Abstract
Aims
MicroRNAs (miRNAs), small non-coding RNAs, have been implicated as regulators of multiple phases of atherothrombosis, and some reports have suggested altered levels in coronary artery in-stent restenosis (ISR). We recently demonstrated that miR-93-5 p was able to discriminate between patients with stable coronary artery disease (CAD) and those with no CAD, after adjusting for traditional risk factors (RFs). Thus, we wanted to determine if circulating miRNAs could predict coronary ISR.
Objective
To determine if circulating miRNAs have diagnostic capability for determining ISR in a cohort of matched patients with and without ISR.
Approach and results
To determine if miRNA plasma levels are elevated in coronary ISR, we conducted a study comprising 78 patients (39 with no ISR and 39 with ISR) and measured plasma miRNAs in each. We then determined the predictive ability of differential miRNAs, adjusting for Framingham Heart Study (FHS) RFs, and stent length and diameter, to discriminate between ISR and no ISR. After correction for multiple testing, two miRNAs—miR425-5p and miR-93-5 p—were differential between patients with ISR and patients without ISR. Only miR-93-5 p remained a strong independent predictor of ISR after correction for FHS RFs (OR 6.30, p=0.008) and FHS RFs plus stent length and diameter (OR 4.80, p=0.02) and improved discriminatory power for ISR over FHS RFs alone in receiver operator characteristic curve analysis.
Conclusion
This novel finding that miR-93-5 p independently predicts ISR extends our recent observation that miR-93-5 p predicted CAD after adjustment for traditional CAD RFs. These data suggest further potential diagnostic utility.
Keywords: MicroRNA, coronary stent, restenosis
Key messages.
What is already known about this subject?
Coronary artery stent restenosis remains the most common complication after coronary stent implantation. There are currently no reliable non-invasive biomarkers for coronary artery instent restenosis.
What does this study add?
This study extends our recent finding showing miR-93-5 p to be a circulating biomarker for coronary atherosclerosis by revealing it is also a robust biomarker of coronary instent restenosis.
How might this impact on clinical practice?
This study reveals the potential of miR-93-5 p to guide further investigation and treatment of coronary instent restenosis by leveraging its diagnostic potential to prioritise those more likely to have this complication.
Introduction
In-stent restenosis (ISR) remains the most common complication after coronary stent implantation, occurring in 7%–13% of patients receiving drug-eluting stents (DESs).1 Unfortunately, there is currently no clinical blood test to detect ISR.
MicroRNAs (miRNAs) are small non-coding RNAs that are key regulators of many cellular events in the pathogenesis of atherothrombosis.2–5 Recently, it has been demonstrated that miRNAs are highly expressed in vascular tissues with regulatory roles in vascular dysfunction, ischaemic angiogenesis, reendothelialisation and vascular restenosis by modulating expression of target genes and thereby regulating key cellular events.6–8
Furthermore, many miRNAs have been reported to be differentially expressed among patients with coronary artery disease (CAD) compared with controls, for example, miR-146a, miR-133a and miR-208a.9–11 MiR-93 was previously reported to be increased in patients with CAD,12 and another study reported it to be the most consistently changed miRNA in patients with peripheral arterial disease and to be a potent modulator of cell proliferation and perfusion post hind limb ischaemia in a mouse model.13
It has been suggested that plasma miRNAs are potential biomarkers for ISR6; however, previous studies did not adjust for traditional risk factors (RFs) while assessing miRNAs in this context.6 Currently, the most common modality used for diagnosis of ISR is coronary angiography, which is invasive. Another frequent modality is CT coronary angiography, which exposes patients to significant radiation and contrast agents. A non-invasive blood biomarker of ISR is therefore highly desirable.
Aim
To determine the diagnostic potential of circulating miRNAs in ISR.
Objective
To determine if circulating miRNAs can add diagnostic utility to established ISR RFs in patients with ISR and matched patients without ISR.
