .236 |
| Relative power (W/kg) | 35.7±5.9 | 36.4±6.8 | 34.7±9.1 | 38.4±8.1 | .187 | .926 | .916 | --- |
| Vertical velocity (m/s) | 1.38±0.2 | 1.39±.2 | 1.38±.3 | 1.48±.3 | .023* | .645 | .174 | .233 |
| EME (posterior tibial nerve stimulation) | ||||||||
| MPTP/mV | 6.8±3.7 | 6.2±2.3 | 6.4±2.8 | 4.9±2.6 | .071 | .843 | .458 | --- |
| Ga. medialis (Dm/mm) | 8.4±2.8 | 6.7±2.0 | 6.9±2.7 | 6.7±1.6 | .076 | .278 | .094† | .133 |
| Ga. medialis EME (mV/mm) | 1.4±.5 | 1.2±.4 | 1.35±.9 | 1.7±.9 | .461 | .521 | .095† | .136 |
| TMG data | ||||||||
| Biceps femoris Tc (ms) | 42.0±7.8 | 44.1±7.2 | 42.3±10 | 38.9±11 | .606 | .503 | .028† | .210 |
| Biceps femoris Dm (mm) | 5.9±2.6 | 6.0±2.6 | 6.5±2.8 | 4.8±1.9 | .036* | .769 | .031† | .204 |
| Ga. lateralis Tc (ms) | 25.2±2.8 | 24.3±2.9 | 34.2±12 | 30.1±11 | - | .858 | - | --- |
| Ga. lateralis Dm (mm) | 4.2±1.3 | 4.1±1.6 | 4.8±1.1 | 4.2±1.5 | .268 | .533 | .324 | --- |
| Ga. medialis Tc (ms) | 29.9±5.0 | 30.3±4.4 | 31.7±7.0 | 27.6±4.8 | .125 | .823 | .075† | .240 |
| Ga. medialis Dm (mm) | 3.9±1.6 | 4.1±1.6 | 4.1±1.8 | 4.2±1.6 | .783 | .837 | .884 | -- |
| Tibialis anterior Tc (ms) | 22.1±8.5 | 22.7±6.7 | 23.8±2.9 | 20.9±2.6 | .569 | .360 | .999 | -- |
| Tibialis anterior Dm (mm) | 2.8±1.4 | 2.8±1.5 | 2.5±1.0 | 2.8±.8 | .439 | .726 | .623 | -- |
| Vastus lateralis Tc (ms) | 25.0±4.1 | 23.4±2.3 | 22.9±3.6 | 23.0±2.0 | .301 | .233 | .307 | -- |
| Vastus lateralis Dm (mm) | 5.4±1.8 | 5.1±1.6 | 4.0±1.5 | 4.1±1.4 | .690 | .064 | .411 | -- |
| Estimated MHC-I (%) | 30.8±17 | 26.6±12 | 24.5±14 | 23.4±8.8 | .374 | .345 | .579 | -- |
| Inflammatory markers | ||||||||
| CRP (mg/L-1) | 1.6±1.1 | 1.2±0.6 | 2.3±1.7 | 3.2±2.1 | .671 | .063 | .122 | -- |
| LDH (µkat/L-1) | 2.5±1.5 | 1.3±1.5 | 2.9±.4 | 2.6±.9 | .026* | .050 | .173 | -- |
MPTP – M-wave peak-to-peak amplitude; Tc – Contraction time; Dm – Maximal radial displacement amplitude; MHC-I – Myosin heavy chain I proportion; CRP – C-reactive protein; LDH – Lactate dehydrogenase; ɳ2- partial eta squared;
Significant time effect (p<.05);
Significant interaction effect (p<.10).
