Abstract
Objectives:
Corticospinal tract excitability and spinal reflex pathways are transiently affected by short applications of static stretching. However, it remains unclear whether the duration and magnitude of these neurophysiological responses can be increased with a longer duration of the applied stretch. The purpose of this study was to investigate alterations in cortical and spinal excitability following five minutes static stretching.
Methods:
Seventeen participants (22.8±2.3 years old) were tested for the tendon tap reflex (T-reflex), Hoffman reflex (H-reflex) and motor-evoked potentials (MEPs) after transcranial magnetic stimulation (TMS) of the ankle flexor muscles in two separate occasions: before and after 5 minute static stretching or 5 minute control period, in a randomized order.
Results:
No changes were observed following the control condition. H/M ratio increased by 16.2% after stretching (P=.036). Furthermore, immediately after stretching it was observed a strong inhibition of the T-reflex (57.6% inhibition, P=.003) that persisted up to five minutes after stretching (16.2% inhibition, P=.013) but returned to baseline following 10 minutes. MEPs were not affected by stretching.
Conclusions:
This study suggests that the neuromuscular responses that follow five minute of static stretching do not influence the excitability of the corticospinal tract and follow a different time course within spinal reflex pathways.
Keywords: Static Stretching, Spinal Reflexes, Corticospinal Excitability, H-Reflex, Motor Evoked Potential
Introduction
When the ankle joint is kept at the end of its range of motion, as for during static stretching, alterations in cortico-spinal excitability and spinal reflexes can be observed. Commonly, motor evoked potentials (MEPs) elicited by TMS and Hoffmann reflex (H-reflex) of soleus muscle are inhibited[1-3]. Inhibitions of MEPs and H-reflex are generally attributed to reduced transmitter release[1,4-6], however, the dissimilar level of stretch required for the inhibitions onsets[2] and the different extents of the inhibitions themselves[7-12] support the implication of separate inhibitory mechanisms[13]. After stretching, these acute alterations are quickly reversed[14] and both MEPs and H-reflex are usually reported to be promptly restored as soon as the joint is repositioned to its neutral angle[2,7,15-17]. Differently, the inhibition of stretch and T-reflex, attributed to reduced muscle spindle sensitivity[34], persists up to several minutes following stretching[9,12,13]. In a recent work though, we showed the also the H-reflex remains altered (facilitated) for a few seconds (~30 seconds) following stretching and MEPs seem to tend in the same direction[13]. It could be hypothesised that these results might be amplified by increasing the applied stimulus (intensity and/or duration of the stretch). Since the intensity of the stretch in the mentioned study[13] was already the maximal tolerable by the participants, only the duration variable could be further investigated. This hypothesis was partially examined by Opplert and colleagues[17] which tested the effects of 1, 2, 3, 4 and 10 x 30 seconds stretching on mechanical properties and H-reflex in the plantar flexor muscles. The authors reported no stretching duration effects. However, it has to be pointed out that data were collected within a period of time exceeding the “sensitive window” of about 30 seconds[13], therefore the methodology applied by Opplert et al.[14] to quantify spinal excitability would have unlikely highlighted an effect. Nevertheless, the study by Opplert and colleagues[17] suggested that stretching applied for longer duration does not increase the size of the sensitive time window within which an alteration in H-reflex excitability can be seen. However, it remains unexplored whether the magnitude of the effect within 30 seconds after stretching is susceptible to stretching duration. Moreover neither cortico-spinal excitability nor T-reflexes were studied leaving the picture of stretching induced neurophysiological changes incomplete.
The aim of the present work is therefore to investigate the effect of 5 x 60 seconds stretching on both cortico-spinal excitability (MEPs by TMS) and spinal reflex excitability (H-reflex and T-reflex). We expect to observe a strong inhibition of the T-reflex and a facilitation of both MEPs and H-reflex. Because the T-reflex recovers slowly to its baseline values[9,13], in order to look at the recovery trend, we performed further T-reflex measurements at 5 and 10 minutes after stretching.
Eperimental procedures
Ethical approval
The study was conformed to the standards set by the Declaration of Helsinki and approved by the research ethics board of the University of Graz. Written informed consent was obtained from all volunteers before the onset of the experimental procedures.
Participants
Seventeen university students (age 22.8±2.3 years, 8 male and 9 female, body mass 68.7±15.3 kg, stature 174.0±11.8 cm) with no history of neurological disorders volunteered for the experiment. Participants were required to abstain from any strenuous physical activity on the testing day as well as to refrain from taking caffeine-containing substances and smoking within 2 h before the testing session.
