Abstract
STUDY QUESTION
What are the impacts of elevated testosterone (T) and an obesogenic western-style diet (WSD), either independently or together, on fertility and metabolic adaptations of pregnancy in primates?
SUMMARY ANSWER
Testosterone increases the time to achieve pregnancy, while a WSD reduces overall fertility, and the combination of testosterone and WSD additionally impairs glucose tolerance and causes pregnancy loss.
WHAT IS KNOWN ALREADY
Both hyperandrogenemia and obesity are hallmarks of polycystic ovary syndrome, which is a leading cause of infertility among women worldwide. Female macaques receiving T and WSD beginning at puberty show increased metabolic, ovarian and uterine dysfunction in the non-pregnant state by 3 years of treatment.
STUDY DESIGN, SIZE, DURATION
The same cohort of female rhesus macaques continued treatments from the time of puberty (2.5 years) to 4 years, including this fertility trial. There were four groups (n = 9–10/group): controls (C), T-treated (T; average total serum level 1.35 ng/ml), WSD-treated, and combined T and WSD-treated (T + WSD) females.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Females, which were typically having menstrual cycles, were paired for 4 days with a proven male breeder following the late follicular rise in circulating estradiol (≥100 pg/ml). The presence of sperm in the reproductive tract was used to confirm mating. Animals went through up to three successive rounds of mating until they became pregnant, as confirmed by a rise in circulating mCG during the late luteal phase and ultrasound evidence of a gestational sac at Day 30 post-mating (GD30). Placental vascular parameters were also measured at GD30. Metabolic measurements consisted of fasting levels of blood glucose and insulin at approximately GD30, 60, 90 and 115, as well as an intravenous (iv) glucose tolerance test (GTT) at GD115.
MAIN RESULTS AND THE ROLE OF CHANCE
While all animals in the C and T groups eventually became pregnant, T-treated females on average had a greater interval to achieve pregnancy (P < 0.05). However, only ~70% of animals in the WSD and T + WSD groups became pregnant (P < 0.004). One pregnancy in T + WSD group resulted in an anembryonic pregnancy which miscarried around GD60, while another T + WSD female conceived with a rare identical twin pregnancy which required cessation due to impending fetal loss at GD106. Thus, the number of viable fetuses was less in the T + WSD group, compared to C, T or WSD. Placental blood volume at GD30 was reduced in all treatments compared to the C group (P < 0.05). Maternal P4 levels were elevated in the WSD (P < 0.03) group and E2 levels were elevated in T + WSD animals (P < 0.05). An increase in serum A4 levels throughout gestation was observed in all groups (P < 0.03) except WSD (P = 0.3). All groups displayed increased insulin resistance with pregnancy, as measured from the ivGTT during pregnancy. However, only the T + WSD group had a significant increase in fasting glucose levels and glucose clearance during the GTT indicating a worsened glucose tolerance. WSD treatment decreased female fetuses third trimester weights, but there was an interaction between WSD and T to increase female fetal weight when normalized to maternal weight.
LARGE SCALE DATA
N/A.
LIMITATIONS REASONS FOR CAUTION
The small number of pregnancies in the WSD and T + WSD groups hampers the ability to make definitive conclusions on effects during gestation. Also, the high fertility rate in the controls indicates the cohort was at their breeding prime age, which may impair the ability to observe subtle fertility defects. The low number of fetuses used for male and female analysis requires additional studies.
WIDER IMPLICATIONS OF THE FINDINGS
The current findings strongly suggest that both hyperandrogenemia and obesity have detrimental effects on fertility and gestation in primates, which may be directly relevant to women with polycystic ovary syndrome.
STUDY FUNDING/COMPETING INTEREST(S)
All ONPRC Cores and Units were supported by NIH Grant P51 OD011092 awarded to ONPRC. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) of the National Institutes of Health (NIH) under Award Number P50HD071836 (to R.L.S.). The authors have no competing conflict of interests to disclose.
Keywords: hyperandrogenemia, obesity, PCOS, fertility, glucose tolerance, maternal obesity, gestational diabetes
Introduction
Polycystic ovary syndrome (PCOS) is the leading cause of infertility among reproductive-age women worldwide (Goodarzi and Azziz, 2006; Sirmans et al., 2014), but the underlying mechanism of this infertility remains unclear. Among the PCOS population, there is significant heterogeneity in the presentation of the disorder, which likely hampers the ability to define the cause(s) of infertility. While elevated androgens are widely considered a hallmark of the disease, this symptom is not required for diagnosis of PCOS using the Rotterdam criteria (Rotterdam, 2004). Hyperandrogenism may contribute to higher rates of pregnancy complications in normal weight women with PCOS, including greater risk of preterm birth, gestational diabetes, and cardiovascular complications (de Wilde et al., 2017; Sterling et al., 2016). Many of the analyses have been performed with data from infertility patient populations seeking therapies, which may provide a confounding factor (Roesner et al., 2017). Additionally, large-scale studies of pregnancy outcomes in women with PCOS are complicated by the high rate of obesity in this population (Palomba et al., 2016).
Metabolic dysfunction is another symptom commonly observed in a significant subpopulation of PCOS patients. Rates of insulin resistance and/or obesity in PCOS patients is 50–80% (Legro et al., 2001; Ovalle and Azziz, 2002), which is notably much higher than that observed in control populations. Obesity during pregnancy is associated with worsened metabolic outcomes, particularly hyperinsulinemia, gestational diabetes mellitus and endothelial dysfunction compared to controls (Ramsay et al., 2002; Chu et al., 2007; Catalano, 2010; Guelinckx et al., 2008). Obesity is also associated with numerous complications of pregnancy including increased rates of preeclampsia, spontaneous abortion and emergency cesarean section (Balen and Anderson, 2007; Sebire et al., 2001). Weight loss in obese PCOS patients improves fertility as does treatment with the insulin sensitizer metformin, indicating that insulin resistance particularly influences fertility in PCOS patients (Pasquali et al., 1997).
These data indicate that hyperandrogenemia and obesity may both exert negative effects on fertility, but the role each plays in PCOS pathophysiology, and whether there is interaction between these two factors remains unclear. Teasing apart the differential effects of androgen and obesity is particularly difficult to do clinically because of the heterogeneity of these symptoms in the PCOS patient population. Recently, we developed a non-human primate model to specifically examine the effects of elevated androgens and an obesogenic western-style diet (WSD), and the interaction between these two factors, on both reproductive and metabolic health. Treatment beginning at puberty, to mimic parameters in adolescent girls at risk for PCOS (McCartney et al., 2007), demonstrated that testosterone (T) exposure, regardless of diet, negatively impacted decidualization in the uterus (Bishop et al. 2017b), which could potentially impair implantation during pregnancy. In addition, both T and/or WSD increased the occurrence of a PCO-like ovarian morphology and the numbers of poor quality/degenerated oocytes recovered from preovulatory follicles (Bishop et al. 2017a, 2017b). Also, the combination of T + WSD caused increased weight gain, fat mass gained (especially in the android region) and mild insulin resistance compared to either treatment alone, which could also hamper fertility (True et al., 2017). Despite continued menstrual cyclicity (with some changes in hormonal patterns and ovarian vascularity (Bishop et al. 2017b)), we hypothesized that the ovarian and uterine dysfunction would also hinder fertility. The current study sought to extend the previous findings in this primate model to determine what effects hyperandrogenemia and/or WSD have on fertility and gestational parameters.
Materials and Methods
Animals
All animal procedures were approved by the Oregon National Primate Research Center’s Institutional Animal Care and Use Committee (IACUC) and comply with the Animal Welfare Act and the APA Guidelines for Ethical Conduct in the Care and Use of Non-human Animals in Research. The animal model and treatments used in the current study were described previously (True et al., 2017). Briefly, female rhesus macaques receiving treatments beginning at ~2.5 years old (peripubertal) resulted in four groups (n = 9–10/group): control animals receiving cholesterol-filled silastic implants and normal monkey chow with 15% of calories from fat (C; Purina 5000/5052), animals receiving testosterone-filled silastic implants and normal monkey chow (T), animals receiving cholesterol-filled silastic implants and a WSD with 36% of calories from fat (WSD; Purina 5L0P), and animals receiving testosterone-filled silastic implants and a WSD (T + WSD). Detailed descriptions of implant preparation and subcutaneous placement, T-treatment as well as diet treatment were provided previously (True et al., 2017). All animals had ad libitum access to food during the day and food was removed at night. Previous characterization did not find differences in caloric intake between the four treatment groups (True et al., 2017). Average serum testosterone levels in the T and T + WSD group before initiation of current study was 1.35 ± 0.01 ng/ml, representing an ~4–5-fold increase over levels observed in control females (True et al., 2017). Serum T levels were monitored weekly during gestation as previously reported (True et al., 2017). Levels were maintained by testosterone implants in T and T + WSD females at 1.3 ± 0.9 ng/ml during gestation (range: 0.9–1.9 ng/ml, data not shown). Animals were on treatment for ~4 years at the end of the fertility study.