GM muscle EME index
The results showed a tendency toward a time x group interaction in the GM EME index (time x group: p=.095; ɳ2=.133), where EME increased by 22.9% (p=.002) only in PLYO. Due to different measurement setups of EME assessment (posterior tibial nerve stimulation) we performed a separate GM Dm analysis where we found an interaction effect (time x group: p=.094; ɳ2=.136). A Bonferroni post hoc revealed a lower Dm compared to control (p=0.01). There was no time (p=.070) or interaction (p=.458) effect observed for MPTP amplitude (Table 3).
Inflammatory response
Data on blood inflammatory markers indicated no significant differences between groups or assessment time points for CRP (time x group: p=.122), while the LDH concentration of the CTRL group significantly decreased (time effect: p=.026) (Table 3). However, this portion of data should be interpreted with caution due to the small number of specimens collected. Six participants were unable to supply a blood sample during baseline data collection (including two from PLYO and four from CTRL) out of the total number of 23 participants.
TMG parameters
In [Table 3], significant interaction effects (time x group) for Tc of BF (p=.028; ɳ2=.210) and GM p=.075, ɳ2=.240) were observed. A Bonferroni post hoc revealed that Tc decreased by –5.7% (p=.001) and –9.6% (.001) in BF and GM in PLYO, respectively. No significant time x group interactions were observed for Tc of other muscles (GL group differences after ANCOVA: p=.858; TA time x group: p=.360; VL time x group: p=.233), nor did the calculated estimation of MHC-1 proportion (Equation 1) change in VL (time x group: p=.579). A significant interaction effect (time x group) was observed in BF Dm (p=.031; ɳ2=.204), where Dm decreased by –20.8% (p=.001) in the PLYO group exclusively.
Regression analysis
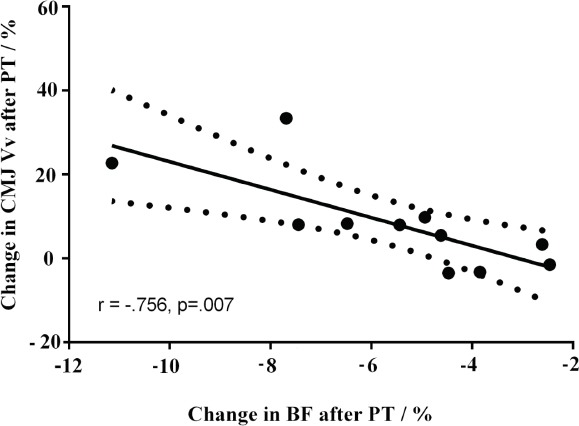
[Figure 2] shows how linear regression analysis confirmed an inverse correlation between the relative change in Vv and BF Tc (r= –.756; p=.007), explaining 57% of common variance in the PYLO group. There were no correlations between change in any Tc data and CMJ height improvements (p>.05).
Figure 2.
Pearson correlation (r) between the relative change in countermovement jump (CMJ) take-off velocity (Vv) and biceps femoris contraction time (Tc). Solid and dashed lines represent the fitted linear model, with 95% confidence intervals.
Discussion
This study investigated the effects of an 8-week plyometric training (PT) session on countermovement jump (CMJ) height, take-off velocity, and Tensiomyography (TMG) derived contractile parameters in seniors. We found that an 8-week session of supervised PT in seniors improved CMJ height in the PLYO group exclusively. In parallel, Tc decreased in three lower-limb muscles (from 5.7% to 28.9%), thereby suggesting a muscle-specific (improvements were observed in GL, GM, and BF, but not in VL and TA) response to PT. Although Tc decrease was not correlated to CMJ height improvement, the shorter Tc was negatively correlated to improvement in Vv. PT was instrumental in greater GM muscle EME. Since we could not confirm Tc decrease in VL, there were also no MHC-I shifts in VL after PT when estimated from TMG parameters (Equation 1).
