Abstract
Squamous cell carcinoma (SCC) of the skin is a keratinocyte malignancy characterized by tumors presenting on sun-exposed areas with surgery being the mainstay treatment. Despite advances in targeted therapy in other skin cancers, such as basal cell carcinoma and melanoma, there have been no such advances in the treatment of SCC. This is partly due to an incomplete knowledge of the pathogenesis of SCC. We have recently identified a protein kinase C-associated kinase (PKK) as a potential tumor suppressor in SCC. We now describe a novel conditional PKK knockout mouse model, which demonstrates that PKK deficiency promotes SCC formation during chemically induced tumorigenesis. Our results further support that PKK functions as a tumor suppressor in skin keratinocytes and is important in the pathogenesis of SCC of the skin. We further define the interactions of keratinocyte PKK with TP63 and NF-κB signaling, highlighting the importance of this protein as a tumor suppressor in SCC development.
The protein kinase C-associated kinase (PKK) is a novel tumor suppressor in skin keratinocytes. Keratinocyte-specific deletion of PKK leads to increased proliferation in keratinocytes and tumor growth in a mouse model of squamous cell carcinoma of the skin in humans.
Introduction
Protein kinase C-associated kinase (PKK), also known as the receptor-interacting protein kinase 4 (RIP4), is a serine threonine kinase (1,2). RIP kinases share a conserved N-terminal kinase domain but have different C-terminal protein:protein interaction motifs. PKK has C-terminal ankyrin repeats similar to RIP5 (3). RIP kinases are widely expressed and function in signaling activated by cell stressors: RIP kinases have a crucial role in both signaling to the NF-κB family of transcription factors and the induction of programmed cell death (4–6). In contrast, PKK has been found to have unique functions in the epidermis. Genetic deletion of PKK in mice results in neonatal lethality secondary to defects in keratinized stratified epithelium that includes anal, oral and esophageal atresia (7). PKK knockout mice had thickened epidermis with upregulation of K14 expression in the granular layer and increased expression of K1 in the thickened epidermis. Filaggrin, loricrin and involucrin were aberrantly expressed in the spinous and granular layers, suggesting keratinocyte terminal differentiation is delayed (7). Consistent with this important role in epidermal development, mutations in PKK cause Bartsocas–Papas syndrome, a disorder defined by multiple skin webs and craniofacial defects (8–10). Mutations in PKK associated with Bartsocas–Papas syndrome have been shown to alter NF-κB signaling (8). In some studies with humans, it has also been suggested that ∆Np63α regulates NF-κB proteins, and PKK and NF-κB subunits are both critical components in controlling epidermal development (8,11). Given the role of PKK in epidermal differentiation and NF-κB and TP63 signaling, we hypothesized that PKK might also regulate keratinocyte transformation. However, neonatal lethality of the PKK knockout mouse has limited our ability to study the function of PKK in vivo.
Squamous cell carcinoma (SCC) is a malignant tumor with an incidence of 2–4% of the European population (12). Multiple risk factors have been identified for SCC, including ultraviolet light exposure, chronic inflammatory disease of the skin, chronic immunosuppression, burns, radiotherapy and decreased skin melanin (12–14). Although most patients who have early diagnosis and surgical excision have a good prognosis, patients with more advanced disease (e.g. tumors >4 cm in diameter) (15) and those with metastatic disease have a 3 years survival of only 33% (15,16). We found that tumor tissue from patients undergoing Mohs surgery contained decreased levels of PKK mRNA compared with normal, adjacent skin (17). Furthermore, depletion of PKK in keratinocytes using RNA interference increased proliferation in vitro, promoting the cell cycle, without affecting the levels of apoptosis (17). In addition, increased tumor size was observed in PKK knockdown keratinocytes in xenotransplantation models (17). It has also been reported that PKK expression is decreased in aggressive SCCs of the tongue (18). PKK expression was also decreased in hepatocellular carcinoma, and overexpression of PKK in transformed fetal hepatocytes led to cessation of anchorage independent growth (19). In contrast, increased PKK expression in ovarian and cervical tumors is thought to promote tumorigenesis (20,21). Thus, the role of PKK in tumorigenesis is probably tissue-specific.
We have found that cutaneous SCC (cSCC) exhibits a comparatively high rate of potentially deleterious mutations in PKK. Furthermore, the rate of PKK mutation in cSCC is higher than that in other tumor types. This suggests that PKK function may be particularly important in the suppression of keratinocyte transformation or cSCC development. Therefore, because germline PKK deletion is lethal, we have developed a strategy allowing selective ablation of PKK in keratinocytes. Using this mouse model, in combination with a well-established two-stage chemical carcinogenesis protocol, we have found that skin tumor development is increased in the absence of keratinocyte PKK. Furthermore, loss of PKK results in decreased canonical NF-κB signaling and increased nuclear TP63. The feedback loop between TP63 and NF-κB is complex with each member regulating the apoptotic activities of the other (22). This study provides genetic evidence to support the idea that PKK functions as a tumor suppressor in cSCC.
Materials and methods
Reverse transcription–polymerase chain reaction
Due to unavailability of commercial, efficient anti-mouse PKK antibody, reverse transcription–polymerase chain reaction (RT–PCR) was performed for PKK expression.
After TRIzol (Invitrogen, CA) extraction of total keratinocyte RNAs, quantitative RT-PCR was performed as previously described (51). Template cDNAs was generated using using oligo (dT) and superscript II reverse transcriptase (Invitrogen). The sequences for real-time quantitative RT-PCR primers used were PKK 5-(Forward) CGACAGGGAACGAATGGAGC, 5’-(Reverse) AGACATGCAATGCAGGAAGTT; Keratin 1 5’-(Forward) ATT TCT GAG CTG AAT CGT GTG ATC, 5’-(Reverse) CTT GGC ATC CTT GAG GGC ATT; Keratin 10 5’-(Forward) TGA TGT GAA TGT GGA AAT GAA TGC, 5’ (Reverse) GTA GTC AGT TCC TTG CTC TTT TCA; Involucrin 5’-(Forward) GGG TGG TTA TTT ATG TTT GGG TGG, 5’(Reverse)-GCC AGG TCC AAG ACA TTC AAC; GAPDH 5’-(Forward) GAA ATC CCA TCA CCA TCT TCC AGG, 5’-(Reverse) GAG CCC CAG CCT TCT CCA.
Quantitative real-time quantitative RT-PCR was performed using an ABI System Detector 7300 (Applied Biosystems, Foster City, CA) under the following thermocycler conditions: stage 1, 95°C for 30 s, 1 cycle; stage 2: 95°C for 5 s and 58°C for 31 s, 40 cycles. GAPDH was used as an internal control for the expression levels of target genes. Relative mRNA levels were calculated in terms of the average cycle number of PCR amplification (CT value) for the target gene, where Δ = CT (PKK gene sample) − CT (GAPDH sample). The formula: 2-ΔΔCt was used to calculate relative expression fold in each sample relative to the control, which was set as 1.