Fertility trial
Females were monitored daily for onset of menses/vaginal bleeding as previously reported (Bishop et al. 2017a, 2017b). From 4 to 6 days following onset of menses until introduction to the male, serum levels of estradiol (E2) were monitored daily to estimate impending ovulation. Once serum levels rose to ≥100 pg/ml, (indicating selection of a dominant preovulatory follicle), the female was pair-housed with a mature male of proven fertility for 4 days. Behavior was monitored closely to ensure compatibility between pairs, and females were swabbed vaginally each day during pairing to identify sperm within the female reproductive tract. Only pairings resulting in sperm within the tract were considered successful mating attempts.
A serum sample was collected on the day the female was removed from the male to confirm a drop in E2 levels and rise in serum progesterone (P4) levels ≥1 ng/ml, indicating likely ovulation (Young et al., 2003). Serum P4 levels were then monitored every 2–3 days until either (i) serum P4 remained above 1 ng/ml for 30 days post-mating, indicating onset of pregnancy or (ii) P4 levels dropped below 1 ng/ml with concurrent menses, indicating implantation had not occurred. If a female did not conceive during her first mating, she would be monitored again for rise in serum E2 and re-paired. The duration of the fertility trial was 4 months during the rhesus breeding season (December–March); during this time period females were mated up to three times (following a demonstrated rise in E2 ≥100 pg/ml).
If pregnancy was suspected, the female was fasted overnight at 30 days post-mating (near GD30; ~mid first trimester), sedated with ketamine (10 mg/kg) and a blood sample was obtained for metabolic and steroid analyses. Subsequently, anesthesia was maintained by inhaled isoflurane/O2 for 3D/4D ultrasonography (GE Voluson E8, GE Healthcare, Chicago, IL) using the RNA5-9-D 4D Micro-convex Transducer to: (i) visualize the gestational sac (when present) and (ii) estimate the gestational age of the fetus (using crown-rump length method). Placental vascular parameters were also analyzed by contrast-enhanced ultrasonography (Keator et al., 2011). Rhesus monkeys have a bi-lobed placenta; previous studies employing contrast-enhanced ultrasound in rhesus females during the early first trimester demonstrated that the primary lobe where the umbilical cord appears to attach forms first, and a secondary/accessory lobe forms a few days later (Keator et al., 2011). The primary lobe, on average, has greater vascularity than the secondary structure. Blood volume (BV) to the bi-lobed macaque placenta was determined for both the dominant (presumably primary) and non-dominant (presumably secondary) lobes using custom software as previously described (Keator et al., 2011). Regions of interest (lobes) were defined by microbubble contrast flow and compared to still images of similar focal points (see Supplementary Figure S1 and Supplementary Movie S1). In one WSD, one T and one T + WSD pregnancies, the non-dominant/secondary lobe was not clearly visualized and therefore BV was only measured in the one dominant/primary lobe of the forming placenta. After these measurements were obtained (for the pregnancies listed in Table I), it was noted that one C group female developed a worsening skin condition which required bi-weekly sedation for skin therapy and therefore was excluded from gestational analyses.
Table I.
Time-mated breeding results
| Group | Pregnant first mating (%)@ | Pregnant all matings (up to 3 max; %)♣ | # Viable pregnancies (GD130/135) |
|---|---|---|---|
| C (n = 10) | 7 (70%) | 10 (100%)a | 10 (100%) |
| T (n = 10) | 4 (40%) | 10 (100%)a | 10 (100%) |
| WSD (n = 10*) | 7 (70%) | 7 (70%)b | 7 (70%) |
| T + WSD (n = 9**) | 3 (33%)^ | 6 (67%)^b | 4 (44%) |
@Overall effect of androgen P < 0.04
♣Overall effect of WSD P < 0.04
Different lower case letters indicate differences between individual treatment groups P < 0.05.
*1 WSD female not cycling (no rise in E2, no P4 not mated); one WSD female only mated 2× (not pregnant) due to behavioral concerns.
**1 T + WSD female possible biochemical pregnancy but no post-E2/LH surge rise in P4, not re-mated.
^1 T + WSD pregnancy anembryonic (miscarried ~GD60) and one twin pregnancy terminated at ~GD105 due to impending premature delivery and fetal demise.
Fetuses were collected at pregnancy termination between gestational Days 130–135 corresponding to the early third trimester. Dams were fasted overnight and sedated with 10–15 mg/kg ketamine with 0.1 ml glycopyrolate followed by transverse hysterotomy resulting in removal of the fetus. The fetus was immediately transferred to the necropsy suite and administered sodium pentobarbital (25–30 mg/kg iv) via the umbilical artery. Once a deep plane of anesthesia, and the absence of reflexes have been achieved, the aorta is severed and the final cause of death is exsanguination. Prior to exsanguination, fetal measurements of weight, crown-rump length, abdominal circumference and head circumference were performed. Sex determination was based on fetal gonads. Sample sizes for female fetuses were n = 4 for C group, n = 7 for T group, n = 4 for WSD group and n = 2 for T + WSD group. Sample sizes for male fetuses were n = 5 for C group, n = 3 for T group, n = 3 for WSD group and n = 2 for T + WSD group.
Longitudinal measurements during pregnancy
To assess metabolic function, circulating levels of both fasting glucose and insulin measures were assessed in each female across pregnancy. Animals were fasted overnight and sedated with ketamine (10 mg/kg) or Tiletamin HCl and Zolazepam HCl mixture (TelazolTM Zoetis Inc, Kalamazoo, MI; 4–5 mg/kg) for blood collection at GD30 (as described above), 60 and 90. On GD115, sedation was achieved with Telazol (3–5 mg/kg) for an accompanied glucose tolerance test (see below). Glucose was measured immediately using the OneTouch Ultra Blood Glucose Monitor (LifeScan), and the remainder of the blood was placed in heparinized tubes on ice for measurements of insulin.
After GD30, serum levels of P4 were measured weekly until time of pregnancy termination (GD130–135, mid-third trimester). Serum was collected at GD30, 60, 90 and 115 during metabolic/ultrasound procedures described above and at the time of pregnancy termination for measurements of E2 and androstenedione (A4).
Intravenous glucose tolerance test
At GD115 (near the end of the second trimester), an intravenous glucose tolerance test (ivGTT) was performed as described previously (True et al., 2017). Briefly, animals were fasted overnight and sedated with Telazol (3–5 mg/kg). A glucose bolus (50% dextrose solution) was administered at a dose of 0.6 g/kg via the saphenous vein. Baseline blood samples were obtained prior to the glucose injection, and 1-ml blood samples were collected at 1, 3, 5, 10, 20, 40 and 60 min later by venipuncture (typically saphenous vein). Glucose was measured immediately using the OneTouch Ultra Blood Glucose Monitor (LifeScan), and the remainder of the blood was placed in heparinized tubes on ice for measurements of insulin.
Hormone and lipid assays
All hormone assays were performed by the Endocrine Technologies Support Core (ETSC) at the Oregon National Primate Research Center (ONPRC) as reported previously (True et al., 2017). All quality controls and calibrations provided by the company, as well as ETSC monkey serum standards, were analyzed with test samples.
Total serum estradiol (E2), progesterone (P4) and testosterone (T) were analyzed using a Roche Cobas e411 automated clinical platform (Roche Diagnostics, Indianapolis, IN). The assay ranges for the E2, P4 and T assays were 5–4300 pg/ml, 0.03 to −60 ng/ml and 0.025–15 ng/ml, respectively. Intra- and inter-assay CVs for the Roche assays in the ETSC are consistently <7%. Serum T was previously validated against liquid chromatography mass spectrometry (LC–MS) for this group of animals (True et al., 2017). Androstenedione (A4) was measured used an LDN ELISA with a range of 0.1–10 ng/ml and intra-assay variation of 6.8% and inter-assay variation of 13%.
Macaque chorionic gonadotropin (mCG) was measured in at least three samples collected between GD9-30 post-mating (spanning the interval of peak mCG production during macaque pregnancy (Atkinson et al., 1975)) by radioimmunoassay using anti-ovine LH sera as originally reported in (Hobson et al., 1975; Hodgen et al., 1974) and validated by ETSC at ONPRC. The assay range is 0.01–5 ng/sample, and intra-assay CV was 5.7%. Peak mCG values of > 12 ng/ml following mating indicated an embryo was likely present and the female was possibly pregnant.