The magnitude of CMJ height improvement after PT is consistent with previously observed outcomes after the same PT in a young population (∆12%)[21], and higher than after 12 weeks of explosive resistance exercise in 60-year-old participants (∆8%)[33]. However, our data on the magnitude of CMJ height improvement are somewhat opposed to the findings of Hoffrén-Mikkola et al.[20]. Indeed, Hoffrén-Mikkola reported a dramatic increase (∆56%) in CMJ height after 11 weeks of PT in the elderly. The latter investigation by design was predominantly focused on sedentary participants (whereas our participants were physically active 2-3 times per week), and this may well explain the two-fold greater level of muscle power and vertical jump ability improvements in the Hoffrén-Mikkola study compared to the present data. Nevertheless, in terms of absolute, post-intervention CMJ improvements, data were matched between the studies.
Our study was the first to examine adjustments in muscle contractile properties following PT in seniors. A similar pattern of Tc decreases was observed when the present findings were compared to those of younger participants involved in the same PT[21], with the exception of adaptation of VL muscle. More precisely, the magnitude of change generated by 8 weeks of PT was larger and more dramatic in the younger population (ranging from –8.7%, ɳ2=.790 for VL to –32.9%, ɳ2=.621 for TA muscle) than that in the present findings (Table 3; ranging from –5.7%, ɳ2=.210 for BF to –9.6%, ɳ2=.240 for GM). Also, in terms of absolute values, Tc was longer in the elderly when compared to younger participants (e.g., in BF: 38.9±10.7 ms vs. 25.3±5.6 ms, respectively); that is in line with the aging study, thereby suggesting a slower rate of muscle contraction in the elderly[6]. Indeed, our study confirmed the cross-sectional findings of Šimunič et al.[6], who found an age-related slowing in three leg muscles (GM, VL and BF) irrespective of sport participation, where power master athletes had the shortest Tc, indicating that regular power training diminishes the slowing of skeletal muscles during aging. Our findings were also indirectly confirmed in a recent comparative bed-rest study[2], where authors reported lower explosive power in the knee extensors in parallel with a slower rate of muscle function recovery (from single fiber to whole body level) in older adults when compared to young adults. To further support this, a cross-sectional study of Thompson et al.[34] reported that the elderly display a lower level of plantar flexor muscle force and rate of force development (~30%) when compared to young or middle-aged men, while Rodríguez-Ruiz et al.[35] observed a lower contraction velocity of the BF in aged adults when compared to younger and middle-aged adults (p=.020).
A narrative review of Malisoux et al.36 summarized that jumping performance, among other factors, is improved throughout a greater take-off Vv, owing to the faster contraction velocity or greater muscle force development. In the present study, the decreases in Tc were observed in BF and GM, where BF Tc improvements were correlated to the improvements of CMJ take-off Vv. Indeed, the correlation findings depicted in [Figure 2] support the aforementioned postulates by displaying the relative changes in BF Tc, explaining a great portion of variation (r2= 57%) in CMJ take-off Vv improvement of lower-limbs. This finding provides a robust indication that a superior contractile capacity favors greater explosive power output (and especially during the push-off phase, as evident from Vv adjustment) of lower-limbs as a result of PT, even in senior adults. There are several underlying mechanisms to explain the muscle-specific Tc findings. Firstly, Wilkerson et al.[37] found muscle-specific adaptations to PT, primarily due to greater neuromuscular performance improvements, which favor the relative contribution of hamstrings when compared with quadricep muscles in female athletes, and this adds, at least to some extent, to the explanation of muscle-specific adaptations to PT in our study. More importantly, an in vitro comparative study of D’Antona et al.[38] demonstrated profound effects of aging on muscle contractile properties and VL muscle MHC distribution. Indeed, the specific force and maximal shortening velocity of single muscle fiber were slower in aged participants when compared to the young, and, surprisingly, irrespective of their MHC composition. Thus, D’Antona et al.[38] advocated for muscle plasticity throughout aging and outlined the possibility of contractility modifications via physical (neuromuscular) activity in the elderly, thereby implying the dual nature of regulation pathways for MHC composition and muscle contractile capacity[39]. However, despite the suggestions of D’Antona et al.[38], and contrary to our initial hypothesis, after PT no changes in VL (Tc or MHC-I) were observed; however, Tc data indicate that such changes occurred at least in GM and BF muscles. The lack of correlations between Tc decrease and CMJ height improvements contradicts the moderate positive correlations observed among these variables in our previous PT study in younger adults[21]. This collaborates well with recent biopsy findings of Power et al.[9], where the authors reported that the preservation of muscle fiber number rather than contractile capacity is likely to enhance the unique athletic performance of master athletes. Taken together, it seems reasonable to assume that the lack of correlation between Tc decrease and CMJ height improvement predominantly mirrors the motor units loss (especially fast motor units loss)[3,40], since explosive movements, such as CMJ, require additional recruitment of fast motor units during the take-off phase. To support this, Anderson[4] defined the period of 60-70 years of age as a critical threshold point, where measurable changes in neuromuscular function occur, as found also in Tc[6]. Nonetheless, factors other than Tc adjustments generated the CMJ height improvement.
