Abstract
Background
Low serum magnesium (SMg) has been linked to increased mortality and cardiovascular disease (CVD) in the general population. We examined whether this association is similar in participants with versus without prevalent chronic kidney disease (CKD) in the multiethnic Dallas Heart Study (DHS) cohort.
Methods
SMg was analyzed as a continuous variable and divided into tertiles. Study outcomes were all-cause death, cardiovascular (CV) death or event, and CVD surrogate markers, evaluated using multivariable Cox regression models adjusted for demographics, comorbidity, anthropometric and biochemical parameters including albumin, phosphorus and parathyroid hormone, and diuretic use. Median follow-up was 12.3 years (11.9–12.8, 25th percentile–75th percentile).
Results
Among 3551 participants, 306 (8.6%) had prevalent CKD. Mean SMg was 2.08 ± 0.19 mg/dL (0.85 ± 0.08 mM, mean ± SD) in the CKD and 2.07 ± 0.18 mg/dL (0.85 ± 0.07 mM) in the non-CKD subgroups. During the follow-up period, 329 all-cause deaths and 306 CV deaths or events occurred. In a fully adjusted model, every 0.2 mg/dL decrease in SMg was associated with ∼20–40% increased hazard for all-cause death in both CKD and non-CKD subgroups. In CKD participants, the lowest SMg tertile was also independently associated with all-cause death (adjusted hazard ratio 2.31; 95% confidence interval 1.23–4.36 versus 1.15; 0.55–2.41; for low versus high tertile, respectively).
Conclusions
Low SMg levels (1.4–1.9 mg/dL; 0.58–0.78 mM) were independently associated with all-cause death in patients with prevalent CKD in the DHS cohort. Randomized clinical trials are important to determine whether Mg supplementation affects survival in CKD patients.
Keywords: cardiovascular disease, chronic kidney disease, magnesium, mineral metabolism, mortality
INTRODUCTION
Chronic kidney disease (CKD) is a global public health problem that affects more than 26 million Americans [1, 2]. There are few specific and effective strategies to retard CKD progression and prevent or ameliorate extra-renal complications. Cardiovascular disease (CVD) remains the leading cause of death in patients with CKD, accounting for ∼50% of deaths [3, 4]. Risk factors contributing to the excess cardiovascular (CV) events due to cardiomyopathy and vasculopathy in the CKD population include hypertension, diabetes, tobacco smoking, anemia, bone mineral disturbances, dyslipidemia and older age [5, 6]. Besides early detection and risk stratification, novel therapies are desperately needed for preserving kidney function and minimizing CV complications in CKD patients.
Magnesium (Mg), the second-most abundant intracellular cation after potassium, is essential for human health as it is involved in virtually every biological function in the cell. Despite a prominent role of Mg in the intracellular compartment, total body Mg deficiency is determined clinically solely by measuring total serum Mg (SMg) concentration, which ranges between 1.6 and 2.2 mg/dL (0.66–0.90 mM) in healthy individuals. Normal SMg levels are tightly regulated by the concerted action of the intestinal uptake from food; the bone, which stores Mg in its hydroxyapatite form; and the kidneys, regulating urinary Mg excretion [7]. In the general population, both a low Mg intake and low SMg levels are associated with an increased incidence of hypertension [8], type 2 diabetes [9], metabolic syndrome [10] and CVD [11–16]. In addition, observational studies have shown that low SMg levels may predict the incidence and progression of CKD [17–19] and adverse outcomes in the general population [12, 20–23] and in patients with either advanced CKD or undergoing hemodialysis [24–28]. However, the association of SMg levels and adverse outcomes has not been fully explored in patients with early CKD.
Therefore, the main goal of the present study is to examine the association of SMg levels with all-cause death, CV death or event, and surrogate markers of CVD in the Dallas Heart Study (DHS) participants with and without CKD. The DHS cohort is unique in its availability of a comprehensive CV assessment and the inclusion of multiethnic participants with early stages of CKD.
MATERIALS AND METHODS
Study population
The DHS is a multiethnic, population-based, cohort study of Dallas County adults aged 30–65 years in which intentional oversampling of African Americans was performed. Written informed consent was provided by all participants, and the University of Texas (UT) Southwestern Institutional Review Board approved the study. The design and detailed methods of DHS Phase 1 (DHS-1) have been previously described [29]. DHS-1 was conducted in three visits, which occurred between 2000 and 2002. In DHS Phase 2 (DHS-2), participants who completed DHS-1 underwent follow-up testing during a single visit to UT Southwestern Medical Center between 2007 and 2009. Participants were subsequently followed for pre-defined clinical events and death. For this study, we excluded participants with no SMg measurements at DHS-1, resulting in a final cohort of 3551 participants.
Evaluation of kidney function
Microalbuminuria was defined based on the urinary albumin/creatinine ratio (ACR) in a first-morning void sample. Estimated glomerular filtration rate (eGFR, mL/min/ 1.73 m2 body surface area) was determined with the four-variable Modification of Diet in Renal Disease (MDRD) [30] and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [31] equations, the latter for sensitivity analyses. Using the National Kidney Foundation guidelines, CKD was defined as eGFR <60 mL/min/1.73 m2 and/or ACR ≥17 mg/g in men and ≥25 mg/g in women [32]. In sensitivity analysis, CKD was also defined as eGFR <60 mL/min/1.73 m2 and/or ACR ≥30 mg/g according to the latest KDIGO guidelines [33].