Insulin concentrations in monkey plasma were determined using a chemiluminescence-based automatic clinical platform (Roche Diagnostics Cobas e411, Indianapolis, IN); this assay was previously validated in non-human primates (True et al., 2017; Varlamov et al., 2010). The range of the insulin assay is 0.2–1000 uIU/ml. The intra- and inter-assay variations were <7%.
Additional serum was collected on GD130–135 immediately prior to surgical pregnancy termination and assayed for lipids. Cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglyercides were measured on a Horiba Pentra 400 chemistry analyzer run by Oregon National Primate Research Center’s Clinical Pathology section. The coefficients of variation reported by the manufacturers are: cholesterol inter-assay CV 0.5–1.5% and intra-assay CV 2.5–3%, HDL inter-assay CV 0.5–3% and intrassay CV of 1.6–2.5%, LDL inter-assay CV 0.7–3% and intra-assay CV 4–6.5%, and triglyceride inter-assay CV of 0.8–3% and intra-assay CV of 1.4–2%.
Statistics
AUC (calculated from zero), fetal correlations to maternal weight and Mann–Whitney comparisons between viable and non-viable/non-pregnant groups were performed in Prism Graphpad 7.0. Number of conceptions after one successful mating attempt and overall pregnancy rate were analyzed by generalized linear modeling/ type III Chi-Squared Analyses of SAS with main factors of androgen (T), WSD and interaction (T by WSD; version 6.1, SAS Institute Inc. Cary, NC, USA). Days to pregnancy in the fertility trial were analyzed by Life Tables/Kaplan–Meier method of SAS with censoring for variable non-pregnant status (females who failed to become pregnant during study interval). Days of implantation bleeding, blood volume of GD30 placenta, GTT measurements, chemistry panel measurements, and fetal measurements were analyzed by Linear Models Function of SAS with variables T, WSD and T by WSD. Repeated measures during gestation were analyzed by Mixed Models function of SAS (factors: gestational day (GD), T, WSD, T by WSD, GD by T, GD by WSD). When main factors were significant (P < 0.05), pairwise analyses were performed by Least Squared Means function with Tukey–Kramer adjustment for multiple comparisons.
Results
Fertility results
After first mating, 7 of 10 females in both the C and WSD groups conceived (70%; Table I) as confirmed by presence of a gestational sac at ~GD30 and mCG values > 12 ng/ml. However, only 4 of 10 T (40%) and 3 of 9 (33%) of T + WSD females conceived at first mating (effect of testosterone: P < 0.04; Table I). Of 39 monkeys in this study, only one female (WSD group) was not paired at least once with a fertile male since she was anovulatory (no rise in E2 > 100 pg/ml, no P4 > 1 ng/ml during each menstrual cycle). One WSD female was only paired twice due to behavioral issues unrelated to breeding, and did not become pregnant in either attempt. One T + WSD female was only paired once due to multiple anovulatory cycles; E2 levels peaked at >400 before mating, but P4 levels remained low (<1 ng/ml) until onset of menses indicating a disruption of the luteal phase and likely failed ovulation. Notably, a serum mCG level near assay cutoff of >12 ng/ml for pregnant animals was measured in this female (11.6 ng/ml), but a gestational sac was not present. This was the only possible biochemical pregnancy in the study, and because pregnancy status is uncertain for this female she is not included in the overall pregnancy rate for the T + WSD group. When the 4-month fertility trial was completed, all 10 of 10 C and T females became pregnant (100%), compared to only 7 of 10 WSD (70%) and 6 of 9 T + WSD (67%) animals (effect of diet: P < 0.004; Table I). While a similar number of C and T females achieved pregnancy, the T-treated females required a greater interval of time to achieve pregnancy (C 63 ± 5, T 76 ± 6, WSD 55 ± 3 and T + WSD 80 ± 6 days to pregnancy; chi-square values: Log-Rank P < 0.0015, Wilcoxon P < 0.05; Supplementary Figure S2A).
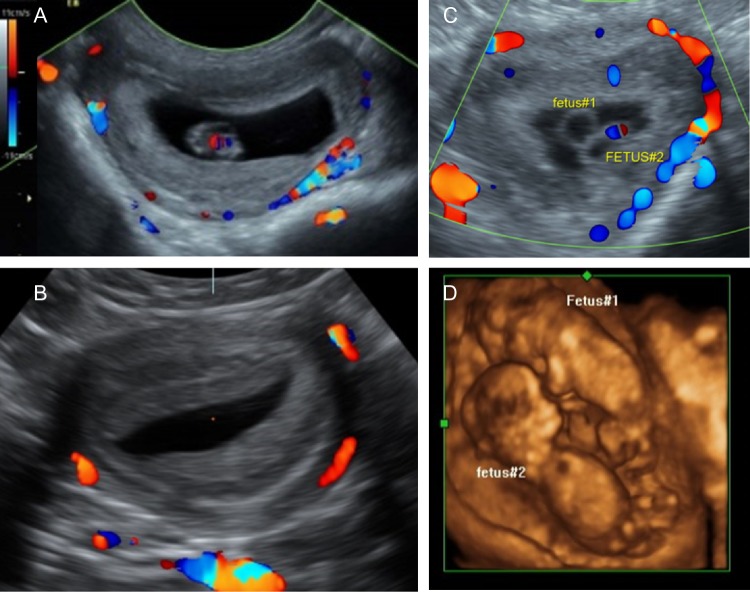
Abnormal conceptuses were noted in two T + WSD pregnancies by ultrasound at GD30 (Fig. 1A–D). Compared to the expected gestational sac with a GD30 fetus (Fig. 1A), one T + WSD-treated female had a sac with decidualized placental tissue but no visible fetus (Fig. 1B). This anembryonic pregnancy disappeared by GD60 when repeated ultrasounds visualized a closed uterus (not shown). Another T + WSD-treated female had a gestational sac with two yolk sacs/embryos (Fig. 1C and D). This was determined to be a rare (for rhesus macaques) monozygotic diamniotic twin pregnancy. This identical twin pregnancy was monitored every 2 weeks throughout gestation, but by GD106 (second trimester) both fetuses developed evidence of dilated fetal bladders, low amniotic fluid, and lower urinary tract obstructions, plus the dam presented with vaginal bleeding. Due to the presence of impending labor and fetal demise, and to preserve the health of the dam, the pregnancy was terminated. These two pregnancies were excluded from all additional analyses. Therefore, the number of pregnancies with viable fetuses (Table I) was less in the T + WSD group (4/9), compared to the C (10/10), T (10/10) and WSD (7/10).
Figure 1.
Ultrasound analyses of pregnant rhesus females at time of pregnancy confirmation (~gestational Day 30; GD30). (A) Power flow color Doppler image of typical gestational sac containing ~GD30 rhesus fetus. (B) Atypical gestational sac identified in one T + WSD female at ~GD30 with forming placental disks but absence of any identifiable fetal tissues leading to diagnosis of anembryonic pregnancy. (C) Atypical gestational sac identified in one T + WSD female containing two yolk sacs/fetal bodies at GD29. (D) 3D image of monozygotic diamniotic twin pregnancy identified in panel C at GD59.
Early gestation
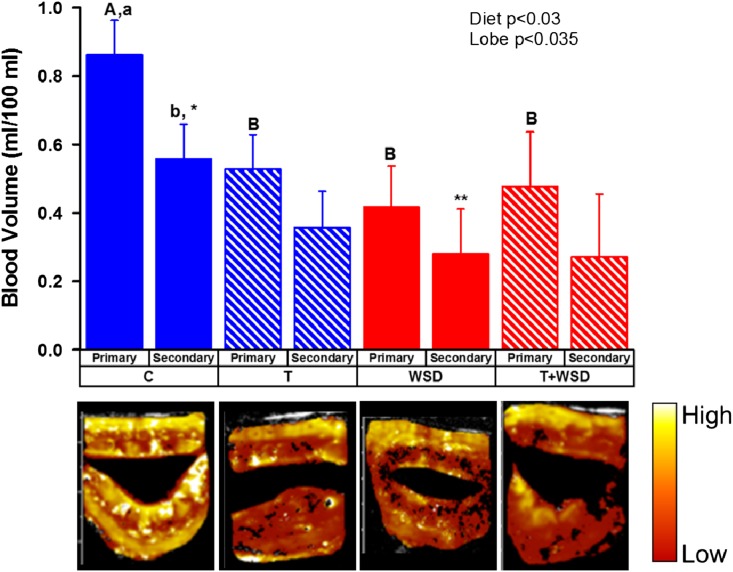
In females who maintained viable fetuses until GD130–135, the number of days of post-mating vaginal bleeding (implantation bleeding, an indication of successful implantation) was reduced in WSD-treated females (effect of diet P < 0.002; Supplementary Figure S2B). Placental blood volume (BV) was measured by contrast-enhanced ultrasound at GD30 in the bi-lobed structure (e.g. Control placenta, Supplementary Movie S1, Supplementary Figure S1). A significant difference in BV was detected by placental lobe (effect of lobe, P < 0.035; Fig. 2); this was due to a significant difference in BV between the presumptive primary and secondary lobes of C females only (1.5-fold difference, P < 0.05; Fig. 2). In contrast, there were no significant differences in BV detected between presumptive primary and secondary lobes in the T, WSD and T + WSD groups (all P > 0.3). While an overall significant reduction in BV was detected by consumption of the WSD (effect of diet, P < 0.03; Fig. 2), this was primarily due to reduced BV in the dominant/primary lobe of the placenta in all treatment groups compared to the C group (all P < 0.05 vs C; Fig. 2).
