ABSTRACT
Background
African-American (AA) women have poorer pregnancy outcomes, and studies in nonpregnant women suggest a different etiology of weight gain in AA compared with white women. We hypothesized that physiologic factors such as low energy expenditure and physical activity would be present in AA compared with white women in pregnancy.
Objective
We aimed to identify physiologic risk factors for disordered energy balance in AA and white women early in pregnancy.
Design
This was a cross-sectional study in 66 pregnant women with obesity, between 14 and 16 wk of gestation. Energy intake was calculated using the intake-balance method. Energy expenditure was measured in free-living conditions [total daily energy expenditure (TDEE)] over 7 d with the use of doubly labelled water and during sleep [sleeping EE (SleepEE)] in a room calorimeter. Body composition was measured by air displacement plethysmography and physical activity by accelerometers. Markers of metabolic health were obtained from fasting blood and urine.
Results
AA (n = 34) and white (n = 32) women were comparable in age (mean ± SEM: 27.7 ± 0.6 y), enrollment body mass index [mean ± SEM (in kg/m2): 36.9 ± 0.7], and body fat (mean ± SEM: 45.0% ± 0.6%). AA women had more fat-free mass (P = 0.01) and tended to be more insulin-resistant (homeostasis model assessment of insulin resistance, P = 0.06). Energy intake was significantly lower in AA than in white women (2499 ± 76 compared with 2769 ± 58 kcal/d, P = 0.001), although absolute TDEE was comparable (AA: 2590 ± 77 kcal/d; white: 2711 ± 56 kcal/d; P = 0.21). After adjusting for body composition, TDEE was significantly lower in AA women (−231 ± 74 kcal/d, P = 0.003), as was SleepEE (−81 ± 37 kcal/d, P = 0.03). Physical activity, substrate oxidation, and metabolic biomarkers (triiodothyronine and thyroxine concentrations, catecholamine excretion) were not significantly different between groups.
Conclusions
Body mass–adjusted energy expenditure is significantly lower in AA than in white pregnant women. Energy intake recommendations for pregnancy do not consider this difference and may therefore overestimate energy requirements in AA women. This may lead to unintentional overeating and contribute to the disparity of excess gestational weight gain and postpartum weight retention that is more prevalent in AA women. This trial was registered at clinicaltrials.gov as NCT01954342.
Keywords: doubly labeled water, energy expenditure, pregnancy, race, room calorimeter
INTRODUCTION
The prevalence of obesity in reproductive-age US women has steadily increased over the past 3 decades and in 2016 reached ∼40%, an all-time high (1). Of particular concern is the discordance in obesity between races. The prevalence of obesity in non-Hispanic-black and African-American (AA) women is 1.5 times as high as in their non-Hispanic white counterparts (57% compared with 33%) (1). Aside from maternal obesity, pregnancy in AA women is more likely complicated by adverse outcomes including gestational diabetes, preeclampsia, stillbirth, preterm birth, cesarean section, and postpartum weight retention (2, 3).
Pregnancy is increasingly acknowledged as a defining period in which offspring health is primed. Therefore, a plausible hypothesis to explain the disparity in infant health outcomes between AA and white individuals may be related to an adverse intrauterine environment. Whereas studies have attempted to pinpoint behavioral (2) and cultural factors (4) to explain poorer pregnancy outcomes in AA women, the role of physiologic factors, particularly those with the capability to influence body weight regulation such as gestational weight gain, retention of weight in the postpartum period, and obesity development in offspring, has been the focus of few investigations.
Understanding the potential racial disparity in the physiologic drive for weight gain during pregnancy is important because prospective and cross-sectional observational studies in nonpregnant women have shown that increased weight gain in AA women is linked to physiologic anomalies such as low resting energy expenditure (EE) (5, 6), impaired cardiometabolic fitness (VO2max) (7, 8), and low physical activity levels (9, 10). During pregnancy, these factors could also favor disparate weight gain trajectories and predispose AA women to pregnancy complications. To our knowledge there is no study which was designed to compare energy metabolism (expenditure and intake) between AA and white women in pregnancy. Understanding behavioral and physiologic differences in pregnant women can lead to specific interventions to directly address the racial disparity of poorer maternal and infant health in AA women.
The aim of this study was to perform comprehensive metabolic phenotyping in AA and white women in early pregnancy with the goal to ascertain potential risk factors for unhealthy weight gain trajectories. We hypothesized that, similar to nonpregnant women, AA women have low EE and physical activity when compared with white women.
METHODS
Study design
This is a cross-sectional analysis undertaken as part of an ongoing prospective observational study assessing the determinants of gestational weight gain in 72 pregnant women with obesity (clinicaltrials.gov: NCT01954342). EE, physical activity, substrate oxidation, and endocrine mediators were measured in obese pregnant women between 13 and 16 wk of gestation at Pennington Biomedical Research Center. Energy intake was measured with the use of the intake-balance method by including body composition measurements at the end of the second trimester. Participants provided written informed consent for participation in the study which was approved by the Institutional Review Board at the Center.
Participants and recruitment
Enrolled women were obese (BMI ≥30), between 18 and 40 y old, had a confirmed singleton, viable pregnancy, and were medically cleared for participation by their primary care obstetrician and the medical investigator. For the purpose of this cross-sectional analysis, only women identified as non-Hispanic and “African-American or black” (n = 34) or “white” (n = 32) were included. Women were excluded for other race (4 Hispanic, 1 biracial, 1 Asian), smoking, alcohol or drug use, pre-existing hypertension, diabetes (glycated hemoglobin ≥6.5%), contraindication to magnetic resonance imaging (implanted metal objects, claustrophobia), HIV or AIDS, severe anemia (hemoglobin <8 g/dL and/or hematocrit <24%), psychological or eating disorders, contraindicated medications or supplements, planned termination of pregnancy, or bariatric surgery. Participants were recruited between January 2015 and January 2017 through community advertisements, social media referrals, and by local obstetricians and midwives (11). Education level and household income were self-reported at the screening visit. Household income was computed as a percentage of the federal poverty line according to family size. Gestational age, fetal sex, and birth weight were confirmed from abstraction of the prenatal chart. Fetal body composition was assessed using air displacement plethysmography (PEA POD, COSMED USA, Inc., Concord, CA) within 10 d of birth.