Study design
The participants visited the laboratory on two separate occasions with a minimum of 24 hours between the two appointments, each lasting about 3 hours. In randomized order, during one testing session they received the treatment and in the other they acted as their own control. The experiment consisted in the measurement of H-reflex, T-reflex and MEPs induced by transcranial magnetic stimulation (TMS) before (pre) and immediately after a control period (control_00) or immediately after stretch (stretch_00). For the T-reflex further assessments were performed 5 and 10 minutes following control (control_05/10 respectively) and 5 and 10 minutes following stretching (stretch_05/10 respectively), therefore the T-reflex measurements lasted about 12 minutes more than measurements of H-reflex and MEPs responses. We decided not to measure H-reflex and MEPs responses after 5 and 10 minutes from stretching because we knew from existing literature (for MEPs2,10,11; for review on H-reflex19) that the effect of stretching on these responses lasts only few seconds.
Stretch consisted of 5 times 60 seconds stretching of the plantar flexors to the maximal individual ankle dorsiflexion (group range 18.6-39.7°) with no rest in between stretching bouts, except for the time needed to return to 10 deg plantar flexion position and back again to the maximal dorsiflexion position. The value of maximal dorsiflexion, once determined at baseline, was kept constant throughout the experiment. The control period consisted in maintaining the leg in the testing position for 300 seconds without any stretching being applied. Either intervention (control or stretch) was repeated before each of the three different sequences of stimulations (H-reflex, T-reflex or TMS in random order) and 20 minutes rest were allowed between the end of one sequence of stimulation and the next (Figure 1).
Figure 1.
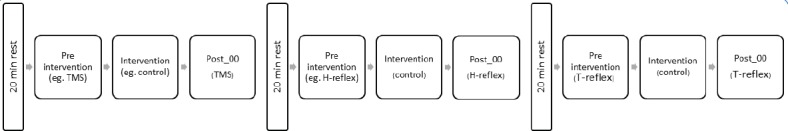
Experimental flow chart. In this flow chart is represented an example for a control session. From left to right: For every experimental session the “Pre intervention” was preceded by 20 minutes rest (10 standing, 10 sitting). In this example the sequence started with TMS measurements performed before and after the intervention. Twenty minutes (10 standing, 10 sitting) from the end of TMS measurements another of the two remaining reflexes was measured, in this case H-reflex. Twenty minutes from the end of H-reflex measurements T-reflex was studied. T-reflex was also examined 5 and 10 minutes after the intervention, this is not represented in the flow chart for simplifying the visualization.
Experimental procedures
Subjects were sitting on an isokinetic dynamometer (CON-TREX MJ, CMV AG, Duebendorf, Switzerland) with the standard setup for ankle joint movement individually adjusted. Participants had their right knee fully extended and the foot resting on the dynamometer footplate, the ankle joint aligned with the dynamometer rotation shaft and the ankle angle set at 10° plantar flexion deviating from a neutral position at 90°. Volunteers sat with the trunk at 110° and the head supported by a cushion (dentafix®, pro medico HandelsGmbH, Graz, Austria) that once positioned could be deflated allowing the formation of a stable form molded on the volunteers’ head and neck shapes. By using a remote control, the volunteers were instructed to adjust the dorsiflexion isokinetic rotation operated by the dynamometer around the foot plate until the point of perceived maximal dorsiflexion. Participants were asked to keep their knee extended and to relax during the procedures.
Once the maximal individual dorsiflexion was defined, subjects left the dynamometer and were prepared for surface electromyographic recording (EMG). Subsequently, position and stimulation intensity for the H-reflex and TMS were determined and two complete H-M recruitment curves were collected. Following this initial procedure the volunteers were allowed 20 minutes rest, 10 minutes standing and 10 minutes sitting comfortably. After this time the volunteers returned in the testing position described above and were instructed to relax completely and either keep their eyes closed or gaze at a 4 meter distance point for the beginning of the measurement. This consisted in a trigger-driven sequence of 12 stimulations to evoke one of the three neurophysiological responses (T-reflex, H-reflex or MEP) (pre-intervention measurement), followed by either the control or stretching procedure (intervention) and concluded with the repetition of the sequence of 12 stimulations (post-intervention measurement). Twenty minutes after the termination of the post-intervention measurement of one the responses, the trigger sequence was started again for the measurement of another response; the order was randomized. The trigger sequence was produced by a computer program. Subsequently, the volunteers were asked to stand up for 10 minutes and then sat down again for another 10 minutes before repeating all the procedure for evoking a different reflex/response. The experiment was carried on in the same way a third time to test the remaining response. The experimental procedure sequence is described in [Figure 1].