Complementarily to Tc, we have investigated the Dm, an indirect measure of passive muscle stiffness[28] and muscle atrophy[41]. The Dm decreased by –20.7% (ɳ2=.204) only in BF muscle (Table 3). The lower Dm should be interpreted as improved passive muscle tension, since higher Dm was found after a 35-day bed rest[28] as well as after the first day of a 35-day bed rest[41], suggesting that the Dm is sensitive to early-atrophic changes that are not evident yet from imaging techniques, which gives Dm a high clinical relevance, especially in older populations, as they atrophy more than their younger counterparts with a much slower rate of recovery[2]. Our findings in BF could be explained by hypertrophic processes leading towards increased muscle resting tension, higher muscle tone, and increased passive muscle stiffness, which might originate inter alia from shifts in MHC composition[20]. The Dm decrease could be explained by the fact that BF is a non-postural muscle that had a lower habitual load before the study, especially in older adults. In younger adults PT induced decreased Dm not only in BF, but also in GM and GL, as two muscles with very intense load during PT[21]. Typically, muscle stiffness, among other characteristics of muscle architecture (e.g., fascicle length and pennation angle), decreases throughout aging[42]. To support previous claims, a comparative study by Hoffren et al.[43], looking at muscle architecture changes, revealed that aging results in shorter fascicle length, shorter contractile velocity, and power yielding deterioration in repetitive jump efficiency in the elderly as compared to young people. According to Hoffren et al.[43], reaction force, and electromyography (EMG these changes were mediated by the diversity in activation patterns between young and elderly adults. Previous papers established that age-specific detrimental adjustments in muscle stiffness are regulated by the central nervous system (CNS), where degenerative changes decrease neural activation and muscle activation in aged populations, primarily due to higher antagonist activation[3,43]. To further support this, previous investigations into PT effects in well-trained athletes depicted substantially different muscle architecture adjustments following 5 weeks of sprint/jump training, implying a decrease in the pennation angle in parallel with a fascicle length and thickness increase in VL muscle[44]. Nonetheless, when compared to the young, seniors in the present study had a markedly lower magnitude of change in terms of absolute and relative Dm values. In brief, it was found that the extent of Dm decrease after the 8-week PT was greater, largely affecting the lower-limb muscles in younger populations (ranging from –14.9%, ɳ2=.418 for GM to –31.9%, ɳ2=.485 for GL muscle) when compared with seniors (only BF decreased by 20.7%, ɳ2=.204). The only study of an older population that investigated stiffness after PT was done by Hoffrén-Mikkola et al.[20] and reported higher stiffness in GL; however, for the same muscle we did not find changes in Dm. This could be explained via imposed training load during PT, where Hoffrén-Mikkola et al.[20] used high volume PT training (with ~4200 foot contacts over an 11 week investigation period), while our study was shorter (conducted over a period of 8 weeks) and involved PT with ~2200 foot contacts. Thus, the PT volume may partly explain Dm adjustments, since higher loading magnitude is a prerequisite of muscle stiffness modulation[45]. Also, it is well-established that a dose-response relationship exists between muscle stiffness improvement and number of jumps performed[46].