Independent variable/predictor
The exposure of interest was SMg level analyzed as a continuous variable or according to statistical tertiles. Fasting SMg was measured in the UT Southwestern General Clinical Research Center (GCRC) lab for DHS-1 and at Quest Diagnostics for DHS-2 as part of the standard chemistry panel (normal SMg range, GCRC: 1.7–2.8 mg/dL; Quest Diagnostics: 1.5–2.5 mg/dL). SMg at DHS-2 was solely utilized for cross-sectional correlations with CV surrogate markers (see Table 5).
Table 5.
Cross-sectional correlations of SMg levels with CV surrogate markers at DHS-1 and DHS-2
| Spearman Correlation coefficients Prob > r under H0: Rho = 0 |
|||||
|---|---|---|---|---|---|
| PWV | SBP | DBP | LV mass | HOMA-IR | |
| SMg DHS-1 | 0.003 | −0.06 | −0.05 | 0.04 | −0.10 |
| 0.87 | <0.001 | 0.001 | 0.10 | <0.001 | |
| SMg DHS-2 | – | −0.13 | −0.11 | −0.04 | −0.12 |
| – | <0.001 | <0.001 | 0.20 | <0.001 | |
DHS-1, DHS Phase 1 (2000–02); DHS-2, DHS Phase 2 (2007–09); PWV (DHS-1, n = 2630); SBP (DHS-1, n = 3498; DHS-2, n = 2200); DBP (DHS-1, n = 3498; DHS-2, n = 2200); LV mass (DHS-1, n = 1388; DHS-2, n = 1388); HOMA-IR (DHS-1, n = 2983; DHS-2, n = 2091).
Primary study outcomes
The primary study outcomes were all-cause death and a composite of CV death and nonfatal CV events. Subjects were followed over a median period of 12.3 years (11.9–12.8, 25th percentile–75th percentile) and 11.1 years (10.3–11.6, 25th percentile–75th percentile) for all-cause death (n = 3551, 0 missing) and CV death or event (n = 3040, 511 missing), respectively. All-cause death was adjudicated through 31 December 2013, from the National Death Index, whereas CV death was adjudicated through 31 December 2012. Deaths were classified as cardiovascular based on the International Classification of Diseases-10 codes I00 to I99 [34]. A blinded DHS committee adjudicated nonfatal CV events through 31 December 2012. CV events were coded as myocardial infarction (MI), post-percutaneous coronary intervention MI, unstable angina, coronary artery bypass grafting, percutaneous coronary intervention, stroke, transient ischemic attack, cerebrovascular revascularization, peripheral artery revascularization, congestive heart failure and atrial fibrillation.
Secondary study outcomes
Coronary artery calcification
In DHS-1, electron beam computed tomography (EBCT) image acquisition was prospectively gated to the electrocardiogram at 80% of the RR interval using an Imatron C-150XP EBCT scanner (Imatron Inc., San Bruno, CA, USA), 30 cm FOV, 512 matrix, sharp reconstruction kernel and a 3-mm slice with a table increment of 3 mm. In DHS-2, multi-detector computed tomography (MDCT) was performed on a single scanner (Toshiba Aquilon 64-Slice MDCT, Toshiba Medical Systems Inc., Tustin, CA, USA). Scoring was performed using the Agatston method as previously described [35].
Coronary artery calcification (CAC) incidence was assessed in 1657 individuals with CAC scores available from both DHS-1 and DHS-2. Among subjects with CAC of <100 Agatston units at baseline (DHS-1, n = 1540), incidence of CAC was determined at DHS-2 as ≥100 Agatston units (n = 166), as this cut-off corresponds to moderate to high 10-year risk of cardiovascular events [36, 37].
Pulse wave velocity
Participants in DHS-1 underwent cardiovascular magnetic resonance (CMR) using a 1.5 Tesla whole-body system (Intera, Philips Medical Systems, Best, The Netherlands). Aortic arch pulse wave velocity (PWV) was assessed using a breath-hold, velocity-encoded, phase-contrast gradient echo sequence acquired perpendicular to the course of the ascending aorta 4 cm above the aortic valve plane [38]. PWV was calculated by dividing arch distance (d) by transit time (t): PWV = d/t (DHS-1, n = 2630) [38].
Left ventricular mass
CMR was performed on a 1.5 Tesla system in DHS-1 [39] and on a 3 Tesla system in DHS-2 (Achieva, Philips Medical Systems). Cine images were acquired using prospective electrocardiogram gating and turbo field echo sequencing at both time points. Left ventricular mass (LV mass) was calculated from short-axis sequences using QMass software (Medis, Medical Imaging Systems, Leiden, The Netherlands) for image analysis.