Surface electromyography
EMG from carefully prepared skin (shaved, abraded and cleaned with alcohol) was collected from the soleus (SOL) and tibialis anterior (TA). Electrodes (Blue Sensor N, Ambu A/S, Ballerup, Denmark) for recording H-reflex from the SOL muscle were placed in monopolar configuration (as suggested by Hadoush et al.18); electrodes on TA were placed in standard bipolar configuration at an interelectrode distance of 20 mm. Ground electrode was placed over the tibial bone medial surface. In order to avoid phase shift no low pass filter was applied. Limitation of the bandwidth with 60 kHz was determined by the isolation amplifier.
Stimulations
All stimulations were performed with the ankle joint at 10° plantar flexion (PF) with an inter stimuli interval of a random value between 7 and 9 seconds.
H and M waves measured in SOL were elicited by constant current electrical stimulation (KeyPoint® 2-channel) delivered to the tibial nerve by rectangular pulses of 1.0 ms duration. The anode (5x9cm, STIMEX adhesive gel electrode) was placed on the patellar tendon and the cathode was placed in the popliteal fossa overlying the nerve at a position that provided the greatest H wave amplitude at the smallest stimulus intensity possible. The cathode electrode (Blue Sensor N, Ambu A/S, Ballerup, Denmark) was glued on the skin in order to prevent any movement during the experimental procedures. The stimulation intensity was selected during the two recruitment ramps and adjusted to obtain a value at which the H wave was still in its ascending phase and an M wave was visible. This intensity, which was usually close to the Hmax, was then used for all the measurements (control and stretching). During the experiment, the current delivered by the stimulator was slightly adjusted when needed to ensure constant amplitude of the M wave[19].
Tendon T-reflex was elicited by a motor (Type GDRX 075, Magnet-Schultz, Germany) driven hammer hitting the Achilles tendon about 3-4 cm above its insertion on the calcaneus delivering a contact peak force of 35N+/-10N. An electrical output from the motor provided information about its rotation allowing hammer displacement and acceleration to be monitored.
Motor evoked potentials in response to single pulse TMS were recorded from SOL (as target muscle) and from TA (as reference muscle) of the right leg. TMS was performed with Magstim 200, (Magstim Company Ltd., UK) using a double cone coil (110 mm coil diameter). The coil was placed 1-2 cm left of the longitudinal fissure on the M1 area and slightly shifted to the left side in order to obtain the largest response from the contralateral right SOL. Resting motor threshold was determined as the minimum stimulator intensity able to evoke MEPs of at least 50 µV amplitude in more than 50% out of ten consecutive trials[20]. To ensure a constant coil positioning throughout the experiments, subjects were wearing EEG caps on which the optimal coil position was marked with a soft pen. MEPs were elicited with stimulation intensity equal to 120% of the resting motor threshold.
During all the stimulations background EMG activity was monitored online to ensure that both agonist and antagonist muscles were relaxed. The absence of background EMG activity was doubled checked off line in a time window of 200 ms before the stimulation.
Data analysis
Electromyography, torque, displacement, trigger and motor output signals were synchronized (DEWESoft™ 7.0 recording system, DEWETRON GmbH, Austria), digitized with a sampling frequency of 10 KHz, stored on a PC and analysed using custom-made algorithms developed in Matlab (R2014b).
The 12 H waves recorded in each series were checked for consistency and those related to an M wave showing peak to peak amplitude exceeding the target stimulation intensity by ±2 standard deviations were discarded[10,11]. Because of this criterion 1 subject had to be excluded. Reflex excitability was quantified as average H/M ratio of the remaining waves within each series. Further analysis of MEPs and H-reflex was performed within a time frame of 40 seconds immediately following stretching; due to the inter stimuli interval adopted, this analysis included only 5 stimulation per participant.
T-reflex waves and MEPs with peak to peak amplitude exceeding ±2 standard deviations within their own recording series were discarded[11,21]; all the remaining waves were retained and peak to peak values were used for statistical analysis.