Materials and methods
Study subjects
This study was performed according to the Ethical Principles of the Declaration of Helsinki. Patients presenting to the Mater Misericordiae University Hospital, Dublin, who met the inclusion criteria and provided informed consent, were enrolled in consecutive series in the study. ISR and non-ISR groups were derived from patients undergoing elective coronary angiography, not having presented with myocardial infarction or unstable angina. Recruitment criteria were established as per the STARD 2015 guidelines.14 All patients undergoing elective coronary angiography who had prior coronary stents meeting eligibility criteria were flagged as potential candidates for admission to the study. ISR was defined as greater than 50% stenosis within the stented segment by quantitative coronary analysis.15 16 Symptoms suggestive of ischaemia included central crushing chest pain radiating to the arm or jaw, increased by exercise and relieved by rest or sublingual glyceryl trinitrate. Abnormal exercise treadmill testing consisted of ST-depression on ECG during exercise, accompanied by chest discomfort. Angiography was performed when these findings were present (classical symptoms or positive exercise treadmill testing), and symptomatology was ascribed to ISR when the ISR was present in the absence of other, confounding significant coronary artery stenosis. The ‘no ISR’ group consisted of patients without ISR and no other significant epicardial coronary artery disease; the cause of their symptoms was unclear. Fully informed consent was obtained from all patients. All patients with a diagnosis of malignancy were excluded from the study. For each case of ISR, a control subject without ISR matched for age and diabetes was chosen.
Plasma sample collection
On gaining arterial access for angiography, blood draws were taken into EDTA tubes (Becton Dickinson, USA), before administration of intravenous heparin, verapamil or glyceryl trinitrate, in order to avoid interaction with miRNA expression. The blood draw was immediately centrifuged and the plasma aspirated and stored at −80°C.
Measurement of miRNA levels
All samples were sent on dry ice to Exiqon (Skelstedet, Denmark) for miRNA analysis and miRNAs measured as previously described,17–20 using a miRCURY LNATM Universal RT microRNA PCR array that was customised to contain 23 microRNAs previously reported to play a role in cardiovascular disease.17 Briefly, all microRNAs were polyadenylated and reverse transcribed into cDNA in a single reaction step. cDNA and Exilent SYBR Green mastermix were transferred to qPCR panels preloaded with primers, using a pipetting robot. Amplification was performed in a Roche Lightcycler 480. All data were normalised to correct for potential overall differences between the samples.
Statistical analysis
Data were analysed using the the ‘gplots’, ‘devtools’, ‘stats’, ‘pROC’ and ‘OptimalCutpoints’ packages of R statistical software and Prism Graphpad V.6. Shapiro-Wilk tests were used to ensure data were normally distributed. The miRNA data was log transformed and again tested to confirm normal distribution. Student’s t-tests and analysis of variance were used to determine statistical differences when the data were normally distributed. Wilcoxon tests were used to detect statistical differences when the data were not normally distributed. Multivariate logistic regression models were constructed with stepwise addition of FHS RFs (age, gender, smoking history, lipids, diabetes mellitus, hypertension and history of hypercholesterolaemia,21 stent and diameter (known predictors of ISR)) for each miRNA. Benjamini-Hochberg tests were used to account for multiple testing, with p<0.05 as significance level. Receiver operator characteristic curves (ROCs) were performed for: (a) FHS RF+stent characteristics (length and diameter); (b) miR-93-5p+covariates in (a).
We performed a power calculation to estimate sample size needed to determine a statistically significant difference. We based this on previously reported miRNAs differential in ISR (miR-143 and miR-145), where the effect size was 1.5–56. Therefore, we used the conservative effect size of 1.5 to calculate our necessary sample size. Using an alpha level of 0.05, we determined that a sample size of 11 patients per group was necessary to determine a significant difference between groups at 90% power. Due to uncertainty of the size effect and to allow further analysis in multivariate models, we recruited 39 patients per group. Power calculations were performed using G*Power V.3.1.22
Results
Table 1 outlines the patient characteristics. The groups were matched for age and diabetes status. The ISR group did not significantly differ from the non-ISR group in any of the patient characteristics or RFs, except for percentage of current smokers, who numbered more in the ISR group. We adjusted for smoking, along with the other traditional RFs, in multivariate analysis. Mean duration from stent implantation to diagnosis of ISR/non-ISR by coronary angiography was 4.9 years.
Table 1.
Patient characteristics
| Characteristic | No ISR (n=39) | ISR (n=39) | P value |
| Age (years), mean±SD | 67±8 | 67±10 | 0.99 |
| Male gender, n (%) | 28 (72) | 30 (77) | 0.38 |
| BMI (kg/m2), mean±SD | 27.8±2.8 | 27.8±3.6 | 0.99 |
| Coronary risk factors | |||
| Hypertension, n (%) | 23 (59) | 21 (54) | 0.47 |
| Family history of CAD, n (%) | 24 (62) | 27 (69) | 0.93 |
| Hx of hypercholesterolaemia, n (%) | 26 (67) | 26 (67) | 0.99 |
| Current smoker, n (%) | 2 (5) | 3 (8) | 0.99 |
| Past smoker, n (%) | 14 (36) | 21 (54) | 0.008 |
| Diabetes mellitus, n (%) | 10 (26) | 10 (26) | 0.99 |
| Medications | |||
| Statins, n (%) | 39 (100) | 36 (92) | 0.08 |
| Stent details | |||
| Stent length, mm, mean±SD | 39.3±42.4 | 48.1±37.6 | 0.35 |
| Stent diameter, mm, mean±SD | 3.04±0.4 | 2.9±0.4 | 0.17 |
| Total stents, n | 41 | 42 | 0.87 |
| DES, n (%) | 35 (90) | 35 (83) | 1.0 |
| BMS, n (%) | 6 (15) | 7 (17) | 0.76 |
Note: two patients had both DES and BMS.
