Abstract
Background
Use of potentially inappropriate medications (PIM) among people with dementia is common. We assessed the patterns of medication use from 1-year before dementia diagnosis, to 1-year after dementia diagnosis, compared with patterns of medication use in people without dementia.
Methods
We conducted longitudinal study using the National Alzheimer’s Coordinating Center data. Adults aged 65 years and older newly diagnosed with dementia (n = 2,418) during 2005–2015 were year, age, and sex matched 1:1 with controls. Generalized estimating equation models weighted for missingness and adjusted for 15 participant characteristics were fit.
Results
Among participants with dementia, number of medications reported 1-year prediagnosis was 8% lower than at diagnosis year (p < .0001) and 11% higher 1-year postdiagnosis compared with year of diagnosis (p < .0001). Among participants with dementia, the odds of PIM exposure, assessed using the 2015 Beers Criteria, was 17% lower 1-year prediagnosis (p < .0001) and 17% higher 1-year postdiagnosis (p = .006) compared with year of diagnosis. Among controls, there were approximately 6% more medications reported between consecutive years (p < .0001 each comparison) and the odds of PIM exposure increased 11% between consecutive years (p = .006 and p = .047). At each annual follow-up, participants with dementia had lower odds of PIM exposure than their controls (prediagnosis p < .0001, at diagnosis p = .0007, postdiagnosis p = .03, respectively). There were no differences in exposure to anticholinergic medications.
Conclusions
Number of medications and PIM use increased annually for participants with and without dementia. Persistent challenge of increasing PIM use in this group of older adults is of major concern and warrants interventions to minimize such prescribing.
Keywords: Medications, Potentially inappropriate medications (PIM), Dementia diagnosis, Generalized estimating equations, Weighting for missingness
Older adults with dementia compared with those without dementia are likely to use more medicines and experience harm (1). Of particular concern is use of potentially inappropriate medications (PIMs), as older adults with dementia compared with those without dementia are more likely to experience a pronounced sensitivity to adverse drug events. A number of reasons may account for PIM use among people with dementia including inadequate guidelines, lack of time during physician–patient encounters, diminished decision-making capacity, difficulties with comprehension and communication, and difficulties in establishing goals of care (2).
There is an increasing body of evidence on PIM use among selected populations of older people with dementia residing in nursing homes, community, and the acute care settings. According to an explicit list of medications with questionable benefit in people with advanced dementia, 53.9% of nursing home residents received at least one medication of questionable benefit (3). A recent Scottish study found that patients with dementia had greater comorbidity and polypharmacy than other primary care patients, even after adjusting for age and sex (4). These findings are consistent with a Danish patient registry study in which dementia patients had 50% higher direct treatment costs compared with matched controls, in addition to greater morbidity and greater use of medications prior to dementia diagnosis (5). A large-scale observational study across eight European countries including community-dwelling and nursing home populations of adults with dementia found that 60% had at least one PIM prescription, assessed using the European Union (7)-PIM list, and 26.4% at least two (6). Moreover, use of two PIMs was associated with increased risk of fall-related injury and hospitalization over 3 months. Use of PIMs is also common among older adults with dementia admitted to hospital. In the acute care setting, a systematic review of observational studies investigating PIM use in older inpatients with cognitive impairment found that PIM prevalence ranged from 20.6% to 80.5% (7).
Although a number of studies have examined the use of PIM medications and high-risk medications among older adults with dementia (4,6,7), and use of antipsychotics in relation to the diagnosis of Alzheimer’s disease (AD) (8), at present, there is lack of evidence as to whether a diagnosis of dementia may affect medication use, specifically if a diagnosis of dementia would increase the likelihood of PIM use. This is of clinical importance as it may help to identify whether interventions to improve PIM prescribing should be implemented following dementia diagnosis. Therefore, the aim of this study was to assess the patterns of medication use 1-year before dementia diagnosis and 1-year after dementia diagnosis compared with patterns of medication use among people without dementia.
Methods
Study Design and Population
The National Alzheimer’s Coordinating Center (NACC) was established in 1999 through the funding by the National Institute on Aging. Since the inception of the Uniform Data Set (UDS) in 2005, past and present Alzheimer’s Disease Centers (ADCs) throughout the United States collected self-reported information and conducted standardized cognitive and behavioral assessments in participants with the full range of cognitive functioning, from normal to dementia. Participants were recruited through clinician or self-referral (patients or family members) or through active community recruitment strategies following ADC-specific recruitment protocols. Detailed descriptions of the cohort and various instruments and assessments used for data collection are available elsewhere (9–11). Briefly, NACC UDS data include information on sociodemographic characteristics, family history, medical history, and medication use. In addition, participants underwent cognitive and neuropsychological evaluations using validated instruments (9,11).
