Abstract
STUDY QUESTION
What are the reasons for or against the future clinical application of germline genome modification (GGM)?
SUMMARY ANSWER
A total of 169 reasons were identified, including 90 reasons for and 79 reasons against future clinical application of GGM.
WHAT IS KNOWN ALREADY
GGM is still unsafe and insufficiently effective for clinical purposes. However, the progress made using Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)- CRISPR-associated system (Cas) has led scientists to expect to overcome the technical hurdles in the foreseeable future. This has invited a debate on the socio-ethical and legal implications and acceptability of clinical applications of GGM. However, an overview of the reasons presented in this debate is missing.
STUDY DESIGN, SIZE, DURATION
MEDLINE was systematically searched for articles published between January 2011 and June 2016. Articles covering reasons for or against clinical application of intentional modification of the nuclear DNA of the germline were included.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Two researchers independently extracted the reported reasons from the articles and grouped them into categories through content analysis.
MAIN RESULTS AND THE ROLE OF CHANCE
The systematic search yielded 1179 articles and 180 articles were included. Most papers were written by professionals in ethics, (science) journalism and biomedical sciences. Overall, 169 reasons were identified, including 90 reasons for, and 79 reasons against future clinical application of GGM. None of the included articles mentioned more than 60/169 reasons. The reasons could be categorized into: (i) quality of life of affected individuals; (ii) safety; (iii) effectiveness; (iv) existence of a clinical need or alternative; (v) costs; (vi) homo sapiens as a species (i.e. relating to effects on our species); (vii) social justice; (viii) potential for misuse; (ix) special interests exercising influence; (x) parental rights and duties; (xi) comparability to acceptable processes; (xii) rights of the unborn child; and (xiii) human life and dignity. Considerations relating to the implementation processes and regulation were reported.
LIMITATIONS, REASONS FOR CAUTION
We cannot ensure completeness as reasons may have been omitted in the reviewed literature and our search was limited to MEDLINE and a 5-year time period.
WIDER IMPLICATIONS OF THE FINDINGS
Besides needing (pre)clinical studies on safety and effectiveness, authors call for a sound pre-implementation process. This overview of reasons may assist a thorough evaluation of the responsible introduction of GGM.
STUDY FUNDING/COMPETING INTEREST(S)
University of Amsterdam, Alliance Grant of the Amsterdam Reproduction and Development Research Institute (I.D.), and Clinical Center, Department of Bioethics, National Institutes of Health Intramural Research Program (S.H.). There are no competing interests.
Keywords: genetic engineering/CRISPR-Cas systems/mutation/germ cells/genome, human/humans/reproductive techniques/ethics/healthcare quality, access, evaluation/review
Introduction
The prospect of intentional modification of the human germline has been both a source of excitement and unease for decades. Although tools for genome modification have been available for some time (zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs)), their technical limitations rendered considerations about clinical applications of germline genome modification (GGM) theoretical (Lunshof, 2016). However, the discovery of clustered regularly interspaced short palindromic repeats (CRISPR)—CRISPR-associated system (Cas)9 (CRISPR-Cas9), for its specificity, efficiency, low-costs and ease in use, has represented a major step forward from previously available engineering tools (Jinek et al., 2012; Cong et al., 2013). Five groups have recently reported GGM of (non-viable) human embryos (Liang et al., 2015; Kang et al., 2016; Fogarty et al., 2017; Ma et al., 2017; Tang et al., 2017). These experiments revealed the techniques are still unsafe and insufficiently effective for clinical purposes. Our lack of understanding about e.g. gene interactions and possible unintended consequences causes particular concern (IBC, 2015). However, scientists expect to overcome many of these technical hurdles in the foreseeable future (Ishii, 2017, Lunshof 2016; Olson, 2016; Smith et al., 2012). Indeed, although questioned by some experts (Egli et al., 2017), remarkable progress has been reported, including high on-target specificity without off-target effects; although half of the embryos still had the mutation and more studies are needed to ensure reproducibility and safety (Ma et al., 2017).
Three types of applications of GGM have been described, some more contentious than others (Chan et al., 2015). First, GGM could correct disease-causing gene(s), to prevent diseases such as cystic fibrosis (Schwank et al., 2013). Mostly, GGM would then represent an alternative to current reproductive options, such as PGD, to prevent the considered disease in the future child (Bosley et al., 2015). Second, GGM could introduce a modification that reduces the risk of acquiring diseases, such as HIV (Kang et al., 2016). Third, GGM could introduce non-medical enhancements to improve the quality of life of the resulting child, such as increasing muscle mass (Proudfoot et al., 2015).
Many authors and professional societies have called for a debate about the socio-ethical and legal implications before the technical limitations currently preventing clinical introduction are overcome (AMS, 2015; IBC, 2015; NASEM, 2017). The result has been a fierce and on-going debate at international conferences and in academic literature and popular media (Baltimore et al., 2015; Bosley et al., 2015; Lanphier et al., 2015). Whereas some consider it our moral duty to alleviate suffering by eliminating diseases or even applying non-medical enhancements, others foresee apocalyptic scenarios including the destruction of humanity (Smith et al., 2012). However, an overview of the reasons provided on both sides is missing. This article aims to provide an overview of, and framework for, the reasons in favor and against applying GGM clinically.
Materials and Methods
A systematic review of reasons was performed, which is a model to systematically identify the reasons provided in the literature on a normative position, claim or phenomenon (Strech and Sofaer, 2012). We followed PRISMA recommendations (Moher et al., 2009).
Search strategy
MEDLINE was systematically searched; the search string is provided as supplemental data (Supplementary Information Full Search String). The reference lists of eligible articles were perused for additional articles.
Article selection
Articles published in English between January 2011 and June 2016 were eligible for inclusion, including all article types (e.g. opinion articles), except for original biological research. Articles covering intentional modification of the nuclear DNA of the germline (i.e. embryo, zygote, gametes or precursor cells of gametes) were eligible and included if they discussed reasons for or against clinical application. Two researchers (S.H. and L.B.) independently considered inclusion through screening titles, abstracts and if necessary, full-texts.
Meta-synthesis
Meta-synthesis, rather than meta-analysis was performed considering the type of data (Hendriks et al., 2015). Two reviewers (S.H. and I.D. or L.B.) independently performed the data collection and analysis; discrepancies were discussed until meeting consensus.
Data extraction
Several steps were taken to structure the identified reasons. First, we distinguished between reasons for and against clinical application of GGM. We did not describe the extent to which the authors endorse the mentioned reasons. The reasons were inductively grouped into categories by content analysis. This included multiple readings, highlighting meaningful units, grouping meaningful units into categories and comparing meaningful units between categories to integrate the categories (Hycner, 1985; Graneheim and Lundman, 2004).
Considerations regarding the implementation processes and regulation were also indexed.
Per article, we reported the disciplines represented by the authors (as identified through their listed affiliations) and, if relevant, the type of study participants.
Finally, as the first experiments of human GGM may have changed the nature of the debate (Mathews et al., 2015), we used Fisher’s exact tests to analyse differences in how frequent domains were reported before and after 2015.
Results
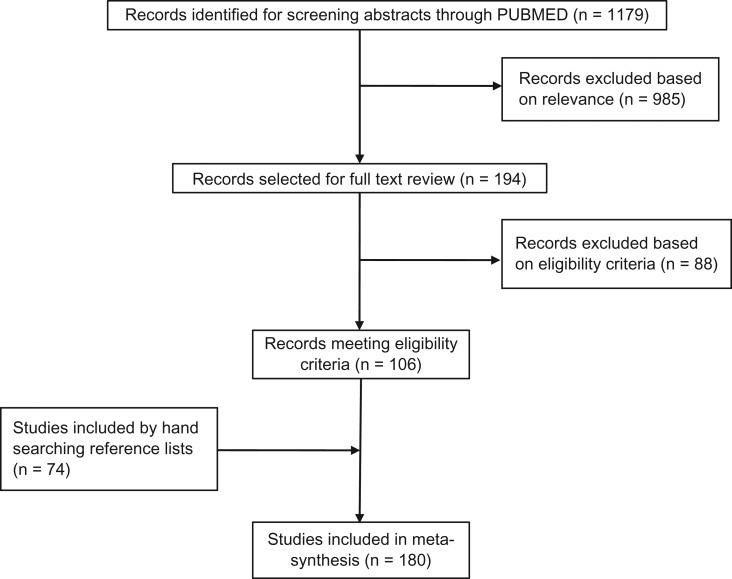
The systematic search yielded 1179 articles (Fig. 1). Based on eligibility, 106 articles were included. We found 74 additional articles perusing reference lists. In total, 180 articles were included. Most articles were published in 2015 (n = 120). In total, 32 articles were published in 2011–2014; 28 between January and June of 2016.
Figure 1.
Flowchart of the study selection process.
Represented stakeholders
The authors represented the following fields: ethics (n = 64/180), (science) journalism (n = 59/180), biomedical sciences (n = 49/180), law/policy (n = 22/180), social sciences (n = 11/180), entrepreneurship (n = 7/180) and economics (n = 2/84; Table I). A total of 19 articles represented (professional) societies. Parents of children with genetic diseases co-authored two articles. One article analysed an Internet forum on genome therapy. Most articles represented views of one stakeholder group (n = 128/180). The most common collaboration was between ethicists and biomedical scientists (n = 12/180).
Table IV.
All included articles by reference number as listed in Tables I–III and Supplementary Information Table S1.
Table I.
Stakeholder groups that have been used as sources (i.e. authors or study participants) in the articles.
| Stakeholder group | N | Referencesa,b |
| Stakeholder group | ||
|---|---|---|
| Professionals in ethics | 64 | 1–64 |
| Professionals in (science) journalism | 59 | 60,65–122 |
| Professionals in biomedical sciences | 49 | 1–3,5,8,16–18,23,26,28,30,32,41,44,56–58,60,62,123–151 |
| Professionals in law and policy | 22 | 2,4,5,8,14,15,24,25,29,44,54,56,60,64,133,152–158 |
| Professionals listed as representing societies | 19 | 5,8,19,23,131,159–172 |
| Professionals in social sciences | 11 | 9,29,62,130,156,173–178 |
| Professionals in economics | 2 | 152,153 |
| Patient representatives (parents of children with genetic anomalies) | 2 | 148,179 |
| The general public | 1 | 32 |
| Professionals in (biomedical) entrepreneurshipc | 7 | 2,5,19,44,131,136,180 |
| Number of stakeholder groups represented per article | ||
| Representing one stakeholder group | 128 | 6,7,10–13,20–22,27,31,33–40,42,43,45–53,55,59,61,63,65–129,132,134,135,137–147,149–151,154,155,157,158,173–180 |
| Representing ethics and biomedical sciences | 12 | 1,3,16–18,23,26,28,30,41,57,58 |
| Representing ethics and law and policy | 7 | 4,14,15,24,25,54,64 |
| Representing ethics and one other stakeholder group | 2 | 9,19 |
| Representing biomedical sciences and one stakeholder group | 5 | 130,131,133,136,148 |
| Representing law/policy and one other stakeholder group | 3 | 152,153,156 |
| Representing three or more stakeholder groups | 9 | 2,5,8,29,32,44,56,60,62 |
| Representing societies without specification of involved stakeholders | 14 | 159–172 |
aAs identified by the listed affiliation.
bNumbers indicate the appropriate reference (Table IV).
cSelf-reporting representing a commercial company.