Figure 2.
Placental blood volume (BV) in forming placenta at ~GD30. Primary and secondary lobes were identified by fill characteristics. False-colored representative images from each treatment group are depicted under chart. Diet and lobe effects were determined by mixed-model analyses and are included as text inserts within the graph. Different uppercase letters indicate significant differences between treatment groups (P < 0.05, C vs T + WSD P = 0.05), while lower case letters indicate differences between lobes (P < 0.05). Asterisks denote trend between secondary lobe of C and WSD groups (P = 0.10).
Metabolic measurements
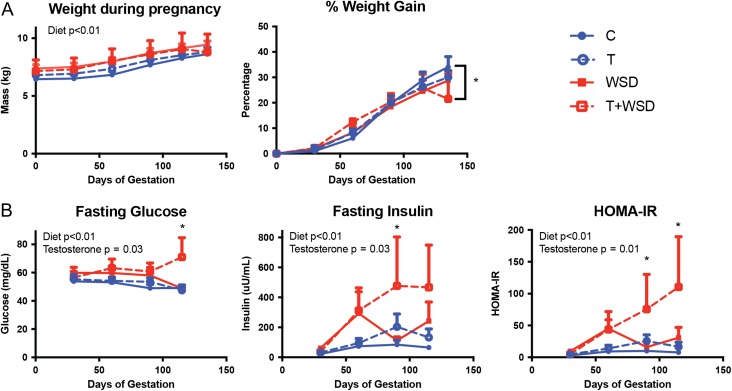
As expected, body weight increased in all groups during pregnancy (effect of gestation: P < 0.01; Fig. 3A). Exposure to WSD-treatment (WSD and T + WSD groups) further increased raw weight over gestation (effect of diet: P < 0.01); however, there were no significant differences between the four groups at any time point. In contrast, the percent weight gain did not show any effects of WSD or T treatment. Further analysis revealed that T + WSD animals had gained significantly less weight at GD135 compared to C animals (P = 0.02); however, inspection of data revealed this was primarily due to weight loss by one individual in the T + WSD group. This individual female began to lose weight at the end of the second trimester.
Figure 3.
Weight gain and measures of insulin sensitivity during gestation. (A) Raw weight and normalized percent weight gain beginning immediately after time-mated breeding (Day 0). (B) Fasting glucose and insulin were measured approximately every 30 days during gestation and used to calculate the homeostatic model assessment of insulin resistance (HOMA-IR; glucose × insulin/405). Diet and testosterone effects were determined by mixed-model analysis are included as text within the graphs. Bracketed * in % weight gain denotes significant difference between C and T + WSD group. All other * denote statistically significant difference between T + WSD group and C, T and WSD groups.
Fasting glucose levels remained relatively stable throughout pregnancy; however, both WSD (effect of diet: P < 0.01) and T (effect of testosterone: P = 0.03) had overall effects to increase fasting glucose across gestation (Fig. 3B). Significantly higher levels of fasting glucose were detected in the T + WSD group compared to all other groups at GD115 (all P < 0.05). Fasting insulin levels increased in all groups over the duration of pregnancy (effect of gestation: P = 0.01), consistent with the well-documented increase in insulin secretion observed during pregnancy (Catalano et al., 1991; Kuhl, 1991). Similar to fasting glucose, fasting insulin levels were also significantly increased by WSD (effect of diet: P < 0.01) and T (effect of testosterone: P = 0.03) during gestation (Fig. 3B). Significantly elevated fasting insulin levels were detected in the T + WSD group compared to all other groups at GD90, and T + WSD females remained elevated compared to C and T groups on GD115 (all P < 0.05). Similar to fasting insulin, the homeostatic model assessment of insulin resistance (HOMA-IR; glucose × insulin/405) increased during pregnancy in all groups (effect of gestation: P = 0.02; Fig. 3B). There was a significant effect of WSD (effect of diet: P < 0.01) and T (effect of testosterone: P = 0.01) to further increase HOMA-IR across gestation. Significantly higher HOMA-IR was detected in the T + WSD group compared to all other groups at GDs 90 and 115 (all P < 0.05).
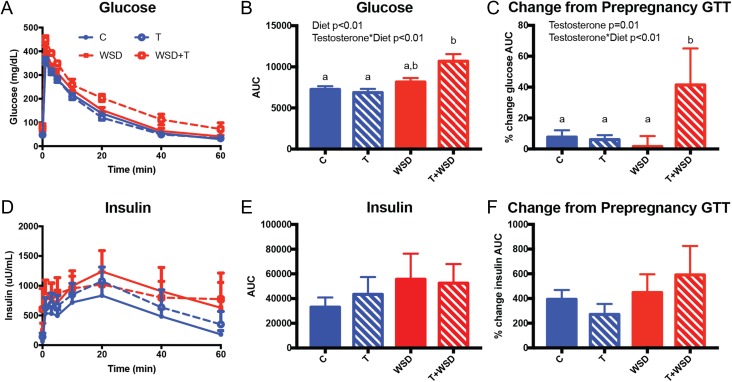
To further assess glucose homeostasis and peripheral insulin sensitivity, ivGTT were performed at GD115 (late second trimester; Fig. 4A and D). There was an overall effect of WSD to increase glucose AUC (effect of diet: P < 0.01), and an interaction of testosterone and WSD to further increase AUC (effect of testosterone × diet: P = 0.01), with the T + WSD group having elevated AUCs compared to C and T groups (both P < 0.01; Fig. 4A and B). Insulin AUC was similar across the four groups during pregnancy (Fig. 4D and E). GTTs from the second trimester were compared to prepregnancy GTTs (previously reported in (True et al., 2017)), and there was a significant effect of T-treatment (effect of testosterone: P = 0.01) to increase the percent change in glucose AUC during pregnancy and a significant interaction for the combination of T + WSD to further increase the percent change in glucose AUC (diet × testosterone: P < 0.01; Fig. 4C). A significantly increased change in pregnancy glucose AUC was induced by T + WSD treatment compared to all other groups (all P < 0.05; Fig. 4C). All groups demonstrated an increase in the insulin AUC during pregnancy, but there were no group differences in the magnitude of this increase (Fig. 4F).
Figure 4.
Intravenous glucose tolerance tests (ivGTT) performed at gestational Day 115 (late second trimester). (A) A glucose bolus was administered at time = 0 and subsequent glucose values were measured for 60 min. (B) Glucose area under the curve (AUC) was calculated from zero, and (C) the percent change in glucose AUC compared to a ivGTT performed at 3 years (previously reported in True et al. (2017)) was calculated. (D) Serum insulin measurements during the GTT and (E) insulin AUC from zero as well as (F) the percent change in insulin AUC compared to a pregnancy ivGTT. Diet, testosterone and testosterone × diet interaction effects were determined by linear model analysis are included as text within the graphs. Different letters denote statistically significant differences between treatment groups determined by post-hoc analysis.
Pregnancy outcomes were compared to prepregnancy metabolic measurements to determine whether existing metabolic health was associated with infertility and/or poor gestational outcomes. Prepregnancy fasting glucose, insulin and HOMA-IR were all higher in the group of animals that did not become pregnant or lost their pregnancy prior to GD135, regardless of treatment group (Table II). Body weight, percent body fat, BMI, GTT glucose AUC and GTT insulin AUC all tended to be higher in the non-viable/non-pregnant group as well, although these differences were not significantly different (P > 0.05). Animals in the non-viable/non-pregnant group were all consuming a WSD, which is expected to worsen insulin sensitivity; therefore, we also compared viable vs non-viable/non-pregnant metabolic factors only within the WSD-groups (WSD, T + WSD). Fasting insulin, HOMA-IR and GTT glucose AUC trended higher (P = 0.06–0.1) in the non-viable/non-pregnant group even when compared to viable pregnancies only in the WSD-treated groups (data not shown).
Table II.