Variable definitions
Race/ethnicity, history of MI and medication usage were self-reported. Prevalent hypertension was defined by medical treatment for hypertension, a systolic blood pressure (SBP) of ≥140 mmHg or a diastolic blood pressure (DBP) of ≥90 mmHg [40]. Five consecutive blood pressure (BP) readings were recorded at 1-min intervals, and the average of the third, fourth and fifth measurements was used for analysis (SBP: DHS-1, n = 3498; DHS-2, n = 2200; DBP: DHS-1, n = 3498; DHS-2, n = 2200). Prevalent type 2 diabetes was defined by medical treatment for diabetes, a fasting blood glucose (BG) ≥126 mg/dL or a non-fasting BG level ≥200 mg/dL [41]. The homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated with the University of Oxford’s HOMA Calculator© (DHS-1, n = 2983; DHS-2, n = 2091) [42]. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. All laboratory parameters were measured from the fasting blood and urine samples obtained at a second in-home visit during DHS-1. Diuretic use consisted of thiazide-like diuretics, loop diuretics, potassium-sparing diuretics and/or aldosterone antagonists. Dietary supplements use consisted of any combination or single treatment with Mg, calcium, active vitamin D and/or multivitamins.
Statistical analysis
Trend through SMg tertiles for quantitative baseline characteristics were analyzed by Jonckheere–Terpstra test, and were tested by Cochran–Armitage trend test for categorical baseline characteristics. Comparisons between CKD and non-CKD subgroups in the same SMg tertile were performed applying the Chi-square test for categorical variables, t-test for Gaussian-distributed continuous variables and Wilcoxon Rank-Sum test for non-Gaussian distributed data. Data were presented as the mean ± standard deviation (SD) or median (25th–75th percentile) for continuous variables and as the percentages for categorical variables. To investigate the relationship between PWV, SBP, DBP, LV mass and HOMA-IR, and SMg levels at DHS-1 and DHS-2, Spearman correlation analysis was performed. The survival risk for all-cause death and for the composite of CV death or event per SMg tertile was estimated using Kaplan–Meier analysis. Multivariable Cox proportional hazard regression models were constructed to calculate the hazard ratio (HR) and 95% confidence interval (CI) for all-cause death, CV death or event, and CAC score ≥100 Agatston units in the entire DHS cohort, and in the CKD and non-CKD subgroups. We constructed four multivariable models: Model 1 adjusted for age, gender and race/ethnicity; Model 2 adjusted for variables in Model 1 plus BMI and serum phosphorus, calcium, bicarbonate, albumin, intact parathyroid hormone (iPTH), total cholesterol and high-density lipoprotein (HDL); Model 3 adjusted for variables in Model 2 plus eGFR, prevalent hypertension, prevalent type 2 diabetes, and the use of diuretics and dietary supplements. All models were fitted, HRs of SMg and its 95% CIs were determined for each 0.2 mg/dL unit decrease in SMg or low SMg tertile in reference to high SMg tertile. To examine the functional form of the association between SMg and all-cause death, we fitted unadjusted cubic splines. All statistical analyses used two-sided α-values, at the significance level of 0.05. For interaction analyses, a P-value of <0.10 was considered statistically significant. All analyses were performed using SAS Version 9.4, Cary, NC, USA and GraphPad Prism 6.0.
RESULTS
Baseline characteristics
Of 3557 patients who had fasting blood collections at DHS-1, baseline SMg data were available for 3551 subjects. SMg was normally distributed with a mean ± SD value of 2.07 ± 0.18 mg/dL (0.85 ± 0.07 mM) in the entire cohort, and 2.08 ± 0.19 mg/dL (0.85 ± 0.08 mM) in the CKD and 2.07 ± 0.18 mg/dL (0.85 ± 0.07) in the non-CKD subgroups. Based on the four-variable MDRD eGFR <60 mL/min/1.73 m2 and/or the presence of microalbuminuria (urinary ACR ≥17 mg/g in men and ≥25 mg/g in women), 306 (8.6%) subjects had prevalent CKD, 36 by eGFR criteria, 227 by ACR criteria and 43 by both eGFR and ACR criteria. Baseline characteristics of non-CKD and CKD study participants according to SMg tertiles are summarized in Table 1. CKD patients were older, and a greater proportion was non-Hispanic black and had history of type 2 diabetes, hypertension or myocardial infarction as compared with those without CKD (Table 1). Consequently, the use of diuretics and renin-angiotensin system (RAAS) inhibitors was significantly higher in the CKD subgroup. CKD patients also had higher iPTH and C-reactive protein (CRP) levels, and slightly higher serum calcium and phosphorus levels. In both, non-CKD and CKD subgroups, higher BMI and lower serum albumin and bicarbonate levels were observed in the low SMg tertile. Similarly, the prevalence of type 2 diabetes was significantly higher in the low SMg tertile, both in CKD and non-CKD participants (Table 1).
Table 1.