Reasons for and against clinical application of GGM
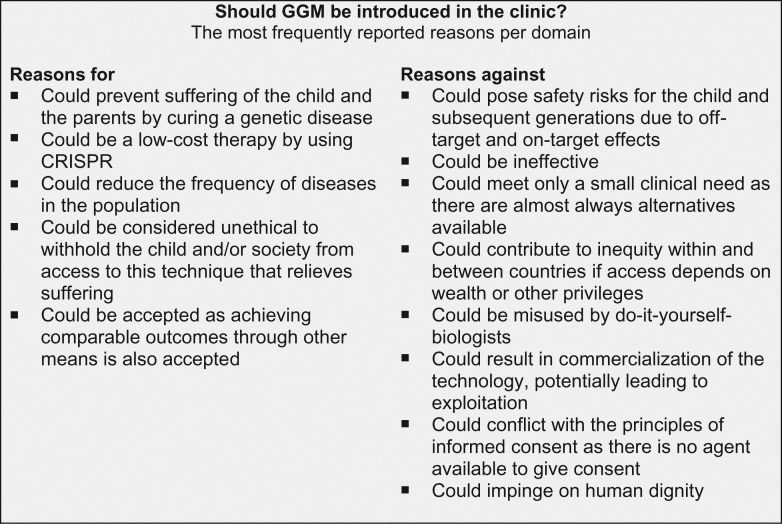
We identified 169 reasons, including 90 reasons for, and 79 reasons against future clinical application of GGM (Table II). The articles reported a maximum of 60/169 reasons (Smith et al., 2012). The reasons could be categorized into 13 domains (i) quality of life of affected individuals, (ii) safety, (iii) effectiveness, (iv) existence of a clinical need or alternative, (v) costs, (vi) homo sapiens as a species, (vii) social justice, (viii) potential for misuse, (ix) special interests, (x) parental rights and duties, (xi) comparability to acceptable processes, (xii) rights of the unborn child, (xiii) human life and dignity (Table II). Before 2015 (i.e. the first human GGM), three domains were mentioned more frequently: parental rights and duties (47 vs 20%, P = 0.003), comparability to acceptable processes (59 vs 30%, P = 0.002), and human life and dignity (47 vs 30%, P = 0.01) (Supplementary Table S1). The domains effectiveness (56 vs 77%, P = 0.03) and special interests (13 vs 32%, P = 0.03) were more frequently mentioned after 2015. Figure 2 displays the most frequently reported reasons per domain.
Table II.
Arguments in favour and against clinical applications of germline genome modification.
| Domain | Side | Argument | N | Referencea |
|---|---|---|---|---|
| Quality of life of affected individuals | Positive | Could prevent suffering of the child and the parents by curing a genetic disease | 169 | 1–8,10–44,46–51,53–62,64–95,97–132,134–137,139,140,142–150,152–172,174–176,178–180 |
| Could prevent potential suffering of the child by reducing the risk of diseases | 29 | 13,15,17,23,28,34–36,41,43–45,58,60,61,67,100,108,117,120,125,129,131,144,145,158,160,161,163 | ||
| Could improve the quality of life of the child by enhancing his/her non-medical traits | 104 | 1,3,6–10,12,13,15–20,23,26–29,31,32,34–37,39,41–48,50,51,53,55,58–60,63,64,67,68,70,72,73,76,77,79,81,83,85,88–90,95,98–100,103,105,106,108,111,115–120,124,125,127,128,130,131,133,136,138–146,154–158,160–165,171,176,178 | ||
| Could provide progeny with an evolutionary advantage | 1 | 161 | ||
| Could improve the quality of life of healthcare providers by increasing their job satisfaction | 1 | 38 | ||
| Could not prevent parents from all opportunities for guiding their children in overcoming difficulties | 2 | 7,63 | ||
| Could have predictable effects on quality of life | 1 | 59 | ||
| Negative | Could cause discord in the parent–child relationship | 7 | 6,17,36,39,46,63,128 | |
| Could hinder parents in supporting their child as a result of the large differences between them | 2 | 1,51 | ||
| Could withhold parents from guiding their children in overcoming difficulties | 2 | 7,63 | ||
| Could not have the expected positive effects on the quality of life of the child and/or the parents | 17 | 6,9,10,31,35–37,45,46,51,58,59,64,91,95,117,146 | ||
| Safety | Positive | Could be safe for the child by using CRISPR which is able to induce specific modifications | 43 | 3,14,16,23,28,37,38,43,48,55,60,62,70,76,77,79,81,89,90,93,95,100,104–106,108,110,113,117,119,121,122,124,137,145,146,151,155,162,163,170,178,180 |
| Could be safe for the child by using preimplantation genetic screening to assess off-target effects | 13 | 1,2,17,28,35,43,44,57,60,121,125,131,139 | ||
| Could be safe for the child by reversing errors using the same technology | 8 | 7,43,44,50,64,90,124,127 | ||
| Could be safe for the child by further development of the technique | 4 | 38,43,89,117 | ||
| Could be safe for the child by modifying precursor gametes, which builds in natural checkpoints | 2 | 43,139 | ||
| Could be safe for the child by introducing common genes of which unforeseen effects are unlikely | 1 | 124 | ||
| Could decrease the life-long treatment burden of the child as he/she will not need further therapy | 2 | 19,123 | ||
| Could decrease the life-long treatment burden of the child as he/she will not need PGD to prevent passing on the disease to future offspring | 2 | 34,44 | ||
| Could have safety risks for the child that are justified based on the expected benefits for that child | 35 | 1,2,9,11,12,17,21,25,28,32,34–37,39,42,43,57,59,61,64,81,83,90,113,117,121,127,131,145,155,157,160,163,175 | ||
| Could have safety risks for the child that are justified based on the overall benefits to mankind | 3 | 36,37,59 | ||
| Could have safety risks for the child of which acceptability would be difficult to determine | 8 | 9,15,20,54,57,60,143,163 | ||
| Could be more safe for the child than previously introduced novel techniques | 16 | 6,9,12,21,25,28,33–36,39,42,43,61,117,163 | ||
| Could be more safe for the child than sexual reproduction | 17 | 9,11,12,20,39,43,56,61,64,72,85,113,124,139,157,163,175 | ||
| Could be more safe for the child than somatic genome modification | 6 | 48,55,71,124,129,160 | ||
| Could allow couples to circumvent the maternal risks of terminating the pregnancy | 1 | 38 | ||
| Could allow couples to circumvent the psychological distress of terminating the pregnancy | 3 | 38,134,139 | ||
| Could allow couples to circumvent the maternal risks and the burden of having multiple IVF cycles for PGD | 1 | 38 | ||
| Could allow couples to circumvent the maternal risks and the burden of IVF if in vitro-derived gametes are used | 2 | 35,134 | ||
| Negative | Could pose safety risks for the child and subsequent generations due to off-target and on-target effects | 153 | 1–21,23–26,28–32,34–49,51,52,54–57,59–62,64,66–79,81,83–95,97–100,102,103,105–108,110,111,113–132,134–137,139–142,145–150,152,155–165,168,169,171–173,175,177–180 | |
| Could increase risks for the child by requiring the use of IVF | 1 | 141 | ||
| Could result in the child suffering from psychological distress | 11 | 6,7,17,32,43,46,47,51–53,58 | ||
| Could result in the child suffering from a social stigma | 2 | 35,131 | ||
| Could result in unpredictable safety risks for the child and subsequent generations | 76 | 2,7–12,16–20,26,29,31,34–40,43,44,46,47,49,51,54,55,61,64,70–72,76,77,86–91,95,100,102,103,106,107,115–117,127,129–132,136,137,144,146,148,152,157,160,162–165,169,171,172,176–178,180 | ||
| Could be difficult to ensure safety before clinical application | 13 | 8,17,28,35–37,39,47,51,55,117,152,177 | ||
| Could be difficult to ensure safety by using preimplantation genetic screening to assess off-target effects | 4 | 17,57,128,139 | ||
| Could be difficult to ensure the long-term follow-up required for assessing safety | 9 | 1,8,17,28,40,60,79,127,164 | ||
| Could pose safety risks for the intended parents | 4 | 58,60,134,160 | ||
| Could propose safety risks and burdens for the intended parents by requiring IVF | 7 | 1,35,43,56,59,63,91 | ||
| Could increase maternal pregnancy risks by increasing risks for the child | 1 | 56 | ||
| Could require a developmental process that exposes people who have supplied materials for research to risks | 1 | 174 | ||
| Effectiveness | Positive | Could be effective | 16 | 2,8,17,18,35,37,43,55,93,100,105,106,121,126,142,150 |
| Could be efficient | 28 | 1,4,14,16,17,23,26,28,38,40,48,55,57,60,64,95,102,104,136,137,139,145,149–151,162,163,165 | ||
| Could be easy to carry out by using CRISPR | 60 | 2–4,17–19,23,28,40,44,48,50,55,57,60,62,67,68,71,72,76–79,81,83,87,89,90,92,93,95,98–100,102,105,106,108,109,111,112,117,119,121,126,137,140,145,146,149,150,155,158,163,169–171,178,180 | ||
| Could be more effective than using somatic therapy | 11 | 8,17,35,36,43,75,100,117,123,129,139 | ||
| Could be more effective than using current alternatives (e.g. PGD) | 10 | 13,17,18,34,35,67,95,134,137,144 | ||
| Could be difficult to determine acceptable levels of effectiveness | 1 | 60 | ||
| Negative | Could be ineffective | 73 | 1,2,4,8,10–13,15,17,19,23–25,29,32,35,38,40,41,44,45,47,48,50,57,60,62,70,71,73,74,77,81–83,86,87,90–92,94,97–100,102,103,106,107,110,113,115,118,122,124,127–129,131,133,137,139,148,150,152,159,160,162–165,175 | |
| Could be inefficient | 22 | 17,18,28,43,44,50,65,72,75,81,92,117,120,125,128,134,145,149,150,152,170,175 | ||
| Could be difficult to carry out the techniques | 2 | 87,134 | ||
| Could be ineffective as causal mutations are in many cases unknown | 22 | 26,28,35,44,48,56,64,85,86,91,98,100,108,117,134,139,141,145,151,155,156,163 | ||
| Could be ineffective as many diseases/traits are too complex to modify | 21 | 13,17,26,28,35,42,44,56,63,72,79,108,117,123,134,141,144,157,158,163,165 | ||
| Could be ineffective as many causal mutations arise de novo | 2 | 26,163 | ||
| Could be difficult to ensure effectiveness by using preimplantation genetic screening to assess mosaicism | 3 | 1,28,44 | ||
| Existence of a clinical need or alternative | Positive | Could meet an unmet clinical need for obtaining genetic parenthood in case of certain parental genetic predispositions (i.e. inability to select not affected embryo)* | 31 | 1,12,13,17,18,24,31,34,38,43,44,55,64,85,95,98–100,114,117,124,127,137,139,144,149,155,160,164,175,179 |
| Could meet an unmet clinical need for obtaining genetic parenthood in case of protecting against polygenic disease (i.e. inability to select not affected embryo)* | 4 | 28,34,35,43 | ||
| Could meet an unmet clinical need for obtaining genetic parenthood in case of introducing protective alleles that the parents do not have* | 2 | 43,129 | ||
| Could have unprecedented potential for eliminating heterozygous carriers from the population | 3 | 28,34,144 | ||
| Could have unprecedented potential for improving the species with non-human traits* | 9 | 6,32,44,47,64,72,145,162,175 | ||
| Could be preferable over current alternatives by circumventing the creation of embryo’s that will be destructed in PGD | 12 | 28,34,55,66,85,98,100,127,129,134,139,155 | ||
| Could be preferable over current alternatives by reducing the need for oocyte donors | 3 | 1,18,38 | ||
| Could be preferable over current alternatives by preventing the ethical issues related to termination of pregnancy | 2 | 139,155 | ||
| Negative | Could meet only a small clinical need as there are almost always alternatives available | 56 | 1,10,13,17–19,26,28,31,32,34,36,37,43,44,47,48,55,60,67,70–73,79,81,84,85,88,95,100,103,106,108,114,117,118,121,125,127,129,131,134,137,140,144,146,155,160,162–164,169,175,178,180 | |
| Could create a demand that would not have existed without the existence of the technique | 9 | 4,13,51,71,91,95,119,137,161 | ||
| Could be preferable over alternatives to only a limited number of people | 1 | 155 | ||
| Costs | Positive | Could be a low-cost therapy by using CRISPR | 35 | 2,3,28,38,40,43,44,48,50,55,60,62,68,72,80,87,90,92,95,98–100,102,106,119,126,127,137,149,150,152,155,158,163,180 |
| Could be a low-cost therapy by improvements from further research | 1 | 178 | ||
| Could be a low-cost therapy by commercialization | 1 | 55 | ||
| Could reduce healthcare costs for individuals and/or society caused by people living with the disorders | 8 | 31,35,44,48,55,62,117,163 | ||
| Could allow people to contribute to society more economically | 1 | 117 | ||
| Could create jobs in healthcare | 1 | 24 | ||
| Could increase costs that are justified based on the benefits | 1 | 174 | ||
| Negative | Could increase healthcare costs by being a high-cost therapy | 7 | 1,10,17,44,117,136,148 | |
| Could increase healthcare costs by causing side-effects that require therapy | 1 | 157 | ||
| Could increase healthcare costs by prolonging life | 1 | 44 | ||