Note: patients were matched for age and diabetes status.
BMI, body mass index; BMS, bare metal stents; CAD, coronary artery disease; DES, drug-eluting stents.
After Benjamini-Hochberg correction for false discovery rate at α=0.05 (figure 1A), miR425-5p and miR-93-5 p levels were significant higher in the ISR group compared with the non-ISR group. The normalised expression levels (ISR vs NISR) of miR425-5p were 0.39±0.47 versus 0.16±0.52, respectively, p=0.04 and for miR-93-5 p were 1.3±0.44 versus 1.0±0.38, respectively, p=0.003 (see figure 1A).
Figure 1.
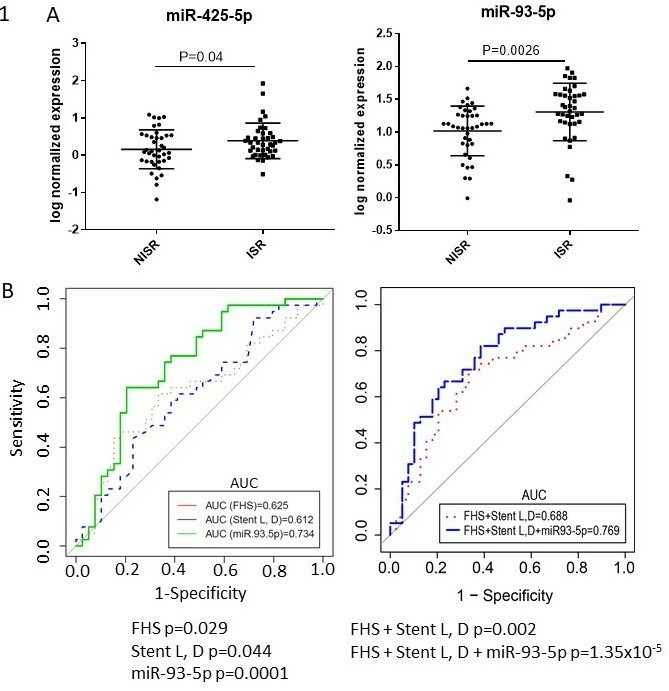
(A) MiR425-5p (left panel) and miR-93-5p (right panel) significantly increased in in-stent restenosis (ISR) compared with non-ISR. Mean and SD with jitter (dot) overlay of normalised microRNA (miRNA) expression level (y axis) versus group (x axis). Each dot represents a miRNA level for an individual patient. (B) Left panel: receiver operator characteristic curves (ROCs) of Framingham Heart Study (FHS) risk factors (dotted red line) versus stent length and diameter (dotted blue line) versus miR-93-5p (green line) revealed miR-93-5p had the best performance, C-statistic 0.73, p=0.0001. Right panel: ROCs of FHS risk factors+stent length (L) and diameter (D) (dotted red line) and FHS+stent L, D+miR-93-5 p (blue line), showing superior discriminatory ability of FHS risk factors and stent length and diameter to detect ISR when combined with miR-93-5p. Stepped lines depict sensitivity (%) as a function of 1−specificity (%). The straight diagonal black line depicts the identity line.
Known RFs for ISR include diabetes,23 which we had already matched for, and stent length and diameter.24 Although traditional cardiovascular RFs are not specifically associated with ISR per se, they are important drivers of overall coronary artery plaque development. To determine if miRNA expression levels were independently differential in ISR versus non-ISR groups, we performed stepwise logistic regression incorporating Framingham Heart Study (FHS) RFs and stent length and diameter (known predictors of ISR). Only miR-93-5p remained an independent predictor of ISR after stepwise addition of all FHS RFs (OR 6.30, p=0.008). After further adjustment for stent length and diameter, miR-93-5p remained an independent predictor of ISR (OR 4.80, p=0.02). To determine if miR-93-5p could be used to discriminate between ISR and non-ISR, we performed ROC analysis. The discriminatory ability of FHS RFs versus stent length and diameter versus miR-93-5p revealed miR-93-5p had the best performance, C-statistic 0.73, p=0.0001 (figure 1B left panel). The discriminatory ability of FHS RFs combined with stent length and diameter to detect ISR (area under the curve (AUC)=0.69, p=0.002) was enhanced by addition of miR-93-5p to the model (AUC=0.77, p=1.33E–5) (figure 1B right panel).