Baseline characteristics of non-CKD and CKD study participants at DHS-1 according to SMg tertiles (mg/dL)
| Characteristics | Non-CKD (n = 3245) |
CKD (n = 306) |
||||||
|---|---|---|---|---|---|---|---|---|
| Tertiles of SMg (mg/dL) |
Tertiles of SMg (mg/dL) |
|||||||
| Low (1.1–1.9) | Medium (2.0–2.1) | High (2.2–3.4) | P trend | Low (1.4–1.9) | Medium (2.0–2.1) | High (2.2–2.6) | P trend | |
| n = 736 | n = 1461 | n = 1048 | n = 118 | n = 109 | n = 79 | |||
| Demographics | ||||||||
| Age (years) | 43.1 ± 10.3 | 42.9 ± 9.95 | 44.8 ± 10.0 | <0.001 | 46.8 ± 9.0* | 47.7 ± 10.5* | 50.0 ± 9.3* | 0.02 |
| Gender (male) | 29.1 | 43.0 | 54.2 | <0.001 | 43.2* | 56.0* | 58.2 | 0.03 |
| Race | <0.001a | 0.01a | ||||||
| Hispanic | 14.1 | 16.7 | 20.3 | — | 8.5* | 21.1* | 7.6* | — |
| Non-Hispanic black | 64.5 | 50.8 | 38.7 | — | 77.1* | 62.4* | 65.8* | — |
| Non-Hispanic white | 20.4 | 30 | 38.7 | — | 12.7* | 14.7* | 25.3* | — |
| BMI (kg/m2) | 31.9 ± 8.6 | 30.4 ± 7.4 | 29.7 ± 6.5 | <0.001 | 33.9 ± 8.9 | 31.8 ± 8.3 | 31.2 ± 6.8 | 0.03 |
| Comorbidities | ||||||||
| Prevalent type 2 diabetes | 19.6 | 7.1 | 4.9 | <0.001 | 44.9* | 31.2* | 21.5* | <0.001 |
| Prevalent hypertension | 41.7 | 34.2 | 37.4 | 0.14 | 73.7* | 67.9* | 72.2* | 0.72 |
| SBP | 127 ± 19 | 126 ± 18 | 127 ± 18 | 0.58 | 142 ± 25* | 141 ± 27* | 142 ± 24* | 0.86 |
| DBP | 79 ± 10 | 79 ± 10 | 79 ± 10 | 0.45 | 86 ± 13* | 84 ± 13* | 84 ± 13* | 0.21 |
| History of MI | 5.0 | 2.0 | 3.0 | 0.03 | 6.8 | 7.3* | 11.4* | 0.27 |
| Medications | ||||||||
| Diuretics | 11.7 | 7.7 | 8.5 | 0.04 | 25.4* | 18.4* | 25.3* | 0.85 |
| RAAS inhibitors | 13.9 | 8.8 | 8.2 | <0.001 | 26.3* | 23.9* | 26.6* | 0.99 |
| PPI | 2.9 | 2.8 | 2.7 | 0.71 | 5.4 | 2.8 | 7.7* | 0.56 |
| Dietary supplements | 12.6 | 15.3 | 18.3 | 0.001 | 11.0 | 14.7 | 19.0 | 0.12 |
| Laboratory values | ||||||||
| eGFR (mL/min/1.73 m2) | 106.4 ± 23.9 | 101.4 ± 22.5 | 97.0 ± 20.4 | <0.001 | 93.2 ± 37.4* | 94.4 ± 33.2* | 76.6 ± 52.0* | <0.001 |
| Microalbuminuria (mg/g) | 3.0 (1.9–5.2) | 2.7 (1.7–4.6) | 2.6 (1.8–4.1) | 0.002 | 63.0 (29.5–227.2)* | 46.0 (28.7–98.7)* | 29.0 (18.2–72.7)* | <0.001 |
| Phosphate (mg/dL) | 3.2 ± 0.6 | 3.2 ± 0.6 | 3.2 ± 0.6 | 0.79 | 3.3 ± 0.7 | 3.4 ± 0.6* | 3.6 ± 1.0* | 0.05 |
| Calcium (mg/dL) | 9.2 ± 0.4 | 9.2 ± 0.4 | 9.3 ± 0.6 | <0.001 | 9.3 ± 0.4 | 9.3 ± 0.4 | 9.4 ± 0.5* | 0.02 |
| Bicarbonate (mEq/L) | 26.8 ± 2.3 | 27.0 ± 2.2 | 27.5 ± 2.3 | <0.001 | 26.4 ± 2.5 | 27.0 ± 2.7 | 27.3 ± 2.8 | 0.01 |
| iPTH (pg/mL) | 35.4 (25.7–47.4) | 37.4 (28.0–50.2) | 37.5 (28.8–51.6) | 0.001 | 39.3 (27.4–59.3)* | 39.8 (25.1–59.3) | 52.2 (36.1–67.5)* | 0.02 |
| Albumin (g/dL) | 3.8 ± 0.3 | 4.0 ± 0.3 | 4.1 ± 0.3 | <0.001 | 3.7 ± 0.4* | 3.9 ± 0.3* | 4.0 ± 0.4* | <0.001 |
| Total cholesterol (mg/dL) | 172 ± 38 | 181 ± 37 | 186 ± 41 | <0.001 | 176 ± 46 | 179 ± 41 | 189 ± 46 | 0.05 |
| HDL (mg/dL) | 51 ± 15 | 50 ± 15 | 48 ± 14 | <0.001 | 50 ± 17 | 48 ± 14 | 50 ± 17 | 0.75 |
| CRP (mg/L) | 3.5 (1.2–8.6) | 2.7 (1.1–6.6) | 2.5 (1.1–5.9) | <0.001 | 4.0 (1.9–12.0)* | 3.9 (1.6–7.9)* | 4.3 (1.8–9.4)* | 0.80 |
Data are presented as mean ± standard deviation, median (25th percentile–75th percentile), or percent for categorical variables.