Conditional PKK knockout mouse
PKKfl/fl mouse has previously been described (27). A targeting construct was generated in which exon 2 of the PKK gene was flanked by loxP sites. The knockout mice were genotyped by genomic PCR with primers: For: TGGTAGAGATGATTCCACCCATTTG and Rev: CTAACTATCCAAGGGCCGTCACTCT. For detection of PKK deletion, the primers: For 5’-GGGTCTAGTGGGTGGTTAATGGGTA and Rev 5’-CCAAAGCCATACAACCTGGTACAAA were used. To generate PKKEKO, PKKfl/fl mice were mated with the Keratin14-ER-Cre transgenic mice (Jackson Laboratory, Bar Harbor, ME. Cat. #005107). For deletion, (PKKEKO) mice were treated twice with tamoxifen [intraperitoneal (IP) injection]. PKK deletion was assessed using curetted back skin and PCR as above. Mice were housed in the University of Rochester and Rochester General Hospital Research Institute in compliance with Institutional Animal Care and Use Committee guidelines at each institution (Protocol #2015-004, RGH IACUC).
Epidermal thickness
Three different locations on two representative mice from a group of 20 or more were measured using a micrometer. The average thickness in microns was calculated and presented in the bar graph. Standard error of means is shown.
Immunohistochemistry and immunofluorescence
Immunofluorescence studies were performed as described previously (49). Four micrometer sections of formalin-fixed and paraffin-embedded tumor samples were used for IF and H&E staining. Following deparaffinization and rehydration, the sections were antigen retrieval with 0.01 M citrate buffer (pH 6.0) for 20 min. The primary antibodies such as cytokeratin 5 (1:250 dilution), cytokeratin 10 (1:200 dilution), cytokeratin 14 (1:250 dilution), involucrin (1:300 dilution) and cyclin D antibody (1:300), from Santa Cruz Biotechnology, then were added and the slides were incubated for overnight. After wash with PBS for three times (each for 5 min), the sections were incubated with secondary antibodies (Alexa Fluor 568-contugated or Alexa Fluor 488-contugated IgG, Invitrogen. 1:400 dilution) for 1 h. After the sections were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) for 5 min, the cover slips were mounted and imaged.
Cultured cells were plated on cover slips for 24 h and then fixed with cold 4% paraformaldehyde. Keratin 1/10 antibody (LH1, sc-53251), keratin 5 antibody (RCK-103, sc-32721), keratin 14 antibody (C-14, sc-17104), keratin 17 antibody (H-41, sc-366511), involucrin (H-20,sc-28557), phospho-p65 antibody (Cell Signaling #8214), p63 antibody (H-137, sc-8343), Ki-67 antibody (Cell Signaling, #12075), p65 antibody (Cell Signaling, #8242), cyclin D (M-20,sc-718) and staining were performed with a standard IF stain protocol. Nuclei were counterstained with DAPI. All studies were repeated on at least two samples of any piece of tissue and confirmed on at least two different samples (e.g. different cell culture plates and different mice).
Western blots and immunofluorescence
Epidermis of back skin from the control and PKK mutant mice treated with 12-O-tetradecanoylphorbol 13-acetate (TPA) for different time points or treated with dimethylbenz(a)anthracene (DMBA)/TPA for 13 weeks were used for western blot analysis: P21 (C-19, Santa Cruz), cdk4 (Santa Cruz, c-22), p63 antibody (4A4, sc-8431), IkBα antibody (Abcam, E130, ab32518), p-P65 antibody (Cell Signaling, #8214) and c-Rel (Santa Cruz, sc-70). GAPDH (Santa Cruz, G-9) was used as a loading control. Nuclei or whole cell extracts were isolated and evaluated by western blotting as described previously (52).
Retrovirus constructs and transduction
Knockdown was assessed by both RT-PCR and western blotting of PKK. Keratinocytes derived from SCCs of mice or immortalized cell lines (HaCaT) were grown in keratinocyte growth medium without calcium and transduced with retroviral vectors exactly as described previously (17). HaCaT cells were provided by Lisa Buck. The histology of the HaCaT were closely monitored to ensure that there was no contamination of the cell cultures with other cell types. Short tandem repeat profiling is performed every few years (or sooner if necessary) to authenticate the cell lines in our laboratory. This utilizes PowerPlex 18D System by Promega. The cells were last tested in the past year. Targeting sequences: shPKK-1, 5′-GGCCCACCTTCCAAGAAATTA; shPKK-2, 5′- CGTTCGTTTCTCGTTGCCTAA; and control sequence, 5′-GTTCTCCGAACGTGTCACG. Calcium was added to a final concentration of 1.2 mM to induce differentiation of HaCat cells. Cells were treated for 24 h and then harvested for immunohistochemistry and quantitative PCR.
Chemical carcinogenesis
Experiments were performed as described previously (51). In brief, the backs of 6-week-old mice were shaved and, after 1 week, mice, which were observed to have a resting hair cycle, were treated with 25 μg 7,12-dimethyl-1,2-benzanthracene (DMBA; Sigma, St. Louis, MO) dissolved in 100 microliters of acetone. One week after initiation, mice were treated topically with 10 μg phorbol ester (TPA; Sigma) twice a week. Papilloma development was recorded biweekly. Numbers of papilloma were compared using standard of means analysis. The data were examined by a Fisher’s exact test. The data present represent eight PKKEKO and seven control mice. One of the controls died early due to anesthesia toxicity. It had developed no more papilloma at that point in time than mice in both groups. The white CD-1 (Tg(KRT14-cre/Esr1) was from a single founder mouse and backcrossed to the black C57BL/6 (C57BL6;129F10-PKKtm1) founder mouse.
Keratinocyte proliferation assays from SCC
This assay was adapted as described previously (32,53). Primary keratinocytes were cultured after isolation by dispase treatment of shaved mouse skin. Cells were maintained in Ca2+-free keratinocyte medium (Lonza, CC-3112) at 32°C in a 5% CO2 incubator. When cultures reached at least 75% confluence, they were replated into 96 well plates at 2 × 104 cells per well. The MTT assay was performed as described previously. Cells were treated at different concentrations with DMBA for 2 days. MTT reagent was added to each well followed by incubation for 2 h. Then, DMSO was added to each well. Spectrophotometric absorbance of each sample was measured at 540 nm using a microplate reader (Bio-Rad, Hercules, CA). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and DMBA were purchased from Sigma Chemical Co. (St. Louis, MO).
DNA sequencing for H-ras mutation
Total genomic DNA was isolated from 40 μm sections of formalin-fixed and paraffin-embedded tumor samples using a genomic DNA isolation kit (Catalog number AM1975, Invitrogen). Standard PCR reactions to identify mutation at codon 61 of H-ras were performed using mouse H-ras specific primers: P1for51 ctaagccgtgttgttttgcagg, P2rev5’ cacctatggctagcccgtgag, P3for51ctcctaccggaaacaggtggt and P4rev cttcgaggacatccatcagtac. The purified PCR product was directly sequenced at the core facility at the medical center of the University of Rochester.