Statistical analysis
Results were checked for normal distribution by Shapiro-Wilk test. For TMS and H-reflex paired sample T-test or Wilcoxon test was used to compare the variation pre-control vs pre-stretch and within conditions (pre-post control and pre-post stretch). For T-reflex data were not normally distributed and the Friedman Test was used for assessing the effect of stretching at 4 time points (pre, post_00/05/10) in two conditions (control/stretch) and Wilcoxon signed ranks tests with Bonferroni-Holm adjustment was used for pairwise comparisons within condition. Statistically significant level was set at 0.05; all statistical analysis was completed using PASW Statistic 18.0.0.
Results
H-reflex
Group H/M ratio absolute values did not change from pre to post within control condition (22.5±23.9 and 22.8±25.3 respectively) but increased from 24.8±25.1 to 26.6±24.5 from pre to post within stretch condition (P=.02, Z=-2.327). [Figure 2] shows recording from a representative subject: H/M ratio increased by 2.8% within control and by 13.8% within stretching condition. Group analysis expressed as average of individual percentage of variation pre-post intervention between conditions confirmed this result (P=.036, t=-2.307) resulting in an average increase in H/M ratio of 16.2±21.5% after stretching versus no changes (0.9±10.5%) after control (Figure 3). The analysis performed within the first 40 seconds after stretching showed an increase in H/M ratio of 12.1±25.0% after stretching versus no changes 4.6±12.8% after control (P=.09, Z=-1.079), however this analysis was performed only on those participants for which all the first 5 stimulations were retained after inspection for stimulation consistency. As a consequence only 11 volunteers were considered in this analysis.
Figure 2.
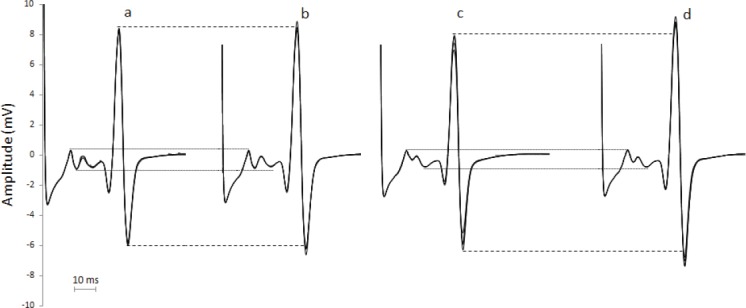
Raw EMG recording of H-reflex from a representative subject. Each track represents four superimposed waves recorded pre and post control (“a” and “b” respectively) and pre and post stretching (“c” and “d” respectively).
Figure 3.
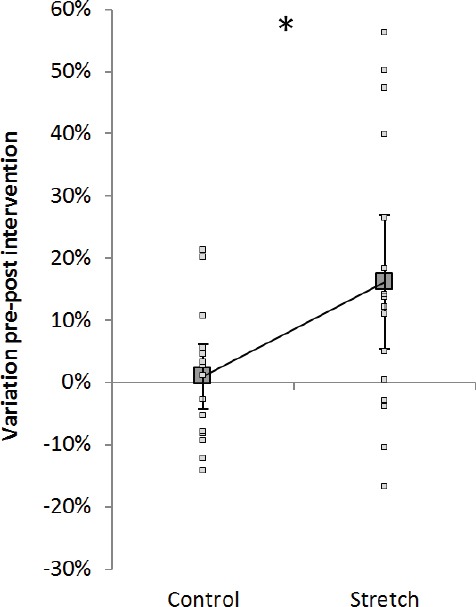
H-reflex variation. Group average ± SD (big grey connected squares) and individual values (small white squares) for variation pre-post control (left) and pre-post stretch (right). Dotted line refers to the results of the volunteer used as example for [Figure 1]. *=P<.05.
T-reflex
Three subjects did not show a T-reflex response and were therefore discarded from the analysis. [Figure 4] shows the percentage of variation of T-reflex amplitude from pre to post control (A) and from pre to post stretching (B) at the three time points investigated. Reflex amplitude was influenced by stretching (χ23 =23.852, P=.001). Pairwise comparisons highlighted no differences between the two pre measurements (pre-control vs pre-stretch) as well as no differences between pre control and the three measurements post control. In contrast, immediately after stretching the T-reflex was on average 57.6% smaller (±32.2% range 7.7-100%) (P=.003, Z=-2.934) and the inhibition observed 5 minutes following stretching (16.2±19.9%) was still significant (P=.013, Z=-2.490). After 10 minutes the values returned to baseline (P=.534, Z-.622).