Anthropometry and body composition
To assess eligibility for participation, enrollment BMI (in kg/m2) was calculated at the screening visit (mean ± SEM: 11.3 ± 0.3 wk of gestation) from body weight (measured to the nearest 0.1 kg in a clinic gown, ScaleTronix5200, White Plains, NY) and height (measured to the nearest 0.1 cm standing without shoes with the head held in the Frankfurt-plane using a wall-mounted stadiometer). Obesity was defined according to enrollment BMI (class I: 30 ≤ BMI < 35, class II: 35 ≤ BMI < 40, class III: BMI ≥ 40).
At outcome assessment visits (14.7 ± 0.1 and 25.1 ± 0.1 wk of gestation), weight was measured in the morning after an overnight fast while wearing a clinic gown. The weight of the gown was subtracted. Body volume was measured by air displacement plethysmography using the BOD POD (COSMED USA, Inc., Concord, CA) with thoracic gas volume corrected for −100 mL per trimester (12). Fat mass (FM) density was estimated as 0.9 kg/L and fat-free mass (FFM) density was based on gestational age using a published exponential regression (13). Body fat was calculated per Siri's equation with FFM density adjusted for gestation age in weeks (14).
Total daily EE
Total daily EE (TDEE) was measured using doubly labeled water over 7 d (15). Two baseline urine samples were collected before participants were dosed with labeled water (1.25 g of 10% enriched H218O and 0.10 g of 99.9% enriched 2H2O per kilogram of body weight). Enrichments of 2H and 18O were analyzed in baseline and post-dose urine samples (+4.5 h, +12 h, +6 d, and +7 d after dosing) by isotope ratio mass spectrometry (16). CO2 production rate (rCO2) was calculated according to Schoeller et al. (15) with the ND:NO ratio calculated as the mean of each participant value and the group mean. TDEE was calculated as the product of rCO2 by the energy equivalent of a liter of CO2 (5.66 kcal/mol or 126.7 kcal/L CO2) for a respiratory quotient of 0.866 (17).
To normalize EE data for individual differences in body composition, linear regression models of EE were developed for the entire cohort (n = 72) with the use of FFM and FM as determinants of EE (18). Age was not a significant predictor of EE in this cohort and was therefore not included in the regression model. Linear regression analysis is superior to the traditional ratio approach (EE per kilogram of weight), since the relation between EE and body mass has a nonzero y-intercept that varies between individuals (18). Using the group-derived regression equations, FFM and FM of each individual were then used to predict EE for each woman. The difference between measured and predicted EE (termed residual EE) allows for a comparison of EE between groups because differences in EE due to body composition are accounted for. Residual EE thereby reflects the EE that is not explained by individual FFM and FM.
The adjusted TDEE was compared with EE derived from a current maternal energy intake model (19). Using individual age, body weight, and height, the model provides an estimate for prepregnancy TDEE and predicts gestational weight gain in relation to energy intake.
Energy intake and diet quality
Energy intake was objectively measured using the intake-balance method (19, 20) where the mean TDEE (measured at 14.7 ± 0.1 wk of gestation and predicted at 25.1 ± 0.1 wk of gestation) was summed with the measured change in body energy stores (771 kcal/kg FFM and 9500 kcal/kg FM) throughout the same time period (21).
Diet composition was assessed throughout the 7-d doubly labeled water measurement with a food photography method via the SmartIntake (Pennington Biomedical Research Center, Baton Rouge, LA) phone application (22, 23). Briefly, participants captured images of meals or food items consumed and plate (food) waste. Images automatically transmitted in real-time via the phone application were reviewed as a set by the participant and study staff at the conclusion of the assessment period to document potential missing data. Dieticians determined portion sizes from a library of 7000 reference food images and the nutritional characteristics of each food were determined from the USDA Food and Nutrient Database for Dietary Studies 2011–2012 (24) and manufacturers’ nutrient information. Before analysis, days of obvious underreporting (<60% TDEE, n = 141/316, 45%) were excluded (25). Macronutrient composition, expressed as % of total self-reported energy intake, was applied to total daily energy intake to quantify intake in kilocalories per day.
Sleeping and resting EE
Sleeping and resting EE were measured in a room calorimeter during 1 overnight stay (26). After refraining from exercise, caffeine, and alcohol for 36 h, participants entered the metabolic chamber at 1800 and exited at 0700 the next morning. A standard dinner was served at 1900 which provided 30% of the daily estimated energy requirement (19) as 30% fat, 55% carbohydrate, and 15% protein. Lights were out from 2230 until 0600 the next morning. Urine was collected overnight into a pooled urine sample to measure urinary nitrogen excretion. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured continuously and EE was calculated using the Weir equation with protein determined from 12-h urinary nitrogen excretion extrapolated to 24 h. Sleeping EE (SleepEE) was assessed between 0200 and 0500 for minutes when radar sensors detected no activity in the previous 15 min. Resting metabolic rate (RMR) was measured for 20 min while the participant was supine on the bed in the chamber, 25 min after waking. The average EEs during the measurements of SleepEE and RMR were extrapolated to 24 h.
Physical activity
Physical activity was quantified by 3 metrics. First, activity-related EE (AREE) was computed for each subject from a linear regression model of TDEE with SleepEE as the determinant (27) with the resulting calculated AREE being positive for individuals with higher physical activity than average and negative for individuals with lower physical activity than average. Such a value is independent of metabolic body size since it is normalized for SleepEE. Second, physical activity level was calculated as the ratio of TDEE to RMR. Third, participants wore an accelerometer (SenseWear Armband, BodyMedia Inc.) over the 24-h period which included the chamber stay, and the time spent sedentary as well as in moderate, vigorous, and very vigorous activity was recorded by the device.