Prepregnancy metabolic measurements grouped by pregnancy outcomes.
| Viable pregnancy (n = 30) | Non-viable/non-pregnant (n = 8) | Mann–Whitney P-value | |
|---|---|---|---|
| Weight (kg) | 7.2 ± 0.2 | 8.6 ± 0.9 | 0.13 |
| Percent body fat | 29.9 ± 2.0 | 38.4 ± 5.2 | 0.09 |
| BMI | 27.4 ± 0.8 | 33.1 ± 3.0 | 0.06 |
| Fasting glucose (mg/dl) | 51.3 ± 1.2 | 58.0 ± 2.3 | 0.01 |
| Fasting insulin (uU/ml) | 25.7 ± 3.2 | 123.6 ± 39.7 | 0.02 |
| HOMA-IR | 3.4 ± 0.5 | 17.7 ± 5.8 | 0.01 |
| GTT glucose AUC | 6741 ± 247 | 6949 ± 539 | 0.87 |
| GTT insulin AUC | 9210 ± 919 | 20 393 ± 7475 | 0.14 |
Viable pregnancy defined as pregnancy that endured to gestational Day 135 out of total 160. Non-viable/non-pregnant is defined as animals with pregnancy loss that occurred before Day 135 and those that did not become pregnant in the fertility trial.
Maternal steroid and lipid levels
Serum E2 levels increased during pregnancy to peak in all females at GD115, but then declined at GD135 (effect of gestation P < 0.01; Fig. 5A). While an overall significant effect of WSD was detected (effect of diet: P = 0.003) this was due to increased levels of E2 in T + WSD females at GD115 compared to C and T groups. Serum E2 remained elevated in T + WSD group females until pregnancy termination at GD130–135 (WSD vs C, WSD vs T at GD115 and 130/135; all P < 0.05).
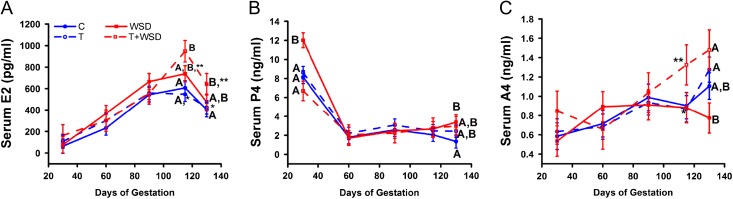
Figure 5.
Serum hormone levels in pregnant rhesus dams during gestation. Serum testosterone estradiol (E2; panel A), progesterone (P4; panel B), and androstenedione (A4; panel C) levels were measured at gestational day (GD) 30, 60, 90, 115 and 135 as described in text. Blue filled circles and solid lines denote C group values, while blue open circles and dashed lines indicate T group values. Red filled squares and solid lines denote WSD group values, while red open squares and dashed lines indicate T + WSD values. Different uppercase letters identify significant differences between groups within GD (P < 0.05), while different lower case letters indicate significant differences within treatment groups by GD (P < 0.05). Asterisks denote trend for difference between treatment groups within GD (P = 0.06–0.1).
Serum P4 rapidly declined 3-7-fold between GD30 and 60 in all groups and remained between 2 and 3 ng/ml throughout the remainder of gestation (effect of gestation: P < 0.01; Fig. 5B). A significant interaction between T and WSD was found for serum P4 levels during gestation (effect of testosterone × diet: P < 0.03). Levels were highest in the WSD group in the first trimester (GD30), while the lowest levels were measured in T + WSD females (WSD vs T + WSD, P < 0.01). At the time of pregnancy termination, P4 levels were again higher in WSD compared to control (C) females (P = 0.05).
C, T and T + WSD group females all displayed increased serum levels of A4 during gestation (gestational day P < 0.01; GD30 vs GD135 P < 0.01, 0.01, and 0.03), while serum A4 levels remained invariable in WSD group females during gestation (GD30 vs 135 P = 0.3; Fig. 5C). An overall significant effect of T was detected (effect of testosterone: P = 0.05) on serum A4 levels throughout gestation, which was primarily due to increased average levels measured in T + WSD group females at GD115 and 135.
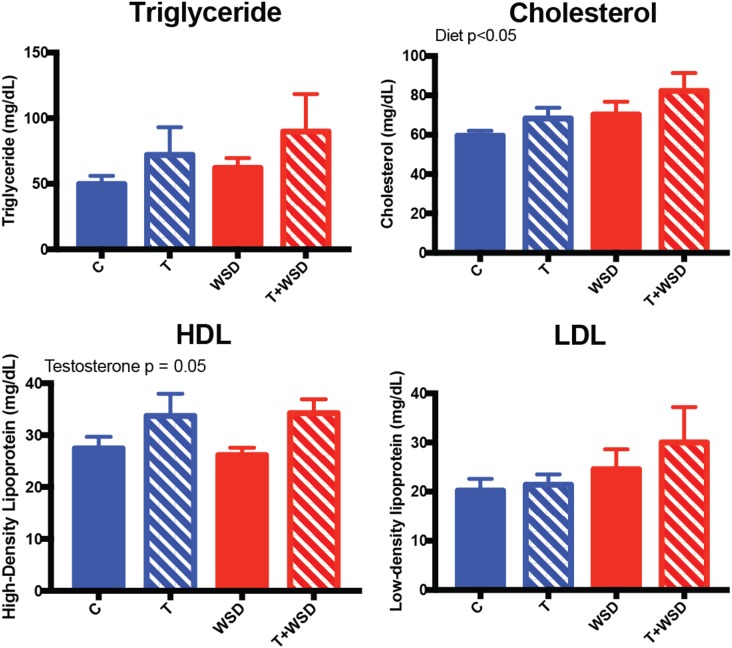
Analyses of lipid levels in blood samples collected from dams immediately prior to pregnancy termination (GD130–135) indicated that cholesterol levels were significantly increased in WSD groups (effect of diet: P = 0.05; Fig. 6). However, there were no significant differences between the four groups. HDL levels were significantly greater with T-treatment (effect of testosterone: P = 0.05), but again there were significant differences between the four groups. Triglyceride and LDL levels appeared similar across the four groups.
Figure 6.
Serum lipid hormone measurement at gestational Days 130–135. Fasting blood collection immediately prior to cesarean section was collected and assayed for triglyceride, cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL). Effect of diet and testosterone was determined by linear model analysis and is included as text within the graphs.
Fetal measurements
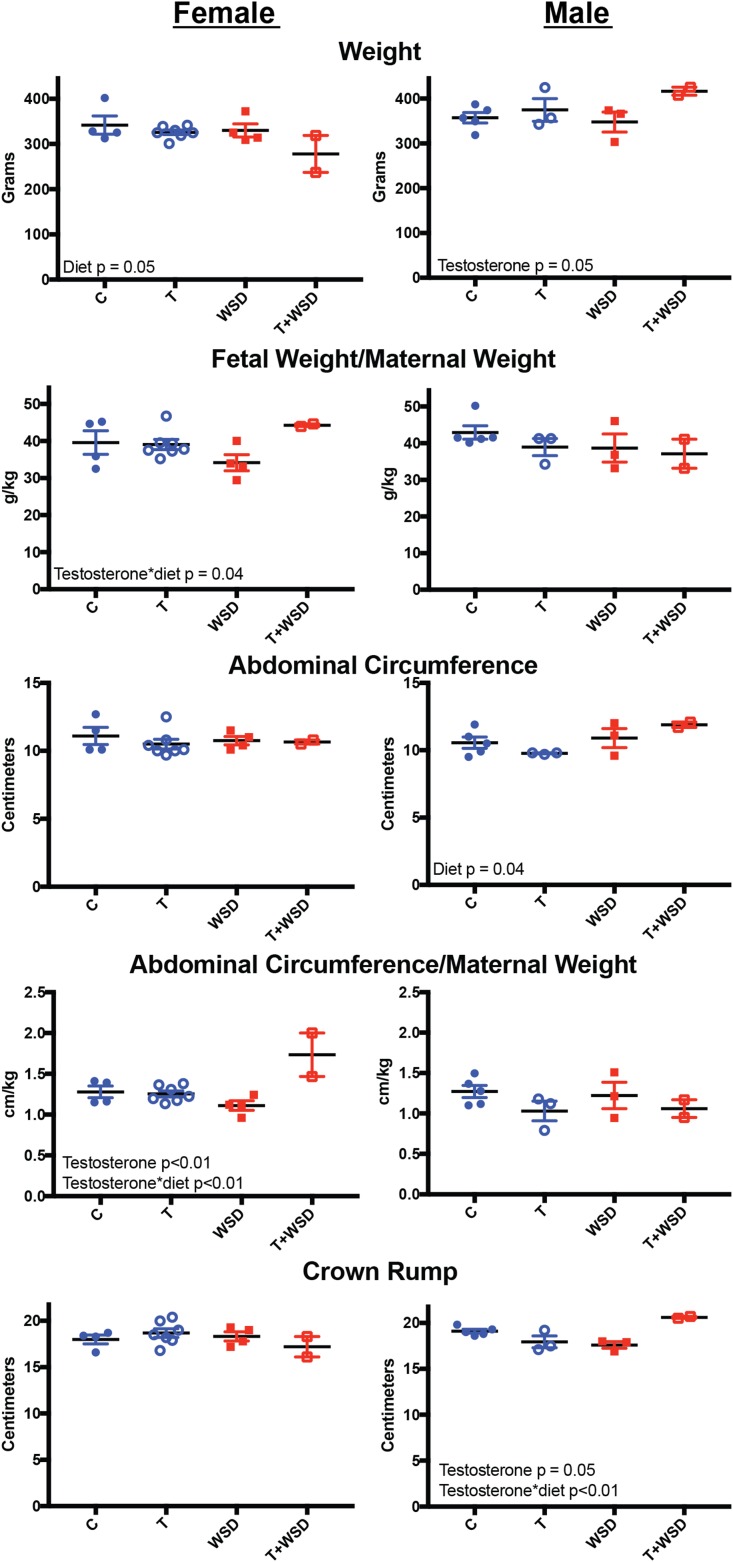
Fetuses collected at pregnancy termination were measured to examine whether maternal WSD and/or testosterone treatment had any effect on gross measurements of size (Fig. 7). Maternal diet decreased female fetus weight (effect of diet: P = 0.05), but post-hoc analysis for group differences was not possible (for either sex) due to small sample sizes. Maternal testosterone exposure increased male fetus weight (effect of testosterone: P = 0.05). Fetal weight correlated with maternal weight (slope: 0.02 ± 0.01, R2: 0.42, P < 0.01; data not shown); therefore, we also investigated normalized fetal weight (fetal weight/maternal weight). There was a significant interaction between maternal testosterone and diet to increase normalized female weight (effect of testosterone × diet: P = 0.04). Neither maternal testosterone or WSD exposure impacted normalized fetal weight in male fetuses.