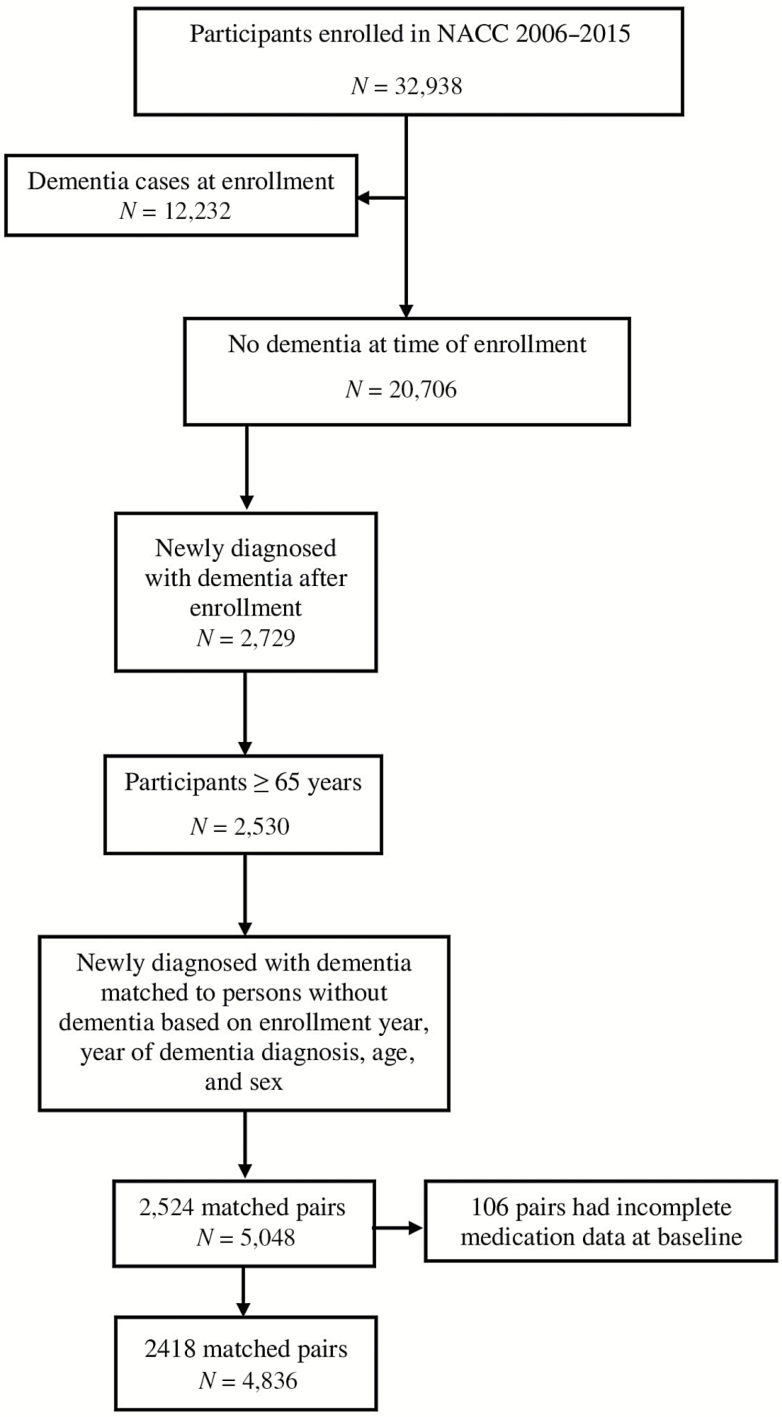
For this study, we used enrollment and yearly follow-up visit data collected between UDS inception and the December 2015 data freeze by 34 ADCs. Our design matched participants with incident dementia to participants without dementia to estimate the patterns of medication use from the year before the diagnosis, to the year of diagnosis, and the year after diagnosis. Of the 32,938 community-dwelling participants enrolled at ADCs during study period, 2,729 participants were newly diagnosed with dementia during cohort follow-up (ie, free of dementia at enrollment), of which 2,530 were 65 years and older (Figure 1). We matched participants diagnosed with incident dementia to participants without dementia (controls) using incidence density sampling. Risk sets were created by year of dementia diagnosis (index year), such that a participant who was selected as a control could be later diagnosed with dementia. People with dementia were exactly matched to the controls by enrollment year, year at diagnosis, age, and sex. Using this algorithm, 2,524 out of 2,530 incident dementia cases were matched with a control. Of these, in 106 pairs, medication data was incomplete. Therefore, the analytic sample consisted of 2,418 matched pairs (4,836 participants) as shown in Figure 1. In the matched sample, 2,396 dementia cases and 2,409 controls had at least one follow-up assessment after the index visit.
Figure 1.
Selection of study population.