Results
Mutation of PKK in human cSCC
We previously reported that PKK expression is decreased in cSCC of humans and that shRNA-mediated depletion of PKK increases tumor growth in xenograft models of cSCC (17). Therefore, we analyzed The Cancer Genome Atlas (TCGA) through the cBIO Portal for Cancer Genomics (23,24). We queried the 145 available datasets (http://www.cbioportal.org/data_sets.jsp) for mutations in PKK (RIPK4). Interestingly, PKK (RIPK4) was most frequently mutated in cSCC, with a mutation frequency that was more than twice that of any other cancer dataset analyzed (Supplementary Figure S1a, available at Carcinogenesis Online). In cSCC, 24% of tumors had missense mutations in RIPK4 (Supplementary Figure S1b, available at Carcinogenesis Online). Furthermore, most mutations were located within the kinase and ankyrin repeat domains (Supplementary Figure S1c, available at Carcinogenesis Online). Mutations in these domains of PKK have previously been shown to cause loss of function in patients with Bartsocas–Papas syndrome (8). The cSCC data deposited in TCGA (25) are consistent with another analysis of aggressive metastatic cSCC that reported a similar PKK mutation rate with changes clustered within the kinase and ankyrin repeat domains (26). Although the analysis of existing sequencing data suggests that PKK is significantly mutated in cSCC, it remains unclear, especially given the high rate of mutagenesis in cSCC, whether loss of PKK function contributes to keratinocyte transformation or SCC progression.
Creation of the PKK conditional knockout mouse
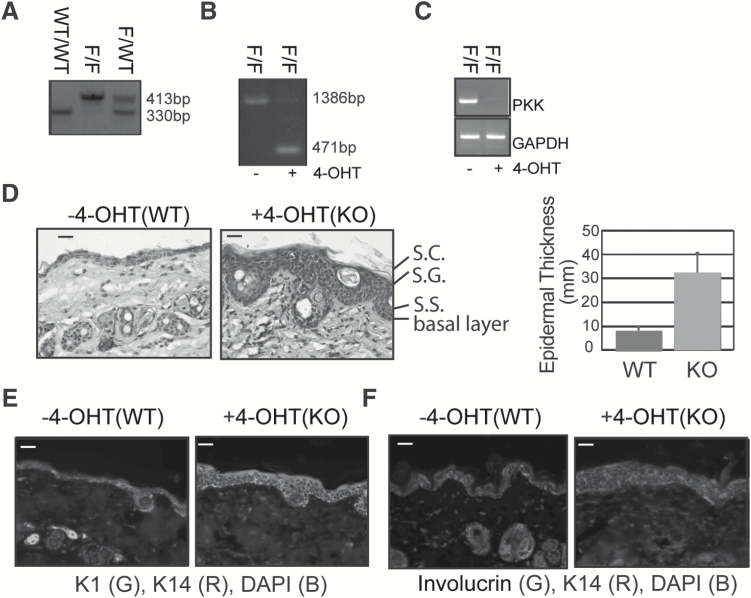
In order to study the role of PKK specifically in the skin, a conditional genetic model was developed. The homozygous PKKfl/fl mouse (27) was bred onto a Keratin-14-ER-Cre transgenic mouse TgKRT14-cre/Esr1 (28), in which the Cre-ER fusion protein is under the control of the keratin 14 (K14) promoter and activated upon administration of 4-OHT or the prodrug tamoxifen. Mice with floxed-PKK alleles were born at normal Mendelian ratios (Figure 1A) and efficient, specific deletion of the PKK locus in epidermis and loss of PKK protein in isolated keratinocytes was achieved by IP treatment with tamoxifen (Figure 1B and C).
Figure 1.
Conditional deletion of PKK in keratinocytes. (A) Genotyping of tail DNA identifies the C57BL6;129F10-PKKtm1 floxed allele (F) as 413 base pairs compared with the wild-type 330 base pair allele. (B) Genomic DNA from C57BL6;129F10-PKKtm1(Tg(KRT14-cre/Esr1) back skin that was either untreated or painted with 4-hydroxytamoxifen (4-OHT). Deletion of PKK is confirmed by a 471 base pair transcript. (C) PCR of PKK and GAPDH from dispase-derived epidermis from C57BL6;129F10-PKKtm1(Tg(KRT14-cre/Esr1) back skin that was either untreated or treated with tamoxifen IP. (D) Hemotoxylin and eosin stained back skin from C57BL6;129F10-PKKtm1(Tg(KRT14-cre/Esr1) treated with tamoxifen or vehicle only. Bar graph quantifying increased epidermal thickness in C57BL6;129F10-PKKtm1(Tg(KRT14-cre/Esr1 back skin compared with control. S.C., stratum corneum; S.G., stratum granulosum; S.S., stratum spinosum. Scale bar is 25 μm. (E) and (F) Immunofluoresence microscopy of C57BL6;129F10-PKKtm1(Tg(KRT14-cre/Esr1) back skin treated with vehicle or tamoxifen and stained against keratinocyte differentiation markers. DAPI is a nuclear counterstain. Scale bar is 50 μm.
Knockout of PKK in keratinocytes leads to increased epidermal thickness but does not affect keratinocyte differentiation
Targeted knockout of PKK in keratinocytes (PKKEKO) by IP injection of tamoxifen (25 mg/kg) twice over 1 week resulted in mice of the same size, with skin that phenotypically appeared normal. However, hematoxylin and eosin (H&E) staining revealed significant epidermal thickening in PKKEKO mice (Figure 1D and E) similar to that in the PKK knockout mouse (7). PKKEKO mouse skin had an expanded basal layer with expression of the basal keratinocyte marker K14 expression extending above the basal layer (Figure 1E and F). Similar results were found with the basal keratinocyte marker Keratin 5 (not shown). However, keratin 1, which is found in suprabasal keratinocytes of the spinous and granular layer, showed a similar expression pattern in PKKEKO and control skin. This was confirmed with H&E analysis showing acanthosis with basal, spinous, granular and stratum corneum layers present in the thickened epidermis (Figure 1E). Involucrin, a late marker of keratinocyte differentiation typically present in the upper granular layer, was similar in the control and in PKKEKO skin (Figure 1F).
Similarly, loss of PKK by siRNA knockdown of PKK in the HaCaT keratinocyte cell line, cultured in calcium-free medium, did not affect differentiation (Supplementary Figure S2, available at Carcinogenesis Online). Expression levels of keratins, 5, 10, 14, or involucrin were similar in normal and PKK-deficient HaCaT keratinocytes. (Supplementary Figure S2a, available at Carcinogenesis Online) mRNA expression levels of differentiation markers after calcium-induced differentiation were also unaffected by PKK knockdown (Supplementary Figure S2b, available at Carcinogenesis Online). These results suggest that keratinocyte intrinsic PKK is not required for suprabasal differentiation, but supports a role for PKK in regulation of keratinocyte proliferation.