| Could lead to significant indirect costs for society through inciting large-scale changes | 2 | 10,136 | ||
| Could entail issues of distributive justice relating to investing in this rather than other issues | 7 | 43,48,56,82,91,158,178 | ||
| Could increase medical tourism if there will be differences in costs | 1 | 175 | ||
| Homo sapiens as a species | Positive | Could reduce the frequency of diseases in the population | 58 | 8,10–12,18,20,21,25,26,28,29,31,34–37,43,44,48,51,55,56,58,61,62,64,67,90,91,98,100–103,106,107,109,110,117,118,121–123,126,127,130,131,137,142,144,145,148,154,158,163,171,178,179 |
| Could allow modified individuals to contribute more to society | 8 | 7,9,11,59,61,117,146,161 | ||
| Could safeguard the survival of our species by allowing modified individuals to contribute more | 8 | 9,11,12,31,35,61,123,163 | ||
| Could have limited impact as consequences are restricted to individual and its descendants | 4 | 20,21,64,157 | ||
| Could be used for eugenics, however, this is not necessarily morally wrong | 9 | 7,35,42,58,146,156,161,163,178 | ||
| Could have large-scale consequences, however, human resilience will likely prevent fall-outs | 1 | 64 | ||
| Could have limited impact as widespread use is unlikely | 5 | 10,28,35,44,91 | ||
| Could have limited effect on diversity as there are many traits | 1 | 10 | ||
| Could have limited effect on the gene pool | 4 | 21,28,35,44 | ||
| Could have no affect on the germline | 1 | 124 | ||
| Could have no affect on future generations if modified individuals do not reproduce | 1 | 157 | ||
| Could lead to a slippery slope, however, this should not be a decisive argument against using this technology | 6 | 34,35,43,103,116,124 | ||
| Could lead to worst-case scenarios, however, this should not be a decisive argument against using this technology | 7 | 10,24,34,51,60,103,157 | ||
| Negative | Could have potentially disastrous consequences leading to dystopias and the demise of our species | 24 | 7,13,14,17,31,34,43,44,50,51,64,71,88,90,117,120,132,154,158,163,164,174,175,178 | |
| Could weaken the resilience of our species by reducing generational turnover through human life extension | 1 | 31 | ||
| Could weaken the resilience of our species by reducing the diversity of the gene pool | 5 | 6,10,32,44,64 | ||
| Could lead to eugenics | 47 | 1,6,7,10,17,21,24,26,28,29,31,35,44,48,50,53,58,70,79,81,85,87,90,99,115–118,126,127,129,130,138,139,141,146,154,156,158,161,163–165,174–176,178 | ||
| Could incite a slippery slope towards unacceptable scenarios | 31 | 2,13,16–19,31,34–36,41,43,44,51,68,69,71,76,81,89,99,102,116–118,127,131,133,138,175,178 | ||
| Could harm biodiversity and ecosystems | 6 | 29,43,50,62,174,175 | ||
| Could alter cultural attitudes and values | 10 | 31,35,44,49,50,53,58,128,138,152 | ||
| Could increase the medicalisation of reproduction | 2 | 128,131 | ||
| Could incite a rat race | 4 | 10,58,158,176 | ||
| Could reduce the valuable diversity in our society | 6 | 6,10,32,42,91,179 | ||
| Could lead to social dilemmas | 4 | 9,10,13,48 | ||
| Could have limited success in the elimination of diseases as this would require modifying heterozygous embryos | 2 | 26,144 | ||
| Could have undesirable effects on society (unspecified) | 11 | 32,47,83,85,91,103,126,131,136,160,171 | ||
| Social justice | Positive | Could prevent the injustice of being dealt a poor genetic hand | 6 | 35,43,45,55,62,64 |
| Could decrease segregation by providing disadvantaged groups with preferential access | 1 | 7 | ||
| Could lead to equity and access to care issues, however, this should not be a decisive argument against using this technology | 5 | 7,21,43,60,154 | ||
| Could reduce the acceptability of disability, however, this should not be a decisive argument against using this technology | 1 | 35 | ||
| Could lead to generational inequity, however, this should not be a decisive argument against using this technology | 1 | 35 | ||
| Negative | Could contribute to inequity within and between countries if access depends on wealth or other privileges | 45 | 1,3,6,7,10,11,17,21,26,28,32,35,43,44,48,50,55,57,58,60,62,76,77,79,84,89,91,117,127,131,136,138,152,156,158,160,162–165,174–176,178,179 | |
| Could contribute to inequity within and between countries through choices in the development of potential modifications | 6 | 32,55,60,62,175,176 | ||
| Could create a ‘genobility’ | 7 | 7,32,43,47,89,121,178 | ||
| Could lead to generational inequity | 3 | 35–37 | ||
| Could reduce the acceptability of disability | 21 | 1,11,13,22,32,35,36,50,55,58,62,84,91,121,160,163–165,175,176,178 | ||
| Could contribute to inequity (unspecified) | 6 | 10,36,37,50,121,175 | ||
| Potential for misuse | Positive | Could pose no biosecurity risk | 1 | 20 |
| Could be too complex to carry out for ‘garage’-biologists | 4 | 44,78,87,109 | ||
| Could be misused, however, this should not be a decisive argument against using this technology | 3 | 43,56,63 | ||
| Negative | Could pose a biosecurity risk | 12 | 9,24,34,48,50,54,62,80,109,160,174,175 | |
| Could be misused in ways that would be difficult to detect | 1 | 55 | ||
| Could be misused by parents with wrong incentives | 3 | 37,44,63 | ||
| Could be misused by do-it-yourself-biologists | 17 | 23,40,44,48,68,78,80,83,87,99,102,109,112,116,126,175,180 | ||
| Could result in (governmental) coercion forcing people to use these technologies | 11 | 10,21,32,35,37,59,77,127,162,164,178 | ||
| Could result in indirect coercion through social norms forcing people to use these technologies | 6 | 21,35,44,55,62,91 | ||
| Could result in indirect coercion through funding forcing people to use these technologies | 4 | 35,91,127,163 | ||
| Could be misused (general) | 16 | 1,17,18,34,49,50,52,56,86,106,121,125,127,143,157,163 | ||
| Special interests | Positive | Could incite commercial interests that are aligned with public interests | 3 | 87,117,155 |
| Negative | Could result in commercialization of the technology, potentially leading to exploitation | 38 | 4,35,41,48–50,54–56,62,75,87,88,90,92,100–102,105,106,111,115–119,121,127,131,138,149,156,158,160,161,163,175,176 | |
| Could incite pressure from patients that leads to premature and/or inappropriate applications | 11 | 3,50,54,56,60,62,72,112,156,161,163 | ||
| Could incite (commercial) interests of clinics that lead to premature and/or inappropriate applications | 8 | 44,60,69,88,99,103,120,143 | ||
| Could incite (commercial) interests of researchers that lead to premature and/or inappropriate applications | 8 | 60,75,90,99,107,142,158,165 | ||
| Could incite special interest that have undue influence on policy-makers | 2 | 62,156 | ||
| Parental rights and duties | Positive | Could be considered part of parents’ right of reproductive liberty | 16 | 1,10,11,13,21,22,35–38,47,72,131,156,161,178 |
| Could improve reproductive autonomy | 9 | 1,13,18,28,35,36,117,137,178 | ||
| Could constitute part of the parental duty to make decisions for their unborn child as he/she cannot yet make these | 3 | 11,61,162 | ||
| Could result in irreversible negative outcomes when abstaining from its use | 2 | 43,61 | ||
| Could be considered unethical to withhold the child and/or society from access to this technique that relieves suffering | 32 | 4,7,10–12,22,23,31,34,35,37–39,43–48,51,59,61,64,87,91,117,129,154,158,161,174,178 | ||
| Negative | Could surpass the limits of reproductive liberty | 5 | 6,10,22,40,131 | |
| Could be considered part of parents’ right of reproductive liberty, however, this is not important | 1 | 37 | ||
| Could make an appeal to the parental duty to protect child against uncertainties of experimental techniques | 3 | 36,37,47 | ||
| Could make no appeal on a parental duty to perfect children as there is no such duty | 3 | 35,37,59 | ||
| Comparability to acceptable processes | Positive | Could be accepted as achieving comparable outcomes through other means is also accepted | 33 | 6,7,9–12,17,20–22,33,36,38,40,43–46,50,55,58,59,61,63,72,90,91,117,139,145,156,161,178 |
| Could be considered natural as genes are modified in nature too | 10 | 11,20,28,43,56,64,85,90,139,175 | ||
| Could be considered to meet the human drive to exercise control | 6 | 7,17,26,63,117,178 | ||
| Could be considered as restoring nature | 2 | 17,28 | ||
| Could be considered unnatural, however, unnatural is not inherently wrong (i.e. naturalistic fallacy) | 13 | 7,11,35,43,53,61,63,64,85,117,119,154,178 | ||
| Negative | Could intervene to an extent that only nature is allowed | 26 | 6,7,12,13,17,31,32,35,37,49,53,55,62–64,99,115,117,118,141,155,161,162,164,177,178 | |
| Could intervene to an extent that only God is allowed | 13 | 6,7,12,13,17,43,48,51,56,64,100,155,161 | ||
| Could be considered unjustified as it is a preventive procedure | 4 | 17,18,25,107 | ||
| Could be compared to accepted current practices, however, these may also be unethical | 1 | 46 | ||
| Rights of the unborn child | Positive | Could implicate the non-identity problem | 10 | 6,11,12,22,35,36,39,43,55,61 |
| Could lead to no relevant non-identity problem | 4 | 22,35,43,55 | ||
| Could be done without implying that acceptance of a child is conditional | 1 | 22 | ||
| Could leave the right to freedom of the child unaffected | 6 | 6,22,36,44,45,58 | ||
| Could conflict with the principles of informed consent, however, parents always make choices for their children | 12 | 1,11,12,28,36,40,53,57,61,72,124,127 | ||
| Negative | Could impinge on the right to freedom of the child | 17 | 7,12,36,40,43–48,52,53,58,72,130,160,175 | |
| Could conflict with the principles of informed consent as there is no agent available to give consent | 28 | 1,11,12,14,17,18,28,36,38,40,43,44,48,53,56,57,61,70,75,99,106,117,125,127,131,158,165,169 | ||
| Could conflict with the principles of informed consent as information about the technique is insufficiently available | 6 | 3,32,36,40,127,176 | ||
| Could imply that the child is not unconditionally accepted | 1 | 22 | ||
| Human life and dignity | Positive | Could be congruent with societal values as the public will sympathize with disease carriers | 6 | 1,51,84,91,117,175 |
| Could be congruent with religious values | 6 | 7,12,32,63,161,175 | ||
| Could be congruent with human dignity as an embryo does not have a moral status | 5 | 38,43,58,84,155 | ||
| Could be incongruent with some perceptions of human dignity but as long as what constitutes human dignity is unclear, this should not be a decisive argument against using this technology | 6 | 22,38,39,53,60,165 | ||
| Could be opposed based on perceptions of a higher purpose of disease, however, this should not be a decisive argument against using this technology as suffering serves no purpose | 1 | 62 | ||
| Could incite a (temporary) yuk-response, however, this should not be a decisive argument against using this technology | 6 | 7,21,90,99,100,156 | ||
| Could incite religious objections, however, this should not be a decisive argument against using this technology | 3 | 43,82,121 | ||
| Negative | Could impinge on human dignity | 31 | 1,6,13–16,18,22,27,28,35,36,38,43,46,48,54,61,67,100,115–117,125,131,138,152,161,165,175,178 | |
| Could conflict with the moral status of a human embryo, which implies they should not be modified and/or created for the purpose of research | 22 | 1,13,17,18,22,23,28,34,39,41,43,53,55,58,60,84,155,160,161,163,165,175 | ||
| Could incite religious objections | 13 | 20,32,43,44,63,64,82,116,129,138,155,165,175 |
aNumbers indicate the appropriate reference (Table IV).