BMI, body mass index; BP, blood pressure; MI, myocardial infarction; RAAS, renin-angiotensin system; PPI, proton pump inhibitors; eGFR, estimated glomerular filtration rate; iPTH, intact parathyroid hormone; HDL, high-density lipoprotein; CRP, C-reactive protein. eGFR was calculated according to the MDRD study equation. Microalbuminuria was calculated based on the urinary albumin/creatinine ratio (ACR). CKD was defined as eGFR <60 mL/min/1.73 m2 and/or ACR >17 mg/g in men and >25 mg/g in women. Dietary supplements consisted of any combination or single treatment with Mg, calcium, active vitamin D and/or multivitamins. Conversion factors for units: phosphate in mg/dL to mmol/L, ×0.3229; calcium in mg/dL to mmol/L, ×0.2495; cholesterol in mg/dL to mmol/L, ×0.02586; HDL in mg/dL to mmol/L, ×0.02586.
Chi-Square test.
P < 0.05 compared with non-CKD in the same tertile group.
Associations between SMg and all-cause mortality or CVD outcomes
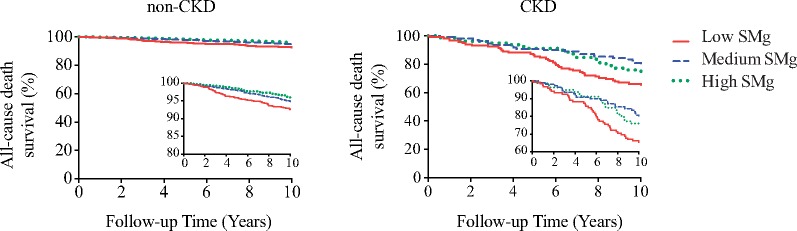
The DHS cohort design is presented in Supplementary Figure S1. The entire cohort, and CKD and non-CKD subgroups were examined with univariable and multivariable analyses using MDRD eGFR together with ACR for CKD/non-CKD subgroup stratification (Tables 2–4). All-cause death was adjudicated in the entire cohort (n = 3551), with a median follow-up of 12.3 years (11.9–12.8, 25th percentile–75th percentile), whereas CV death or event data were available for 3040 participants (511 missing), with a median follow-up of 11.1 years (10.3–11.6, 25th percentile–75th percentile). During the follow-up period, 329 (9.3%) total deaths occurred, of which 58 (1.6%) were attributed to CVD (Supplementary Table S1). In addition, 273 (9.0%) nonfatal CV events were adjudicated (25 of these patients died; Supplementary Table S1). There was a borderline significant interaction between the CKD status and SMg levels on all-cause death (P = 0.15) but not on CV death or event (P = 0.97). The unadjusted Kaplan–Meier curves (Figure 1) revealed that the risk of all-cause death increased significantly in the low SMg tertile in both CKD (Log-Rank P = 0.01; HR 1.69; 95% CI 1.02–2.08, versus high SMg) and non-CKD (Log-Rank P < 0.001; HR 2.00; 95% CI 1.40-2.80, versus high SMg) participants (Figure 1). The risk of the composite of CV death or event across SMg tertiles was not different in both CKD and non-CKD subgroups (Log-Rank P = 0.59 and P = 0.92, respectively; data not shown).
Table 2.
Adjusted HRs for study outcomes in the entire cohort, and CKD and non-CKD subgroups using SMg as a continuous variable per 0.2 mg/dL decrease
| Model 1 |
Model 2 |
Model 3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HRa | 95% CI | P-value | HRa | 95% CI | P-value | HRa | 95% CI | P-value | ||
| All-cause death | ||||||||||
| Entire cohort | 1.49 | 1.32–1.68 | <0.001 | 1.35 | 1.18–1.54 | <0.001 | 1.31 | 1.14–1.49 | <0.001 | |
| CKD | 1.26 | 1.04–1.53 | 0.02 | 1.25 | 1.00–1.56 | 0.05 | 1.37 | 1.08–1.75 | 0.01 | |
| Non-CKD | 1.47 | 1.27–1.70 | <0.001 | 1.33 | 1.13–1.57 | 0.001 | 1.22 | 1.04–1.45 | 0.02 | |
| CV death or event | ||||||||||
| Entire cohort | 1.19 | 1.05–1.35 | 0.006 | 1.13 | 0.99–1.28 | 0.08 | 1.07 | 0.94–1.21 | 0.33 | |
| CKD | 1.14 | 0.92–1.41 | 0.23 | 1.11 | 0.88–1.40 | 0.37 | 1.04 | 0.81–1.33 | 0.55 | |
| Non-CKD | 1.12 | 0.97–1.30 | 0.13 | 1.07 | 0.91–1.26 | 0.39 | 1.01 | 0.86–1.18 | 0.90 | |
| Incident CAC | ||||||||||
| Entire cohort | 1.32 | 1.08–1.61 | 0.007 | 1.25 | 1.00–1.56 | 0.05 | 1.05 | 0.84–1.32 | 0.67 | |
| CKD | 2.05 | 1.00–4.17 | 0.05 | 1.71 | 0.53–5.56 | 0.37 | 1.47 | 0.37–5.88 | 0.59 | |
| Non-CKD | 1.23 | 0.99–1.52 | 0.06 | 1.24 | 0.98–1.56 | 0.08 | 1.05 | 0.82–1.33 | 0.71 | |
CKD, chronic kidney disease; CV, cardiovascular; CAC, coronary artery calcification; HR, hazard ratio; 95% CI, 95% confidence interval. eGFR was calculated according to the MDRD study equation. CKD was defined as eGFR <60 mL/min/1.73 m2 and/or ACR >17 mg/g in men and >25 mg/g in women.