Figure 7.
Fetal measurements at gestational Days 130–135. Early third trimester fetuses were measured for weight, abdominal circumference (cm), and crown-rump length (cm) following pregnancy termination. Fetal weight and abdominal circumference correlated with maternal weight at pregnancy termination; therefore, normalized fetal weight and abdominal circumference were also calculated. Individual graphs were generated for female (left column) and male fetuses (right column). Effect of diet and testosterone was determined by linear model analysis and is included as text within the graphs.
Female abdominal circumference was not affected by maternal testosterone or WSD (Fig. 7). Male abdominal circumference was significantly increased by maternal WSD (effect of diet: P = 0.04). Similar to body weight, abdominal circumference also correlated with maternal weight (slope: 0.17 ± 0.03, R2: 0.52, P < 0.01; data not shown) and normalized values were also investigated (abdominal circumference/maternal weight). Testosterone increased normalized abdominal circumference in female fetuses (effect of testosterone: P < 0.01); and this was further increased by a testosterone and diet interaction (effect of testosterone × diet: P < 0.01). Maternal treatments had no effect on normalized abdominal circumference in male fetuses.
Maternal treatments also had no effect on crown-rump measurements in female fetuses (Fig. 7). Testosterone had a significant effect on crown-rump measurements in male fetuses (effect of testosterone: P = 0.05) depending on maternal diet. There was a significant interaction of testosterone and diet to increase crown rump (effect of testosterone × diet: P < 0.01). Head circumference was also measured but did not show any differences in female or male fetuses (data not shown). Neither crown rump nor head circumference correlated with maternal weight; therefore, normalized values were not determined.
Discussion
After 3-and-a-half years of characterizing the effects of early androgen and WSD exposure, beginning at menarche, on metabolic and reproductive function in female rhesus macaques, the current study sought to determine how these treatments and resulting symptoms alter fertility. While T-treatment increased the interval to achieve pregnancy, WSD-treatment reduced the number of pregnancies achieved during the four months of the fertility trial. Moreover, only the combination of T and WSD exposure was associated with pregnancy loss. Consistent with these observations was the finding that animals who had difficulty becoming pregnant or maintaining pregnancy also had elevated signs of insulin resistance prior to pregnancy. During gestation, both T and WSD appeared to decrease placental blood flow and alter pattern of steroid hormone production. Consistent with observations in the non-pregnant state, T + WSD worsens metabolic function during pregnancy and T + WSD pregnant females show modest hyperglycemia both in basal conditions and following a glucose challenge in the late second trimester, while all other groups were able to maintain relative euglycemia. These findings indicate that androgen, WSD and the combination are each contributing factors that may reduce the overall chance of a successful pregnancy.
The current model is particularly useful because of the presence of T exposure in the absence of an obesogenic diet, which facilitates discrimination between effects caused by androgens alone, an obesogenic diet alone, and the combination of these two factors. Current findings of diminished placental blood volume early in gestation in all treatment groups and altered steroid hormone levels in the third trimester with both T and WSD treatments are initial indications that both androgens and an obesogenic diet may have independent, but detrimental, effects on placental function. This hypothesis is consistent with previously published work demonstrating altered placental function in a monkey model of gestational obesity (Frias et al., 2011), and may indicate that the detrimental effects of high-fat diet consumption are present prior to the onset of obesity. This study also found differences between WSD non-obese dams and WSD obese dams, indicating potentially independent effects of WSD-consumption and obesity. Maternal obesity is known to cause alterations in placental function in women. Specifically, maternal obesity is associated with increased placental size, placental inflammation, and pregnancy complications attributed to placental dysfunction such as preeclampsia (Catalano, 2010; Farley et al., 2009; Guelinckx et al., 2008; Weiss et al., 2004; Zhu et al., 2010). PCOS patients also have increased risk of preeclampsia (de Vries et al., 1998; Katulski et al., 2015), but whether this is due to the high rates of obesity in this population or some independent contribution from hyperandrogenemia is unclear (Haakova et al., 2003). Further time-mated breeding experiments on this cohort of females will be performed to see if reduction in BV to the dominant/primary structure is a defining characteristic of the early gestational period in T, WSD and T + WSD pregnancies.
Animals consuming a WSD, regardless of T-treatment, had the lowest rates of pregnancy. To determine whether prepregnancy metabolic function was a predictor of fertility success, we segregated animals by whether or not they had a successful viable pregnancy. Interestingly, fasting insulin and glucose levels were elevated in animals with non-viable pregnancies and who failed to become pregnant. The non-viable/non-pregnant group was enriched for WSD-consuming animals; therefore, we also examined whether insulin sensitivity was associated with worsened fertility when only examining animals from the WSD and T + WSD groups. HOMA-IR remained elevated in the non-viable/non-pregnant group (P = 0.06), indicating that the animals with the worst insulin resistance had the poorest fertility outcomes even amongst animals that were all consuming an obesogenic diet. Interestingly, the T + WSD group had the poorest metabolic function during gestation, consistent with their decreased insulin sensitivity observed previously in the non-pregnant state (True et al., 2017). These findings are consistent with the observation that worsened prepregnancy insulin sensitivity is associated with a higher risk of the development of gestational diabetes (Clark et al., 1997). Importantly, worsened glucose homeostasis during pregnancy is associated with an increased risk for the development of type 2 diabetes (Bellamy et al., 2009; Buchanan et al., 1998; Kim et al., 2002; Lee et al., 2007), and follow-up studies in the current model will determine if insulin sensitivity recovers following pregnancy in the four treatment groups.
Hyperinsulinemia is prevalent in women with PCOS, and appears to be a main factor impacting pregnancy outcome in these patients (Chang et al., 2013; De Leo et al., 2011; Vanky et al., 2004). An in-vitro maturation/IVF/embryo transfer trial of PCOS patients found those with insulin resistance had reduced pregnancy rates compared to patients without insulin resistance (Chang et al., 2013). Interestingly, this defect was associated specifically with implantation defects, since oocyte quality and pre-implantation embryogenesis were unaffected. Previous work from the current macaque model demonstrated decreased markers of uterine decidualization and receptivity in the T-treated animals of the current cohort, regardless of diet (Bishop et al. 2017a, 2017b). Notably, although a greater interval was required to achieve pregnancy, all T-treated animals became pregnant during the fertility trial. But, unlike many lean women diagnosed with PCOS (Diamanti-Kandarakis and Dunaif, 2012), these T-only treated females are not yet insulin resistant (True et al., 2017). Our results are more similar to a recent report that non-clinically referred women with PCOS who are lean and not overweight show only mild hyperinsulinemia and euglycemia (Dumesic et al., 2016). If implantation problems were present in this group, they were not strictly related to insulin resistance, which was not previously observed in the T-alone group, and were mild enough that they could be overcome eventually given the 100% pregnancy rate.