Clinical chemistry
Urinary nitrogen, creatinine, norepinephrine, and epinephrine excretion were measured in a 12-h pooled urine sample collected overnight during the chamber stay (Bio Rad Microplate reader, DLD Diagnostika, Hamburg, Germany). A fasting blood sample was collected for glucose (DXC600, Beckman Coulter Inc., Brea, CA), insulin, and thyroid hormones (triiodothyronine, thyroxine, and thyroid-stimulating hormone—TSH) (Immulite 2000, Siemens, Broussard, LA).
Statistics
Data are reported as means ± SEMs. To test for differences between AA and white women, an independent sample t test was performed on the least square means. For categorical variables, a chi-square test was performed. To compare EE between groups, after adjustment for body mass and composition, regression analyses were developed on the entire study cohort (n = 72) for SleepEE, RMR, and TDEE measured in early pregnancy and FFM and FM were the independent determinants of EE. A 1-sided t test was performed to analyze whether residual EE was different from 0. If residual EE was different between races, we tested for an interaction between race and FFM to test whether race was a main effect or if the effect was related to race differences in FFM. Estimates of TDEE were analyzed for fixed effects [race: AA compared with white, method: measured compared with modeled (19)] and a race-by-method interaction using linear mixed models for repeated measures. All analyses were carried out by a biostatistician (RAB) using SAS/STAT software, Version 9.4 of the SAS System for Windows (SAS Institute Inc., Cary, NC). All tests were evaluated using the significance level α = 0.05.
RESULTS
Subject characteristics
Sixty-six women (34 AA, 32 white) classified as obese (BMI: 36.9 ± 0.7), aged 27.7 ± 0.6 y (range 18–38 y) were phenotyped between the 14th and 16th wk of pregnancy (Table 1) and had follow-up body weight and body composition measured 2 mo later between weeks 25 and 27 of pregnancy. For the entire cohort, 55% had ≥1 previous live birth, which was significantly more prevalent in the AA women (68% compared with 40%, P = 0.02). The 2 groups were not different with respect to age, adiposity, blood pressure, fasting glucose, or insulin (Table 1). AA women however had larger FFM than white women (P = 0.01) and higher glycated hemoglobin (P = 0.001). In addition, AA women had lower socioeconomic status as indicated by a lower level of education and household income (P < 0.05). Infant gender was not different between AA and white women, and birth weights of the infants were similar between groups. AA women delivered ∼1 wk earlier than white women, but labor type (no labor, spontaneous, or induced) and delivery mode (spontaneous vaginal, operative vaginal, cesarean with labor, or cesarean without labor) were comparable (P = 0.77 and P = 0.29, respectively).
TABLE 1.
A comparison of subject characteristics between African-American and white pregnant women1
| African American | White | ||
|---|---|---|---|
| Variable | n = 34 | n = 32 | P |
| Maternal demographics | |||
| Age, y | 27.4 ± 0.8 | 28.1 ± 0.9 | 0.55 |
| Gestation age, wk | 14 5/7 ± 1/7 | 14 4/7 ± 1/7 | 0.23 |
| Parity (0, 1, ≥2), n | 11, 10, 13 | 19, 10, 3 | 0.02 |
| Poverty:income ratio | 2.2 ± 0.3 | 4.4 ± 0.4 | <0.001 |
| Education, AU | 4.1 ± 0.2 | 4.6 ± 0.2 | 0.02 |
| Enrollment BMI (class I, II, III), n | 13, 11, 10 | 16, 11, 5 | 0.38 |
| Enrollment BMI, kg/m2 | 37.9 ± 1.0 | 35.8 ± 1.0 | 0.15 |
| Body composition | |||
| Weight, kg | 102.7 ± 3.2 | 96.6 ± 3.3 | 0.19 |
| Fat-free mass, kg | 56.9 ± 1.4 | 51.6 ± 1.4 | 0.01 |
| Fat mass, kg | 45.8 ± 2.2 | 45.0 ± 2.3 | 0.81 |
| Fat mass, % | 44.2 ± 0.7 | 45.8 ± 0.8 | 0.15 |
| Weight gain, 2nd trimester, g/wk | 287 ± 48 | 355 ± 47 | 0.31 |
| Cardiometabolic factors | |||
| HbA1c, % | 5.6 ± 0.1 | 5.3 ± 0.1 | 0.001 |
| Glucose, mg/dL | 89 ± 2 | 88 ± 1 | 0.67 |
| Insulin, mU/mL | 17.7 ± 1.5 | 13.8 ± 1.3 | 0.06 |
| HOMA-IR, AU | 4.0 ± 0.4 | 3.1 ± 0.3 | 0.06 |
| Systolic blood pressure, mm Hg | 108 ± 1 | 108 ± 2 | 0.95 |
| Diastolic blood pressure, mm Hg | 67 ± 1 | 67 ± 1 | 0.96 |
| Infant demographics | |||
| Infant sex (F/M), n | 14, 18 | 17, 15 | 0.45 |
| Birth weight, g | 3354 ± 101 | 3533 ± 74 | 0.15 |
| Body fat, % | 12.6 ± 1.3 | 11.3 ± 1.1 | 0.42 |
| Gestation age at delivery, wk | 38 3/7 ± 3/7 | 39 5/7 ± 1/7 | 0.006 |
1Data are means ± SEMs unless otherwise indicated. Maternal demographics and HbA1c were assessed at the screening visit; infant demographics were abstracted from the prenatal chart; and body composition and cardiometabolic factors were assessed between 13 and 16 wk of gestation. P indicates the statistical significance of the difference between groups by independent sample t tests on the least square means. AU, arbitrary units; HbA1c, glycated hemoglobin.
Comparison of energy intake and diet quality between AA and white pregnant women
Energy intake measured by the intake-balance method was significantly lower in AA than in white pregnant women throughout the second trimester (AA: 2499 ± 76, white: 2769 ± 58 kcal/d, P = 0.001, Figure 1A). The proportions of calories consumed from carbohydrate (AA: 47% ± 2%, white: 45% ± 2%, P = 0.48), fat (AA: 37% ± 1%, white: 39% ± 1%, P = 0.29), and protein (AA: 16% ± 1%, white: 17% ± 1%, P = 0.81) were however not different between the 2 groups of women.