*Argument specific to germline genome modification.
Figure 2.
The most frequently reported reasons per domain.
Quality of life of affected individuals
Seven reasons for GGM referred to improving the quality of life of affected individuals. GGM could prevent suffering of the child and the parents by curing a genetic disease, prevent potential suffering of the child by reducing the risk of diseases, or improve the quality of life of the child and the parents by enhancing his/her non-medical traits. Articles argued GGM could provide progeny with an evolutionary advantage. Moreover, it could improve the job satisfaction of healthcare providers (as they care about their patients whose well-being is improved). Furthermore, it was argued GGM would have predictable effects on quality of life, and would not withhold parents from opportunities for guiding their children in overcoming difficulties.
In contrast, four arguments were raised that GGM, when successful, would not improve the quality of life of affected individuals. Specifically, despite reaching the desired outcome, GGM could cause discord in the parent–child relationship, hinder parents in supporting their child because of the large differences between them, withold parents from guiding their children in overcoming difficulties, and could not have the expected positive effects on the quality of life of the child and/or the parents.
Safety
Overall, 18 arguments for GGM related to safety. Some articles discussed that GGM could be safe for the child by applying the following strategies: using CRISPR which is able to induce specific modifications, using PGS to assess off-target effects, reversing errors using the same technology, further development of the technique, modifying precursor gametes (which would build in natural checkpoints), and/or by introducing common genes of which unforeseen effects are unlikely. Additionally, articles reasoned that GGM could decrease the child’s life-long treatment burden as he/she will not need further therapy or PGD to prevent passing on the disease to future offspring. Some argued that safety risks for the child could be justified based on the expected benefits for that child, or based on the overall benefits to mankind. The difficulty of determining acceptable levels of risk for the child was raised. It was suggested GGM could be more safe for the child than previously introduced techniques, sexual reproduction or somatic genome modification. Additionally, it could allow couples to circumvent the maternal risks and psychological distress of pregnancy termination, the maternal risks and the burden of multiple IVF cycles for PGD and/or the maternal risks and the burden of IVF if in vitro-derived gametes are used.
Twelve concerns about safety were expressed. Articles argued that GGM could pose safety risks for the child and subsequent generations due to off-target and on-target effects (i.e. the targeted gene protecting against the targeted disease but increasing the risk on a different disease). Furthermore, it would require using IVF, which by itself increases risks for the child. It could also result in the child suffering from psychological distress or social stigma. Concerns were expressed that the safety risks could be unpredictable and it could be difficult to ensure safety before clinical application or to assess safety by using PGS to assess off-target effects. Furthermore, ensuring the long-term follow-up required to assess safety could be challenging. Some reasoned that GGM could pose safety risks for the intended parents, the need for IVF would involve additional safety risks and burdens, and higher health risks for children would increase obstetric risks. Finally, some stressed that the process of developing GGM may expose people supplying research materials to risks.
Effectiveness
Six reasons for GGM related to effectiveness. Some articles argued that GGM could be effective, efficient, and easy to carry out by using CRISPR. Several authors stressed that effectiveness should be interpreted in the context of somatic genome modification, or current alternatives such as PGD, both of which may be less effective. Determining acceptable minimal limits of effectiveness could be challenging.
Seven reasons against GGM related to effectiveness. It could be ineffective, inefficient, or difficult to carry out the techniques. Articles reasoned that GGM could be ineffective as causal mutations are in many cases unknown, many diseases/traits are too complex to modify, and many causal mutations arise de novo. Finally, some stressed that ensuring effectiveness through assessing mosaicism by PGS could be difficult.
Existence of a clinical need or alternative
Eight arguments in favour of GGM built on an unmet clinical need. Some articles discussed that GGM could meet an unmet need for obtaining genetic parenthood in case of certain parental genetic predispositions (e.g. both homozygous and therefore it would not be possible to select a not affected embryo), protecting against polygenic diseases, and introducing protective alleles that the parents do not have. Additionally, GGM could have unprecedented potential for eliminating heterozygous carriers from the population and improving the species with non-human traits. Finally, it could be preferable over current alternatives: by circumventing the creation of embryo’s that will be destructed in PGD, by reducing the need for oocyte donors and by preventing the ethical issues related to pregnancy termination.
Three arguments against GGM referred to the clinical need being insufficient. Specifically, GGM could: meet only a limited clinical need as alternatives are almost always available, create a demand that otherwise would not have existed, and be preferable over alternatives to only few people.
Costs
Seven financial reasons were given for GGM. It could be a cheap therapy by using CRISPR, with improvements of further research, and by commercialization. Furthermore, curing children would prevent costs of (life-long) therapy and care for individuals and/or society, and would allow these individuals to contribute more economically. Additionally, it could create jobs in healthcare. Finally, some argued that the benefits justify the costs.
Six reasons against using GGM referred to costs. It could increase healthcare costs by: being an expensive therapy, causing side-effects that require therapy, and prolonging life. Additionally, it could lead to significant indirect costs for society through inciting large-scale changes (e.g. modifications increasing stature may require redesigning buildings to accommodate taller individuals). Furthermore, articles reasoned that investing in GGM rather than other issues (e.g. people currently suffering from these diseases) raises questions about distributive justice, and pricing differences may incite medical tourism.
Homo sapiens as a species
A total of 13 arguments in favour of GGM referred to benefits to our species. Articles suggested that GGM could reduce the frequency of, or eradicate, diseases in the population. It may allow modified individuals to contribute more to society and thereby even safeguard the survival of our species. Some argued that even potential eugenic purposes would not necessarily be unethical. Furthermore, although there may be large-scale consequences, human resilience will prevent fall-outs. Others reasoned negative impacts would be limited as consequences are restricted to the individuals and their descendants. Furthermore, some discussed that widespread use of GGM was unlikely, therefore limiting the potential societal impact. Specifically, effects on the gene pool and diversity would be limited as there are many traits. Additionally, GGM may not affect the germline (i.e. by modifying embryonic stem cells in ways that are not passed on to future generations) or may not affect future generations if modified individuals do not reproduce. Finally, some argued that the potential for worst-case scenarios or a slippery slope towards unacceptable scenarios are not limited to GGM and may be controlled, or otherwise should not constitute a decisive argument against GGM.
Overall, 13 concerns about GGM referred to our species. Some argued GGM could have disastrous consequences leading to dystopias and the demise of our species. For example, the resilience of our species could be weakened by reducing the gene pool’s diversity and/or by reducing generational turnover through human life extension. Additionally, GGM could lead to eugenics, and to a slippery slope towards unacceptable scenarios. It may also harm biodiversity and ecosystems. GGM may alter cultural attitudes and values, increase the medicalisation of reproduction, and incite a rat race. It may lead to reducing valuable diversity in our society. Furthermore, it may present social dilemmas (i.e. a conflict between individual and collective interests). Additionally, some reasoned that eliminating diseases from the population would be unlikely as this would require large-scale modification of heterozygous embryos. Finally, some authors warn against unspecified undesirable societal effects.
Social justice
Five benefits of GGM in improving equality were named. It could prevent the injustice of being dealt a poor genetic hand, or even decrease segregation by providing disadvantaged groups with ‘headstart’ programmes or preferential access as a form of affirmative action. Alternatively, some argued that potential issues related to equity and access to care, reducing acceptability of disability, and creating generational inequity are not limited to GGM and may be controlled, or otherwise should not constitute a decisive argument against GGM.
Six concerns about exacerbating issues relating to social justice were expressed. GGM could contribute to inequity within and between countries if access depends on wealth or other privilege, and/or through choices in the development of potential applications. It may create some form of a ‘genobility’ or lead to generational inequity (i.e. the first modified generation being disproportionally exposed to risks). Additionally, GGM may reduce the acceptability of disability. Finally, some warned against unspecified inequality issues.
Potential for misuse
Three arguments in favour of GGM related to its potential misuse. Articles reasoned that clinical application of GGM would not pose biosecurity risks, and misuse by do-it-yourself-biologists would be unlikely. Furthermore, the potential for misuse is not limited to GGM and may be controlled, hence it should not constitute a decisive argument against using GGM.
Eight concerns about misuse of GGM were named. The potential for posing a biosecurity hazard and the difficulty to detect misuse of the technology were stressed. GGM could be misused by parents with wrong incentives and by do-it-yourself-biologists. The potential for (governmental) coercion forcing people to use these technologies was addressed, as well as the potential for indirect coercion through social norms or funding. Finally, some warned against unspecified misuses.
Special interests
In favour of GGM, some authors referred to special interests. Specifically, they noted that commercial interests could be aligned with public interests in preventing the fall-out of potential harms.
Five articles voiced concerns about exploitation by special interests. They argued that potential commercialization of GGM could lead to exploitation. Additionally, special interests/pressure from patients, clinics and/or researchers may lead to premature or innapprporiate applications. Finally, special interests could have undue influence on policy-makers.
Parental rights and duties
Five reasons for GGM related to parental rights and duties. Articles reasoned that using GGM is part of the intended parents’ reproductive liberty, and would improve reproductive autonomy. Moreover, intended parents have a duty to make decisions about their unborn children and abstaining from GGM cannot be reversed. Finally, some considered it unethical to withhold the child and/or society from access to this technique to relieve suffering.
Four concerns were raised relating to parental rights and duties. Some considered GGM to surpass the limits of intended parents’ reproductive liberty. Others stated that even if part of parents’ reproductive liberty, this right is not important. Furthermore, parents have a duty to protect their children against uncertainties of experimental techniques. Finally, some argued that there is no parental duty to have perfect children and, consequently, there is no duty to apply GGM.
Comparability to acceptable processes
Five reasons in favour of GGM drew comparisons to existing and accepted processes. Some articles reasoned that GGM could be accepted as achieving comparable outcomes through other means is also accepted. Furthermore, it could be considered: as natural, considering genes are modified in nature too; as meeting our human drive to exercise control; and as restoring the natural state. Finally, even if modification is considered unnatural, unnatural is not inherently wrong (i.e. naturalistic fallacy).
Four concerns related to comparability of existing and accepted processes. These concerns included the arguments that only nature or God should intervene to the extent of GGM. Furthermore, some articles stressed that the intervention would take place before confirming the expression of the disease, and therefore could not be justified. Finally, some reasoned that comparability to current practices is a flawed argument since these may also be unethical.