Discussion
We describe for the first time the differential expression of miR-93-5p between patients with ISR and patients without ISR and found that miR-935p was a strong independent predictor of ISR. The study has limitations: although prospectively designed, it is a case–control study and potentially subject to confounding. To minimise this risk, we recruited a larger sample size, matched for diabetes and adjusted for known predictors of ISR and also traditional Framingham RFs. Another limitation is the lack of validation in an independent cohort. However, we previously demonstrated a role for miR-93-5p in CAD where we reported it was the strongest independent predictor of CAD in a cohort of 100 patients.17 Only one other study has reported a relationship between miR-93 and CAD12: He et al reported a positive association of serum miR-93 with CAD, a positive correlation between miR-93 and serum cholesterol and a negative association with ABCA1, in a Chinese cohort of 35 cases.12 The CAD and control groups in this study had significant differences in diabetes, hypertension and lipid levels, which were not adjusted for. They hypothesised that miR-93 repressed ABCA1 expression by directly targeting 3′ UTR, resulting in increased cholesterol levels leading to increased coronary atherosclerosis.12 Our subsequent study,17 adjusted for traditional RFs, further established and strengthened the link between miR-93-5p and CAD.
Recent work has identified miR-21, miR-100, miR-143 and miR-145 to be differentially expressed in ISR.6 There were some important differences with our study. For example, the ethnicity of the cohorts was different (ours was Caucasian, He et al was Chinese). Importantly, the control group in He et al consisted of patients without any coronary stents and without any coronary artery disease—this is a critical difference, and their comparison conceivably would enrich for miRNA markers of coronary artery disease (which miR-21, miR-100, miR-143, and miR-145 are), rather than ISR per se.
Extending this finding, our novel data reported herein linking miR-93-5p to ISR add to our, and others’, previous reports of a relationship between miR-93-5 p and CAD.12 17 Our results suggest that miR-93-5p has further cardiac biomarker potential as a predictor of ISR, and implies an miR-93-5p-related mechanism common to development of both coronary atherosclerosis and coronary ISR, which needs to be further explored.
While other miRs have been reported to play a role in ISR, for example, miR-145, miR-146, miR-221/222, the potential of these miRNAs as therapeutic agents in ISR remains to be seen.25 26 However, miR-21 knockout mice did have reduced ISR, and consequently, targeting miR-21 may be a promising target for treatment of ISR.26
Footnotes
JFO and AN contributed equally.
Contributors: JOS and GJB conceived and designed the study, obtained ethics approval and wrote the manuscript. AN collected and processed the samples, analysed the images, consented the patients and wrote the manuscript. CM provided critical insight and helped write the manuscript. EFF analysed the data and helped write the manuscript. PY determined appropriate statistical analysis and performed statistical analysis.