The GM muscle EME efficiency improved by ∆23% (ɳ2=.136) in the PLYO group, primarily due to lower Dm in GM after PT, while MPTP was unchanged with a tendency toward decrease in both groups (Table 3), as both groups attended organized exercise sessions with the same frequency and duration. Since we could not confirm MPTP change, the increased EME could be interpreted the same way as the Dm decrease. The combination of EMG and mechanical signals provides insight into the electromechanical coupling in skeletal muscle[47].
There are a few limitations to the present study that must be acknowledged. Peak values of RPE were 11±3 (i.e., fairly light) and this low effort may partially explain the limited and muscle-specific adaptations, since motor unit activation in the elderly appears to be intensity dependent[48]; however, it is well known that RPE is mainly sensitive to endurance actions. Importantly, RPE was a safety measure we took, since the PT in seniors is not a habitual, daily routine and it involves intense powerful movements. The safety of the senior participants was our primary concern, since we replicated the training load from our previous study designed for young healthy adults, which yielded a significant response in muscle contractile capacity and vertical jump height. In addition, we evaluated biochemical markers of muscle damage and inflammation to provide indirect indices of exercise protocol safety.
In conclusion, this study clearly supports the benefits of 8 weeks of moderate load week PT in enhancing explosive actions, Tc, and Dm with a superior muscle EME index of selected lower-limbs in seniors. Thus, the preset findings advocate for the application of EME index as a viable tool to screen for muscle adaptation(s) following training, since PT was instrumental to a ∆23% improvement in the EME index of the PLYO group. It appears that supervised PT favors the restoration of muscle function throughout aging. However, the adaptation(s) following PT were rather asymmetrical and muscle specific. This bears important implications in terms of quality of life throughout aging, since the present findings may aid in the development of possible countermeasures to attenuate the profound effects of aging on muscle contractile capacity by designing more specific exercise interventions for seniors. Taken together, we recommend the implementation of supervised and moderate load PT in active populations of seniors to attenuate the detrimental effects of aging and to possibly govern the current fall prevention strategies by improving the electromechanical efficiency of lower limb muscles.
Author contributions
DZ and BŠ designed all experimental procedures; DZ, AP, BŠ, KK, and FU collected data throughout all experimental procedures; DZ, AP, and BŠ analyzed the data; while KK and FU were responsible for verifying data accuracy. KK was in charge of participant allocation. DZ and BŠ were in charge of paper write-ups, while AP and FU critically revised the manuscript. All authors approved the final version of the manuscript for publication.
Acknowledgments
This work was supported by the Slovenian Research Agency under the projects: “Assessment of blood parameters and extracellular vesicles for optimization of sport results”, grant number: 20420-01/15, and “The effect of exercise with variable load on skeletal muscle in older adults: randomized crossover trial”, grant number: Z7-9420.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Hvid LG, Aagaard P, Ørtenblad N, Kjaer M, Suetta C. Plasticity in central neural drive with short-term disuse and recovery - effects on muscle strength and influence of aging. Exp Gerontol. 2018;106:145–153. doi: 10.1016/j.exger.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Pišot R, Marusic U, Biolo G, Mazzucco S, Lazzer S, Grassi B, Šimunič B. Greater loss in muscle mass and function but smaller metabolic alterations in older compared with younger men following 2 wk of bed rest and recovery. J Appl Physiol. 2016;120:922–929. doi: 10.1152/japplphysiol.00858.2015. [DOI] [PubMed] [Google Scholar]