Rights of the child
The rights of the child were reflected in five reasons in favour of GGM. Some articles argued that considerations considering harm to the unborn child are irrelevant if the child would not have been born otherwise and would have a life worth living (the ‘non-identity problem’). However, others explain the ‘non-identity problem’ may not be relevant here or does not provide a sound argument. Other articles reasoned that GGM would not impinge on the child’s freedom, nor imply conditional acceptance of a child. Finally, some discussed that even if conflicting with informed consent, parents always make choices for their children and this should thus not be a decisive argument against GGM.
Four worries were voiced about the rights of the child. GGM could impinge on the child’s freedom (i.e. violate his/her right to an open future). Furthermore, it could conflict with informed consent as there is no agent available to give consent and as information about GGM is insufficiently available. Finally, using GGM may imply that the child is not unconditionally accepted.
Human life and dignity
Seven reasons in favour of GGM related to human life and dignity. Some argued that GGM may actually be congruent with: societal values, as the public will sympathize with disease carriers; human dignity, as embryos do not have a moral status; and religious values, as God enabled the use of this technology and modified individuals may serve God better. Alternatively, it was asserted that the following arguments should not be decisive against using this technology: arguments based on human dignity, since what constitutes human dignity remains unclear; the perception that suffering/disease has a higher purpose; a yuk-response (i.e. a negative emotional response); and/or religious objections.
Three reasons against GGM related to human life and dignity. Articles reasoned that GGM would impinge on human dignity, and specifically, that human embryos should not be created or modified for the purpose of research, because that conflicts with the moral status of the embryo. Furthermore, religious objections were expressed.
Considerations regarding the implementation processes and regulation
Many authors expressed considerations regarding implementation processes and regulation (Table III).
Table III.
Considerations with regard to the implementation processes and appropriate regulation.
| Domain | Consideration | N | Referencea |
|---|---|---|---|
| Process of determining acceptability | There is a need to involve stakeholders in an open discussion, including experts as well as non-experts | 93 | 2,4,5,8,11,13–15,17–19,21,23,26,29,32,38,41,44,48–50,52,54,55,60,62,67,70–72,74,77–82,84–88,90,91,94,96–98,100,102,103,107,112,116–118,120,121,126,128,131–134,137,139,142,143,145–148,152,153,155,158–165,167,168,170,171,173–177 |
| It may be difficult to define what medical conditions qualify for modification | 31 | 1,2,15,17,23,31,32,44,55,58,60,62,64,72,74,88,89,95,101,105,117–119,126,136,143,158,160,161,163,173 | |
| It may be difficult to define the difference between a medical condition and human variability | 13 | 6,28,44,51,72,84,91,160,163,175,176,178,179 | |
| It may be difficult to define the difference between a medical condition and enhancement | 13 | 7,20,36,37,43,44,51,95,154,164,171,176,178 | |
| It may be difficult to define the difference between human and non-human traits | 1 | 158 | |
| It may be difficult to define the difference between somatic and germline cells | 1 | 49 | |
| Need for regulation | There is no need for regulation | 4 | 10,12,99,174 |
| There is a need to prevent overregulation, which may prevent proper research and debate | 5 | 14,24,44,97,124 | |
| There is a need to prevent overregulation, which may incite unwarranted public fears | 2 | 24,49 | |
| There is a need for regulation | 101 | 1,4,5,10–15,17–19,23,27–31,33–36,38,41,44,46,48–50,52,54,55,60,62,65–67,70,72,74,76,77,79,81–84,86–91,94,98–101,103,104,106,107,112–118,120,121,126–128,138,140,142,146,148,152,153,155–158,160,162–165,168–172,174,175,177–180 | |
| There is a need for regulation to prevent a public outcry resulting in the prohibition of all applications of genome modification | 24 | 3,17–19,41,44,54,62,67,71,72,76,79,83,87–89,91,97,100,103,127,133,173 | |
| Regulation should be regional as it should acknowledge cultural values | 11 | 20,44,60,77,81,84,95,136,155,157,160 | |
| Regulation should be international as regional choices would affect all countries | 19 | 1,23,48,50,62,70,76,81,88,89,96,112,142,157,160–163,165 | |
| Regulation should be international as to prevent medical tourism | 8 | 4,21,44,124,160,163,165,175 | |
| Regulation should be flexible to keep up with rapidly evolving technologies | 14 | 14,15,18,40,54,55,60,62,89,95,152,153,160,179 | |
| It may be difficult to enforce regulation (in some countries) | 41 | 1,3,7,14–17,20,21,23,38,44,48–50,55,60,62,65,71,72,76,80,83,85,87–89,97,101,107,113,114,117,124,126,127,156,161,163,178 | |
| It may be difficult to define how and who should make decisions on regulation | 19 | 21,23,26,28,32,49,55,62,81,112,113,115,131,152,153,160,161,164,177 |
aNumbers indicate the appropriate reference (Table IV).
In determining acceptability, authors expressed the need to involve expert and non-expert stakeholders in an open discussion. Furthermore, they argued that defining what medical conditions qualify for modification could be challenging. Additionally, defining the difference between: medical conditions and human variability (e.g. hearing loss), medical conditions and enhancement, human and non-human traits, and somatic and germline cells, may be difficult. Regarding regulation, some opposed setting up regulation as they argued intended parents and their clinicians/scientists should decide on acceptability. Some warned against overregulation, which may prevent proper research and debate and/or may incite unwarranted fears among the public. In contrast, many argued in favour of regulating GGM and referred to what they considered appropriate existing regulations, or the need for additional oversight. Some articles argued for regulating GGM to prevent a public outcry resulting in the prohibition of somatic genome modification. Some reasoned that regulation should be regional, to acknowledge cultural values. Others argued that it should be international, as regional choices would affect all countries, and having these regional differences would incite medical tourism. Articles discussed that regulation should be flexible to adapt to rapidly evolving technologies. Finally, concerns were expressed that enforcing regulations may be challenging in some countries, e.g. because they govern by guidelines or professional codes without effective enforcement mechanisms. Finally, some expressed unclarity about how and who ought to make regulatory decisions.
Discussion
This review provides, to our knowledge, the first systematic review on the ethics of GGM, identifying 90 reasons for, and 79 reasons against its future clinical application. Previous, non-systematic, articles presented a maximum of 60/169 reasons. This review represents a valuable addition to previous literature by providing an overview of, and framework for, the reasons put forward in this debate.
Limitations
There were several methodological challenges. First, different terminology is used and articles on GGM were poorly indexed, resulting in a broad search strategy and relying heavily on perusing reference lists. Second, unlike more traditional systematic reviews, we could not assess risk of bias in the included studies, as there are no quality criteria for performing a meta-analysis of opinion papers (Hendriks et al., 2015). Third, synthesis required the reviewers to interpret the articles. Despite using two reviewers, the authors’ meaning may have been misinterpreted. Additionally, we identified stakeholders’ disciplines by their listed affiliations, which is a conservative interpretation of their expertise. Fourth, by systematically reviewing the literature, we aimed to provide a more complete overview of reasons. However, we cannot ensure completeness as relevant reasons may have been omitted in the reviewed literature (Strech and Sofaer, 2012). Moreover, the large volume of literature impelled us to limit the scope of our search for feasibility. Presuming that most arguments used in earlier debates, e.g. those in the 70s (incited by recombinant DNA technology), 80s and 90s (incited by the Human Genome Project) have reappeared in the current discussions (Lunshof, 2016), we only included papers published between 2011 and 2016. We also excluded original biological studies, hoping to still cover insights from biomedical experts as they (co)authored n = 53 non-biological studies. Additionally, our search was limited to MEDLINE, although we supplemented this by perusing reference-lists of identified papers. However, we acknowledge that these choices may have resulted in missing relevant reasons. Finally, to reduce the risk of bias, all reasons mentioned in the literature are described. However, neither describing reasons, nor reporting the frequencies of articles reporting on them, should be confused with a claim of which reasons are more sound, legitimate, or more important than others (Strech and Sofaer, 2012).
Findings in the context of literature
By summarizing and quantifying the identified reasons, the results section served descriptive ethics. We provide some additional considerations.
At the core of many reasons for GGM is the importance of genetic parenthood. If genetic parenthood would not be as important, achieving the goals of GGM (i.e. preventing a genetic disease, reducing the risk of diseases and/or inducing non-medical enhancements in a future child) would be safer and more effective through, e.g. selecting a suitable partner or sperm donor. Although infertile patients value genetic parenthood, they may not persue it if that involves significant risks, costs or limited success rates (Hendriks et al., 2017, 2018). Investigating the relative importance of genetic parenthood may be key in determining the value of GGM (Cohen, 2017; Hendriks et al., 2018).
We differentiated between safety for the child and effectiveness. These differ when considering an embryo carrying a mutation as the starting point; i.e. effectiveness referring to the probability of curing the disease, and safety referring to not causing additional harm. However, for patients considering options for having healthy children, safety and effectiveness may be perceived as equivalent. Clarifying this may help communicating with the public.
Scholars have suggested that the reasons for and against GGM are not new, but have also been used for other novel technologies such as PGD (Tonkens, 2011a; Harris, 2016). Indeed, we identified few reasons that are specific to GGM. These include improving the species with non-human traits and combining genetic parenthood with desired medical or non-medical traits that the intended parents cannot pass on. However, arguments being non-specific to GGM, does not diminish the need for reflection, as a difference in degree may be a difference in kind.
We found that effectiveness and special interests were more frequently mentioned after the first human GGM reports, which could relate to the experiments’ low success rates. Special interests becoming a concern as some groups are actually working on GGM and fighting over securing patents (Ledford, 2017). Parental rights and duties, comparability to acceptable processes, and human life and dignity were discussed less frequently after the first experiments. We speculate that considerations about duties to perform GGM and its comparability to accepted practices is more relevant in theory and when the technique has advanced to being safe and effective. Furthermore, the experiments invited more accessible, but less in-depth, media attention.
Implications
Frameworks for evaluating ethical considerations of new technologies distinguish three steps: (i) identifying the relevant topics to consider, (ii) appraisal and analysis of the relevant topics and (iii) decision-making on (conditions) for implementation (Assasi et al., 2014).
This review contributes to the first step by providing an overview of the previously identified topics. However, our results also show that this first step is not saturated as non-expert perspectives are called for but insufficiently studied (Baltimore et al., 2015; Chan et al., 2015). Further research may identify novel reasons/topics by focusing on public and patients’ perspectives. The domains identified here may present a framework for gathering and classifying new topics.
Additionally, future research may provide input for the second step by appraising the identified topics/reasons. Although all identified reasons deserve consideration, extra attention may be drawn to those where authors disagreed upon (e.g. whether the potential for a slippery slope should constitute a reason not to introduce GGM), issues authors flagged as unresolved and challenging (e.g. defining the difference between medical conditions and enhancements), and the underlying values and concepts (e.g. obtaining genetic parenthood). This may involve both normative analysis and stakeholder consultation (Assasi et al., 2014).
Regarding the third step, the decision-making on the introduction of GGM, we found that most articles stressed the need for regulation (Bosley et al., 2015; Chan et al., 2015). This corresponds to a broader plea for regulating novel techniques (Schatten, 2002; Strasberg and Ludbrook, 2003; Dondorp and de Wert, 2011). The current regulatory landscape covering GGM is diverse and complex (Isasi and Knoppers, 2015; Isasi et al., 2016). Indeed, authors stressed that the appropriate regulatory process remains unclear (Lunshof, 2016). As such, we recommend further analysis of the regulatory process, including aspects raised by the articles such as the decision-making approach itself, the level of decision-making (i.e. international or national), ways of operationalizing the requested regulatory flexibility, and maintaining public trust.