Altered signaling and proliferation in PKK deficient epidermis
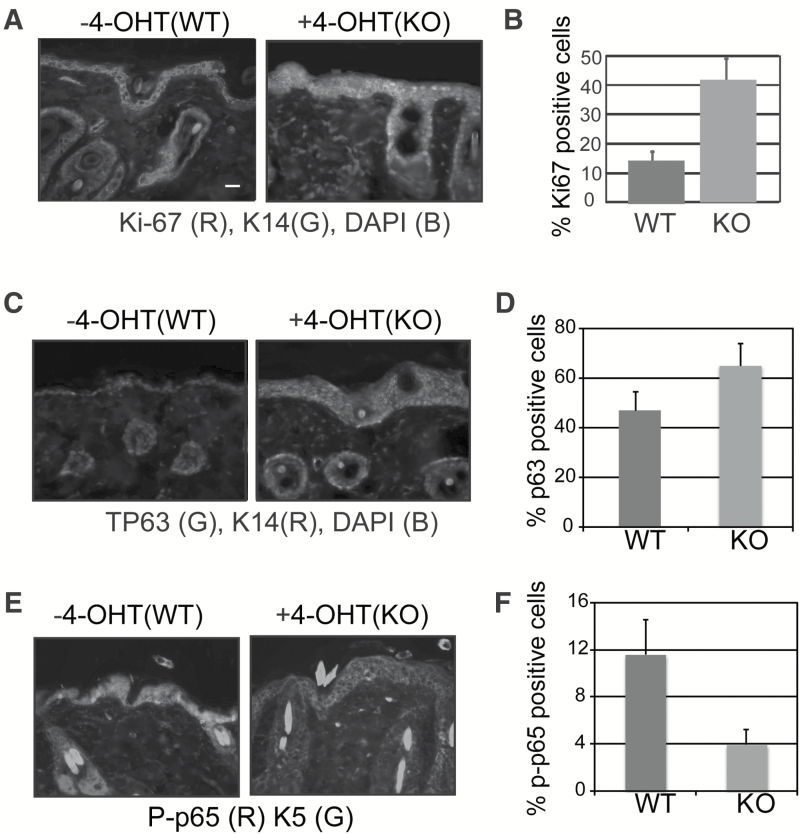
The thickening of the epidermis in mice with conditional deletion of PKK in keratinocytes (Figure 1D) suggests an increase in cell proliferation. Therefore, we sought to confirm the role of PKK in regulating keratinocyte proliferation using the conditional knockout model. To directly examine keratinocyte proliferation, we performed Ki-67 staining in PKKEKO skin. We previously reported that siRNA-mediated suppression of PKK promotes keratinocyte proliferation without altering apoptosis (17). Consistent with these findings, we observed increased Ki-67 staining in the interfolllicular epidermis of PKKEKO skin compared with control skin (Figure 2A and B). Thus, cell intrinsic PKK suppresses proliferation of keratinocytes in intact murine epidermis.
Figure 2.
Deletion of PKK alters epidermal keratinocyte proliferation and signaling. (A) Immunofluoresence microscopy of C57BL6;129F10-PKKtm1(Tg(KRT14-cre/Esr1) back skin treated with vehicle or tamoxifen and stained against the basal keratinocyte differentiation marker keratin 14 and the proliferation marker Ki-67. Scale bar is 25 μm. (B) Ki-67 nuclear staining from three representative areas from WT and PKKEKO mouse skin was calculated. Standard error of means is shown. P ≤ 0.005. (C) and (D) Immunofluoresence microscopy of C57BL6;129F10-PKKtm1(Tg(KRT14-cre/Esr1) back skin treated with vehicle or tamoxifen stained against the basal keratinocyte differentiation marker keratin 5 or 14 and (c) TP63 or (e) phospho-p65. (D) and (F) TP63 and phospho-p65 staining from three representative areas from WT and PKKEKO mouse skin was calculated. Standard error of means is shown. P ≤ 0.01.
We have previously shown that reducing PKK expression with siRNA altered signaling through TP63 and NF-κB and increased keratinocyte proliferation (17). Keratinocyte overexpression of TP63 induces epidermal hyperproliferation (29). Therefore, we examined expression and nuclear localization of TP63 in PKKEKO epidermis. PKK deletion in keratinocytes leads to increased TP63 expression and nuclear localization compared with normal mouse keratinocytes (Figure 2C and D). Consistent with the K14 staining, nuclear TP63 staining was seen in multiple suprabasal layers. We also investigated canonical NF-κB in PKK deficient keratinocytes. siRNA knockdown of PKK in cultured human SCC keratinocytes led to decreased TPA-induced NF-κB activity and decreased IKK activity (17). Staining for phosphorylated p65 showed that PKKEKO skin had decreased total and nuclear p-p65 levels compared with control skin (Figure 2E and F). Therefore, similar to what we have previously shown in vitro, and consistent with the observed hyperplasia (Figure 1D), skin from PKKEKO mice shows increased nuclear TP63 and decreased nuclear phospho-p65. Increased proliferation, decreased canonical NF-κB signaling and increased nuclear TP63 all suggest that keratinocyte tumorigenesis might be increased in PKKEKO mice.
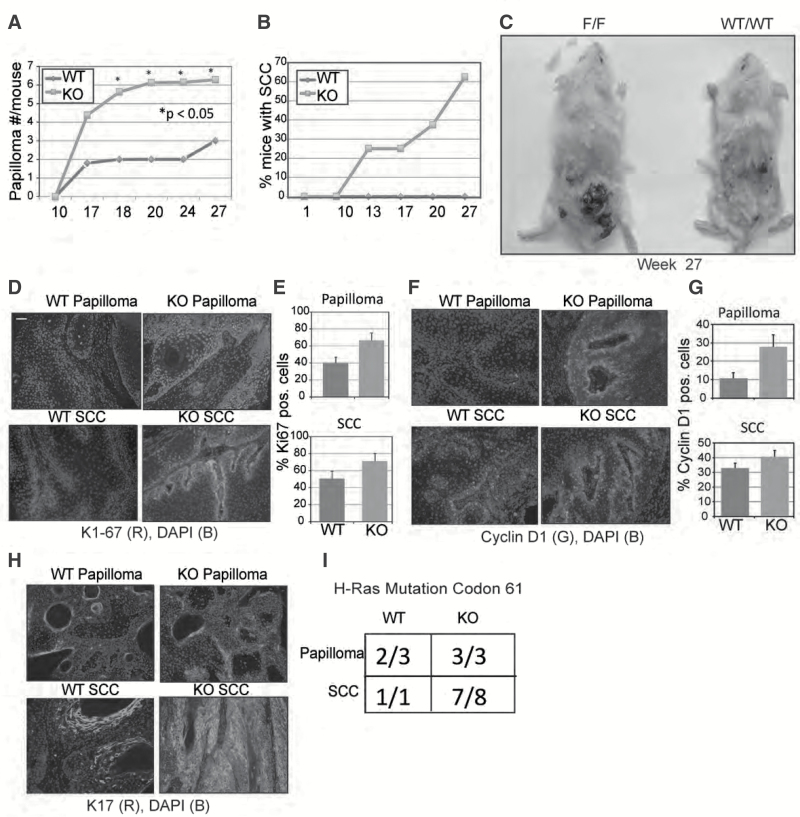
Deletion of PKK in basal keratinocytes promotes tumors after DMBA/TPA induced carcinogenesis
The high rate of PKK mutagenesis in cSCC (25,26) increased tumor growth in PKK-depleted SCC (17) and decreased PKK expression in human cSCC (17); all suggest that PKK may be a bona fide, and perhaps tissue specific, tumor suppressor. Therefore, we examined chemical-induced tumorigenesis in PKKEKO mice. Mice were treated with DMBA once and then TPA twice weekly for 27 weeks. PKKEKO mice showed increased papilloma formation compared with controls (Figure 3A). Over 27 weeks of study, nine squamous cell carcinomas were observed in PKKEKO skin, with tumors in five of eight PKK-deficient mice compared with none in the control group (Figure 3B). The papillomas were grossly larger in the PKKEKO mice (Figure 3C). Even by week 54, only one control mouse had developed SCC. We harvested week 27 papillomas and SCC from PKKEKO mice and week 27 papillomas and week 54 SCC from wild-type mice. Similar to our observations in PKK-deficient epidermis, we found that papillomas and SCC excised from PKKEKO mice showed increased Ki-67 staining (Figure 3D). Counting positive nuclei at 40× magnification in at least three representative areas of each tumor showed that papillomas and SCC in control mice stained positive for Ki-67 in 40% and 50% of counted nuclei, respectively, whereas papillomas and SCCs from PKKEKO mice stain positive for nuclear Ki-67 in 63% and 70% of cells, respectively (Figure 3E).