- 3.Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging:strength training as a countermeasure. Scand J Med Sci Sports. 2010;20:49–64. doi: 10.1111/j.1600-0838.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- 4.Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports. 2003;13:40–47. doi: 10.1034/j.1600-0838.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- 5.Porter MM, Vandervoort AA, &Lexell J. Aging of human muscle:structure, function and adaptability. Scand J Med Sci Sports. 1995;3:129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 6.Šimunic B, Pišot R, Rittweger J, Degens H. Age-related Slowing of Contractile Properties Differs between Power-, Endurance- and non-athletes;a Tensiomyographic Assessment. J Gerontol A Biol Sci Med Sci. 2018 doi: 10.1093/gerona/gly069. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 7.Grimby G, Danneskiold-Samsøe B, Hvid K, &Saltin B. Morphology and enzymatic capacity in arm and leg muscles in 78–81 year old men and women. Acta Physiol Scand. 1982;115:125–34. doi: 10.1111/j.1748-1716.1982.tb07054.x. [DOI] [PubMed] [Google Scholar]
- 8.Nilwik R. Snijders, T Leenders, Groen BBL, van Kranenburg J, Verdijk LB, Van Loon LJC. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Geronto. 2013;48:492–498. doi: 10.1016/j.exger.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Power GA, Minozzo FC, Spendiff S, Filion ME, Konokhova Y, Purves-Smith MF, Rassier DE. Reduction in single muscle fiber rate of force development with aging is not attenuated in world class older masters athletes. Am J Physiol Cell Physiol. 2016;310:C318–C327. doi: 10.1152/ajpcell.00289.2015. [DOI] [PubMed] [Google Scholar]
- 10.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Swain DP. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults:Guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 11.Lopez P, Pinto RS, Radaelli R, Rech A, Grazioli R, Izquierdo M, Cadore EL. Benefits of resistance training in physically frail elderly:a systematic review. Aging Clin Exp Res. 2017;30:889–899. doi: 10.1007/s40520-017-0863-z. [DOI] [PubMed] [Google Scholar]
- 12.Walker S, Haff GG, Häkkinen K, Newton RU. Moderate-load muscular endurance strength training did not improve peak power or functional capacity in older men and women. Front Physiol. 2017;26:743–750. doi: 10.3389/fphys.2017.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher JP, Steele J, Gentil P, Giessing J, Westcott WL. A minimal dose approach to resistance training for the older adult;the prophylactic for aging. Exp Gerontol. 2017 doi: 10.1016/j.exger.2017.09.012. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.Walker S, Santolamazza F, Kraemer W, Häkkinen K. Effects of Prolonged Hypertrophic Resistance Training on Acute Endocrine Responses in Young and Older Men. J Aging Phys Act. 2015;23:230–236. doi: 10.1123/japa.2013-0029. [DOI] [PubMed] [Google Scholar]
- 15.Steib S, Schoene D, Pfeifer K. Dose-response relationship of resistance training in older adults:A meta-analysis. Med Sci Sports Exerc. 2010;42:902–914. doi: 10.1249/MSS.0b013e3181c34465. [DOI] [PubMed] [Google Scholar]
- 16.Bottaro M, Machado SN, Nogueira W, Scales R, Veloso J. Effect of high versus low-velocity resistance training on muscular fitness and functional performance in older men. Eur J Appl Physiol. 2007;99:257–264. doi: 10.1007/s00421-006-0343-1. [DOI] [PubMed] [Google Scholar]