Dementia Diagnosis
Participants in our study were deemed to have dementia if they met the standard criteria for dementia of the AD type or for other non-AD-related dementias based on comprehensive neuropsychiatric battery or cognitive assessment with a trained ADC clinician, as previously published (11–13). A diagnosis of AD was considered if the participant met the criteria for dementia and had probable AD as the primary clinical diagnosis based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) criteria (14).
Medication Exposure
Medication exposure was measured using a systematic medication review approach (ie, the participant or a family member was asked to bring all the medications or a list of medications to the annual assessment) to capture prescription and over-the-counter medications for the 2-week window preceding the yearly visit (11). Medication use was assessed using three approaches: (a) number of medications at each visit; (b) exposure to anticholinergic drugs measured using the updated version of the Anticholinergic Drug Scale (ADS) (15). For this study, we only considered ADS medication exposure as opposed to the ADS, as less than 45% of participants at baseline reported any ADS medication; and (c) PIM exposure measured using the updated 2015 Beers Criteria list of medications independent of diseases (16).
Participant Characteristics
Sociodemographic characteristics included type of residence (single-family residence, retirement community, assisted living or nursing home, or other), race (white, black, or other), level of education (high-school degree or less, college education, or graduate education), living situation (lives alone, lives with spouse or partner, lives with relatives or friend, lives with group, or other), level of independence (able to live independently, requires assistance, or completely dependent) years of cigarette smoking and body mass index. Comorbidities included severity of depressive symptoms measured using the Geriatric Depression Scale (0–4, 5–9, 10–15), the presence of psychiatric disorder (delirium, attention deficit hyperactive disorder, anxiety, bipolar disorder, bulimia, depression, dysthemia, episode of AD), the presence of behavioral symptoms (delusions, hallucinations, and agitation or aggression), cardiovascular disease (myocardial infarction, atrial fibrillation, angioplasty, past coronary artery bypass grafting surgery, current pace maker implantation, congestive heart failure, or other cardiovascular disease), stroke, diabetes, urinary incontinence (absent, recent/active, remote/inactive, or unknown), and transient ischemic attacks. Cognitive evaluation information was assessed using the Clinical Dementia Rating (CDR) global score conducted through an interview by a clinician (17). In our study to account for the severity of dementia, we included the CDR score categorized as no impairment (CDR = 0), questionable impairment (CDR = 0.5), mild impairment (CDR = 1), and moderate to severe impairment (CDR = 2 or 3).
Statistical Analysis
Baseline characteristics were summarized with means and SD for continuous variables and the difference between the groups was tested using t test. Categorical variables were summarized with frequencies and percentages, and the differences were tested using Pearson chi-square test. Number of medications reported 1-year prediagnosis, at diagnosis visit (index year), and 1-year postdiagnosis were summarized using means and percentiles. Exposure to any ADS medication and exposure to any PIM medication were summarized with frequencies and percentages.
To account for missing data due to attrition (eg, death or loss to follow-up), we used weighted generalized estimating equation (GEE) models to investigate patterns of medication use in the year prior to a diagnosis of dementia, at the time of the diagnosis (index year), and the year after the diagnosis. The GEE models account for the within-person correlation and the weights account for attrition (18). To select covariates associated with missing data, we fitted a backward selection logistic regression model with all observed potential predictors of attrition and retained covariates with p value less than or equal to 0.2. The weights, defined as the inverse probability of attrition, were estimated from a logistic regression model with an attrition indicator as the dependent variable and time of visit, medication value at previous visit, dementia diagnosis, sex, transient ischemic attacks, stroke, diabetes, and presence of psychiatric disorder as the covariates.
Each of the weighted outcome models was adjusted for age, sex, living situation, type of residence, psychiatric disorder, cardiovascular disease, transient ischemic attack, stroke, diabetes, years of cigarettes smoking, global dementia rating, body mass index, severity of depression, urinary incontinence, time of visit, and the interaction between dementia diagnosis and time of visit. This interaction allowed comparisons between those with and without dementia, as well as between yearly observations for those with and without dementia. All models were selected based on quasi-information criteria. For the outcome of the number of medications, a weighted Poisson GEE model with a log link function and an exchangeable within-person correlation was used to estimate adjusted relative risks (aRR) as risk ratios. For the outcomes of exposure to any ADS medications and exposure to any PIMs, a weighted logistic GEE regression model with logit link function and exchangeable working correlation was used to estimate adjusted odds ratios (aOR). Data analysis was performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
The descriptive statistics for baseline characteristics by incident dementia diagnosis are presented in Table 1. The mean age of the sample participants was 79 years, with the majority being white, living with a spouse or partner in a single-family residence. The prevalence of the major comorbidities, mainly cardiovascular disease, was similar in participants with and without dementia. Medication exposures according to dementia diagnosis 1-year before diagnosis, at diagnosis, and 1-year after diagnosis are presented in Table 2. At diagnosis year, exposure to PIMs was identified in 66.0% of people with dementia compared with 71.8% of people without dementia. A list of the most prevalent PIMs is provided in Supplementary Table 1.