Figure 3.
Chemical carcinogenesis in the PKK knockout mouse. (A) Chemical carcinogenesis studies with C57BL6;129F10-PKKtm1(Tg(KRT14-cre/Esr1) mice that were either wild-type (non-floxed PKK allele) (WT) or containing the floxed PKK allele and treated with tamoxifen (IP) (KO). Papillomas >3 mm in diameter were counted through 27 weeks of the study. There were eight mice in each group. (B) Squamous cell carcinomas were counted through 27 weeks in the knockout and through 54 weeks in the wild-type. There were no squamous cell carcinomas observed in the wild-type mice at weeks 27 and 9 observed in the knockout. (C) Wild-type compared with knockout mice at week 27 during chemical carcinogenesis. (D) Immunofluorescence microscopy staining of Ki-67 of papillomas and SCC from wild-type and PKKEKO skin. (E) Ki-67 nuclear staining was quantitated by counting at three representative areas of papillomas or SCCs at 40× magnification. (F) Immunofluorescence microscopy staining of cyclin D1 of papillomas and SCC from wild-type and PKKEKO skin. (G) Quantification of cyclin D1 staining was performed by counting at three representative areas of papillomas or SCCs at 40× magnification. (H) Immunofluorescence microscopy staining of keratin 17 of papillomas and SCC from wild-type and knockout mouse skin. (i) Mutational analysis of Hras gene at codon 61 of papilloma and SCC from wild-type or knockout keratinocytes. Scale bar is 50 μm.
We have previously shown that reducing PKK levels in keratinocytes results in upregulation of cyclin D1 (17). Therefore, we also examined cyclin D1 staining in papillomas and SCC excised from PKKEKO and control mice (Figure 3F). Cyclin D1 expression was increased, especially in mutant papillomas (Figure 3G). A marked increase in the expression of keratin 17 (K17), another marker of keratinocyte proliferation (30), was also observed in PKKEKO SCC and papillomas compared with control (Figure 3H).
DMBA treatment of mouse skin leads to activating mutations in H-ras at codon 61 and codons 12/13 (31). Therefore, we tested samples of papillomas and SCCs from the DMBA/TPA treated knockout and control mice in order to determine whether they contained H-ras mutations at codon 61 (CAA → CTA). Seven of eight PKKEKO and one of one control mice had SCCs with codon 61 mutations of H-ras. Most papillomas also had codon 61 mutations (Figure 3I). Thus, loss of PKK increases papilloma and SCC formation in a two-stage model of chemical carcinogenesis.
PKK deletion in keratinocytes promotes tumorigenesis in mouse skin
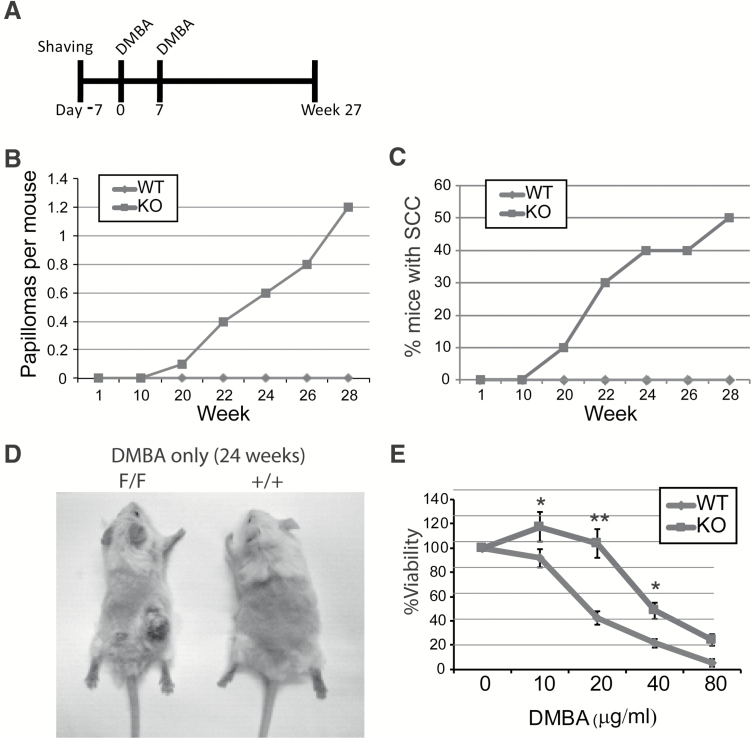
Given that PKK deficiency did not cause spontaneous cSCC but did promote tumor formation in two-staged chemical carcinogenesis, we next wanted to determine which stage of tumorigenesis is inhibited by PKK. Therefore, we investigated the effects of loss of PKK on treatment of mice with the tumor initiator DMBA alone (Figure 4A). DMBA treatment alone does not typically lead to papilloma or SCC formation in mice, and we did not observe any papilloma (Figure 4B) or SCC (Figure 4C) formation in control mice (10 mice) under our experimental condition. In contrast, in PKKEKO mice (10 mice), we observed both papilloma (Figure 4B) and SCC (Figure 4C and D) tumor formation after treatment with DMBA alone. To assess whether PKK loss can replace the tumor initiator DMBA in chemical carcinogenesis experiments, we treated both PKK-deficient (10 mice) or control mice (10 mice) with the tumor promoter TPA alone twice weekly. No SCCs or papillomas were observed in PKKEKO or control mice after 27 weeks of continuous treatment with TPA alone (data not shown). Therefore, loss of PKK function alone does not cause tumor initiation, whereas loss does lead to tumor promotion.
Figure 4.
DMBA alone induces SCC in PKKEKO mice. (A) Chemical carcinogenesis study utilizing only a tumor initiator, DMBA, was performed. Mice were shaved 1 week prior to starting DMBA treatment. After 2 weeks of once weekly DMBA treatment, mice were followed biweekly for 27 weeks. There were 10 mice per group. The percentage of SCC in the knockout mice were counted. (B) and (C) No papillomas or SCCs formed in the control treated mice at 27 weeks. Papillomas and SCC were observed in the PKKEKO mice. (D) Photograph showing wild-type (+/+) and knockout (F/F) mice after DMBA only treatment at 24 weeks. (e) MTT proliferation assay of wild-type and knockout keratinocytes culture with different concentrations of DMBA. All samples were performed in triplicate. *P ≤ 0.05, **P ≤ 0.005.
To further study the effect of DMBA on the growth behavior of the keratinocytes with PKK deficiency, primary keratinocytes were obtained from control and PKKEKO mice and studied with a yellow tetrazolium MTT proliferation assay (32,33). This assay measures the cell viability and proliferation of cultured cells. The reduction of tetrazolium salts during proliferation is assayed spectrophotometrically (Figure 4E). Analysis of treated cells using an MTT assay revealed that at lower DMBA concentrations, PKKEKO primary mouse keratinocytes had enhanced proliferation in response to DMBA exposure compared with the control cells, indicating a survival advantage of PKK-deficient keratinocytes following DMBA treatment.