- 17.Bento PCB, Pereira G, Ugrinowitsch C, Rodacki ALF. Peak torque and rate of torque development in elderly with and without fall history. Clin Biomech. 2010;25:450–454. doi: 10.1016/j.clinbiomech.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Markovic G, Mikulic P. Neuro-musculoskeletal and performance adaptations to lower-extremity plyometric training. Sports Med. 2010;40:859–895. doi: 10.2165/11318370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Moran J, Ramirez-Campillo R, Granacher U. Effects of jumping exercise on muscular power in older adults:a meta-analysis. Sports Med. 2018:1–15. doi: 10.1007/s40279-018-1002-5. [DOI] [PubMed] [Google Scholar]
- 20.Hoffrén-Mikkola M, Ishikawa M, Rantalainen T, Avela J, &Komi PV. Neuromuscular mechanics and hopping training in elderly. Eur J Appl Physiol. 2015;115:863–877. doi: 10.1007/s00421-014-3065-9. [DOI] [PubMed] [Google Scholar]
- 21.Zubac D, Šimunič B. Skeletal Muscle Contraction Time and Tone Decrease After 8 Weeks of Plyometric Training J Strength Cond Res. 2017;31:1610–1619. doi: 10.1519/JSC.0000000000001626. [DOI] [PubMed] [Google Scholar]
- 22.Šimunič B, Degens H, Rittweger J, Narici MV, Mekjavic I, Pisot R. Noninvasive Estimation of Myosin Heavy Chain Composition in Human Skeletal Muscle. Med Sci Sports Exerc. 2011;43:1619–1625. doi: 10.1249/MSS.0b013e31821522d0. [DOI] [PubMed] [Google Scholar]
- 23.Esposito F, Limonta E, Cé E, Gobbo M, Veicsteinas A, Orizio C. Electrical and mechanical response of finger flexor muscles during voluntary isometric contractions in elite rock-climbers. Eur J Appl Physiol. 2009;105:81–92. doi: 10.1007/s00421-008-0877-5. [DOI] [PubMed] [Google Scholar]
- 24.Akataki K, Mita K, Itoh Y. Repeatability of mechanomyogram (MMG) from voluntary isometric contraction of biceps brachii muscles. Jpn J Phys Fit Sport. 1998;47:489–498. [Google Scholar]
- 25.Orizio C. Comments on the letter “Accelerometer and mechanomyogram”. J Biomech. 2002;35:385. doi: 10.1016/s0021-9290(01)00130-0. [DOI] [PubMed] [Google Scholar]
- 26.Paravlić A, Zubac D, &Šimunič B. Reliability of the twitch evoked skeletal muscle electromechanical efficiency:A ratio between tensiomyogram and M-wave amplitudes. J Electromyogr Kinesiol. 2017;37:108–116. doi: 10.1016/j.jelekin.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Valenčič V. Direct measurement of the skeletal muscle tonus. Artif Organs. 1990;21:240–242. doi: 10.1111/j.1525-1594.1997.tb04658.x. [DOI] [PubMed] [Google Scholar]
- 28.Pišot R, Narici MV, Šimunič B, De Boer M, Seynnes O, Jurdana M, Mekjavič IB. Whole muscle contractile parameters and thickness loss during 35-day bed rest. Eur J Appl Physiol. 2008;104:409–414. doi: 10.1007/s00421-008-0698-6. [DOI] [PubMed] [Google Scholar]
- 29.Šimunič B. Between-day reliability of a method for non-invasive estimation of muscle composition. J Electromyogr Kinesiol. 2012;22:527–530. doi: 10.1016/j.jelekin.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Delagi E, Iazzetti J, Perotto A, Morrison D. Anatomical guide for the electromyographer:the limbs and trunk. J Anatomy. 2011 [Google Scholar]
- 31.Dumitru D, Amato AA, Zwarts M. Special nerve conduction techniques. In: Dumitru D, Amato AA, Zwarts M, editors. Electrodiagnostic Medicine. second ed. Hanley & Belfus; 2001. p. 247. [Google Scholar]
- 32.Pierce CA, Block RA, Aguinis H. Cautionary note on reporting eta-squared values from multifactor Anova designs. Educ Psychol Meas. 2004;6:916–920. [Google Scholar]