FIGURE 1.
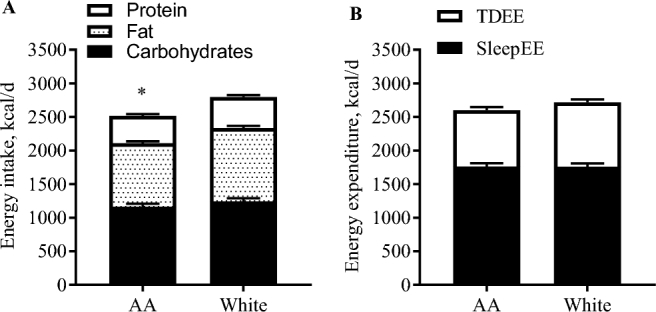
Comparison of energy intake and EE between AA and white women in early pregnancy. Data are presented as means ± SEMs. (A) Energy intake was calculated by the intake-balance method, combination between doubly labeled water and changes in fat-free and fat mass (AA: n = 26; white: n = 31); and macronutrient composition using food photography (AA: n = 25; white: n = 26). (B) EE was measured over 7 d using doubly labeled water (TDEE) and once overnight (0200–0500) in whole-room calorimetry (SleepEE). *Significant difference in total energy intake between AA and white women (P < 0.05), whereas macronutrient composition was comparable between the groups. AA, African American; EE, energy expenditure; SleepEE, sleeping energy expenditure; TDEE, total daily energy expenditure.
Comparison of EE between AA and white pregnant women
For the entire cohort, absolute free-living EE over 7 d (TDEE) was 2650 ± 50 kcal/d. SleepEE was 1768 ± 35 kcal/d which comprised 66% ± 1% of TDEE. EE during rest (RMR) was 1821 ± 36 kcal/d and 2% ± 1% more than SleepEE. The remaining EE (587 ± 31 kcal/d), mostly attributed to activity and the thermic effect of food, comprised 22% ± 1% of TDEE. No significant differences were observed between the groups for any measure of EE when expressed as absolute values. When considering the races separately, TDEE was 2590 ± 77 kcal/d in AA and 2711 ± 56 kcal/d in white women (P = 0.21; Figure 1B). SleepEE was 1778 ± 51 kcal/d in AA and 1758 ± 49 kcal/d in white women (P = 0.78), and EE attributed to activity and the thermic effect of food was 537 ± 43 kcal/d and 639 ± 45 kcal/d, respectively (P = 0.11).
TDEE correlated with body weight (r = 0.47, P < 0.001), FFM (r = 0.55, P < 0.001, Figure 2A), and FM (r = 0.35, P = 0.004). After adjusting TDEE for individual differences in FFM and FM, residual TDEE (Figure 2B) was significantly different from 0 kcal/d for both AA and white women (AA: −108 ± 58 kcal/d, P = 0.04; white: +125 ± 47 kcal/d, P = 0.02). Residual EE was 233 ± 74 kcal/d or 9% ± 3% lower in AA than in white women (Figure 2B, P = 0.003).
FIGURE 2.
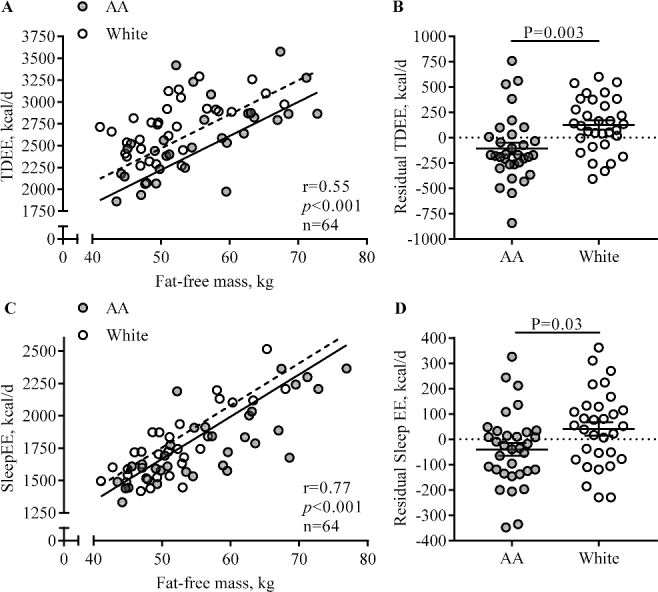
Comparison of EE adjusted for body composition between AA and white pregnant women with obesity. Each data point represents 1 participant, and the lines are means ± SEMs. Residual EE reflects the difference between the EE predicted from a regression equation and EE that was measured. Correlations between fat-free mass and energy expenditure (A: TDEE; C: SleepEE) and the associated regression lines are shown for AA (TDEE: n=32; SleepEE: n=33; filled circles, straight lines) and white (TDEE: n=32; SleepEE: n=31; open circles, dotted lines) women separately, because for both TDEE and SleepEE, we observed a main effect for race and no race × FFM interaction. The main effect for race on TDEE (B) and SleepEE (D) is represented as residual EE, which reflects the difference between the EE predicted from a regression equation (EE as dependent of fat-free mass and fat mass) and measured EE. AA, African American; EE, energy expenditure; SleepEE, sleeping energy expenditure; TDEE, total daily energy expenditure.
Similarly, SleepEE correlated with FFM (r = 0.77, P < 0.001, Figure 2C), FM (r = 0.72, P < 0.001), and weight (r = 0.81, P < 0.001). Residual SleepEE (Figure 2D) was 81 ± 37 kcal/d or 5% ± 2% lower in AA than in white women (P = 0.03), indicating lower metabolism per kilogram of mass.