Model 1 was adjusted for age, gender and race/ethnicity. Model 2 was adjusted for variables in Model 1 plus BMI, serum phosphorus, calcium, bicarbonate, albumin, iPTH, total cholesterol and HDL. Model 3 was adjusted for variables in Model 2 plus eGFR, prevalent hypertension, prevalent type 2 diabetes, and use of diuretics and dietary supplements.
For the outcome of incident CAC, data are presented as odds ratio (OR).
Table 4.
Adjusted HRs for study outcomes in the non-CKD subgroup according to SMg tertiles (mg/dL)
| Model 1 |
Model 2 |
Model 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| All-cause death | |||||||||
| Low SMg | 2.09 | 1.47–2.98 | <0.001 | 1.54 | 1.03–2.33 | 0.04 | 1.30 | 0.86–1.96 | 0.22 |
| Medium SMg | 1.38 | 0.99–1.92 | 0.06 | 1.26 | 0.88–1.82 | 0.21 | 1.24 | 0.86–1.79 | 0.25 |
| High SMg | Ref. | — | — | Ref. | — | — | Ref. | — | — |
| CV death or event | |||||||||
| Low SMg | 1.29 | 0.91–1.84 | 0.16 | 1.22 | 0.83–1.79 | 0.31 | 1.06 | 0.72–1.57 | 0.77 |
| Medium SMg | 1.06 | 0.78–1.44 | 0.69 | 0.99 | 0.71–1.37 | 0.95 | 1.01 | 0.73–1.41 | 0.94 |
| High SMg | Ref. | — | — | Ref. | — | — | Ref. | — | — |
SMg, serum magnesium; CV, cardiovascular; HR, hazard ratio; 95% CI, 95% confidence interval. HR of the low versus high SMg level. eGFR was calculated according to the MDRD study equation. CKD was defined as eGFR <60 mL/min/1.73 m2 and/or ACR >17 mg/g in men and >25 mg/g in women.
Model 1 was adjusted for age, gender and race/ethnicity. Model 2 was adjusted for variables in Model 1 plus BMI, serum phosphorus, calcium, bicarbonate, albumin, iPTH, total cholesterol and HDL. Model 3 was adjusted for variables in Model 2 plus eGFR, prevalent hypertension, prevalent type 2 diabetes, and use of diuretics and dietary supplements.
FIGURE 1.
Unadjusted Kaplan–Meier curves for all-cause death in non-CKD (Long-Rank P < 0.001) and CKD (Log-Rank P = 0.01) participants stratified by SMg tertiles. The inserts show the same data on an enlarged Y-axis. eGFR was calculated according to the MDRD study equation. CKD was defined as eGFR <60 mL/min/1.73 m2 and/or ACR ≥17 mg/g in men and ≥25 mg/g in women.
In multivariable time-to-event analyses, every 0.2 mg/dL (0.08 mM) decrease in SMg increased the adjusted hazard for all-cause death by 31% in the entire cohort; 37% in CKD and 22% in non-CKD participants (Table 2). Interactions of SMg with serum albumin (SMg × Alb) and serum phosphorus (SMg × P) on the association between SMg levels and all-cause death were evaluated. Only a significant SMg × Alb interaction (P < 0.001) was found in the CKD subgroup.
After stratification by SMg tertiles, the low SMg tertile was independently associated with all-cause death in the CKD subgroup (adjusted HR 2.31; 95% CI 1.23–4.36, for low versus high tertile; Table 3), whereas in non-CKD participants the association was attenuated in Model 3 (Table 4).
Table 3.
Adjusted HRs for study outcomes in the CKD subgroup according to SMg tertiles (mg/dL)
| Model 1 |
Model 2 |
Model 3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| All-cause death | ||||||||||
| Low SMg | 2.22 | 1.33–3.72 | 0.002 | 1.92 | 1.03–3.60 | 0.04 | 2.31 | 1.23–4.36 | 0.01 | |
| Medium SMg | 0.97 | 0.55–1.72 | 0.92 | 0.91 | 0.45–1.84 | 0.80 | 1.15 | 0.55–2.41 | 0.71 | |
| High SMg | Ref. | — | — | Ref. | — | — | Ref. | — | — | |
| CV death or event | ||||||||||
| Low SMg | 1.47 | 0.85–2.57 | 0.17 | 1.16 | 0.62–2.18 | 0.64 | 0.97 | 0.50–1.89 | 0.93 | |
| Medium SMg | 0.89 | 0.49–1.61 | 0.69 | 0.67 | 0.33–1.37 | 0.27 | 0.52 | 0.24–1.12 | 0.09 | |
| High SMg | Ref. | — | — | Ref. | — | — | Ref. | — | — | |
SMg, serum magnesium; CV, cardiovascular; HR, hazard ratio; 95% CI, 95% confidence interval.