Table 1.
Demographic and Clinical Characteristics of Study Population at Baseline Visit
| Baseline Characteristics | Dementia (N = 2,418) | No Dementia (N = 2,418) | p Valuea |
|---|---|---|---|
| Age, mean (SD) | 78.2 (7.4) | 78.8 (7.5) | NA |
| Female, N (%) | 1,256 (51.9) | 1,256 (51.9) | |
| Race, N (%) | |||
| White | 2,066 (85.4) | 2,013 (83.3) | |
| Black | 258 (10.7) | 347 (14.4) | |
| Other | 87 (3.6) | 56 (2.3) | <.0001 |
| Education, N (%) | |||
| High school or less | 630 (26.1) | 491 (20.3) | |
| College | 1,021 (42.2) | 1,045 (43.2) | |
| Graduate | 760 (31.4) | 874 (36.2) | <.0001 |
| Living status, N (%) | |||
| Lives alone | 658 (27.2) | 878 (36.3) | |
| Lives with spouse or partner | 1,504 (62.2) | 1,350 (55.8) | |
| Other living arrangements | 256 (10.6) | 188 (7.8) | <.0001 |
| Type of residence, N (%) | |||
| Single-family residence | 2,052 (84.9) | 2,046 (84.6) | |
| Retirement community | 239 (9.9) | 267 (11.0) | |
| Assisted living | 77 (3.2) | 26 (1.1) | |
| Other | 50 (2.1) | 79 (3.3) | <.0001 |
| Comorbidities, N (%) | |||
| Psychiatric disorder | 172 (7.1) | 124 (5.1) | .004 |
| Cardiovascular disease | 1,731 (71.6) | 1,748 (72.3) | .586 |
| Transient ischemic attack | 203 (8.4) | 168 (7.0) | .059 |
| Stroke | 204 (8.4) | 131 (5.4) | <.0001 |
| Diabetes | 359 (14.9) | 313 (12.9) | .056 |
| Body mass index (kg/m2), mean (SD) | 24.4 (7.5) | 25.7 (6.9) | <.0001 |
| Years of smoked cigarettes, mean (SD) | 11.0 (16.0) | 11.8 (15.8) | .064 |
| Urinary incontinence, N (%) | |||
| Absent | 1,870 (77.3) | 1,912 (79.1) | |
| Recent/active | 461 (19.1) | 401 (16.6) | |
| Remote/inactive | 84 (3.5) | 96 (4.0) | .069 |
| Global Clinical Dementia Rating, N (%) | |||
| No impairment | 230 (9.5) | 1,606 (66.4) | |
| Questionable impairment | 2,066 (85.4) | 808 (33.4) | |
| Mild impairment | 116 (4.8) | 4 (0.2) | |
| Moderate impairment | 6 (0.3) | 0 (0.0) | <.0001 |
| Geriatric Depression Scale, categories | |||
| 0–4 (normal) | 1,957 (82.5) | 2,193 (91.2) | |
| 5–9 (mild) | 331 (14.0) | 187 (7.8) | |
| 10–15 (moderate to severe) | 75 (3.2) | 22 (0.9) | <.0001 |
| Dementia diagnosis | NA | NA | |
| Alzheimer’s disease | 2,075 (85.8) | ||
| Lewy-body dementia | 136 (5.6) | ||
| Vascular dementia | 92 (3.8) | ||
| Frontotemporal dementia | 30 (1.2) | ||
| Other | 85 (3.5) | ||
Note: NA = not applicable.
a p Values for categorical variables were calculated using Pearson chi-square test and for continuous variables using t test.
Table 2.