Figure 4.
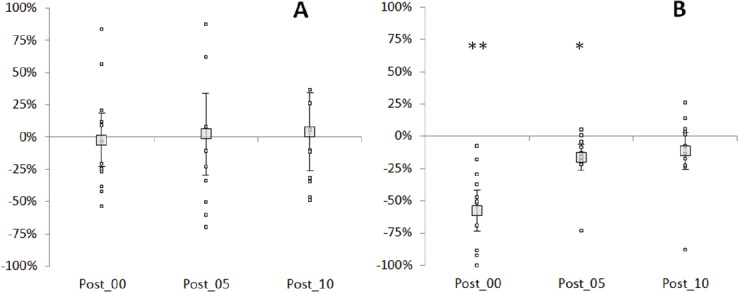
T-reflex variation. Percentage of variation pre-post control (A) and pre-post stretching (B) for each time point after intervention for individual subjects (small markers) and the group mean ± SD (large markers). **=P<.01; *=P<.05.
MEPs
All participants tolerated well the TMS assessments without reporting any side effects during or after the procedure. For SOL at control the resting motor threshold for the stretching condition was 40.00±6.94% of the stimulator output and for the stretch condition 39.76±6.78%. No difference between the resting motor thresholds for the two conditions was found (P=.720) and no change in the post TMS assessments. The mean MEP amplitude at pre stretching was 0.51±0.27 mV and for the control condition 0.40±0.19 mV. No significant differences (P=.102; t=-1.760) between the variation pre-post intervention of two conditions was observed. Similarly to H-reflex, the analysis was performed on a reduced number of participants (N=12) within the first 40 seconds following stretching. Also within this time frame no differences were found (P=.814; Z=-.235).
Discussion
The main finding of this study is that five minute static stretching of the plantar flexor muscles induces a long lasting inhibition of T-reflex, facilitates the H-reflex within 2 minutes and has no effect on motor cortex excitability.
Testing the acute influence of an intervention on the H-reflex excitability is particularly complicated when the effects have a quick reversibility, as it seems the case of stretching. Indeed most of the studies report that stretching influences H-reflex during its application, as though when the joint is at the end of its range of motion, but as soon as the joint is back to its neutral position no effect is normally seen (for review see[22). Differently, we[13] and others[8,23] have shown that the H-reflex is facilitated immediately after stretching, but this effect seemed to last only few seconds. Based on previous reports[17], we were not expecting that the period of time during which the H-reflex facilitation can be seen would have been extended by the longer stretching application (5 min) adopted in the present study, nevertheless we were still expecting to detect a stronger facilitation compared to shorter stretching application. Interestingly, the extent of the reflex facilitation (+16.2%) was very similar to the one we observed after one minute stretching (+18.3%)[13], suggesting that stretching duration has no effect on this parameter.
H-reflex amplitude is affected by both pre and post-synaptic influences[24]. In order to try to explain the effect of stretching on variations in H-reflex size, we need to find elements that can both affect the H-reflex loop pathway and be affected by stretching. Afferents from Golgi tendon organs could represent a candidate, indeed afferents from muscle spindles and from Golgi organs are activated together so that the H reflex is the net result of Ia excitation minus Ib inhibition (for review[25). Under high tension, Golgi tendon organs’ response falls slowly for one minute for then stopping[26]. It could be therefore hypothesised that after five minute stretching group Ib afferents remained inactive or decreased their activity for a period of time. However, this reduction in post-synaptic inhibition should have had a similar effect on MEPs, and this was not the case in the present study. Similarly to Ib afferents, also Renshaw cells feedback inhibition to their homonymous muscles and are affected by stretching, being inhibited by afferents from muscle spindle secondary endings[27]. The hypothesis that stretching would increase H-reflex size through a reduction of recurrent inhibition is supported by the work by Hultborn and Pierrot-Deseilligny[28] that showed that during sustained contractions, as recurrent inhibition discharge was progressively decreasing, the size of the H-reflex increased. The implication of Renshaw cells would also explain the different results between H-reflex and MEPs, in fact the firing rate of the Renshaw cell is in relation to the firing rates of the motoneurons[29], hence small tonic motoneurons will receive a weaker recurrent inhibition compared to fast large phasic motoneurons. Since muscle activation through TMS follows an orderly recruitment[30,31], having used a low stimulation intensity (40.00±6.94%) we likely activated predominantly small motoneurons. Therefore, if recurrent inhibition was inhibited for a period of time after stretching, then this reduced inhibition would be more marked on H-reflex than on MEPs. One other possibility is that the facilitation (or the reduction in inhibition) occurred at a level of the neural network that is activated by peripheral nerve stimulation but not by TMS. In this case a decrease of presynaptic inhibition on the Ia afferents would result in an increase in H-reflex but not in MEPs size. Gregory et al.[8] suggested that the increase in H-reflex they observed after stretching was a consequence of a reduced level of muscle spindle resting discharge (measured on cats) that might have led to a reduction in the inhibition of motoneurons. However this hypothesis would not explain why H-reflex facilitation can be observed only within few seconds after stretching whilst T-reflex remains inhibited for several minutes.