HR of the low versus high tertile of SMg level. eGFR was calculated according to the MDRD study equation. CKD was defined as eGFR <60 mL/min/1.73 m2 and/or ACR >17 mg/g in men and >25 mg/g in women.
Model 1 was adjusted for age, gender and race/ethnicity. Model 2 was adjusted for variables in Model 1 plus BMI, serum phosphorus, calcium, bicarbonate, albumin, iPTH, total cholesterol and HDL. Model 3 was adjusted for variables in Model 2 plus eGFR, prevalent hypertension, prevalent type 2 diabetes, and use of diuretics and dietary supplements.
The association between the low SMg tertile and CV death or event was not statistically significant in both CKD and non-CKD subgroups.
Associations between SMg and surrogate CVD outcomes
In cross-sectional analyses, we did not find a significant correlation between SMg levels and either LV mass or aortic PWV. However, we did find a significant inverse correlation between SMg levels and SBP and DBP measurements (Table 5). A significant inverse correlation was also found between SMg levels and insulin resistance by HOMA-IR (Table 5). Each 0.2 mg/dL (0.08 mM) decrease in SMg increased the adjusted hazard for incident CAC scores ≥100 Agatston units by 32% in the entire cohort, but the association was attenuated with the inclusion of anthropometric and biochemical parameters (Table 2).
Sensitivity analysis
CKD was adjudicated utilizing the CKD-EPI eGFR equation in addition to ACR criteria. All multivariable analyses utilizing SMg both as a continuous variable (per 0.2 mg/dL or 0.08 mM decrease) and divided into tertiles were replicated (Supplementary Tables S2–S4). The results were essentially the same as those generated utilizing the MDRD eGFR equation to define CKD. Furthermore, the analysis was replicated utilizing the CKD KDIGO definition (eGFR <60 mL/min/1.73 m2 and/or ACR ≥30 mg/g; Supplementary Table S5). The results were very similar to the original analysis (for SMg as a continuous variable) and borderline significant for SMg divided in tertiles (Supplementary Table S5), the latter likely related to the smaller number of patients with CKD according to the KDIGO definition (n = 244 versus n = 306).
Exploratory analysis
We further explored the relationship between SMg levels and all-cause death utilizing cubic spline curves. As depicted in Supplementary Figure S2, we confirmed the inverse relationship between SMg levels (X-axis) and the hazard ratio for all-cause death (Y-axis) in the DHS cohort.
DISCUSSION
The main finding of our investigation is that lower SMg levels are independently associated with a higher risk of all-cause death in patients with prevalent early stage CKD. This association is independent of demographics, baseline eGFR, comorbid conditions and the use of diuretics. In non-CKD participants, the strength of the association was attenuated by other biochemical parameters, prevalent type 2 diabetes, prevalent hypertension, and the exposure to diuretics and/or dietary supplements. CKD patients may be more susceptible to low SMg levels due to the prevalence of underlying endothelial dysfunction and comorbidities, which are present even at early stages of CKD [5, 43, 44]. In support of this hypothesis, we observed that patients with prevalent CKD were older and mostly non-Hispanic black; had lower serum albumin; higher CRP levels; and higher prevalence of type 2 diabetes, hypertension and myocardial infarction, compared with their non-CKD counterparts at the beginning of the observation period.
Low extracellular Mg concentrations have been shown to induce endothelial dysfunction, calcification of vascular smooth muscle, and loss of anti-inflammatory and antioxidant capacities [45–47]. In our analysis, SMg inversely correlated with SBP and DBP measurements, and the insulin resistance index HOMA-IR. Despite these significant cross-sectional correlations, every 0.2 mg/dL decrease in SMg was not significantly associated with incident CAC scores ≥100 Agatston units in a fully adjusted model.
Recent studies support our findings by showing that low SMg is a significant predictor of death in pre-dialysis CKD patients or patients undergoing hemodialysis; however, they carry some limitations. Sakaguchi et al. showed that hypomagnesemia is a predictor of CV and non-CV death in a Japanese cohort of hemodialysis patients, but they limited their follow-up to 1 year [26]. A similar US cohort study of hemodialysis patients reported comparable findings but lacked information for specific cause of CV death and the use of oral Mg-containing medications [25]. Similarly, Kanbay et al. found that hypomagnesemia is a risk factor for CV death or event in patients with CKD Stages 3–5, but heart failure hospitalizations were not adjudicated [24]. The present study expands our knowledge on the role of SMg in patients with early stages of CKD, and opens new potential interventional portals targeting these patients. Data from small studies suggest beneficial effects of oral Mg supplementation on CV calcification and surrogate parameters of atherosclerosis in patients with CKD [48–50]. However, no randomized controlled trials have examined the effects of long-term Mg supplementation on surrogate or hard CV outcomes in patients with different stages of CKD.