Funding: This work was supported by a University College Dublin grant awarded to GJB.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: All studies were approved by the Institutional Review Boards of the Mater Misericordiae University Hospitals
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Stettler C, Wandel S, Allemann S, et al. . Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 2007;370:937–48. 10.1016/S0140-6736(07)61444-5 [DOI] [PubMed] [Google Scholar]
- 2. O'Sullivan JF, Martin K, Caplice NM. Microribonucleic acids for prevention of plaque rupture and in-stent restenosis: "a finger in the dam". J Am Coll Cardiol 2011;57:383–9. 10.1016/j.jacc.2010.09.029 [DOI] [PubMed] [Google Scholar]
- 3. O'Sullivan JF, Neylon A, McGorrian C, et al. . MicroRNA expression in coronary artery disease. Microrna 2014;2:205–11. 10.2174/22115366113026660018 [DOI] [PubMed] [Google Scholar]
- 4. Elia L, Quintavalle M, Zhang J, et al. . The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ 2009;16:1590–8. 10.1038/cdd.2009.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lovren F, Pan Y, Quan A, et al. . MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation 2012;126(11 Suppl 1):S81–S90. 10.1161/CIRCULATIONAHA.111.084186 [DOI] [PubMed] [Google Scholar]
- 6. He M, Gong Y, Shi J, et al. . Plasma microRNAs as potential noninvasive biomarkers for in-stent restenosis. PLoS One 2014;9:e112043 10.1371/journal.pone.0112043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ren J, Zhang J, Xu N, et al. . Signature of circulating microRNAs as potential biomarkers in vulnerable coronary artery disease. PLoS One 2013;8:e80738 10.1371/journal.pone.0080738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qin S, Zhang C. MicroRNAs in vascular disease. J Cardiovasc Pharmacol 2011;57:8–12. 10.1097/FJC.0b013e318203759b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fichtlscherer S, De Rosa S, Fox H, et al. . Circulating microRNAs in patients with coronary artery disease. Circ Res 2010;107:677–84. 10.1161/CIRCRESAHA.109.215566 [DOI] [PubMed] [Google Scholar]
- 10. Wang G-K, Zhu J-Q, Zhang J-T, et al. . Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J 2010;31:659–66. 10.1093/eurheartj/ehq013 [DOI] [PubMed] [Google Scholar]
- 11. D'Alessandra Y, Devanna P, Limana F, et al. . Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J 2010;31:2765–73. 10.1093/eurheartj/ehq167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He Y, Lin L, Cao J, et al. . Up-regulated miR-93 contributes to coronary atherosclerosis pathogenesis through targeting ABCA1. Int J Clin Exp Med 2015;8:674–81. [PMC free article] [PubMed] [Google Scholar]
- 13. Hazarika S, Farber CR, Dokun AO, et al. . MicroRNA-93 controls perfusion recovery after hindlimb ischemia by modulating expression of multiple genes in the cell cycle pathway. Circulation 2013;127:1818–28. 10.1161/CIRCULATIONAHA.112.000860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bossuyt PM, Reitsma JB, Bruns DE, et al. . STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351 10.1136/bmj.h5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuntz RE, Baim DS. Defining coronary restenosis. newer clinical and angiographic paradigms. Circulation 1993;88:1310–23. 10.1161/01.CIR.88.3.1310 [DOI] [PubMed] [Google Scholar]
- 16. Alraies MC, Darmoch F, Tummala R, et al. . Diagnosis and management challenges of in-stent restenosis in coronary arteries. World J Cardiol 2017;9:640–51. 10.4330/wjc.v9.i8.640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O Sullivan JF, Neylon A, McGorrian C, et al. . miRNA-93-5p and other miRNAs as predictors of coronary artery disease and STEMI. Int J Cardiol 2016;224:310–6. 10.1016/j.ijcard.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 18. Acharya SS, Fendler W, Watson J, et al. . Serum microRNAs are early indicators of survival after radiation-induced hematopoietic injury. Sci Transl Med 2015;7:287ra69 10.1126/scitranslmed.aaa6593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eulalio A, Mano M, Dal Ferro M, et al. . Functional screening identifies miRNAs inducing cardiac regeneration. Nature 2012;492:376–81. 10.1038/nature11739 [DOI] [PubMed] [Google Scholar]
- 20. Loo JM, Scherl A, Nguyen A, et al. . Extracellular metabolic energetics can promote cancer progression. Cell 2015;160:393–406. 10.1016/j.cell.2014.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D'Agostino RB, Vasan RS, Pencina MJ, et al. . General cardiovascular risk profile for use in primary care: the Framingham Heart study. Circulation 2008;117:743–53. 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 22. Faul F, Erdfelder E, Buchner A, et al. . Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–60. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- 23. Cho JY. Identification of risk factors influencing in-stent restenosis with acute coronary syndrome presentation. Chonnam Med J 2017;53:203–10. 10.4068/cmj.2017.53.3.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stolker JM, Kennedy KF, Lindsey JB, et al. . Predicting restenosis of drug-eluting stents placed in real-world clinical practice: derivation and validation of a risk model from the event registry. Circ Cardiovasc Interv 2010;3:327–34. 10.1161/CIRCINTERVENTIONS.110.946939 [DOI] [PubMed] [Google Scholar]
- 25. Gareri C, De Rosa S, Indolfi C. MicroRNAs for restenosis and thrombosis after vascular injury. Circ Res 2016;118:1170–84. 10.1161/CIRCRESAHA.115.308237 [DOI] [PubMed] [Google Scholar]
- 26. McDonald RA, Halliday CA, Miller AM, et al. . Reducing in-stent restenosis: therapeutic manipulation of miRNA in vascular remodeling and inflammation. J Am Coll Cardiol 2015;65:2314–27. 10.1016/j.jacc.2015.03.549 [DOI] [PMC free article] [PubMed] [Google Scholar]