Decreased NF-κB signaling in PKKEKO mice
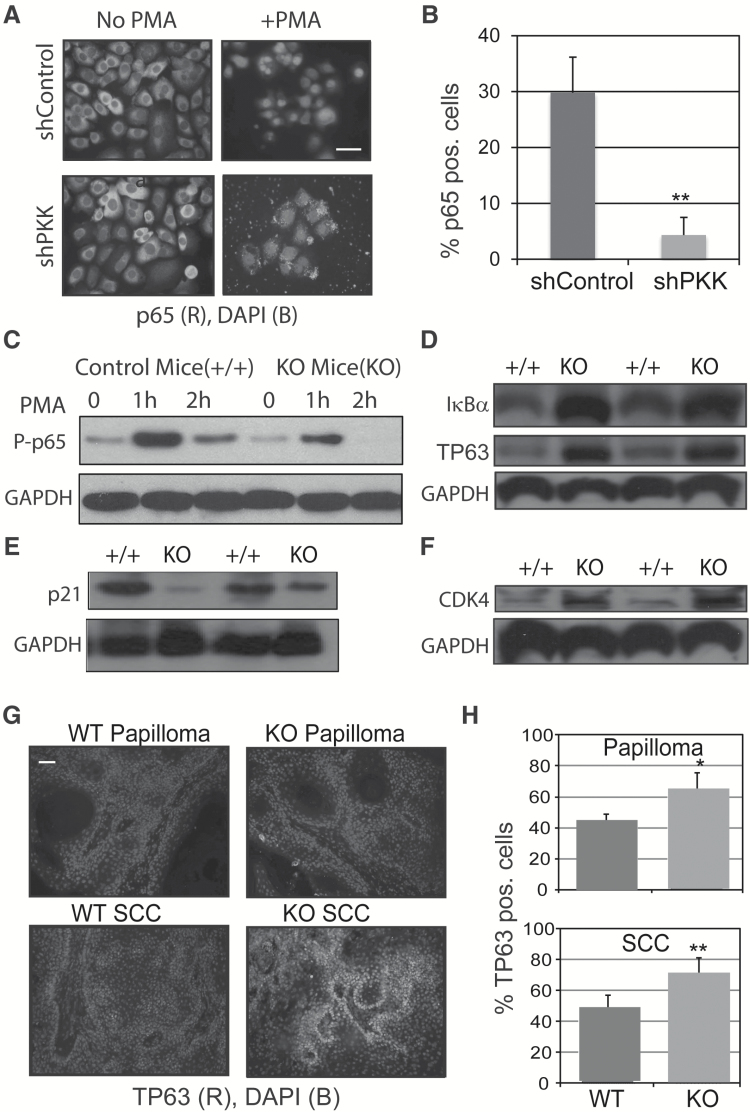
We have previously shown that shRNA knockdown of PKK in keratinocytes cultures leads to decreased IKK and NF-κB activity (17). To further these findings, we examined cultured keratinocytes from human SCCs treated with the NF-κB stimulus, phorbol ester (TPA). Both SCC-1 (Figure 5A) and SCC-2 (data not shown) have previously been described (17). Transduced SCC-1 control cells showed robust TPA-induced nuclear localization of p65, whereas SCC-1 cells expressing shPKK did not activate p65 translocation (Figure 5A and B). This confirms that PKK deficiency leads to inhibition of NF-κB signaling in keratinocytes. We next treated control and PKKEKO mouse skin with TPA for different time points and harvested keratinocytes by curettage. Western blotting of epidermal lysates from control and PKKEKO mice revealed increased phosphorylation of p65 after TPA treatment in control mice compared with PKKEKO skin (Figure 5C). Moreover, the phosphorylation persists after 2 h of TPA treatment in the control epidermis compared with PKKEKO. IκBα was increased in PKKEKO lysates (Figure 5D), consistent with decreased upstream NF-κB signaling in epidermal keratinocytes lacking PKK.
Figure 5.
Altered NF-κB and TP63 signaling in PKK-deleted keratinocytes. (A) Immunofluorescence microscopy of cultured keratinocytes from human SCCs that were treated with vehicle or 20 ng/ml of phorbol ester (TPA). p65 (red) and DAPI (blue). Scale bar is 25 μm. (b) Bar graphs show positive nuclear staining of cultured keratinocytes at 40× magnification. *P ≤ 0.05, **P ≤ 0.01. (C) Immunoblots against phosph-p65 in epidermis treated with TPA from C57BL6;129F10-PKKtm1(Tg(KRT14-cre/Esr1) wild-type or knockout. GAPDH is the loading control. All results were repeated at least two times. (D–F) Immunoblots of (D) IκBα and TP63 (E), p21 (F) and CDK4 of epidermis from control or PKKEKO mouse back skin. (G) Immunofluorescence microscopy of chemically induced tumors from wild-type or knockout mice. TP63 (red) and DAPI (blue). Scale bar is 50 μm. (H) Quantification of TP63 nuclear staining was counted at three representative areas of papillomas or SCCs at 40× magnification and is shown at right. *P ≤ 0.05, **P ≤ 0.01.
An analysis of TP63 expression was also performed. As shown in Figure 5D, TP63 was increased in PKKEKO epidermis compared with control. CDK4 is important in cell cycle progression while p21 inhibits cell cycle. Previously, we have shown that in cultured keratinocytes, with decreased PKK due to shPKK, there is a decrease in p21 and increase in CDK4, correlating with increased keratinocyte proliferation (17). Consistent with these in vitro data, the expression of p21, which was shown to be repressed by TP63 (34,35), was decreased in PKKEKO epidermis compared with control (Figure 5E). Moreover, epidermis from PKKEKO mice contains significantly increased CDK4 (Figure 5F), which correlates well with our observation that PKK deletion induces a thickened epidermis (Figure 1D). As epidermis from PKKEKO mice showed elevated TP63, tumors from PKKEKO mice were next examined. Papillomas and SCCs from DMBA/TPA treated wild-type and PKKEKO mice were examined by fluorescence microscopy. Nuclear TP63 intensity and frequency were increased in the tumors of PKKEKO mice (Figure 5G and H). Staining for phospho-p65 in tumor tissue was low and did not yield sufficient data to compare control and PKKEKO tumors. Taken together, these results strongly support the hypothesis that PKK has tumor suppressor function and that loss of PKK supports tumor progression in cSCC, which is consistent with the observed dysregulation of NF-κB and TP63 in PKKEKO mice.
Discussion
With a novel, skin-directed, inducible knockout mouse model (PKKEKO), we investigated the role of PKK in skin tumor development using a classical DMBA/TPA, two-stage carcinogenesis. Although other relevant models are available, such as the ultraviolet model utilizing genetically altered-hairless mice, the chemical carcinogenesis model is the best defined tumor model in mice and therefore was our choice for studying a novel tumor suppressor (36). Our results demonstrate that PKK functions as a tumor suppressor in keratinocytes. This extends our previous in vitro and in vivo xenotransplant work and highlights the essential and specific role of PKK as a regulator of SCC tumor progression. Moreover, this builds on the expanding landscape showing that PKK can function as a tumor suppressor in other keratinocytes tissues (18) and hepatocytes (19).