Comparison of physical activity between AA and white pregnant women
To quantify AREE, we regressed SleepEE on TDEE (r = 0.76, P < 0.001; Figure 3A). If the EE during sleep and activity were proportional, all data points would lie on the regression line. We observed no main effect of race, and no interaction between FFM and race, and thus AREE (Figure 3B) was not significantly different between AA and white women (AA: −44 ± 42 kcal/d, white: 71 ± 46 kcal/d, P = 0.07). Physical activity level (calculated as TDEE/resting EE) was low among all women and did not significantly differ by race (Figure 3C). Using accelerometry, no group difference was observed in daily steps taken (AA: 3720 ± 252 steps/d, white: 4082 ± 260 steps/d, P = 0.32, Figure 3D) or in the time spent in moderate and vigorous activity (AA: 21 ± 2 min/d, white: 22 ± 6 min/d, P = 0.82).
FIGURE 3.
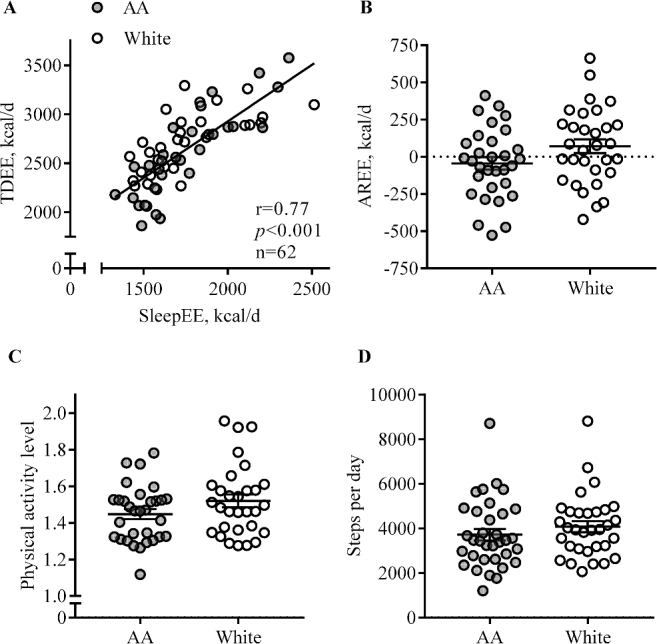
Comparison of physical activity between AA and white pregnant women with obesity. Each data point represents 1 participant, and the lines are means ± SEMs. The regression line (in A) is shown for all AA (n = 31, filled circle) and white (n = 31, open circle) women together, because no main effect for race or race × FFM interaction was observed. (B) AREE reflects the difference between the EE predicted from a regression equation (TDEE as dependent of SleepEE) and measured TDEE. (C) Physical activity level was calculated as the ratio of TDEE to RMR. (D) Steps were measured using SenseWear accelerometers for 24 h (AA: n = 34; white: n = 32). AA, African American; AREE, activity-related energy expenditure; EE, energy expenditure; SleepEE, sleeping energy expenditure; TDEE, total daily energy expenditure.
Metabolic profile
To identify potential metabolic mediators contributing to the difference in EE between AA and white women, we compared substrate oxidation measured during the metabolic chamber, thyroid hormones, and urinary catecholamine excretion. Compared with white women, substrate oxidation, assessed as respiratory quotient over 12 h (AA: 0.873 ± 0.006, white: 0.877 ± 0.005, P = 0.61) and during sleep (AA: 0.852 ± 0.007, white: 0.858 ± 0.007, P = 0.52), was not significantly different in AA women. No significant difference was observed for thyroid hormone concentrations (triiodothyronine: AA: 185 ± 8 ng/dL, white: 183 ± 8 ng/dL, P = 0.86; and thyroxine: AA: 10.1 ± 0.3 μg/dL, white: 9.9 ± 0.3 μg/dL, P = 0.70), but TSH was lower in AA than in white women (TSH: AA: 1.4 ± 0.1 mU/mL, white: 2.1 ± 0.2 mU/mL, P = 0.01). Urinary catecholamine excretion also was comparable between AA and white women (epinephrine: AA: 11.6 ± 1.2 nmol/L, white: 10.7 ± 1.1 nmol/L, P = 0.52; norepinephrine, AA: 188 ± 22 nmol/L, white: 146 ± 13 nmol/L, P = 0.11).
Energy requirements for healthy gestational weight gain
Energy intake recommendations during pregnancy are based on estimates of TDEE. Estimates of TDEE derived from a current maternal energy intake model (19), through the use of individual weight, height, and age of women, were not different between AA and white women (Figure 4). The TDEE predicted by the maternal energy intake model was significantly higher than measured TDEE (+313 ± 39 kcal/d, P < 0.001). The overestimate of TDEE was more pronounced for the AA women than for the white women (AA: +422 ± 55, white: +186 ± 55 kcal/d, P = 0.003), which is due to the lower adjusted TDEE in AA women (P < 0.001).
FIGURE 4.
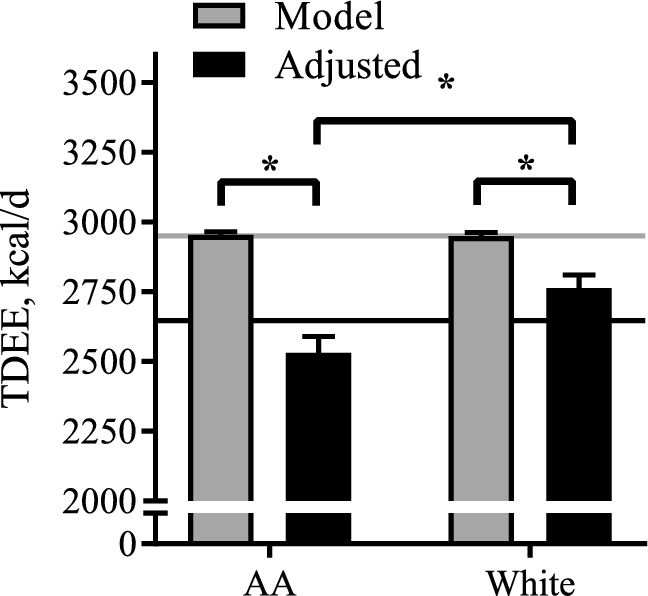
Estimates of TDEE for AA and white women using doubly labeled water and a published maternal energy intake model (19). Data are presented as means ± SEMs, by method of TDEE measurement and race. Estimates for EE are derived from the current maternal energy intake model (Model, grey bars) and from the double isotope dilution method, adjusted for body mass and body composition (Adjusted, black bars). The horizontal lines indicate the overall mean for AA (n = 32) and white (n = 32) women. *Statistically significant difference. Comparisons between AA and white women were analyzed if the interaction term Method × Race was confirmed to be statistically significant (P < 0.05). AA, African American; EE, energy expenditure; TDEE, total daily energy expenditure.