Of note, the high overall number of pregnancies in the C and T group at the end of the trial (100% in both groups) indicates that female macaques in the current study were in their reproductive prime, and were comparable in age to ~18-year-old women (McGee et al., 2014). Furthermore, no behavioral issues were identified during the time-mated breeding trial, and mating successfully occurred based on the presence of sperm in the reproductive tract. The high fertility rate may make it more difficult to detect subtle reproductive deficits. It may also indicate that the ability of WSD to reduce the pregnancy rate in the current cohort of macaques is actually an underestimate of the detrimental impact obesity may have on women seeking infertility treatments in the clinic who range in age from their late twenties to late thirties. The underlying mechanism for decreased fertility success in the WSD-treated groups, despite being in their reproductive prime age, is unclear. This WSD-treatment increased the number of degenerating oocytes in the current cohort of animals (Bishop et al. 2017a, 2017b); therefore, it is possible that diminished oocyte quality resulted in lowered fertility rates in this group.
A unique opportunity of the current non-human primate model is the ability to study the effects of chronic androgen and WSD treatment not only on maternal health and fertility, but also on fetal development. Previous research has indicated many adult diseases may have early developmental origins (Barker, 1998), and the current model offers the unique opportunity to determine how androgens and/or an obesogenic diet may alter fetal physiology. Research in humans, non-human primates and rodents all indicates that maternal obesity and insulin resistance may increase the risk of childhood obesity in their offspring (Boney et al., 2005; Hillier et al., 2007; Rivera et al., 2015; Sullivan et al., 2017). The current study observed that although females from T + WSD dams appeared smaller, when normalized for maternal body weight these fetuses appeared larger. This result was driven largely by the low weights at pregnancy termination in two of the four T + WSD dams, including one that lost a large amount of weight at the end of pregnancy; further studies are needed to determine whether female offspring are more sensitive to maternal testosterone and WSD exposure. In addition to altered metabolic function, there is evidence that maternal environment can regulate offspring reproductive function later in life (Luzzo et al., 2012). Particularly, studies in primates, sheep and rodents demonstrate that prenatal exposure to androgens is capable of mimicking many aspects of PCOS in adulthood, long after androgen exposure has been withdrawn (Abbott et al., 2009; Eisner et al., 2002; Foecking et al., 2005; Steckler et al., 2005). The current model will allow for important detailed investigation into whether low levels of hyperandrogenemia, similar to those observed in PCOS patients, alters fetal development in primates in the presence and absence of obesity.
An important finding of the current study was that while WSD decreased the number of females that became pregnant, the combination of T + WSD was associated with pregnancy loss in two of the six pregnancies. Although the relative numbers are small, both of these losses resulted from alterations to normal embryogenesis, suggesting these were not related to uterine factors. Further studies investigating alterations to oocyte competency by T and/or WSD are ongoing (Bishop et al. 2017a, 2017b). The increase in pregnancy loss in the T + WSD group is also consistent with observations in PCOS women, where rates of miscarriage are reportedly as high as 40% (Jakubowicz et al., 2002). Prior to pregnancy, as discussed, the T + WSD group also demonstrated the most significant metabolic dysfunction (True et al., 2017). Previous work in a non-human primate model of obesity model indicates that consumption of WSD results in uteroplacental dysfunction (Frias et al., 2011) and WSD-consuming dams that were obese had severe pregnancy complications such as placental infarctions and stillbirth compared to their non-obese counterparts. With continued treatment on the WSD, and increased rates of obesity in the WSD-alone group, it will be possible to further dissect whether pregnancy loss in the current model is (i) mainly due to changes in oocyte quality or is worsened by changes to uterine factors (as discussed above) or (ii) solely due to the poor metabolism observed in combined T + WSD treatment.
In summary, these data show that chronic exposure to mildly elevated levels of circulating androgens and consumption of an obesogenic diet beginning at puberty both contribute to impaired fertility plus altered gestational metabolism and steroidogenesis, albeit with different outcomes. The combination of androgen and WSD exposure worsens metabolic function during pregnancy to a greater extent than consumption of the WSD alone and is associated with pregnancy loss. Further studies on this cohort will reveal the impact of treatment reversal on both reproductive and metabolic health.
Supplementary data
Supplementary data are available at Human Reproduction online.
Supplementary Material
Contrast-enhanced ultrasound imaging of the uterus and forming placental disks of a control female at gestational Day 32 (standard ultrasound image depicted in Supplemental Fig. S1). With this low mechanical index (MI) imaging, the presence of the microbubble contrast reagent within the vasculature shows up bright white, while all non-vascular areas are dark. To determine vascular characteristics, the MI is increased for 2 s, bursting the microbubbles (18–20 s), and then the MI is decreased again and tissue re-perfusion is imaged until past saturation point (~28-s post-burst) for accurate estimation of signal plateau. Note that the microbubbles do not cross the placental barrier and do not enter the fetal blood stream at this stage of gestation.
Acknowledgements
We’d like to acknowledge the expertise of the Endocrine Technologies Support Core (ETSC) at the Oregon National Primate Research Center (ONPRC) under the Direction of David Erickson, Ph.D. Thanks to the ONPRC Division of Comparative Medicine’s Surgical Services Unit (SSU), under the direction of Theodore Hobbs, D.V.M. for assisting with these studies. The male macaques were provided by the ONPRC Division of Comparative Medicine Time-Mated Breeding Program under the supervision of Lauren Drew Martin, D.V.M. and Travis Hodge, B.S. Breeding coordination was also provided by the DCM Time-Mated Breeding in collaboration with the ONPRC Assisted Reproductive Technology (ART) Core lead by Carol Hanna, Ph.D. and Cathy Ramsey, B.S. In addition, the ONPRC Tissue Distribution Program aided in fetal necropsy and coordination of tissue-sharing across laboratories.
Authors’ roles
C.A.T. and C.V.B. wrote the article and contributed to study design and analysis. D.L.T., E.C.M., M.C.W. and C.V.B. executed the study. O.D.S. and R.L.S. contributed to study design, article drafting and critical discussion.
Funding
All ONPRC Cores and Units were supported by P51 OD011092 Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) of the National Institutes of Health (NIH) under Award Number P50HD071836 to R.L.S. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol 2009;9:776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson LE, Hotchkiss J, Fritz GR, Surve AH, Neill JD, Knobil E. Circulating levels of steroids and chorionic gonadotropin during pregnancy in the rhesus monkey, with special attention to the rescue of the corpus luteum in early pregnancy. Biol Reprod 1975;3:335–345. [DOI] [PubMed] [Google Scholar]
- Balen AH, Anderson RA. Policy, Practice Committee of the BFS. Impact of obesity on female reproductive health: British Fertility Society, Policy and Practice Guidelines. Hum Fertil (Camb) 2007;4:195–206. [DOI] [PubMed] [Google Scholar]
- Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;2:115–128. [PubMed] [Google Scholar]
- Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;9677:1773–1779. [DOI] [PubMed] [Google Scholar]
- Bishop CV, Hanna C, Ramsey C, Reiter T, Daughtry B, Chavez S, Hennebold JD, Stouffer RL. Elevated androgen and/or consumption of a western-style diet has detrimental effects on rhesus monkey ovulatory follicles and oocytes. Fertility Sterility 2017;108:e70–e71. [Google Scholar]
- Bishop CV, Mishler EC, Takahashi DL, Reiter TE, Bond KR, True CA, Slayden OD, Stouffer RL. Chronic hyperandrogenemia in the presence and absence of a western-style diet impairs ovarian and uterine structure/function in young adult rhesus monkeys. Hum Reprod 2018;33:128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;3:e290–e296. [DOI] [PubMed] [Google Scholar]
- Buchanan TA, Xiang A, Kjos SL, Lee WP, Trigo E, Nader I, Bergner EA, Palmer JP, Peters RK. Gestational diabetes: antepartum characteristics that predict postpartum glucose intolerance and type 2 diabetes in Latino women. Diabetes 1998;8:1302–1310. [DOI] [PubMed] [Google Scholar]
- Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction 2010;3:365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol 1991;6:1667–1672. [DOI] [PubMed] [Google Scholar]