Conclusions
Besides needing (pre)clinical studies on safety and effectiveness, authors call for further ethical analysis and societal debate to define principles and conditions for responsible clinical use of GGM. This overview of the reasons may assist such a thorough evaluation.
Supplementary Material
Authors’ roles
I.D. and L.B. contributed to execution, analysis and critical discussion. M.C. and A.B. contributed to the critical discussion. S.R. contributed to the study design and critical discussion. S.H. contributed to all components of the study. The views expressed are the authors’ own and do not reflect those of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Funding
University of Amsterdam, Alliance Grant of the Amsterdam Reproduction and Development Research Institute (I.D.), and Clinical Center, Department of Bioethics, National Institutes of Health Intramural Research Program (S.H.).
Conflict of interest
None.
References
- Addison C, Taylor-Alexander S. Gene editing and germ-line intervention: the need for novel responses to novel technologies. Mol Ther 2015. a;23:1678–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addison C, Taylor-Alexander S. Gene editing: advising advice. Science 2015. b;349:935. [DOI] [PubMed] [Google Scholar]
- Alvis S. Gene editing in research. Wellcome 2016. https://wellcome.ac.uk/what-we-do/our-work/our-policy-work-gene-editing.
- American S. Altering embryo genes, safely, should not be off-limits. Scientific American 2015. https://www.scientificamerican.com/article/altering-embryo-genes-safely-should-not-be-off-limits/
- Araki M, Ishii T. International regulatory landscape and integration of corrective genome editing into in vitro fertilization. Reprod Biol Endocrinol 2014;12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki M, Ishii T. Providing appropriate risk information on genome editing for patients. Trends Biotechnol 2016;34:86–90. [DOI] [PubMed] [Google Scholar]
- Assasi N, Schwartz L, Tarride J-E, Campbell K, Goeree R. Methodological guidance documents for evaluation of ethical considerations in health technology assessment: a systematic review. Expert Rev Pharmacoecon Outcomes Res 2014;14:203–220. [DOI] [PubMed] [Google Scholar]
- Ayala FJ. Cloning humans? Biological, ethical, and social considerations. Proc Natl Acad Sci USA 2015;112:8879–8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. The purpose of the summit. International Summit on Human Gene Editing: Commissioned Papers Washington: U.S. National Academy of Sciences, U.S. National Academy of Medicine, Royal Society, Chinese Academy of Sciences, 2015, 3–5.
- Baltimore D, Berg P Let’s Hit ‘Pause’ Before Altering Humankind. The Wall St J 2015.
- Baltimore D, Berg P, Botchan M, Carroll D, Charo RA, Church G, Corn JE, Daley GQ, Doudna JA, Fenner M et al. A prudent path forward for genomic engineering and germline gene modification. Science 2015;348:36–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin R. Interrogating equity: a disability justice approach to genetic engineering. International Summit on Human Gene Editing: Commissioned Papers Washington: U.S. National Academy of Sciences, U.S. National Academy of Medicine, Royal Society, Chinese Academy of Sciences, 2015, 48–51.
- BioInsights Is human embryo gene editing using CRISPR/Cas9 on the cards in the UK? BioInsights 2015. http://insights.bio/cell-and-gene-therapy-insights/2015/09/21/is-human-embryo-gene-editing-using-crisprcas9-on-the-cards-in-the-uk/
- Bosley KS, Botchan M, Bredenoord AL, Carroll D, Charo RA, Charpentier E, Cohen R, Corn J, Doudna J, Feng G et al. CRISPR germline engineering—the community speaks. Nat Biotechnol 2015;33:478–486. [DOI] [PubMed] [Google Scholar]
- Bourne H, Douglas T, Savulescu J. Procreative beneficence and in vitro gametogenesis. Monash Bioeth Rev 2012;30:29–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M, Dabrock P. ‘I bet you won’t’: the science-society wager on gene editing techniques. EMBO Rep 2016;17:279–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. DNA editing takes a serious step forward—for better or worse. LA Times 2015.
- Callaway E. Gene-editing research in human embryos gains momentum. Nature 2016;532:289–290. [DOI] [PubMed] [Google Scholar]
- Caplan AL, Parent B, Shen M, Plunkett C. No time to waste—the ethical challenges created by CRISPR. EMBO Rep 2015;16:1421–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal P. Sexual dimorphism and human enhancement. J Med Ethics 2013;39:722–728. [DOI] [PubMed] [Google Scholar]
- Cathomen T, Ehl S. Translating the genomic revolution—targeted genome editing in primates. N Engl J Med 2014;370:2342–2345. [DOI] [PubMed] [Google Scholar]
- Chan S, Donovan PJ, Douglas T, Gyngell C, Harris J, Lovell-Badge R, Mathews DJ, Regenberg A, Hinxton G. Genome editing technologies and human germline genetic modification: The Hinxton Group Consensus Statement. Am J Bioeth 2015;15:42–47. [DOI] [PubMed] [Google Scholar]
- Charo A. The legal/regulatory context. International Summit on Human Gene Editing: Commissioned Papers Washington: National Academy of Sciences, U.S. National Academy of Medicine, Royal Society, Chinese Academy of Sciences, 2015, 13–19.
- Charo RA. On the road (to a cure?)—stem-cell tourism and lessons for gene editing. N Engl J Med 2016;374:901–903. [DOI] [PubMed] [Google Scholar]
- Church G. Perspective: encourage the innovators. Nature 2015;528:S7. [DOI] [PubMed] [Google Scholar]
- Cicerone RJ, Dzau VJ National Academy of Sciences and National Academy of Medicine Announce Initiative on Human Gene Editing. 2015.
- Cicerone RJ, Dzau VJ, Bai C, Ramakrishnan V. On human gene editing: international summit statement by the organizing committee, 2015 In: International Summit on Human Gene Editing A Global Discussion. Washington, DC: The National Academies Press, 2016. [PubMed] [Google Scholar]
- Cohen G. Two views about the gene editing ‘breakthrough’ that are not getting enough attention (IMHO). Harvard Law Bill of Health 2017. https://blogs.harvard.edu/billofhealth/2017/08/04/two-views-about-the-gene-editing-breakthrough-that-are-not-getting-enough-attention-imho/
- Collins FS. Statement on NIH funding of research using gene-editing technologies in human embryos. 2015. National Institutes of Health. https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-nih-funding-research-using-gene-editing-technologies-human-embryos
- Comfort N. Can we cure genetic diseases without slipping into eugenics? The Nation2015. https://www.thenation.com/article/can-we-cure-genetic-diseases-without-slipping-into-eugenics/
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013;339:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbyn Z. CRISPR: Is it a good idea to ‘upgrade’ our DNA? The Guardian News and Media 2015. https://www.theguardian.com/science/2015/may/10/crispr-genome-editing-dna-upgrade-technology-genetic-disease
- Cressey D, Abbott A, Ledford H Scientists apply for license to edit genes in human embryos. Scientific American2015. https://www.scientificamerican.com/article/scientists-apply-for-license-to-edit-genes-in-human-embryos/
- Cressey D, Cyranoski D. Gene editing poses challenges for journals. Nature 2015;520:594. [DOI] [PubMed] [Google Scholar]
- Cyranoski D. Ethics of embryo editing divides scientists. Nature 2015. b;519:272. [DOI] [PubMed] [Google Scholar]
- Cyranoski D, Reardon S Chinese scientists genetically modify human embryos. Nature News 2015. a. Nature Publishing Group.
- Cyranoski D, Reardon S. Embryo editing sparks epic debate. Nature 2015. b;520:593–594. [DOI] [PubMed] [Google Scholar]
- Cyranoski D. Embryo editor. Nature 2015. a;528:461. [Google Scholar]
- Daley GQ, Hyun I, Apperley JF, Barker RA, Benvenisty N, Bredenoord AL, Breuer CK, Caulfield T, Cedars MI, Frey-Vasconcells J et al. Setting global standards for stem cell research and clinical translation: The 2016 ISSCR Guidelines. Stem Cell Reports 2016;6:787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney JJ. Possible people, complaints, and the distinction between genetic planning and genetic engineering. J Med Ethics 2011;37:410–414. [DOI] [PubMed] [Google Scholar]
- Deleidi M, Yu C. Genome editing in pluripotent stem cells: research and therapeutic applications. Biochem Biophys Res Commun 2016;473:665–674. [DOI] [PubMed] [Google Scholar]
- Dondorp W, de Wert G. Innovative reproductive technologies: risks and responsibilities. Hum Reprod 2011;26:1604–1608. [DOI] [PubMed] [Google Scholar]
- Doudna J. Genome-editing revolution: my whirlwind year with CRISPR. Nature 2015. a;528:469–471. [DOI] [PubMed] [Google Scholar]
- Doudna J. Perspective: embryo editing needs scrutiny. Nature 2015. b;528:S6. [DOI] [PubMed] [Google Scholar]
- Dzau VJ, Cicerone RJ. Responsible use of human gene-editing technologies. Hum Gene Ther 2015;26:411–412. [DOI] [PubMed] [Google Scholar]
- Economist T. Genome editing: the age of the red pen. The Economist 2015. a. https://www.economist.com/briefing/2015/08/22/the-age-of-the-red-pen
- Economist T. Germ-line gene therapy: to the crack of doom. The Economist 2015. b. https://www.economist.com/science-and-technology/2015/05/02/to-the-crack-of-doom
- Egli D, Zuccaro M, Kosicki M, Church G, Bradley A, Jasin M. Inter-homologue repair in fertilized human eggs? bioRxiv 2017. https://www.biorxiv.org/content/early/2017/08/28/181255 or the doi: https://doi.org/10.1101/181255 [DOI] [PubMed]
- Ellis K, Terry SF. Dangerous liaisons: connecting CRISPR/Cas9 to clinical science. Genet Test Mol Biomarkers 2015;19:409–410. [DOI] [PubMed] [Google Scholar]
- Elster J. Procreative beneficence: cui bono? Bioethics 2011;25:482–488. [DOI] [PubMed] [Google Scholar]
- Evans BJ. Governance at the institutional and national level. International Summit on Human Gene Editing: Commissioned Papers Washington: U.S. National Academy of Sciences, U.S. National Academy of Medicine, Royal Society, Chinese Academy of Sciences, 2015, 39–43.
- Evitt NH, Mascharak S, Altman RB. Human germline CRISPR-Cas modification: toward a regulatory framework. Am J Bioeth 2015;15:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessenden M. Gene editing in human embryos ignites controversy. Smithsonian 2015.
- Flotte TR. Therapeutic germ line alteration: has CRISPR/Cas9 technology forced the question? Hum Gene Ther 2015;26:245–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty NME, McCarthy A, Snijders KE, Powell BE, Kubikova N, Blakeley P, Lea R, Elder K, Wamaitha SE, Kim D et al. Genome editing reveals a role for OCT4 in human embryogenesis. Nature 2017;550:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T. An ASGCT perspective on the national academies genome editing summit. Mol Ther 2016;24:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T, Jonlin EC, King NM, Torbett BE, Wivel NA, Kaneda Y, Sadelain M. ASGCT and JSGT joint position statement on human genomic editing. Mol Ther 2015;23:1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SM. Some Jewish thoughts on genetic enhancement. J Med Ethics 2011;37:415–419. [DOI] [PubMed] [Google Scholar]
- Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today 2004;24:105–112. [DOI] [PubMed] [Google Scholar]
- Greely HT. Of Science, CRISPR-Cas9, and Asilomar. Law and Biosciences Blog 2015. Stanford Law. https://law.stanford.edu/2015/04/04/of-science-crispr-cas9-and-asilomar/
- Gross M. Bacterial scissors to edit human embryos? Curr Biol 2015;25:R439–R442. [DOI] [PubMed] [Google Scholar]
- Gunson D, McLachlan H. Risk, Russian-roulette and lotteries: Persson and Savulescu on moral enhancement. Med Health Care Philos 2013;16:877–884. [DOI] [PubMed] [Google Scholar]
- Gyngell C, Douglas T. Stocking the genetic supermarket: reproductive genetic technologies and collective action problems. Bioethics 2015;29:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton T. Ethical and societal questions loom large as gene editing moves closer to the clinic. J Am Med Assoc 2016;315:546–548. [DOI] [PubMed] [Google Scholar]
- Harris J. Germline manipulation and our future worlds. Am J Bioeth 2015. a;15:30–34. [DOI] [PubMed] [Google Scholar]
- Harris J. Why human gene editing must not be stopped. The Guardian 2015. b.