Afferent input from dynamic stretching of muscle spindles is also able to modify the output of motor cortical neurons[32], and prolonged input from the spindle receptor group was shown to induce after-effects in motor cortex excitability[33,34]. Also during static stretching of a relaxed muscle, afferent activity from spindles increases, which in the following may drive after-effects in motor cortex excitability. However, the results on MEP amplitudes show that corticospinal excitability in the soleus muscle remained unchanged following 5 minutes stretching. Thus, with respect to the changes found at the spinal level, motor cortical influences on spinal motoneurons are rather unlikely. Lastly some compensatory increase of cortical influence might have occurred. For proving this, paired pulse TMS protocols are required in order to test whether intra-cortico-spinal excitability increases after a period of prolonged stretching.
In agreement with existing literature, T-reflex was strongly inhibited[7,11-13,35]. This result very likely does not reflect a change in the neurological pathway[6] and it is commonly attributed to reduced sensitivity of the muscle spindles[36]. It is worth mentioning that the amount of inhibition observed immediately after five minutes stretching in the present study (57.6%) was less than the one observed in our previous study (63.1%) where only one minute stretching was applied[13]. However, being this result reasonably similar, it might simply be that T-reflex can only be inhibited up to a certain amount regardless the duration of the applied stretch. If instead the amount of inhibition has a tendency to reduce as the stretching time increases, then it could be hypothesised that muscle spindles lose their tension quickly during stretching, but as the stimulus continues without increasing intensity (as for constant angle stretching procedures) they manage to regain some of the slack. Finally, differently to our previous study where stretching procedure was applied for one minute[13], T-reflex inhibition was observed also five minutes following stretching. An immediate suggestion would be that stretching applied for a longer duration has a longer lasting effect. Accordingly, Avela and colleagues[9] showed that stretch reflex size measured on soleus muscle 15 minutes following 1 hour of repeated passive stretching of the triceps surae was on average almost still 60% smaller than that recorded at baseline.
Limitations
In the present work we did not measure the H-reflex and MEPs 5 and 10 minutes following stretching. Not having observed a variation in MEPs immediately after stretching, we exclude that an effect on this parameter could have been delayed by 5 minutes. However, in relation to the H-reflex, we cannot exclude that the observed inhibition could have protracted further.
Another limitation is in relation to not having measured the Mmax throughout and at the end of the experiment. Consequently H-reflex and MEPs before and after stretching could have not been normalised by the Mmax before and after stretching respectively. However, previous works demonstrated no changes in Mmax either during or following static stretching[2,7,37,38], repeated prolonged passive stretching[9,10] or static stretching training[11].
Conclusions
In conclusion, 5 minute static stretching induces an increment in H-reflex and does not influence MEPs. T-reflex inhibition showed a much longer duration.
Acknowledgements
The authors acknowledge the assistance of Paul Kressnik with the data analysis.
Footnotes
F.B. was financially supported by a grant from the Austrian Science Fund (FWF), grant/award number: ‘P 27665’. All other authors have no conflict of interest.