DISCUSSION
The aim of this study was to perform comprehensive metabolic phenotyping of body composition, energy intake, EE, and physical activity using gold-standard techniques in AA and white women with obesity in early pregnancy with the goal to ascertain potential risk factors for unhealthy weight gain trajectories. After adjusting for individual differences in body weight and body composition, AA women expended significantly fewer calories during sleep and in free-living conditions over 7 d in early pregnancy than did their white counterparts. Physical activity was not different between AA and white women. Taken together, AA women with obesity show evidence of metabolic slowing that may in part explain their predisposition to adverse pregnancy outcomes such as excess postpartum weight retention and aberrant obesity development in children born to AA mothers. Energy intake recommendations for pregnant women may significantly overestimate the energy requirements of AA women owing to their lower EE, which may contribute to unintentional overeating and excess gestational weight gain in this population.
Current recommendations for gestational weight gain are the same for all women regardless of race (28), and in epidemiologic studies the incidence of excess gestational weight gain rarely differs between AA and white women (29). However, AA women have a higher propensity for developing pregnancy complications and excess postpartum weight retention (2, 3). Until now, studies have attempted to explain this maternal disparity in terms of differences in health behaviors (2), social environments (4), or attitudes towards pregnancy weight gain (30). For the first time, we here show that physiologic factors, namely a low EE, may also contribute to pregnancy outcomes in AA women and in particular to their impaired weight loss postpartum.
This cross-sectional study of 66 pregnant women incorporates both EE in free-living conditions over 7 d and EE during sleep. Our study shows that EE per unit of metabolic mass is lower in pregnant AA women than in their white counterparts both in free-living conditions and during sleep. The lower EE was not due to a lesser amount of physical activity. We sought to explain the difference in metabolism on the basis of thyroid hormones and sympathetic nervous system activity; however, these were not different between the 2 groups and are therefore unlikely to mediate the observed difference in EE. Similarly, diet composition and substrate oxidation did not significantly differ by race, and thus it is unlikely that an impaired fat oxidation causes lower EE in AA women. Underlying mechanisms that may explain the slower metabolism in AA women are race differences in the composition of the FFM and organ sizes (31). Moreover, AA women may also have lower fitness (7, 32) or increased energy efficiency during low-intensity activities, e.g., walking (33). Unfortunately we are unable to discern these mechanisms with the measures used in our study, and future studies should include such measurements.
Although the observation of a blunted EE in AA women compared with white women is not a new finding for the general population (5, 6), this is the first known report in pregnancy. Clinically, this observation is important because low EE has been shown to predict future weight gain in nonpregnant cohorts (34–37) and, recently, in 2 independent cohorts of pregnant AA and white women (38, 39). Although population-level data do not show a higher incidence of excess gestational weight gain in AA women, there are consistent reports that AA women are less likely to return to their prepregnancy weight (40–43), which could be the consequence of low EE.
Given the importance of pregnancy on infant development and metabolic programming throughout life, metabolic characteristics such as low EE may imprint a similar metabolic phenotype in the offspring. Therefore, low EE in AA mothers may program low EE in their offspring and thus increase the risk for excess weight gain throughout infancy and childhood. This is indeed supported by various reports of low EE in preadolescent and adolescent AA children (44, 45). Childhood obesity is more prevalent among AA children (46), further supporting this hypothesis. Characterization of energy metabolism in women in early pregnancy may therefore identify race-specific risk factors for adverse child health in ethnic minorities.
An important clinical application of EE measurements is to determine energy requirements. Given that the association between gestational weight gain and adverse pregnancy outcomes is not affected by maternal race in epidemiologic studies, current recommendations for gestational weight gain and maternal energy requirements are applied to all women and are not specific for different races. However, based on our findings, we hypothesize that energy intake requirements need to be lower for AA compared with white pregnant women. Although we show that current models overestimate energy requirements for obese women overall, the overestimate is >200 kcal/d larger for AA than for white women. The closer agreement for white women likely corresponds with the fact that studies of EE used to inform current guidelines were derived from predominantly white cohorts (78% white, 10% AA) (19). We calculate that the magnitude of the overestimation in energy requirements could amount to an additional 7 kg of weight gained throughout pregnancy (19). This is particularly problematic because body composition studies in pregnant women demonstrate that excess weight gained in pregnancy is primarily comprised of FM (47). Furthermore, since AA women are more likely to retain weight after pregnancy (48), it is likely that they will enter subsequent pregnancies with more adiposity. Thus, a critical outcome of this study is the identification that energy intake recommendations for pregnant women need to be adjusted to accommodate lower energy metabolism in AA women similar to nonpregnant populations (49) and future studies on this subject should attempt to equally phenotype women from different racial backgrounds. Despite overestimated energy intake recommendations, energy intake was lower in AA women than in white women. Our statistical analysis did not reveal any significant effect of socioeconomic status including level of education or the poverty index on energy intake; however, a greater proportion of the AA women were indeed poorer compared with the white counterparts in this cohort. Other factors that may relate to lower energy intake in AA women such as increased food insecurity (50) are an important area of further investigation.
To our knowledge, this is the first study that has undertaken comprehensive metabolic phenotyping in women early in pregnancy and examined whether there are differences between AA and white women with obesity. The observation that AA women with obesity have a slower metabolism per unit of metabolic mass despite similar levels of physical activity has important implications for current energy intake guidelines for AA women in pregnancy. Current guidelines do not consider low EE for AA women and suggest an energy intake which, if adhered to, would result in excess gestational weight gain. However, whether the phenotypic differences in metabolism between AA and white women explain the higher incidences of adverse pregnancy outcomes and lay the foundation for racial disparities in childhood obesity needs to be further studied.