- Harris J. Germline modification and the burden of human existence. Camb Q Healthc Ethics 2016;25:6–18. [DOI] [PubMed] [Google Scholar]
- Hayden EC. Should you edit your children’s genes? Nature 2016;530:402–405. [DOI] [PubMed] [Google Scholar]
- Heidari R, Shaw DM, Elger BS. CRISPR and the rebirth of synthetic biology. Sci Eng Ethics 2017;23:351–363. [DOI] [PubMed] [Google Scholar]
- Hendriks S, Dondorp W, de Wert G, Hamer G, Repping S, Dancet EA. Potential consequences of clinical application of artificial gametes: a systematic review of stakeholder views. Hum Reprod Update 2015;21:297–309. [DOI] [PubMed] [Google Scholar]
- Hendriks S, Peeraer K, Bos H, Repping S, Dancet EAF. The importance of genetic parenthood for infertile men and women. Hum Reprod 2017;39:2076–2087. [DOI] [PubMed] [Google Scholar]
- Hendriks S, van Wely M, D’Hooghe TM, Mol F, Peeraer K, Repping S, Dancet EAF The relative importance of genetic parenthood. 2018. Submitted. [DOI] [PubMed]
- Henrich DC. Human nature and autonomy: Jürgen Habermas’ critique of liberal eugenics. Ethic Perspect 2011;18:249–268. [Google Scholar]
- Hildt E. Human germline interventions–think first. Front Genet 2016;7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hycner RH. Some guidelines for the phenomenological analysis of interview data. Hum Stud 1985;8:279–303. [Google Scholar]
- IBC Report of the IBC on updating its reflection on the human genome and human rights. In United Nations Educational SaCO and Committee IB (eds). 2015, Paris, France.
- Isasi R, Kleiderman E, Knoppers BM. Genetic technology regulation. Editing policy to fit the genome? Science 2016;351:337–339. [DOI] [PubMed] [Google Scholar]
- Isasi R, Knoppers BM. Oversight of human inheritable genome modification. Nat Biotechnol 2015;33:454–455. [DOI] [PubMed] [Google Scholar]
- Ishii T. Potential impact of human mitochondrial replacement on global policy regarding germline gene modification. Reprod Biomed Online 2014;29:150–155. [DOI] [PubMed] [Google Scholar]
- Ishii T. Germ line genome editing in clinics: the approaches, objectives and global society. Brief Funct Genomics 2017;16:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T. Germline genome-editing research and its socioethical implications. Trends Mol Med 2015;21:473–481. [DOI] [PubMed] [Google Scholar]
- ISSCR ISfSCR The ISSCR Statement on Human Germline Genome Modification. 2015. http://www.isscr.org/professional-resources/news-publicationsss/isscr-news-articles/article-listing/2015/03/19/statement-on-human-germline-genome-modification
- Jacobs H. Rise of the planet. EMBO Rep 2013;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasanoff S, Hurlbut JB, Saha K. CRISPR democracy: gene editing and the need for inclusive deliberation. Issues Sci Technol 2015;32:25–32. [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012;337:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane G. Mastery without mystery: why there is no promethean sin in enhancement. J Appl Philos 2011;28:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Normile D. Bioethics. Embryo engineering study splits scientific community. Science 2015;348:486–487. [DOI] [PubMed] [Google Scholar]
- Kang X, He W, Huang Y, Yu Q, Chen Y, Gao X, Sun X, Fan Y. Introducing precise genetic modifications into human 3PN embryos by CRISPR/Cas-mediated genome editing. J Assist Reprod Genet 2016;33:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M. The ultimate coders: revolutionary new tool can rewrite DNA. GE Reports 2015.
- Kevles DJ. The history of eugenics. International Summit on Human Gene Editing: Commissioned Papers Washington: U.S. National Academy of Sciences, U.S. National Academy of Medicine, Royal Society, Chinese Academy of Sciences, 2015, 9–12.
- Kim M. Now that we can edit our genome, where do we go? Daily Harald2015. http://www.dailyherald.com/article/20150706/entlife/150709982/
- Kim HJ, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev 2014;15:321–334. [DOI] [PubMed] [Google Scholar]
- Kmietowicz Z. Experts back genome editing in human embryo research. Br Med J 2015;351:h4874. [DOI] [PubMed] [Google Scholar]
- Kolata G. Chinese scientists edit genes of human embryos, raising concerns. The New York Times2015.
- Krishan K, Kanchan T, Singh B. Human genome editing and ethical considerations. Sci Eng Ethics 2016;22:597–599. [DOI] [PubMed] [Google Scholar]
- LaBarbera AR. Proceedings of the International Summit on Human Gene Editing: a global discussion-Washington, DC, December 1–3, 2015. J Assist Reprod Genet 2016;33:1123–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFountaine JS, Fathe K, Smyth HD. Delivery and therapeutic applications of gene editing technologies ZFNs, TALENs, and CRISPR/Cas9. Int J Pharm 2015;494:180–194. [DOI] [PubMed] [Google Scholar]
- Lancet T. Genetics in medicine—progress and pitfalls. Lancet 2015;385:2223. [DOI] [PubMed] [Google Scholar]
- Lander ES. Brave new genome. N Engl J Med 2015. a;373:5–8. [DOI] [PubMed] [Google Scholar]
- Lander ES. What we don’t know. International Summit on Human Gene Editing: Commissioned Papers Washington: U.S. National Academy of Sciences, U.S. National Academy of Medicine, Royal Society, Chinese Academy of Sciences, 2015. b, 20–27.
- Lanphier E, Urnov F, Haecker SE, Werner M, Smolenski J. Don’t edit the human germ line. Nature 2015;519:410–411. [DOI] [PubMed] [Google Scholar]
- Larson C, Schaffer A Genome editing. MIT Technology Review 2014.
- Ledford H. Biohackers gear up for genome editing. Nature 2015. a;524:398–399. [DOI] [PubMed] [Google Scholar]
- Ledford H. CRISPR, the disruptor. Nature 2015. b;522:20–24. [DOI] [PubMed] [Google Scholar]
- Ledford H. The landscape for human genome editing. Nature 2015. c;526:310–311.26469023 [Google Scholar]
- Ledford H. Mini enzyme moves gene editing closer to the clinic. Nature 2015. d;520:18. [DOI] [PubMed] [Google Scholar]
- Ledford H. Where in the world could the first CRISPR baby be born? Nature 2015. e;526:310–311. [DOI] [PubMed] [Google Scholar]
- Ledford H. CRISPR: gene editing is just the beginning. Nature 2016;531:156–159. [DOI] [PubMed] [Google Scholar]
- Ledford H. Bitter CRISPR patent war intensifies. Nature News 2017.
- Lentzos F. Mutational technologies: engage public in gene-editing policy. Nature 2015;521:289. [DOI] [PubMed] [Google Scholar]
- Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, Lv J, Xie X, Chen Y, Li Y et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell 2015;6:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M, Esvelt K, Inglesby T. Calls for caution in genome engineering should be a model for similar dialogue on pandemic pathogen research. Ann Intern Med 2015;163:790–791. [DOI] [PubMed] [Google Scholar]
- Lokody I. Genetic therapies: correcting genetic defects with CRISPR-Cas9. Nat Rev Genet 2014;15:63. [DOI] [PubMed] [Google Scholar]
- Lovell-Badge R. Applications of gene editing: germline modification. International Summit on Human Gene Editing: Commissioned Papers 2015. U.S. National Academy of Sciences, U.S. National Academy of Medicine, Royal Society, Chinese Academy of Sciences, Washington, pp. 28–31.
- Lundberg AS, Novak R. CRISPR-Cas gene editing to cure serious diseases: treat the patient, not the germ line. Am J Bioeth 2015;15:38–40. [DOI] [PubMed] [Google Scholar]
- Lunshof J. Regulate gene editing in wild animals. Nature 2015;521:127. [DOI] [PubMed] [Google Scholar]
- Lunshof JE. Human germ line editing-roles and responsibilities. Protein Cell 2016;7:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Marti-Gutierrez N, Park S-W, Wu J, Lee Y, Suzuki K, Koski A, Ji D, Hayama T, Ahmed R et al. Correction of a pathogenic gene mutation in human embryos. Nature 2017;548:413–419. [DOI] [PubMed] [Google Scholar]
- Macer D. Ethical consequences of the positive views of enhancement in Asia. Health Care Anal 2012;20:385–397. [DOI] [PubMed] [Google Scholar]
- Malek J. Use or refuse reproductive genetic technologies: which would a ‘good parent’ do? Bioethics 2013;27:59–64. [DOI] [PubMed] [Google Scholar]
- Malmqvist E. Reprogenetics and the ‘parents have always done it’ argument. Hastings Cent Rep 2011;41:43–49. [DOI] [PubMed] [Google Scholar]
- Mariscal C, Petropanagos A. CRISPR as a driving force: the Model T of biotechnology. Monash Bioeth Rev 2016;34:101–116. [DOI] [PubMed] [Google Scholar]
- Maron DF. All gene-editing research should proceed cautiously, scientists conclude. Scientific American2015. a. https://www.scientificamerican.com/article/all-gene-editing-research-should-proceed-cautiously-scientists-conclude1/
- Maron DF. ‘Improving’ humans with customized genes sparks debate among scientists. Scientific American2015. b. https://www.scientificamerican.com/article/improving-humans-with-customized-genes-sparks-debate-among-scientists1/
- Martikainen M, Pedersen O. Germline edits: Heat does not help debate. Nature 2015;520:623. [DOI] [PubMed] [Google Scholar]
- Mathews DJ, Chan S, Donovan PJ, Douglas T, Gyngell C, Harris J, Regenberg A, Lovell-Badge R. CRISPR: a path through the thicket. Nature 2015;527:159–161. [DOI] [PubMed] [Google Scholar]
- McCarthy M. Scientists call for moratorium on clinical use of human germline editing. Br Med J 2015;351:h6603. [DOI] [PubMed] [Google Scholar]
- Miller HI. Germline gene therapy: we’re ready. Science 2015. a;348:1325. [DOI] [PubMed] [Google Scholar]
- Miller HI. Recasting Asilomar’s lessons for human germline editing. Nat Biotechnol 2015. b;33:1132–1134. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009:6. [PMC free article] [PubMed] [Google Scholar]
- Morange M. Genetic modification of the human germ line: the reasons why this project has no future. C R Biol 2015;338:554–558. [DOI] [PubMed] [Google Scholar]
- Mulder CL, Zheng Y, Jan SZ, Struijk RB, Repping S, Hamer G, van Pelt AM. Spermatogonial stem cell autotransplantation and germline genomic editing: a future cure for spermatogenic failure and prevention of transmission of genomic diseases. Hum Reprod Update 2016;22:561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TF. Selecting the traits of children prior to birth. Virtual Mentor 2012;14:158–161. [DOI] [PubMed] [Google Scholar]
- The Academy of Medical Sciences et al 2015. Genome editing in human cells—initial joint statement. AMS; https://wellcome.ac.uk/sites/default/files/wtp059707.pdf. [Google Scholar]
- NASEM Human Genome Editing: Science, Ethics, and Governance National Academies of Sciences, Engineering, Medicine; National Academy of Sciences; National Academy of Medicine, 2017. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering and Medicine (NASEM). Advisory group for human gene editing initiative named. News from the National Academies. 2015. Washington, DC.