Edited by: A. Ireland
References
- 1.Budini F, Gallasch E, Christova M, Rafolt D, Tilp M. Soleus H-reflex inhibition decreases during 30 seconds static stretching of plantar flexors, showing two recovery steps. Front Physiol. 2018;9:935. doi: 10.3389/fphys.2018.00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guissard N, Duchateau J, Hainaut K. Mechanisms of decreased motoneurone excitation during passive muscle stretching. Exp Brain Res. 2001;137(2):163–9. doi: 10.1007/s002210000648. [DOI] [PubMed] [Google Scholar]
- 3.Robinson KL, McComas AJ, Belanger AY. Control of soleus motoneuron excitability during muscle stretch in man. J Neurol Neurosurg Psychiatry. 1982;45(8):699–704. doi: 10.1136/jnnp.45.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen J, Petersen N, Crone C. Changes in transmission across synapses of Ia afferents in spastic patients. Brain. 1995;118(4):995–1004. doi: 10.1093/brain/118.4.995. [DOI] [PubMed] [Google Scholar]
- 5.Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108(3):450–62. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- 6.Wood SA, Gregory JE, Proske U. The influence of muscle spindle discharge on the human H reflex and the monosynaptic reflex in the cat. J Physiol. 1996;497(1):279–90. doi: 10.1113/jphysiol.1996.sp021767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guissard N, Duchateau J, Hainaut K. Muscle stretching and motoneuron excitability. Eur J Appl Physiol. 1988;58(1-2):47–52. doi: 10.1007/BF00636602. [DOI] [PubMed] [Google Scholar]
- 8.Gregory JE, Mark RF, Morgan DL, Patak A, Polus B, Proske U. Effects of muscle history on the stretch reflex in cat and man. J Physiol. 1990;424(1):93–107. doi: 10.1113/jphysiol.1990.sp018057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avela J, Kyröläinen H, Komi PV. Altered reflex sensitivity after repeated and prolonged passive muscle stretching. J Appl Physiol. 1999;86(4):1283–91. doi: 10.1152/jappl.1999.86.4.1283. [DOI] [PubMed] [Google Scholar]
- 10.Avela J, Finni T, Liikavainio T, Niemelä E, Komi PV. Neural and mechanical responses of the triceps surae muscle group after 1 h of repeated fast passive stretches. J Appl Physiol. 2004;96(6):2325–32. doi: 10.1152/japplphysiol.01010.2003. [DOI] [PubMed] [Google Scholar]
- 11.Guissard N, Duchateau J. Effect of static stretch training on neural and mechanical properties of the human plantar-flexor muscles. Muscle Nerve. 2004;29(2):248–55. doi: 10.1002/mus.10549. [DOI] [PubMed] [Google Scholar]
- 12.Weir DE, Tingley J, Elder GC. Acute passive stretching alters the mechanical properties of human plantar flexors and the optimal angle for maximal voluntary contraction. Eur J Appl Physiol. 2005;93(5-6):614–23. doi: 10.1007/s00421-004-1265-4. [DOI] [PubMed] [Google Scholar]
- 13.Budini F, Gallasch E, Christova M, Rafolt D, Rauscher AB, Markus T. One minute plantar flexors'static stretch transiently increases H-reflex excitability and exerts no effect on corticospinal pathways. Exp Physiol. 2017 doi: 10.1113/EP086374. [DOI] [PubMed] [Google Scholar]
- 14.Budini F, Christova M, Gallasch E, Kressnik P, Rafolt D, Tilp M. Transient increase in cortical excitability following static stretching of plantar flexor muscles. Front Physiol. 2018;9:530. doi: 10.3389/fphys.2018.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vujnovich AL, Dawson NJ. The effect of therapeutic muscle stretch on neural processing. J Orthop Sports Phys Ther. 1994;20(3):145–53. doi: 10.2519/jospt.1994.20.3.145. [DOI] [PubMed] [Google Scholar]
- 16.Yapicioglu B, Colakoglu M, Colakoglu Z, Gulluoglu H, Bademkiran F, Ozkaya O. Effects of a dynamic warm-up, static stretching or static stretching with tendon vibration on vertical jump performance and EMG responses. J Hum Kinet. 2013;39(1):49–57. doi: 10.2478/hukin-2013-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Opplert J, Genty J-B, Babault N. Do Stretch Durations Affect Muscle Mechanical and Neurophysiological Properties? Int J Sports Med. 2016;37(09):673–9. doi: 10.1055/s-0042-104934. [DOI] [PubMed] [Google Scholar]