Description of Medication Exposure, Cognitive Function, Behavioral Symptoms, and Level of Independence Among Participants With and Without Dementia
| 1-Year Before Index Year | At Diagnosis (Index Year) | 1-Year After Index Year | ||||
|---|---|---|---|---|---|---|
| Dementia (N = 2,418) | No Dementia (N = 2,418) | Dementia (N = 2,396) | No Dementia (N = 2,409) | Dementia (N = 1,488) | No Dementia (N = 1,853) | |
| Medication exposures | ||||||
| Number of medications, mean (SD) | 6.8 (4.3) | 6.6 (4.2) | 7.4 (4.1) | 7.2 (4.4) | 8.2 (4.2) | 7.6 (4.2) |
| No medication use, N (%) | 96 (4.0) | 112 (4.6) | 41 (1.7) | 78 (3.2) | 20 (1.3) | 28 (1.5) |
| Exposure to any ADS medications, N (%) | 1,083 (44.8) | 946 (39.1) | 1,170 (48.8) | 973 (40.4) | 770 (51.8) | 764 (41.2) |
| Exposed to PIMs, N (%) | 1,482 (61.3) | 1,663 (68.8) | 1,595 (66.0)a | 1,735 (71.8) a | 1,041 (69.4)b | 1,380 (74.2)c |
| Cognitive function | ||||||
| Clinical Dementia Rating sum of scores, mean (SD) | 1.91 (1.44) | 0.43 (0.78) | 4.29 (2.61) | 0.52 (0.91) | 5.53 (3.38) | 0.70 (1.44) |
| Behavioral symptoms, N (%) | ||||||
| Delusions | 121 (5.00) | 14 (0.58) | 244 (10.09) | 24 (0.99) | 194 (12.93) | 21 (1.13) |
| Hallucinations | 46 (1.90) | 6 (0.25) | 131 (5.42) | 9 (0.37) | 100 (6.67) | 13 (0.70) |
| Agitation or aggression | 502 (20.76) | 207 (8.56) | 683 (28.25) | 239 (9.88) | 454 (30.27) | 184 (9.89) |
| Level of independence, N (%) | ||||||
| Able to live independently | 1,447 (59.8) | 2,214 (91.6) | 837 (34.6) | 2,145 (88.7) | 341 (22.7) | 1,588 (85.4) |
| Requires assistance | 954 (39.5) | 199 (8.2) | 1,501 (62.1) | 265 (10.0) | 1,069 (71.3) | 266 (14.3) |
| Completely dependent | 11 (0.5) | 3 (0.1) | 71 (2.9) | 5 (0.2) | 88 (5.9) | 6 (0.3) |
Note: ADS = Anticholinergic Drug Scale; PIM = potentially inappropriate medication.
a N = 2,418 for those exposed to PIMs. bN = 1,500 for those exposed to PIMs. cN = 1,860 for those exposed to PIMs.
Medication Patterns for Participants Diagnosed With Dementia
Analyses comparing the medication exposures 1-year prediagnosis versus at diagnosis visit (index year) and 1-year postdiagnosis versus at diagnosis visit are presented in Table 3. The number of medications reported 1-year prediagnosis was 8% lower on average relative to the number of medications reported at diagnosis visit (aRR: 0.92; p < .0001). Furthermore, the number of medications reported 1-year postdiagnosis was 11% higher on average relative to the number of medications reported at diagnosis visit (aRR: 1.11; p < .0001). For exposure to ADS medications, there were no significant differences prediagnosis or postdiagnosis relative to at diagnosis visit. The odds of exposure to PIMs reported 1-year prediagnosis was 17% lower than the odds of exposure at diagnosis visit (aOR: 0.83; p < .0001). The odds of exposure to PIMs reported 1-year postdiagnosis was 17% higher relative to the odds of exposure at diagnosis visit (aOR: 1.17; p = .006).
Table 3.
Patterns of Medication Use Before and After Dementia Diagnosis Within Dementia and Time-matched Controls (Without Dementia)
| Medication Exposurea | Annual Comparisons | Dementia | p Value | No Dementia | p Value |
|---|---|---|---|---|---|
| Number of medicationsb,c | aRR (95% CI) | aRR (95% CI) | |||
| 1-year before vs. at diagnosis | 0.92 (0.89, 0.94) | <.0001 | 0.93 (0.91, 0.95) | <.0001 | |
| 1-year after vs. at diagnosis | 1.11 (1.08, 1.14) | <.0001 | 1.06 (1.04, 1.08) | <.0001 | |
| Exposure to ADS medicationsd,e | aOR (95% CI) | aOR (95% CI) | |||
| 1-year before vs. at diagnosis | 0.90 (0.81, 1.00) | .06 | 1.02 (0.94, 1.10) | .68 | |
| 1-year after vs. at diagnosis | 0.98 (0.88, 1.09) | .69 | 1.01 (0.93, 1.10) | .78 | |
| Exposure to PIMsd,e | aOR (95% CI) | aOR (95% CI) | |||
| 1-year before vs. at diagnosis | 0.83 (0.75, 0.91) | <.0001 | 0.89 (0.82, 0.97) | .006 | |
| 1-year after vs. at diagnosis | 1.17 (1.05, 1.32) | .006 | 1.11 (1.00, 1.23) | .047 | |
Note: ADS = Anticholinergic Drug Scale; aOR = adjusted odds ratios; aRR = adjusted risk ratios; CI = confidence interval; GEE = generalized estimating equation; PIM = potentially inappropriate medication.
aThe models were adjusted for age, gender, living situation, type of residence, psychiatric disorder, cardiovascular disease, transient ischemic attack, stroke, diabetes, years smoked cigarettes, global dementia rating, body mass index, urinary incontinence, severity of depression, time of visit, and the interaction between dementia diagnosis and time of visit. bFor number of medications, the risk estimates are aRR. cPoisson GEE model was used for number of medications, weighted by inverse probability of dropout 1-year after diagnosis. dFor exposure to ADS medications and exposure to PIMs, the risk estimates are aOR. eLogistic GEE models weighted by inverse probability of dropout 1-year after diagnosis were used for the exposures to ADS medications and PIMs.