- Nature Gene politics. Nature 2015. a;523:5–6. [DOI] [PubMed] [Google Scholar]
- Nature Germline editing: time for discussion. Nat Med 2015. b;21:295. [DOI] [PubMed] [Google Scholar]
- Nature Splice of life. Nature 2015. c;521:5.25951245 [Google Scholar]
- Nature CRISPR everywhere. Nature 2016. a;531:155.26961638 [Google Scholar]
- Nature Gene intelligence. Nature 2016. b;531:140. [DOI] [PubMed] [Google Scholar]
- Newson AJ, Wrigley A Identifying key developments, issues and questions relating to techniques of genome editing with engineered nucleases. 2015. Nuffield Council on Bioethics.
- Olson S. International Summit on Human Gene Editing: A Global Discussion Washington, DC: The National Academies Press, 2016. [PubMed]
- O’Keefe M, Perrault S, Halpern J, Ikemoto L, Yarborough M. ‘Editing’ genes: a case study about how language matters in bioethics. Am J Bioeth 2015;15:3–10. [DOI] [PubMed] [Google Scholar]
- Palpant NJ, Dudzinski D. Zinc finger nucleases: looking toward translation. Gene Ther 2013;20:121–127. [DOI] [PubMed] [Google Scholar]
- Pergament E. The promise of gene therapy. Curr Opin Obstet Gynecol 2016;28:132–135. [DOI] [PubMed] [Google Scholar]
- Pollack R. A powerful new way to edit DNA. The New York Times2014. https://www.nytimes.com/2014/03/04/health/a-powerful-new-way-to-edit-dna.html
- Pollack R. Eugenics lurk in the shadow of CRISPR. Science 2015;348:871. [DOI] [PubMed] [Google Scholar]
- Porteus MH, Dann CT. Genome editing of the germline: broadening the discussion. Mol Ther 2015;23:980–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell R. In genes we trust: germline engineering, eugenics, and the future of the human genome. J Med Philos 2015;40:669–695. [DOI] [PubMed] [Google Scholar]
- Powell R, Buchanan A. Breaking evolution’s chains: the prospect of deliberate genetic modification in humans. J Med Philos 2011;36:6–27. [DOI] [PubMed] [Google Scholar]
- Proudfoot C, Carlson DF, Huddart R, Long CR, Pryor JH, King TJ, Lillico SG, Mileham AJ, McLaren DG, Whitelaw CB et al. Genome edited sheep and cattle. Transgenic Res 2015;24:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilter JG. The new enhancement technologies and the place of vulnerability in our lives. Theor Med Bioeth 2016;37:9–27. [DOI] [PubMed] [Google Scholar]
- Rajewsky K, Delbruck M. The historical scientific context. International Summit on Human Gene Editing: Commissioned Papers Washington: U.S. National Academy of Sciences, U.S. National Academy of Medicine, Royal Society, Chinese Academy of Sciences, 2015.
- Reagan JA. Taming our brave new world. J Med Philos 2015;40:621–632. [DOI] [PubMed] [Google Scholar]
- Reardon S. Ethics of embryo editing paper divides scientists. Nature News2015. a. https://www.nature.com/news/ethics-of-embryo-editing-paper-divides-scientists-1.17410 [DOI] [PubMed]
- Reardon S. Gene-editing summit supports some research in human embryos. Nature2015. b. https://www.nature.com/news/gene-editing-summit-supports-some-research-in-human-embryos-1.18947
- Reardon S. Global summit reveals divergent views on human gene editing. Nature 2015. c;528:173. [DOI] [PubMed] [Google Scholar]
- Reardon S. Human-genome editing summit to sample global attitudes. Nature2015. d. https://www.nature.com/news/human-genome-editing-summit-to-sample-global-attitudes-1.18879
- Reardon S. NIH reiterates ban on editing human embryo DNA. Nature News2015. e. https://www.nature.com/news/nih-reiterates-ban-on-editing-human-embryo-dna-1.17452
- Reardon S. US science academies take on humangenome editing. Nature News2015. f. https://www.nature.com/news/us-science-academies-take-on-human-genome-editing-1.17581.
- Regalado A. Chinese team reports gene-editing human embryos. MIT Technology Review [On-line]2015. a. https://www.technologyreview.com/s/536971/chinese-team-reports-gene-editing-human-embryos/.
- Regalado A. Engineering the perfect baby. MIT Technology Review [On-line]2015. b. https://www.technologyreview.com/s/535661/engineering-the-perfect-baby/.
- Regalado A. Industry body calls for gene-editing moratorium. MIT Technology Review2015. c. https://www.technologyreview.com/s/535846/industry-body-calls-for-gene-editing-moratorium/
- Regalado A. Scientists call for a summit on gene-edited babies. MIT Technology Review2015. d. https://www.technologyreview.com/s/536021/scientists-call-for-a-summit-on-gene-edited-babies/
- Rivera H. Regarding the article: ‘Human germline genetic modification: scientific and bioethical perspectives. Arch Med Res 2013;44:321–322. [DOI] [PubMed] [Google Scholar]
- Robillard JM, Whiteley L, Johnson TW, Lim J, Wasserman WW, Illes J. Utilizing social media to study information-seeking and ethical issues in gene therapy. J Med Internet Res 2013;15:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojahn SY. Genome surgery. MIT Technology Review2014. https://www.technologyreview.com/s/524451/genome-surgery/
- Sarewitz D. CRISPR: science can’t solve it. Nature 2015;522:413–414. [DOI] [PubMed] [Google Scholar]
- Savic N, Schwank G. Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res 2016;168:15–21. [DOI] [PubMed] [Google Scholar]
- Savulescu J, Gyngell C, Douglas T. Germline edits: trust ethics review process. Nature 2015. a;520:623. [DOI] [PubMed] [Google Scholar]
- Savulescu J, Pugh J, Douglas T, Gyngell C. The moral imperative to continue gene editing research on human embryos. Protein Cell 2015. b;6:476–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten GP. Safeguarding ART. Nat Cell Biol 2002;4:s19–s22. [DOI] [PubMed] [Google Scholar]
- Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 2013;13:653–658. [DOI] [PubMed] [Google Scholar]
- SDB Position Statement from the Society for Developmental Biology on Genomic Editing in Human Embryos. 2015.
- Senior M. UK funding agencies weigh in on human germline editing. Nat Biotechnol 2015;33:1118–1119. [DOI] [PubMed] [Google Scholar]
- Sharma A, Scott CT. The ethics of publishing human germline research. Nat Biotechnol 2015;33:590–592. [DOI] [PubMed] [Google Scholar]
- Sheridan C. CRISPR germline editing reverberates through biotech industry. Nat Biotechnol 2015;33:431–432. [DOI] [PubMed] [Google Scholar]
- Smith KR, Chan S, Harris J. Human germline genetic modification: scientific and bioethical perspectives. Arch Med Res 2012;43:491–513. [DOI] [PubMed] [Google Scholar]
- Smith KR, Chan S, Harris J. Reply: human germline genetic modification: scientific and bioethical perspectives. Arch Med Res 2013;44:323. [DOI] [PubMed] [Google Scholar]
- Smolenski J. CRISPR/Cas9 and germline modification: new difficulties in obtaining informed consent. Am J Bioeth 2015;15:35–37. [DOI] [PubMed] [Google Scholar]
- Sparrow R. In vitro eugenics. J Med Ethics 2014;40:725–731. [DOI] [PubMed] [Google Scholar]
- Specter M. The gene hackers. New Yorker2015. https://www.newyorker.com/magazine/2015/11/16/the-gene-hackers
- Stein R. Critics lash out at Chinese scientists who edited DNA in human embryos. NPR2015. https://www.npr.org/sections/health-shots/2015/04/23/401655818/critics-lash-out-at-chinese-scientists-who-edited-dna-in-human-embryos
- Strasberg SM, Ludbrook PA. Who oversees innovative practice? Is there a structure that meets the monitoring needs of new techniques? J Am Coll Surg 2003;196:938–948. [DOI] [PubMed] [Google Scholar]
- Strech D, Sofaer N. How to write a systematic review of reasons. J Med Ethics 2012;38:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman J. Ethics and germline gene editing. EMBO Rep 2015;16:879–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Zeng Y, Du H, Gong M, Peng J, Zhang B, Lei M, Zhao F, Wang W, Li X et al. CRISPR/Cas9-mediated gene editing in human zygotes using Cas9 protein. Mol Genet Genomics 2017;292:525–533. [DOI] [PubMed] [Google Scholar]
- Tauxe W. Genome editing: 4 big questions. Nature 2015;528:S17. [DOI] [PubMed] [Google Scholar]
- Terry SF. Societal implications: the role of advocacy organisations. International Summit on Human Gene Editing: Commissioned Papers Washington: U.S. National Academy of Sciences, U.S. National Academy of Medicine, Royal Society, Chinese Academy of Sciences, 2015, 36–38.
- Thompson C. CRISPR: move beyond differences. Nature 2015. a;522:415. [DOI] [PubMed] [Google Scholar]
- Thompson C. Governance, regulation, and control: public participation. International Summit on Human Gene Editing: Commissioned Papers Washington: U.S. National Academy of Sciences, U.S. National Academy of Medicine, Royal Society, Chinese Academy of Sciences, 2015. b, 44–47.
- Tonkens R. Parental wisdom, empirical blindness, and normative evaluation of prenatal genetic enhancement. J Med Philos 2011. a;36:274–295. [DOI] [PubMed] [Google Scholar]
- Tonkens R. Good parents would not fulfil their obligation to genetically enhance their unborn children. J Med Ethics 2011. b;37:606–610. [DOI] [PubMed] [Google Scholar]
- Tonkens R. Parental virtue and prenatal genetic alteration research. J Bioeth Inq 2015;12:651–664. [DOI] [PubMed] [Google Scholar]
- Travis J. Genetic engineering. Germline editing dominates DNA summit. Science 2015;350:1299–1300. [DOI] [PubMed] [Google Scholar]
- Vassena R, Heindryckx B, Peco R, Pennings G, Raya A, Sermon K, Veiga A. Genome engineering through CRISPR/Cas9 technology in the human germline and pluripotent stem cells. Hum Reprod Update 2016;22:411–419. [DOI] [PubMed] [Google Scholar]
- Vogel G. Bioethics. Embryo engineering alarm. Science 2015;347:1301. [DOI] [PubMed] [Google Scholar]
- Walters L. Genetics and bioethics: how our thinking has changed since 1969. Theor Med Bioeth 2012;33:83–95. [DOI] [PubMed] [Google Scholar]
- Werner-Felmayer G, Shalev C. Human germline modification—a missing link. Am J Bioeth 2015;15:49–51. [DOI] [PubMed] [Google Scholar]
- Williams P. ‘Precision Medicine: Who’s Getting Rich Off Your Genes?’ 2015. https://madlawprofessor.wordpress.com/2015/04/08/precision-medicine/
- Wirth T, Parker N, Yla-Herttuala S. History of gene therapy. Gene 2013;525:162–169. [DOI] [PubMed] [Google Scholar]
- Witzany G. No time to waste on the road to a liberal eugenics? EMBO Rep 2016;17:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. Applications of CRISPR-Cas9 mediated genome engineering. Mil Med Res 2015;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.