- Chang EM, Han JE, Seok HH, Lee DR, Yoon TK, Lee WS. Insulin resistance does not affect early embryo development but lowers implantation rate in in vitro maturation-in vitro fertilization-embryo transfer cycle. Clin Endocrinol (Oxf) 2013;1:93–99. [DOI] [PubMed] [Google Scholar]
- Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, Dietz PM. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007;8:2070–2076. [DOI] [PubMed] [Google Scholar]
- Clark CM Jr., Qiu C, Amerman B, Porter B, Fineberg N, Aldasouqi S, Golichowski A. Gestational diabetes: should it be added to the syndrome of insulin resistance? Diabetes Care 1997;5:867–871. [DOI] [PubMed] [Google Scholar]
- De Leo V, Musacchio MC, Piomboni P, Di Sabatino A, Morgante G. The administration of metformin during pregnancy reduces polycystic ovary syndrome related gestational complications. Eur J Obstet Gynecol Reprod Biol 2011;1:63–66. [DOI] [PubMed] [Google Scholar]
- de Vries MJ, Dekker GA, Schoemaker J. Higher risk of preeclampsia in the polycystic ovary syndrome. A case control study. Eur J Obstet Gynecol Reprod Biol 1998;1:91–95. [DOI] [PubMed] [Google Scholar]
- de Wilde MA, Lamain-de Ruiter M, Veltman-Verhulst SM, Kwee A, Laven JS, Lambalk CB, Eijkemans MJC, Franx A, Fauser B, Koster MPH. Increased rates of complications in singleton pregnancies of women previously diagnosed with polycystic ovary syndrome predominantly in the hyperandrogenic phenotype. Fertil Steril 2017;2:333–340. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012;6:981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, Thomas AM, Grogan TR, Haykal R, Schooler TA et al. . Hyperandrogenism accompanies increased intra-abdominal fat storage in normal weight polycystic ovary syndrome women. J Clin Endocrinol Metab 2016;11:4178–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril 2002;1:167–172. [DOI] [PubMed] [Google Scholar]
- Farley D, Tejero ME, Comuzzie AG, Higgins PB, Cox L, Werner SL, Jenkins SL, Li C, Choi J, Dick EJ Jr. et al. . Feto-placental adaptations to maternal obesity in the baboon. Placenta 2009;9:752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod 2005;6:1475–1483. [DOI] [PubMed] [Google Scholar]
- Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, Grove KL. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 2011;6:2456–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi MO, Azziz R. Diagnosis, epidemiology, and genetics of the polycystic ovary syndrome. Best Pract Res, Clin Endocrinol Metab 2006;2:193–205. [DOI] [PubMed] [Google Scholar]
- Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev 2008;2:140–150. [DOI] [PubMed] [Google Scholar]
- Haakova L, Cibula D, Rezabek K, Hill M, Fanta M, Zivny J. Pregnancy outcome in women with PCOS and in controls matched by age and weight. Hum Reprod 2003;7:1438–1441. [DOI] [PubMed] [Google Scholar]
- Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care 2007;9:2287–2292. [DOI] [PubMed] [Google Scholar]
- Hobson W, Faiman C, Dougherty WJ, Reyes FI, Winter JS. Radioimmunoassay of rhesus monkey chorionic gonadotropin. Fertil Steril 1975;1:93–97. [PubMed] [Google Scholar]
- Hodgen GD, Tullner WW, Vaitukaitis JL, Ward DN, Ross GT. Specific radioimmunoassay of chorionic gonadotropin during implantation in rhesus monkeys. J Clin Endocrinol Metab 1974;3:457–464. [DOI] [PubMed] [Google Scholar]
- Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Roberts KA, Nestler JE. Effects of metformin on early pregnancy loss in the polycystic ovary syndrome. J Clin Endocrinol Metab 2002;2:524–529. [DOI] [PubMed] [Google Scholar]
- Katulski K, Czyzyk A, Podfigurna-Stopa A, Genazzani AR, Meczekalski B. Pregnancy complications in polycystic ovary syndrome patients. Gynecol Endocrinol 2015;2:87–91. [DOI] [PubMed] [Google Scholar]
- Keator CS, Lindner JR, Belcik JT, Bishop CV, Slayden OD. Contrast-enhanced ultrasound reveals real-time spatial changes in vascular perfusion during early implantation in the macaque uterus. Fertil Steril 2011;4:1316–1321. e1311-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;10:1862–1868. [DOI] [PubMed] [Google Scholar]
- Kuhl C. Insulin secretion and insulin resistance in pregnancy and GDM. Implications for diagnosis and management. Diabetes 1991;40(Suppl. 2):18–24. [DOI] [PubMed] [Google Scholar]
- Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes Care 2007;4:878–883. [DOI] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med 2001;8:607–613. [DOI] [PubMed] [Google Scholar]
- Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One 2012;11:e49217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S et al. . Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab 2007;2:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee WK, Bishop CV, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL. Effects of hyperandrogenemia and increased adiposity on reproductive and metabolic parameters in young adult female monkeys. Am J Physiol Endocrinol Metab 2014;11:E1292–E1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil Steril 2002;6:1095–1105. [DOI] [PubMed] [Google Scholar]
- Palomba S, Santagni S, Gibbins K, La Sala GB, Silver RM. Pregnancy complications in spontaneous and assisted conceptions of women with infertility and subfertility factors. A comprehensive review. Reprod Biomed Online 2016;5:612–628. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Casimirri F, Vicennati V. Weight control and its beneficial effect on fertility in women with obesity and polycystic ovary syndrome. Hum Reprod 1997;12:82–87. [DOI] [PubMed] [Google Scholar]
- Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab 2002;9:4231–4237. [DOI] [PubMed] [Google Scholar]
- Rivera HM, Kievit P, Kirigiti MA, Bauman LA, Baquero K, Blundell P, Dean TA, Valleau JC, Takahashi DL, Frazee T et al. . Maternal high-fat diet and obesity impact palatable food intake and dopamine signaling in nonhuman primate offspring. Obesity (Silver Spring) 2015;11:2157–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesner S, Dietrich JE, Weigert J, Montag M, Toth B, Strowitzki T. Time-lapse imaging reveals differences in growth dynamics of embryos after in vitro maturation compared with conventional stimulation. Fertil Steril 2017;3:606–612.e603. [DOI] [PubMed] [Google Scholar]
- Rotterdam EA-SPcwg Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;1:41–47. [DOI] [PubMed] [Google Scholar]
- Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, Regan L, Robinson S. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord 2001;8:1175–1182. [DOI] [PubMed] [Google Scholar]
- Sirmans SM, Parish RC, Blake S, Wang X. Epidemiology and comorbidities of polycystic ovary syndrome in an indigent population. J Investig Med 2014;6:868–874. [DOI] [PubMed] [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology 2005;7:3185–3193. [DOI] [PubMed] [Google Scholar]
- Sterling L, Liu J, Okun N, Sakhuja A, Sierra S, Greenblatt E. Pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. Fertil Steril 2016;3:791–797.e792. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Rivera HM, True CA, Franco JG, Baquero K, Dean TA, Valleau JC, Takahashi DL, Frazee T, Hanna G et al. . Maternal and postnatal high-fat diet consumption programs energy balance and hypothalamic melanocortin signaling in nonhuman primate offspring. Am J Physiol Regul Integr Comp Physiol 2017;2:R169–R179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True CA, Takahashi DL, Burns SE, Mishler EC, Bond KR, Wilcox MC, Calhoun AR, Bader LA, Dean TA, Ryan ND et al. . Chronic combined hyperandrogenemia and western-style diet in young female rhesus macaques causes greater metabolic impairments compared to either treatment alone. Hum Reprod 2017;9:1880–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanky E, Salvesen KA, Heimstad R, Fougner KJ, Romundstad P, Carlsen SM. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: results of a randomized study. Hum Reprod 2004;8:1734–1740. [DOI] [PubMed] [Google Scholar]
- Varlamov O, Somwar R, Cornea A, Kievit P, Grove KL, Roberts CT Jr.. Single-cell analysis of insulin-regulated fatty acid uptake in adipocytes. Am J Physiol Endocrinol Metab 2010;3:E486–E496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, Saade G, Eddleman K, Carter SM, Craigo SD et al. . Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol 2004;4:1091–1097. [DOI] [PubMed] [Google Scholar]
- Young KA, Chaffin CL, Molskness TA, Stouffer RL. Controlled ovulation of the dominant follicle: a critical role for LH in the late follicular phase of the menstrual cycle. Hum Reprod 2003;11:2257–2263. [DOI] [PubMed] [Google Scholar]
- Zhu MJ, Du M, Nathanielsz PW, Ford SP. Maternal obesity up-regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta 2010;5:387–391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contrast-enhanced ultrasound imaging of the uterus and forming placental disks of a control female at gestational Day 32 (standard ultrasound image depicted in Supplemental Fig. S1). With this low mechanical index (MI) imaging, the presence of the microbubble contrast reagent within the vasculature shows up bright white, while all non-vascular areas are dark. To determine vascular characteristics, the MI is increased for 2 s, bursting the microbubbles (18–20 s), and then the MI is decreased again and tissue re-perfusion is imaged until past saturation point (~28-s post-burst) for accurate estimation of signal plateau. Note that the microbubbles do not cross the placental barrier and do not enter the fetal blood stream at this stage of gestation.