Knockout of PKK in keratinocytes leads to a hypercellular but fully stratified epidermis, consistent with increased proliferation, which we previously documented in keratinocytes lacking PKK (17). Genetic deletion of PKK produces a marked increase in susceptibility to DMBA/TPA-induced skin carcinogenesis (Figure 3B). Based on our previous result (17), which demonstrated that knockdown of PKK by shRNA promotes the cell cycle, independent of DMBA induced mutagenesis, we believe that our results using DMBA/TPA and DMBA alone experiments support a tumor suppressor role for PKK. However, one alternative explanation for the DMBA experiments would be that the increased cell cycling caused by PKK knockout leads to an increased susceptibility of the cycling keratinocytes to DMBA-induced mutagenesis.
PKK function is cell-type dependent, as PKK deficiency is associated with both keratinocyte and hepatocyte proliferation, whereas PKK overexpression in ovarian cells is tumorigenic (17,19,20). PKK is not the first protein that can function as both a tumor promoter and a suppressor, as NF-κB exhibits a similar functional dichotomy (37). Thus, it is especially interesting that loss of PKK has profound effects on NF-κB signaling in keratinocytes. PKK knockdown in mouse and human keratinocytes makes cells less responsive to NF-κB stimuli and there is decreased NF-κB transcriptional activity and decreased phosphorylation of IκBα after TPA treatment in cultured keratinocytes (17) and intact skin (Figure 5). It is clear that PKK is important for NF-κB signaling in keratinocytes and this is in agreement with previous studies (38–40). With its effect on NF-κB signaling and wound healing (5), it will be interesting to determine whether PKK is a key to tumor promotion in settings such as chronic inflammation (SCC in chronic wounds) and viral infections (e.g. hepatocellular carcinoma). PKK has already been shown to be decreased during wound healing, and therefore, chronic PKK decrease may be the tumor promoter that leads to SCC (41).
Examination of keratins 1, 5, 14 and involucrin was normally distributed within the thickened epidermis of PKKEKO skin (Figure 1E and F). Considering that PKK affects the nuclear localization of both TP63 and NF-κB, a lack of effect on epidermal differentiation was surprising (42,43). This suggests that PKK either only affects parts of these pathways that are not essential for epidermal differentiation or that the magnitude of the effect of PKK loss selectively alters tumor suppression while leaving epidermal differentiation intact. It will be important to determine more precisely which specific parts of the NF-κB and TP63 pathways are affected by PKK. For example, although IKKα CARD14 and PKK are all upstream effectors of NF-κB signaling, they have distinct roles in NF-κB signaling and divergent effects on keratinocyte differentiation, inflammation and tumorigenesis, respectively (17,44,45). Nevertheless, we observe a dramatic increase in TP63 expression (Figure 5D) and nuclear localization in the epidermis and epidermal tumors of PKKEKO mice (Figures 3C and 5G). TP63 is over-expressed in many SCCs including skin and those of the head and neck, and lung (46–48). Nuclear TP63 is known to negatively regulate p21 expression (34), suggesting TP63 is important in the increased proliferation of cells after PKK knockdown. Upregulation of cdk4 and cyclin E and downregulation of p21 promote an increase in S phase. In addition to effects on S phase, PKK deficiency leads to a decreased ability to stimulate NF-κB activity, which has previously been shown to increase tumorigenesis in skin (49). Together with previous reports, this work establishes that loss of PKK in keratinocytes is associated with changes in multiple signaling pathways, which promote keratinocyte proliferation and tumor progression (17,18,50).
Perhaps most importantly is the fact that these studies establish PKK functioning as a tumor suppressor in cSCC. Several pieces of data support this conclusion: first, mRNA levels of PKK are decreased in most human SCC tumors we have studied (17); second, PKK is mutated in a significant fraction of aggressive human cSCC (25,26); and, third, loss of PKK in keratinocytes increases keratinocyte proliferation and dramatically increases susceptibility to chemical carcinogenesis. Given the apparently selective role of PKK in cSCC, we propose that this may represent a promising target for development of new therapeutic approaches in metastatic disease.
Supplementary Material
Supplementary material is available at Carcinogenesis online.
Funding
B.P. was funded by Rochester General Hospital Skin Disease Research Fund, National Institutes of Health (K08AR055986) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Psoriasis Foundation Discovery Grant.
Author Contributions
B.P., L.C., M.H., C.A., D.O. and K.G. performed experiments. B.P., L.C., C.A., M.H., D.O., E.G. and M.P. contributed to experimental design and data analysis. B.P., M.H., E.G. and L.C. wrote the manuscript.
Acknowledgements
B.P. and M.S.H. would like to thank Michael Pichichero for helpful guidance with this project.
Conflict of Interest Statement: None declared.
Abbreviations
- cSCC
cutaneous squamous cell carcinoma
- DAPI
4’,6-diamidino-2-phenylindole
- DMBA
dimethylbenz(a)anthracene
- IP
intraperitoneal
- PKK
protein kinase C-associated kinase
- RIP
receptor-interacting protein kinase
- RT–PCR
reverse transcription–polymerase chain reaction
- SCC
squamous cell carcinoma
- TPA/PMA
12-O-tetradecanoylphorbol 13-acetate
- 4-OHT
4-hydroxytamoxifen
References
- 1. Meylan E., et al. (2005)The RIP kinases: crucial integrators of cellular stress. Trends Biochem. Sci., 30, 151–159. [DOI] [PubMed] [Google Scholar]
- 2. Zhang D., et al. (2010)Receptor-interacting protein (RIP) kinase family. Cell. Mol. Immunol., 7, 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kwa M.Q., et al. (2015)Disease-associated mutations in IRF6 and RIPK4 dysregulate their signalling functions. Cell. Signal., 27, 1509–1516. [DOI] [PubMed] [Google Scholar]
- 4. Dzamko N., et al. (2012)An emerging role for LRRK2 in the immune system. Biochem. Soc. Trans., 40, 1134–1139. [DOI] [PubMed] [Google Scholar]
- 5. Adams S., et al. (2010)RIP4 is a target of multiple signal transduction pathways in keratinocytes: implications for epidermal differentiation and cutaneous wound repair. Exp. Cell Res., 316, 126–137. [DOI] [PubMed] [Google Scholar]
- 6. Adams S., et al. (2007)Regulation of NF-kappaB activity and keratinocyte differentiation by the RIP4 protein: implications for cutaneous wound repair. J. Invest. Dermatol., 127, 538–544. [DOI] [PubMed] [Google Scholar]
- 7. Holland P., et al. (2002)RIP4 is an ankyrin repeat-containing kinase essential for keratinocyte differentiation. Curr. Biol., 12, 1424–1428. [DOI] [PubMed] [Google Scholar]
- 8. Kalay E., et al. (2012)Mutations in RIPK4 cause the autosomal-recessive form of popliteal pterygium syndrome. Am. J. Hum. Genet., 90, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitchell K., et al. (2012)Exome sequence identifies RIPK4 as the Bartsocas-Papas syndrome locus. Am. J. Hum. Genet., 90, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gripp K.W., et al. (2013)Exome analysis in clinical practice: expanding the phenotype of Bartsocas–Papas syndrome. Am. J. Med. Genet. A., 161, 1058–1063. [DOI] [PubMed] [Google Scholar]
- 11. Candi E., et al. (2006)p63 is upstream of IKK alpha in epidermal development. J. Cell Sci., 119, 4617–22. [DOI] [PubMed] [Google Scholar]