- 18.Hadoush H, Tobimatsu Y, Nagatomi A, Kimura H, Ito Y, Maejima H. Monopolar surface electromyography:a better tool to assess motoneuron excitability upon passive muscle stretching. J Physiol Sci. 2009;59(3):243–7. doi: 10.1007/s12576-009-0027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Neural adaptation to resistance training:changes in evoked V-wave and H-reflex responses. J Appl Physiol. 2002;92(6):2309–18. doi: 10.1152/japplphysiol.01185.2001. [DOI] [PubMed] [Google Scholar]
- 20.Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots:basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91(2):79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 21.Nogueira-Campos AA, de Oliveira LAS, Della-Maggiore V, Esteves PO, de Carvalho Rodrigues E, Vargas CD. Corticospinal excitability preceding the grasping of emotion-laden stimuli. PloS One. 2014;9(4):e94824. doi: 10.1371/journal.pone.0094824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budini F, Tilp M. Changes in H-reflex amplitude to muscle stretch and lengthening in humans. Rev Neurosci. 2016 doi: 10.1515/revneuro-2016-0001. [DOI] [PubMed] [Google Scholar]
- 23.Mark RF, Coquery JM, Paillard J. Autogenetic reflex effects of slow or steady stretch of the calf muscles in man. Exp Brain Res. 1968;6(2):130–45. doi: 10.1007/BF00239167. [DOI] [PubMed] [Google Scholar]
- 24.Misiaszek JE. The H-reflex as a tool in neurophysiology:Its limitations and uses in understanding nervous system function. Muscle Nerve. 2003;28(2):144–60. doi: 10.1002/mus.10372. [DOI] [PubMed] [Google Scholar]
- 25.Burke D. Clinical uses of H reflexes of upper and lower limb muscles. Clin Neurophysiol Pract. 2016;1:9–17. doi: 10.1016/j.cnp.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews BH. Nerve endings in mammalian muscle. J Physiol. 1933;78(1):1. doi: 10.1113/jphysiol.1933.sp002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fromm C, Haase J, Wolf E. Depression of the recurrent inhibition of extensor motoneurons by the action of group II afferents. Brain Res. 1977;120(3):459–68. doi: 10.1016/0006-8993(77)90399-7. [DOI] [PubMed] [Google Scholar]
- 28.Hultborn H, Pierrot-Deseilligny E. Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. J Physiol. 1979;297(1):229–51. doi: 10.1113/jphysiol.1979.sp013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hultborn H, Katz R, Mackel R. Distribution of recurrent inhibition within a motor nucleus. II. Amount of recurrent inhibition in motoneurones to fast and slow units. Acta Physiol. 1988;134(3):363–74. doi: 10.1111/j.1748-1716.1988.tb08502.x. [DOI] [PubMed] [Google Scholar]
- 30.Bawa P, Lemon RN. Recruitment of motor units in response to transcranial magnetic stimulation in man. J Physiol. 1993;471(1):445–64. doi: 10.1113/jphysiol.1993.sp019909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114(2):329–38. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- 32.Lucier GE, Rüegg DC, Wiesendanger M. Responses of neurones in motor cortex and in area 3A to controlled stretches of forelimb muscles in cebus monkeys. J Physiol. 1975;251(3):833–53. doi: 10.1113/jphysiol.1975.sp011125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forner-Cordero A, Steyvers M, Levin O, Alaerts K, Swinnen SP. Changes in corticomotor excitability following prolonged muscle tendon vibration. Behav Brain Res. 2008;190(1):41–9. doi: 10.1016/j.bbr.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Lapole T, Temesi J, Arnal PJ, Gimenez P, Petitjean M, Millet GY. Modulation of soleus corticospinal excitability during Achilles tendon vibration. Exp Brain Res. 2015;233(9):2655–62. doi: 10.1007/s00221-015-4336-3. [DOI] [PubMed] [Google Scholar]
- 35.Rosenbaum D, Hennig EM. The influence of stretching and warm-up exercises on Achilles tendon reflex activity. J Sports Sci. 1995;13(6):481–90. doi: 10.1080/02640419508732265. [DOI] [PubMed] [Google Scholar]
- 36.Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles:a review. Prog Neurobiol. 1993;41(6):705–21. doi: 10.1016/0301-0082(93)90032-n. [DOI] [PubMed] [Google Scholar]
- 37.Romano C, Schieppati M. Reflex excitability of human soleus motoneurones during voluntary shortening or lengthening contractions. J Physiol. 1987;390:271. doi: 10.1113/jphysiol.1987.sp016699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duclay J, Martin A. Evoked H-reflex and V-wave responses during maximal isometric, concentric, and eccentric muscle contraction. J Neurophysiol. 2005;94(5):3555–62. doi: 10.1152/jn.00348.2005. [DOI] [PubMed] [Google Scholar]