Acknowledgements
We acknowledge administrative support from Porsha Vallo, Elizabeth Sutton, Kelsey Olson, Alexandra Beyer, Alexis O'Connell, and Natalie Comardelle, the technical assistance of Jennifer Rood, Owen Carmichael, Loren Cain, Kimberly Landry, and Brian Gilmore, and the recruitment and retention support from Ralph Dauterive, Evelyn Griffin, and Evelyn Hayes.
The authors' responsibilities were as follows—JM, ER, and LMR: designed the research and wrote the article; JM, LAG, ADA, and MSA: conducted the research; JM, RAB, ER, and LMR: analyzed the data or performed the statistical analysis; LMR: had primary responsibility for final content; and all authors: read and approved the final manuscript. None of the authors have any conflicts of interest to declare.
Notes
Supported by the National Institutes of Health (R01DK099175; LMR) and in part by U54GM104940 and P30DK072476 (ER).
Abbreviations used:
- AA
African-American
- AREE
activity-related energy expenditure
- EE
energy expenditure
- FFM
fat-free mass
- FM
fat mass
- RMR
resting metabolic rate
- SleepEE
sleeping energy expenditure
- TDEE
total daily energy expenditure
- TSH
thyroid-stimulating hormone
REFERENCES
- 1. Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315(21):2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bryant AS, Worjoloh A, Caughey AB, Washington AE. Racial/ethnic disparities in obstetric outcomes and care: prevalence and determinants. Am J Obstet Gynecol 2010;202(4):335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Penn N, Oteng-Ntim E, Oakley LL, Doyle P. Ethnic variation in stillbirth risk and the role of maternal obesity: analysis of routine data from a London maternity unit. BMC Pregnancy Childbirth 2014;14:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whitaker KM, Wilcox S, Liu J, Blair SN, Pate RR. African American and White women's perceptions of weight gain, physical activity, and nutrition during pregnancy. Midwifery 2016;34:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weyer C, Snitker S, Bogardus C, Ravussin E. Energy metabolism in African Americans: potential risk factors for obesity. Am J Clin Nutr 1999;70(1):13–20. [DOI] [PubMed] [Google Scholar]
- 6. Sharp TA, Bell ML, Grunwald GK, Schmitz KH, Sidney S, Lewis CE, Tolan K, Hill JO. Differences in resting metabolic rate between white and African-American young adults. Obes Res 2002;10(8):726–32. [DOI] [PubMed] [Google Scholar]
- 7. Shook RP, Hand GA, Wang X, Paluch AE, Moran R, Hebert JR, Swift DL, Lavie CJ, Blair SN. Low fitness partially explains resting metabolic rate differences between African American and white women. Am J Med 2014;127(5):436–42. [DOI] [PubMed] [Google Scholar]
- 8. Ceaser TG, Fitzhugh EC, Thompson DL, Bassett DR Jr. Association of physical activity, fitness, and race: NHANES 1999–2004. Med Sci Sports Exerc 2013;45(2):286–93. [DOI] [PubMed] [Google Scholar]
- 9. Wilson-Frederick SM, Thorpe RJ Jr, Bell CN, Bleich SN, Ford JG, LaVeist TA. Examination of race disparities in physical inactivity among adults of similar social context. Ethn Dis 2014;24(3):363–9. [PMC free article] [PubMed] [Google Scholar]
- 10. Marshall SJ, Jones DA, Ainsworth BE, Reis JP, Levy SS, Macera CA. Race/ethnicity, social class, and leisure-time physical inactivity. Med Sci Sports Exerc 2007;39(1):44–51. [DOI] [PubMed] [Google Scholar]
- 11. Sutton EF, Cain LE, Vallo PM, Redman LM. Strategies for successful recruitment of pregnant patients into clinical trials. Obstet Gynecol 2017;129(3):554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jensen D, Webb KA, Davies GA, O'Donnell DE. Mechanical ventilatory constraints during incremental cycle exercise in human pregnancy: implications for respiratory sensation. J Physiol 2008;586(19):4735–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Raaij JM, Peek ME, Vermaat-Miedema SH, Schonk CM, Hautvast JG. New equations for estimating body fat mass in pregnancy from body density or total body water. Am J Clin Nutr 1988;48(1):24–9. [DOI] [PubMed] [Google Scholar]
- 14. Siri WE. Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition 1993;9(5):480–91; discussion, 492. [PubMed] [Google Scholar]
- 15. Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol 1986;250(5 Pt 2):R823–30. [DOI] [PubMed] [Google Scholar]
- 16. Martin CK, Heilbronn LK, de Jonge L, Delany JP, Volaufova J, Anton SD, Redman LM, Smith SR, Ravussin E. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 2007;15(12):2964–73. [DOI] [PubMed] [Google Scholar]
- 17. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109(1–2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Melzer K, Schutz Y, Kayser B. Normalization of basal metabolic rate for differences in body weight in pregnant women. Eur J Obstet Gynecol Reprod Biol 2011;159(2):480–1. [DOI] [PubMed] [Google Scholar]
- 19. Thomas DM, Navarro-Barrientos JE, Rivera DE, Heymsfield SB, Bredlau C, Redman LM, Martin CK, Lederman SA, Collins LM, Butte NF. Dynamic energy-balance model predicting gestational weight gain. Am J Clin Nutr 2012;95(1):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gilmore LA, Butte NF, Ravussin E, Han H, Burton JH, Redman LM. Energy intake and energy expenditure for determining excess weight gain in pregnant women. Obstet Gynecol 2016;127(5):884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol 2003;189(5):1423–32. [DOI] [PubMed] [Google Scholar]
- 22. Martin CK, Correa JB, Han H, Allen HR, Rood JC, Champagne CM, Gunturk BK, Bray GA. Validity of the Remote Food Photography Method (RFPM) for estimating energy and nutrient intake in near real-time. Obesity (Silver Spring) 2012;20(4):891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin CK, Han H, Coulon SM, Allen HR, Champagne CM, Anton SD. A novel method to remotely measure food intake of free-living individuals in real time: the remote food photography method. Br J Nutr 2009;101(3):446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agricultural Research Service USDA food and nutrient database for dietary studies 2011-2012. Beltsville, MD: U.S. Department of Agriculture; 2014. [Google Scholar]