- 33.Caserotti P, Aagaard P, Buttrup Larsen J, Puggaard L. Explosive heavy-resistance training in old and very old adults:Changes in rapid muscle force, strength and power. Scand J Med Sci Sports. 2008;18:773–782. doi: 10.1111/j.1600-0838.2007.00732.x. [DOI] [PubMed] [Google Scholar]
- 34.Thompson BJ, Ryan ED, Sobolewski EJ, Conchola EC, Cramer JT. Age related differences in maximal and rapid torque characteristics of the leg extensors and flexors in young, middle-aged and old men. Exp Gerontol. 2013;48:277–282. doi: 10.1016/j.exger.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Ruiz D, García-Manso JM, Rodríguez-Matoso D, Sarmiento S, Da Silva-Grigoletto M, Pisot R. Effects of age and physical activity on response speed in knee flexor and extensor muscles. Eur Rev Aging Phys Act. 2013;10:127–132. [Google Scholar]
- 36.Malisoux L, Francaux M, Theisen D. What do single-fiber studies tell us about exercise training? Med Sci Sports Exerc. 2007;39:1051–1060. doi: 10.1249/mss.0b13e318057aeb. [DOI] [PubMed] [Google Scholar]
- 37.Wilkerson GB, Colston MA, Short NI, Neal KL, Hoewischer PE, Pixley JJ. Neuromuscular Changes in Female Collegiate Athletes Resulting from A Plyometric Jump-Training Program. J Athl Train. 2004;39:17–23. [PMC free article] [PubMed] [Google Scholar]
- 38.D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552:499–511. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canepari M, Pellegrino MA, D'Antona G, Bottinelli R. Skeletal muscle fibre diversity and the underlying mechanisms. Acta Physiol (Oxf) 2010;199:465–76. doi: 10.1111/j.1748-1716.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- 40.Piasecki M, Ireland A, Piasecki J, Stashuk DW, Swiecicka A, Rutter MK, McPhee JS. Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non-sarcopenic older men. J Physiol. 2018;596:1627–1637. doi: 10.1113/JP275520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Šimunic B, Degens H, Rittweger J, Narici MV, Pišot V, Mekjavic IB, Pišot R. Tensiomyographic measurement of atrophy related processes during bed rest and recovery. In European Space Agency. 2013 (Special Publication) ESA SP (Vol. 706 SP) [Google Scholar]
- 42.Mian OS, Thom JM, Ardigò LP, Minetti AE, Narici MV. Gastrocnemius muscle-tendon behaviour during walking in young and older adults. Acta Physiol (Oxf) 2007;189:57–65. doi: 10.1111/j.1748-1716.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- 43.Hoffrén M, Ishikawa M, Komi PV. Age-related neuromuscular function during drop jumps. J Appl Physiol. 2007;103:1276–1283. doi: 10.1152/japplphysiol.00430.2007. [DOI] [PubMed] [Google Scholar]
- 44.Blazevich AJ, Gill ND, Bronks R, Newton RU. Training-Specific Muscle Architecture Adaptation after 5-wk Training in Athletes. Med Sci Sports Exerc. 2003;35:2013–2022. doi: 10.1249/01.MSS.0000099092.83611.20. [DOI] [PubMed] [Google Scholar]
- 45.Hobara H, Inoue K, Omuro K, Muraoka T, Kanosue K. Determinant of leg stiffness during hopping is frequency-dependent. Eur J Appl Physiol. 2011;111:2195–2201. doi: 10.1007/s00421-011-1853-z. [DOI] [PubMed] [Google Scholar]
- 46.Arampatzis A, Peper A, Bierbaum S, Albracht K. Plasticity of human Achilles tendon mechanical and morphological properties in response to cyclic strain. J Biomech. 2010;43:3073–3079. doi: 10.1016/j.jbiomech.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Barry DT, Gordon KE, Hinton GG. Acoustic and surface EMG diagnosis of pediatric muscle disease. Muscle Nerve. 1990;13:286–290. doi: 10.1002/mus.880130403. [DOI] [PubMed] [Google Scholar]
- 48.Kallio J, Aagaard P, Avela J, Komi PV, Selänne H, Linnamo V. Motor unit discharge rate in dynamic movements of the aging soleus. Front Hum Neurosci. 2014;29(8):773–777. doi: 10.3389/fnhum.2014.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]