- 12. Kolk A., et al. (2014)Melanotic and non-melanotic malignancies of the face and external ear - A review of current treatment concepts and future options. Cancer Treat. Rev., 40, 819–837. [DOI] [PubMed] [Google Scholar]
- 13. Armstrong B.K., et al. (2001)The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B., 63, 8–18. [DOI] [PubMed] [Google Scholar]
- 14. Lee D.A., et al. (2009)Nonmelanoma skin cancer. Facial Plast. Surg. Clin. North Am., 17, 309–324. [DOI] [PubMed] [Google Scholar]
- 15. Clark R.R., et al. (2010)A retrospective analysis of histological prognostic factors for the development of lymph node metastases from auricular squamous cell carcinoma. Histopathology, 57, 138–146. [DOI] [PubMed] [Google Scholar]
- 16. Brantsch K.D., et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet. Oncol., 9, 713–720. [DOI] [PubMed] [Google Scholar]
- 17. Poligone B., et al. (2015)PKK suppresses tumor growth and is decreased in squamous cell carcinoma of the skin. J. Invest. Dermatol., 135, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X., et al. (2014)RIPK4 is downregulated in poorly differentiated tongue cancer and is associated with migration/invasion and cisplatin-induced apoptosis. Int. J. Biol. Markers, Jun 25; 29(2), e150–9. [DOI] [PubMed] [Google Scholar]
- 19. Heim D., et al. (2015)Retroviral insertional mutagenesis in telomerase-immortalized hepatocytes identifies RIPK4 as novel tumor suppressor in human hepatocarcinogenesis. Oncogene, 34, 364–372. [DOI] [PubMed] [Google Scholar]
- 20. Huang X., et al. (2013)Phosphorylation of dishevelled by protein kinase RIPK4 regulates Wnt signaling. Science, 339, 1441–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu D.Q., et al. (2015)Increased RIPK4 expression is associated with progression and poor prognosis in cervical squamous cell carcinoma patients. Sci. Rep., 5, 11955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sen T., et al. (2011)Tumor protein p63/nuclear factor kappa-B feedback loop in regulation of cell death. J. Biol. Chem., Dec 16; 286(50), 43204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao J., et al. (2013)Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal., 6, pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cerami E., et al. (2012)The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov., 2, 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Y.Y., et al. (2015)Genomic analysis of metastatic cutaneous squamous cell carcinoma. Clin. Cancer Res., 21, 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pickering C.R., et al. (2014)Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin. Cancer Res., 20, 6582–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen L., et al. (2016)A critical role for the protein kinase PKK in the maintenance of recirculating mature B cells and the development of B1 cells. Immunol. Lett., 172, 67–78. [DOI] [PubMed] [Google Scholar]
- 28. Vasioukhin V., et al. (1999)The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl. Acad. Sci. U.S.A., 96, 8551–8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koster M.I., et al. (2004)p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev., 18, 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Depianto D., et al. (2010)Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat. Genet., 42, 910–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Balmain A., et al. (1983)Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature, 303, 72–74. [DOI] [PubMed] [Google Scholar]
- 32. Mosmann T. (1983)Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods, 65, 55–63. [DOI] [PubMed] [Google Scholar]
- 33. Yusuf N., et al. (2009)Resveratrol enhances cell-mediated immune response to DMBA through TLR4 and prevents DMBA induced cutaneous carcinogenesis. Mol. Carcinog., 48, 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Truong A.B., et al. (2006)p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev., 20, 3185–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sen T., et al. (2010)Regulation of ΔNp63α by NFκΒ. Cell Cycle, 9, 4841–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nassar D., et al. (2015)Genomic landscape of carcinogen-induced and genetically induced mouse skin squamous cell carcinoma. Nat. Med., 21, 946–954. [DOI] [PubMed] [Google Scholar]
- 37. DiDonato J.A., et al. (2012)NF-kappaB and the link between inflammation and cancer. Immunol. Rev., 246, 379–400. [DOI] [PubMed] [Google Scholar]
- 38. Kim S.W., et al. (2008)Protein kinase C-associated kinase is required for NF-kappaB signaling and survival in diffuse large B-cell lymphoma cells. Blood, 111, 1644–1653. [DOI] [PubMed] [Google Scholar]
- 39. Moran S.T., et al. (2003)Protein kinase C-associated kinase can activate NFκB in both a kinase-dependent and a kinase-independent manner. J. Biol. Chem., 278, 21526–21533. [DOI] [PubMed] [Google Scholar]
- 40. Muto A., et al. (2002)Protein kinase C-associated kinase (PKK) mediates Bcl10-independent NF-kappa B activation induced by phorbol ester. J. Biol. Chem., 277, 31871–31876. [DOI] [PubMed] [Google Scholar]
- 41. Chandrakesan P., et al. (2013)Differential effects of β-catenin and NF-κB interplay in the regulation of cell proliferation, inflammation and tumorigenesis in response to bacterial infection. PLoS One, 8, e79432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mills A.A., et al. (1999)p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature, 398, 708–713. [DOI] [PubMed] [Google Scholar]
- 43. Wullaert A., et al. (2011)NF-[kappa]B in the regulation of epithelial homeostasis and inflammation. Cell Res., 21, 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hu Y., et al. (1999)Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science, 284, 316–320. [DOI] [PubMed] [Google Scholar]
- 45. Jordan Catherine T., et al. (2012)Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis. Am. J. Hum. Genet., 90, 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ha L., et al. (2011)Dysregulated DeltaNp63alpha inhibits expression of Ink4a/arf, blocks senescence, and promotes malignant conversion of keratinocytes. PLoS One, 6, e21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lu H., et al. (2011)TNF-alpha promotes c-REL/DeltaNp63alpha interaction and TAp73 dissociation from key genes that mediate growth arrest and apoptosis in head and neck cancer. Cancer Res., 71, 6867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang X., et al. (2011)DeltaNp63 versatilely regulates a Broad NF-kappaB gene program and promotes squamous epithelial proliferation, migration, and inflammation. Cancer Res., 71, 3688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dajee M., et al. (2003)NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature, 421, 639–643. [DOI] [PubMed] [Google Scholar]
- 50. Heim D., et al. (2015)Retroviral insertional mutagenesis in telomerase-immortalized hepatocytes identifies RIPK4 as novel tumor suppressor in human hepatocarcinogenesis. Oncogene, 34, 364–372. [DOI] [PubMed] [Google Scholar]
- 51. Poligone B., et al. (2013)A role for NF-κB activity in skin hyperplasia and the development of keratoacanthomata in mice. PLoS One, 8, e71887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Poligone B., et al. (2002)Elevated NF-kappaB activation in nonobese diabetic mouse dendritic cells results in enhanced APC function. J. Immunol., 168, 188–196. [DOI] [PubMed] [Google Scholar]
- 53. Park E., et al. (2007)Reduction in IκB kinase α expression promotes the development of skin papillomas and carcinomas. Cancer Res., 67, 9158–9168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.