- 25. Most J, Vallo PM, Altazan AD, Gilmore AL, Sutton EF, Cain LE, Burton JH, Martin CK, Redman LM. Assessing the validity of measuring energy intake by remote food photography method as compared to doubly labeled water in pregnant women. Faseb J 2017;31:136.1. [Google Scholar]
- 26. Nguyen T, de Jonge L, Smith SR, Bray GA. Chamber for indirect calorimetry with accurate measurement and time discrimination of metabolic plateaus of over 20 min. Med Biol Eng Comput 2003;41(5):572–8. [DOI] [PubMed] [Google Scholar]
- 27. Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E, Pennington CT. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One 2009;4(2):e4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines Determining optimal weight gain. In: Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press; 2009. [PubMed] [Google Scholar]
- 29. Fontaine PL, Hellerstedt WL, Dayman CE, Wall MM, Sherwood NE. Evaluating body mass index-specific trimester weight gain recommendations: differences between black and white women. J Midwifery Womens Health 2012;57(4):327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Groth SW, Morrison-Beedy D, Meng Y. How pregnant African American women view pregnancy weight gain. J Obstet Gynecol Neonatal Nurs 2012;41(6):798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gallagher D, Albu J, He Q, Heshka S, Boxt L, Krasnow N, Elia M. Small organs with a high metabolic rate explain lower resting energy expenditure in African American than in white adults. Am J Clin Nutr 2006;83(5):1062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ceaser T, Hunter G. Black and White race differences in aerobic capacity, muscle fiber type, and their influence on metabolic processes. Sports Med 2015;45(5):615–23. [DOI] [PubMed] [Google Scholar]
- 33. Hunter GR, McCarthy JP, Bamman MM, Larson-Meyer DE, Fisher G, Newcomer BR. Exercise economy in African American and European American women. Eur J Appl Physiol 2011;111(8):1863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tataranni PA, Harper IT, Snitker S, Del Parigi A, Vozarova B, Bunt J, Bogardus C, Ravussin E. Body weight gain in free-living Pima Indians: effect of energy intake vs expenditure. Int J Obes Relat Metab Disord 2003;27(12):1578–83. [DOI] [PubMed] [Google Scholar]
- 35. Hume DJ, Yokum S, Stice E. Low energy intake plus low energy expenditure (low energy flux), not energy surfeit, predicts future body fat gain. Am J Clin Nutr 2016;103(6):1389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, Boyce V, Howard BV, Bogardus C. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med 1988;318(8):467–72. [DOI] [PubMed] [Google Scholar]
- 37. Astrup A, Gotzsche PC, van de Werken K, Ranneries C, Toubro S, Raben A, Buemann B. Meta-analysis of resting metabolic rate in formerly obese subjects. Am J Clin Nutr 1999;69(6):1117–22. [DOI] [PubMed] [Google Scholar]
- 38. Berggren EK, O'Tierney-Ginn P, Lewis S, Presley L, De-Mouzon SH, Catalano PM. Variations in resting energy expenditure: impact on gestational weight gain. Am J Obstet Gynecol 2017;217(4):445.e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meng Y, Groth SW, Stewart P, Smith JA. An exploration of the determinants of gestational weight gain in African American women: genetic factors and energy expenditure. Biol Res Nurs 2018;20(2):118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Headen IE, Davis EM, Mujahid MS, Abrams B. Racial-ethnic differences in pregnancy-related weight. Adv Nutr 2012;3(1):83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Endres LK, Straub H, McKinney C, Plunkett B, Minkovitz CS, Schetter CD, Ramey S, Wang C, Hobel C, Raju T et al. . Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstet Gynecol 2015;125(1):144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE. Longitudinal changes in adiposity associated with pregnancy. The CARDIA study. Coronary Artery Risk Development in Young Adults study. JAMA 1994;271(22):1747–51. [PubMed] [Google Scholar]
- 43. Waage CW, Falk RS, Sommer C, Morkrid K, Richardsen KR, Baerug A, Shakeel N, Birkeland KI, Jenum AK. Ethnic differences in postpartum weight retention: a Norwegian cohort study. BJOG 2016;123(5):699–708. [DOI] [PubMed] [Google Scholar]
- 44. DeLany JP, Bray GA, Harsha DW, Volaufova J. Energy expenditure in preadolescent African American and white boys and girls: the Baton Rouge Children's Study. Am J Clin Nutr 2002;75(4):705–13. [DOI] [PubMed] [Google Scholar]
- 45. Tershakovec AM, Kuppler KM, Zemel B, Stallings VA. Age, sex, ethnicity, body composition, and resting energy expenditure of obese African American and white children and adolescents. Am J Clin Nutr 2002;75(5):867–71. [DOI] [PubMed] [Google Scholar]
- 46. Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med 2014;370(5):403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN Jr. Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstet Gynecol 1997;90(4 Pt 1):483–8. [DOI] [PubMed] [Google Scholar]
- 48. Walker LO, Freeland-Graves JH, Milani T, Hanss-Nuss H, George G, Sterling BS, Kim M, Timmerman GM, Wilkinson S, Arheart KL et al. . Weight and behavioral and psychosocial factors among ethnically diverse, low-income women after childbirth: I. Methods and context. Women Health 2004;40(2):1–17. [DOI] [PubMed] [Google Scholar]
- 49. Lam YY, Redman LM, Smith SR, Bray GA, Greenway FL, Johannsen D, Ravussin E. Determinants of sedentary 24-h energy expenditure: equations for energy prescription and adjustment in a respiratory chamber. Am J Clin Nutr 2014;99(4):834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Borders AE, Wolfe K, Qadir S, Kim KY, Holl J, Grobman W. Racial/ethnic differences in self-reported and biologic measures of chronic stress in pregnancy. J Perinatol 2015;35(8):580–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


