Mitochondrial NADH dehydrogenase 5 (MT-ND5) Asp393Asn missense mutation is established to cause mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS).1,2 We describe a case and family with this mutation and a divergent phenotype that eluded diagnosis. We suggest an expanded nomenclature, mitochondrial cerebellar ataxia, renal failure, neuropathy, and encephalopathy (MCARNE).
Case report
A 60-year-old naturalist had a slowly progressive loss of feeling in his feet with gait ataxia over 10 years with associated memory declines. Renal failure was diagnosed at age 57 years with a creatinine level of 3.5 mg/dL compared with 5 years earlier at 1.4 mg/dL (0.8–1.3 mg/dL). He did not have hypertension, was not a drinker, and had no stroke history. At age 59 years, he underwent living nonrelative donor kidney transplant. He had hearing loss and required hearing aids by age 45 years.
On cognitive evaluation, he scored 32/38 on Kokmen Short Test of Mental Status with deficits in recall and construction with inability to draw a cube or remember 4 objects at 5 minutes. He had a wide-based gait with inability to perform tandem walking or heel to shin testing and required 2 ski poles to walk. He had a mild ataxic dysarthria with past-pointing on finger-to-nose testing. There were reduced ankle reflexes without the Babinski sign. Pin prick, vibration, and proprioception were reduced at the toes. Upper and lower extremity strength was normal.
Brain MRI showed moderate generalized cerebral and severe cerebellar atrophy without strokes (figure). Nerve conduction studies at age 52 years showed absent medial plantar and reduced ulnar and sural sensory amplitudes with large motor units on needle EMG limited to extremity muscles consistent with a length-dependent axonal polyneuropathy. Myopathic units were not seen. Renal ultrasound revealed small highly echogenic renal parenchyma consistent with chronic kidney disease. Renal biopsy (figure) at age 57 years demonstrated focal segmental and global glomerulosclerosis with moderate chronic tubulointerstitial nephropathy. No mitochondrial ultrastructural abnormalities were found on electron microscopy. Serum vitamin E, copper, ceruloplasmin, fasting glucose, vitamin B12, methylmalonic acid, folate, thyroid-stimulating hormone, serum and urine immunofixation, very-long-chain fatty acid levels, antinuclear antibodies, antibodies to extractable nuclear antigens, tissue transglutaminase antibodies, syphilis IgG, RPR, HIV serology, and peripheral acanthocytosis were normal or negative.
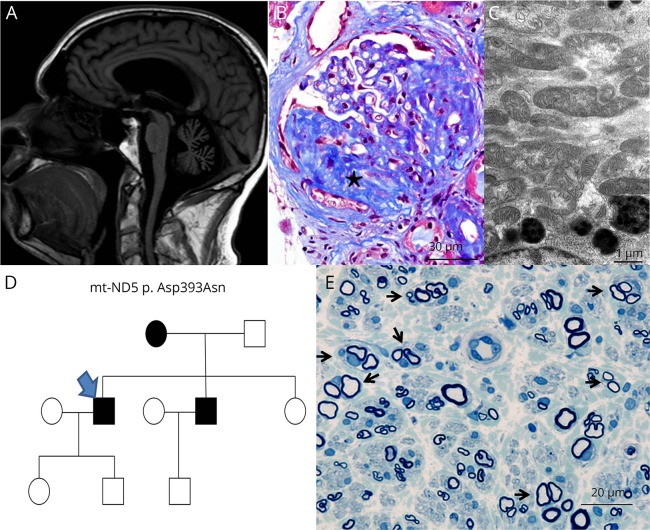
Figure. ND5 and MCARNE phenotype.
(A) Brain MRI sagittal T1 FLAIR showing moderate generalized cerebral and severe cerebellar atrophy without evidence of previous strokes. (B) Kidney biopsy revealed a subset of glomeruli with segmental glomerulosclerosis (marked star) as is seen here at ×400 magnification with Masson trichrome stain. (C) Morphologically, the mitochondria were well within the range of normal variation as is seen in electron micrograph at ×13,000 magnification. (D) Pedigree chart; index case (arrow). (E) Methylene blue epoxy section of the patient's brother's sural nerve biopsy at ×500 magnification showing clusters of regenerating myelinated axons surrounded by concentric Schwann cell processes (arrows) with onion bulb–like formations.
His mother had ataxia and neuropathy. His only brother was diagnosed with “idiopathic neuropathy” at age 50 years with negative evaluations for cause without ataxia or hearing loss. He was considered a possible living kidney donor and excluded when asymptomatic idiopathic nephropathy was found. Creatinine clearance was at 50 mL/min/m2 (normal > 60 mL/min/m2). That brother had undergone a whole sural nerve biopsy, which showed decreased density of myelinated nerve fibers and frequent clusters of regenerating fibers surrounded by concentric Schwann cell processes (figure), similar to other confirmed mitochondrial mutation cases.3 His sister does not have neuropathy or nephropathy at age 72 years. His son, daughter, and brother's son are healthy at age 23, 19, and 27 years, respectively (figure). His sister has no children. Cerebellar ataxia genetic testing for Fragile-X, APTX, SCA1, SCA2, SCA3, SCA5, SCA6, SCA7, SCA8, SCA10, SCA13, SCA14, SCA17, DRPLA, FRDA, AOA1, POLG1, TTPA, SIL1, SETX, and KCNC3 was all unremarkable. Evaluations for acidemia showed a borderline lactate 2.2 mmol/L (normal 0.7–2.1 mmol/L) and pyruvate 0.152 mmol/L (normal 0.03–0.107 mmol/L) level.