Medication Patterns for Participants Without Dementia
Among the matched-control participants without dementia, the number of medications reported 1-year before the index year was 7% lower relative to the number of medications reported a year later (aRR: 0.93; p < .0001; Table 3). Furthermore, the number of medications reported 1-year after the index year was 6% higher relative to the number of medications reported at the index year’s visit (aRR: 1.06; p < .0001). The odds of exposure to ADS medications was not significant at either annual comparison. The odds of exposure to PIMs reported 1-year before the index year was 11% lower than the odds of exposure at the index year’s visit (aOR: 1.11, p = .006). The odds of exposure to PIMs reported 1-year after the index year was 11% higher than the odds of exposure at the index year’s visit (aOR: 1.11; p = .047).
Comparisons of Participants With and Without Dementia at Each Annual Visit
Comparisons of the mean number of medications for participants diagnosed with dementia and their matched controls without dementia are showed in Table 4. There was no significant difference between the 1-year before and at the index year’s visit. However, the number of medications 1-year after the index year for those with dementia was 7% higher than the reported number of medications for those without dementia (aRR: 1.07; p = .004). There was no significant difference in the odds of exposure to any ADS medication at the three annual visits. The odds of exposure to PIMs among participants diagnosed with dementia was 28% lower 1 year before diagnosis (aOR: 0.72; p < .0001), 23% lower at diagnosis visit (aOR: 0.77; p = .0007), and 18% lower 1 year after diagnosis (aOR: 0.82; p = .03) than the odds of exposure among participants without dementia.
Table 4.
Patterns of Medication Use in Participants Diagnosed With dementia Compared With Time-matched Controls (Without Dementia) Before, at, and After Diagnosis
| Medication Exposurea | 1 Y Before Dementia Diagnosis | p Value | At Dementia Diagnosis (Index Year) | p Value | 1 Y After Dementia Diagnosis | p Value |
|---|---|---|---|---|---|---|
| Number of medicationsb,c | aRR (95% CI) | aRR (95% CI) | aRR (95% CI) | |||
| 1.01 (0.97, 1.07) | .55 | 1.03 (0.98, 1.08) | .285 | 1.07 (1.02, 1.12) | .004 | |
| Exposure to ADS medicationsd,e | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |||
| 1.04 (0.89, 1.22) | .61 | 1.17 (0.99, 1.39) | .06 | 1.13 (0.94, 1.37) | .19 | |
| Exposure to PIMse | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |||
| 0.72 (0.63, 0.82) | <.0001 | 0.77 (0.67, 0.90) | .0007 | 0.82 (0.68, 0.98) | .03 |
Note: ADS = Anticholinergic Drug Scale; aRR = adjusted odds ratios; aRR = adjusted risk ratios; CI = confidence interval; GEE = generalized estimating equation; PIM = potentially inappropriate medication.
aThe models were adjusted for age, gender, living situation, type of residence, psychiatric disorder, cardiovascular disease, transient ischemic attack, stroke, diabetes, years smoked cigarettes, global dementia rating, body mass index, urinary incontinence, severity of depression, time of visit, and the interaction between dementia diagnosis and time of visit. bFor number of medications, the risk estimates are aRR. cPoisson GEE model was used for number of medications weighted by inverse probability of dropout 1 y after diagnosis. dFor exposure to ADS medications and exposure to PIMs, the risk estimates are adjusted odds ratios. eLogistic GEE models weighted by inverse probability of dropout 1 y after diagnosis were used for the exposures to ADS medications and PIMs.
Discussion
To our knowledge, this is the first study to compare patterns of PIM use before and after dementia diagnosis with matched controls. Our study has four primary findings. First, overall medication burden among individuals with dementia was significantly higher (7%) 1-year postdementia diagnosis compared with individuals without dementia in the same year. Second, PIMs exposure increased significantly over time in individuals with and without dementia. Third, PIMs exposure was significantly lower for those diagnosed with dementia compared with individuals without dementia at all time points. Fourth, exposure to anticholinergic medications was not significantly different in individuals with dementia when compared with individuals without dementia, and it did not increase over time regardless of cognitive status.
The findings of this study show that overall medication use increased over time among older adults with and without dementia. This is not surprising as it has been well documented that medication use increases with age (19). Moreover, among older adults with dementia, overall medication burden was higher compared with older adults without dementia in the year after diagnosis of dementia. This might be due to the initiation of antidementia medications following the diagnosis of dementia, as well as the fact that the average patient with dementia has several other chronic conditions that require other medications, and typically receives care from five different providers annually (20). However, the finding that older adults with dementia are less likely to use PIMs over time compared with older adults without dementia is somewhat unexpected, and in contrast to prior research (4). It may be that addition of new diagnosis among individuals with other chronic conditions triggers ongoing review of the quality of care by treating clinicians, and subsequent limited prescribing of PIMs to this high-risk patient group. Regardless of this finding, the persistent challenge of increasing PIM use among older adults still remains, and it warrants increasing interventions do reduce such prescribing.
Interestingly, in this study, use of anticholinergic medicines did not differ significantly over time or according to dementia diagnosis status. This finding could be due to a combination of reasons. It may be that clinicians are better at recognizing specific anticholinergic medications, such as antipsychotics and antidepressants as opposed to monitoring all PIMs (eg, proton pump inhibitors, antispasmodics), which includes a range of therapeutic classes. Moreover, it could be due to the tool chosen to measure anticholinergic burden, which are variably defined (21). Although many of PIMs and medications with anticholinergic effects may have an important clinical indication, there is growing evidence that the deleterious use of these medications may also outweigh the benefits among people with dementia. We have previously shown that cumulative exposure to anticholinergic and sedative medications is associated with increased risk of hospitalization and mortality among older adults with AD (22). Use of antipsychotics (23) and antidepressants (24) has been linked to increased risk of hip fracture among older adults with AD. Others have reported that risk of dementia is slightly higher in people with minimal exposure to benzodiazepines but not with the highest level of exposure (25). In another study, cumulative use of anticholinergic medications was associated with an increased risk for dementia (26). As such, although it may not be possible to deprescribe all PIMs or anticholinergic medications, selective deprescribing of medications that have no clear indication or reducing the dose may minimize potential risk associated with these medications. Indeed, a recent study that enrolled participants from the University of Kentucky ADC cohort specifically targeted anticholinergic medications and showed that a patient-centered multidisciplinary intervention can reduce anticholinergic burden (27). In our recent study, we found that individuals with Lewy-body dementia reported higher PIM use than those with AD, whereas those with frontotemporal dementia reported lower PIM use (28). Therefore, tailored deprescribing intervention targeting PIM use among individuals with specific dementia diagnosis may be warranted.
Strengths of this study include that NACC data are collected in a standardized manner at ADCs throughout the United States and validated instruments are used to collect patient-reported information and to conduct in-depth cognitive evaluations in all participants. In addition, we used matching, adjusted analyses for many potential confounders, as well as weighted GEE modeling approach that addresses missing data, thus reducing bias. Alternatively, the missing data can jointly model the medication outcome with the probability of missingness (29). Limitations of using NACC data stems from the collection of data at enrollment and yearly after and medications may have changed throughout the year. The findings of our observational study may not be generalizable to other populations as NACC is not a nationally representative data repository. In particular, our population may not be representative of the entire U.S. population as NACC participants are generally more educated and more likely to receive care in academic hospitals and clinics. Therefore, careful consideration is needed when generalizing our findings to all older adults with and without dementia. Causality in changes in patterns of medication use cannot be attributed to a dementia diagnosis, but may in part be motivated by the dementia diagnosis. Other factors, such as new or worsening severity of conditions or adverse effects of medications, may explain these changes. In relation to medication data, given that medication use asked about current medications taken by the participant (ie, within 14 days of the visit), we could not ascertain medication exposure that occurred between visits. Participants may have been misclassified as non-users if they started and stopped treatment between two consecutive visits. In terms of using the Beers Criteria, we only considered drug list as oppose to drug–disease interactions, which may have underestimated PIM exposure.
Conclusion
Our findings suggest that number of medications increases annually among older adults with and without dementia. Moreover, the use of PIMs increases over time and is significantly lower among older adults with dementia compared with individuals without dementia. However, we did observe an increase in PIM use after dementia diagnosis. Further efforts are clearly needed to support better recognition of PIMs to minimize possible harms, and to ensure that clinicians are being cautious, and are individualizing therapies sufficiently in this high-risk population.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by grants (R01 AG054130 DM, R01 AG047891 to H.A., G.O.A.) and by the Yale Pepper Center (P30 AG021342, P30 AG021342 H.A., G.O.A.), Yale Alzheimer’s Disease Research Center, Data Management and Statistics Core (P50 AG047270 H.A., C.M.R.) all from the National Institutes of Health/National Institute on Aging. D.G. is supported by the Australian National Health Medical Research Council Dementia Leadership Fellowship (1136849) and International Profile Development Fund grant, University of Sydney.
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Conflict of Interest
None reported.