Mitochondrial genome testing was ambiguous from lymphocytes with ∼2% of the DNA sequenced, querying MT-ND5 m.13513G>A Asp393Asn. A sequencing error vs mitochondrial heteroplasmy was raised as the possibilities to explain this result by the performing laboratory. Given the very small percentage of lymphocytes with the variant and because earlier patients with MT-ND5 Asp393Asn had not been identified with this phenotype,1–4 uncertainty remained to the diagnosis. However, when mitochondrial genomic analysis of the kidney was performed, 62% of ∼7,000 DNA reads (depth of coverage) had MT-ND5 Asp393Asn mutation. The mutation provided a unifying diagnosis for cerebellar ataxia, renal failure, neuropathy, and encephalopathy. Low-dose coenzyme 10 and carnitine were started, and yearly neurologic and cardiac evaluations were recommended.
Discussion
MT-ND5 mutation can present with the phenotype of MCARNE. This case expands the reported manifestations of MT-ND5 mutations beyond Leigh syndrome,3,4 Leber hereditary optic neuropathy, and MELAS.5 We suspect that other mitochondrial mutations could present similarly, and the MCARNE phenotype will be important to recognize. Mitochondrial ultrastructural kidney tissue abnormalities are absent, and tubulointerstitial nephropathy can be reported as occurred in our case, and glomerular and cystic kidney changes were not seen in our patient.6 Also highlighted is the importance of having DNA sequencing from the kidney with a high depth of coverage (×7,000 in our case) so as not to miss mitochondrial mutation heteroplasmy.7
Author contributions
P.S. Ng: concept and design, acquisition of data, MRI images, figure legends, analysis and interpretations, and drafting and revisions of the manuscript. M.V. Pinto: acquisition of data, analysis and interpretations, and drafting and critical revisions of the manuscript. J.L. Neff: pathology images, figure legends, and manuscript revisions. L. Hasadsri: data analysis of DNA sequencing and kindred evaluation, mitochondrial test creation, and critical edits in response to reviewers. E.W. Highsmith: acquisition of data, analysis and interpretations, critical revisions of the manuscript, and study supervision. M.E. Fidler: review of pathology images and critical revisions of the manuscript. R.H. Gavrilova: acquisition of data, analysis and interpretations, and critical revisions of the manuscript. C.J. Klein: concept and design, acquisition of data, analysis and interpretations, drafting and critical revisions of the manuscript, and study supervision.
Study funding
This work was supported by the Mayo Clinic Center for Individualized Medicine.
Disclosure
P.S. Ng and M.V. Pinto report no disclosures. J.L. Neff has received research support from Duke University. L. Hasadsri reports no disclosures. Dr Highsmith is deceased; disclosures are not included for this author. M.E. Fidler reports no disclosures. R.H. Gavrilova has served on the scientific advisory board of Mitochondrial Medicine. C.J. Klein has served on the scientific advisory boards of CMTA Research and Therapeutics; has received funding for travel and/or speaker honoraria from Akcea; and serves on the editorial board of Neurology®. Go to Neurology.org/NG for full disclosures.
References
- 1.Santorelli FM, Tanji K, Kulikova R, et al. Identification of a novel mutation in the mtDNA ND5 gene associated with MELAS. Biochem Biophys Res Commun 1997;238:326–328. [DOI] [PubMed] [Google Scholar]
- 2.Sara S, Jorida C, Jiesheng L, et al. The G13513A mutation in the ND5 gene of mitochondrial DNA as a common cause of MELAS or Leigh syndrome: evidence from 12 cases. Arch Neurol 2008;65:368–372. [DOI] [PubMed] [Google Scholar]
- 3.Vital A, Vital C. Mitochondria and peripheral neuropathies. J Neuropathol Exp Neurol 2012;71:1036–1046. [DOI] [PubMed] [Google Scholar]
- 4.Chol M, Lebon S, Bénit P, et al. The mitochondrial DNA G13513A MELAS mutation in the NADH dehydrogenase 5 gene is a frequent cause of Leigh-like syndrome with isolated complex I deficiency. J Med Genet 2003;40:188–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pulkes T, Eunson L, Patterson V, et al. The mitochondrial DNA G13513A transition in ND5 is associated with a LHON/MELAS overlap syndrome and may be a frequent cause of MELAS. Ann Neurol 1999;46:916–919. [DOI] [PubMed] [Google Scholar]
- 6.Seidowsky A, Hoffmann M, Glowacki F, et al. Renal involvement in MELAS syndrome—a series of 5 cases and review of the literature. Clin Nephrol 2013;80:456–463. [DOI] [PubMed] [Google Scholar]
- 7.Ye F, Samuels DC, Clark T, Guo Y. High-throughput sequencing in mitochondrial DNA research. Mitochondrion 2014;17:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]