References
- 1. Parsons C. Polypharmacy and inappropriate medication use in patients with dementia: an underresearched problem. Ther Adv Drug Saf. 2017;8:31–46. doi:10.1177/2042098616670798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reeve E, Bell JS, Hilmer SN. Barriers to optimising prescribing and deprescribing in older adults with dementia: a narrative review. Curr Clin Pharmacol. 2015;10:168–177. [DOI] [PubMed] [Google Scholar]
- 3. Tjia J, Briesacher BA, Peterson D, Liu Q, Andrade SE, Mitchell SL. Use of medications of questionable benefit in advanced dementia. JAMA Intern Med. 2014;174:1763–1771. doi:10.1001/jamainternmed.2014.4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clague F, Mercer SW, McLean G, Reynish E, Guthrie B. Comorbidity and polypharmacy in people with dementia: insights from a large, population-based cross-sectional analysis of primary care data. Age Ageing. 2017;46:33–39. doi:10.1093/ageing/afw176 [DOI] [PubMed] [Google Scholar]
- 5. Frahm-Falkenberg S, Ibsen R, Kjellberg J, Jennum P. Health, social and economic consequences of dementias: a comparative national cohort study. Eur J Neurol. 2016;23:1400–1407. doi:10.1111/ene.13043 [DOI] [PubMed] [Google Scholar]
- 6. Renom-Guiteras A, Thürmann PA, Miralles R, et al. ; RightTimePlaceCare Consortium Potentially inappropriate medication among people with dementia in eight European countries. Age Ageing. 2018;47:68–74. doi:10.1093/ageing/afx147 [DOI] [PubMed] [Google Scholar]
- 7. Redston MR, Hilmer SN, McLachlan AJ, Clough AJ, Gnjidic D. Prevalence of potentially inappropriate medication use in older inpatients with and without cognitive impairment: a systematic review. J Alzheimers Dis. 2018;61:1639–1652. doi:10.3233/JAD-170842 [DOI] [PubMed] [Google Scholar]
- 8. Koponen M, Tolppanen AM, Taipale H, et al. Incidence of antipsychotic use in relation to diagnosis of Alzheimer’s disease among community-dwelling persons. Br J Psychiatry. 2015;207:444–449. doi:10.1192/bjp.bp.114.162834 [DOI] [PubMed] [Google Scholar]
- 9. Beekly DL, Ramos EM, van Belle G, et al. ; NIA-Alzheimer’s Disease Centers. The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18:270–277. [PubMed] [Google Scholar]
- 10. Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi:10.1097/WAD.0b013e318191c7dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi:10.1097/01.wad.0000213865.09806.92 [DOI] [PubMed] [Google Scholar]
- 12. Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol. 2012;71:266–273. doi:10.1097/NEN.0b013e31824b211b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 14. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 15. Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481–1486. doi:10.1177/0091270006292126 [DOI] [PubMed] [Google Scholar]
- 16. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227–2246. doi:10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 17. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 18. Gosho M. Model selection in the weighted generalized estimating equations for longitudinal data with dropout. Biom J. 2016;58:570–587. doi:10.1002/bimj.201400045 [DOI] [PubMed] [Google Scholar]
- 19. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818–1831. doi:10.1001/jama.2015.13766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, Medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52:187–194. [DOI] [PubMed] [Google Scholar]
- 21. Gnjidic D, Tinetti M, Allore HG. Assessing medication burden and polypharmacy: finding the perfect measure. Expert Rev Clin Pharmacol. 2017;10:345–347. doi:10.1080/17512433.2017.1301206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gnjidic D, Hilmer SN, Hartikainen S, et al. Impact of high risk drug use on hospitalization and mortality in older people with and without Alzheimer’s disease: a national population cohort study. PLoS One. 2014;9:e83224. doi:10.1371/journal.pone.0083224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koponen M, Taipale H, Lavikainen P, et al. Antipsychotic use and the risk of hip fracture among community-dwelling persons with Alzheimer’s disease. J Clin Psychiatry. 2017;78:e257–e263. doi:10.4088/JCP.15m10458 [DOI] [PubMed] [Google Scholar]
- 24. Torvinen-Kiiskinen S, Tolppanen AM, Koponen M, et al. Antidepressant use and risk of hip fractures among community-dwelling persons with and without Alzheimer’s disease. Int J Geriatr Psychiatry. 2017;32:e107–e115. doi:10.1002/gps.4667 [DOI] [PubMed] [Google Scholar]
- 25. Gray SL, Dublin S, Yu O, et al. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ. 2016;352:i90. doi:10.1136/bmj.i90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175:401–407. doi:10.1001/jamainternmed. 2014.7663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moga DC, Abner EL, Rigsby DN, et al. Optimizing medication appropriateness in older adults: a randomized clinical interventional trial to decrease anticholinergic burden. Alzheimers Res Ther. 2017;9:36. doi:10.1186/s13195-017-0263-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramsey CM, Gnjidic D, Agogo GO, Allore H, Moga D. Longitudinal patterns of potentially inappropriate medication use following incident dementia diagnosis. Alzheimers Dement (NY). 2018;4:1–10. doi:10.1016/j.trci.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agogo GO, Ramsey CM, Gnjidic D, Moga D, Allore H. Longitudinal associations between different dementia diagnoses and medication use jointly accounting for dropout. Int Psychogeriatr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.