The strengths of our study include the utilization of a large multiethnic population of more than 3000 subjects of which ∼50% were African American, the long-term median follow-up of 12.3 years and the standardized longitudinal data collection methodology with comprehensive CVD assessment. Moreover, the DHS collected a databank of genomic DNA samples of study participants that could be utilized to test whether previously identified genetic variants associated with Mg homeostasis contribute to differences in CV and non-CV mortality in DHS participants [51, 52]. Unique to our study is the inclusion of CKD participants with early stages of CKD, in whom Mg supplementation may potentially contribute to secondary prevention of CKD progression and CVD.
Some limitations are noted in our study. First, the number of all-cause deaths and CV deaths or events that were adjudicated in the entire DHS cohort is small, and other non-CV causes of death were not categorized. Also, a small sample size of CKD participants with available CVD surrogate marker adjudication was available. This small sample size may have limited statistical power and the ability to detect small effects. Second, given the narrow range of serum albumin levels and the sample size limitation, we could not further investigate how the SMg×Alb interaction that was found in the CKD subgroup affects the association of SMg levels with all-cause death at different serum albumin strata. The examination of the SMg×Alb interaction has been proven to be relevant in other cohorts because it somehow reflect the status of overall illness associated with CKD [19, 25, 26]. Finally, this study did not investigate the relationship between time-varying SMg levels and outcome.
Overall, compared with our knowledge on calcium and phosphorus metabolism in CKD patients, the regulation of Mg homeostasis in this population is still relatively poorly understood. In the DHS, SMg levels were not statistically different in CKD and non-CKD study participants, likely because our CKD population comprises mostly early stages of CKD. It is also possible that an undetected status of intracellular Mg deficiency may be more important than total low SMg levels in the pathobiology of CVD since Mg is essential for energy balance [53, 54]. Future research should address the correlation of free intracellular Mg concentrations and SMg levels in CKD and non-CKD patients, and should investigate the role of free intracellular Mg concentrations in the development or progression of comorbidities in CKD [53, 54].
In conclusion, we observed that lower SMg levels were independently associated with increased all-cause death in CKD patients. The examination of the causal relationship between low SMg and CV and non-CV complications in CKD is of paramount importance and provides the rationale for prospective interventional studies of Mg supplementation as an inexpensive therapy to mitigate fatal complications in CKD patients.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr Sonia Garg and Dr Amit Khera for revising the article. The authors also thank Dr S. Susan Hedayati for the constructive comments during the design and execution of the study.
AUTHORS’ CONTRIBUTIONS
S.F. and J.A.N. conceived the study concept and design; S.F., X.L., B.A.H., and J.A.N. acquired, analyzed or interpreted the data; S.F. and J.A.N. drafted the manuscript; N.M.M., K.S., R.D.T., and O.W.M. provided critical revision of the manuscript for intellectual content; S.F., X.L., B.A.H, and J.A.N. performed statistical analysis; R.D.T. and O.W.M. obtained funding; N.M.M., K.S., R.D.T., O.W.M, and J.A.N. supervised the study.
FUNDING
The Dallas Heart Study is supported by grant no. UL1TR001105 from the National Center for Advancing Translational Science of the National Institutes of Health. This work was also supported by the University of Texas Southwestern Medical Center O’Brien Kidney Research Core Center (grant no. P30DK079328). S.F. and J.A.N. were supported by the Ben J. Lipps Research Fellowship Program of American Society of Nephrology Foundation for Kidney Research. J.A.N. was also supported by the Truelson Fellowship Fund at UT Southwestern Charles and Jane Pak Center of Mineral Metabolism and Clinical Research.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Coresh J, Selvin E, Stevens LA. et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–2047 [DOI] [PubMed] [Google Scholar]
- 2. Levey AS, Atkins R, Coresh J. et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 2007; 72: 247–259 [DOI] [PubMed] [Google Scholar]
- 3. Foley RN, Parfrey PS, Sarnak MJ.. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32: S112–S119 [DOI] [PubMed] [Google Scholar]
- 4. Go AS, Chertow GM, Fan D. et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 5. London GM, Marchais SJ, Guerin AP. et al. Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Curr Opin Nephrol Hypertens 2005; 14: 525–531 [DOI] [PubMed] [Google Scholar]
- 6. Stenvinkel P, Carrero JJ, Axelsson J. et al. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 2008; 3: 505–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Baaij JH, Hoenderop JG, Bindels RJ.. Magnesium in man: implications for health and disease. Physiol Rev 2015; 95: 1–46 [DOI] [PubMed] [Google Scholar]
- 8. Peacock JM, Folsom AR, Arnett DK. et al. Relationship of serum and dietary magnesium to incident hypertension: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol 1999; 9: 159–165 [DOI] [PubMed] [Google Scholar]
- 9. Schulze MB, Schulz M, Heidemann C. et al. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med 2007; 167: 956–965 [DOI] [PubMed] [Google Scholar]
- 10. He K, Liu K, Daviglus ML. et al. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation 2006; 113: 1675–1682 [DOI] [PubMed] [Google Scholar]
- 11. Liao F, Folsom AR, Brancati FL.. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 1998; 136: 480–490 [DOI] [PubMed] [Google Scholar]
- 12. Zhang W, Iso H, Ohira T. et al. Associations of dietary magnesium intake with mortality from cardiovascular disease: the JACC study. Atherosclerosis 2012; 221: 587–595 [DOI] [PubMed] [Google Scholar]
- 13. Larsson SC, Orsini N, Wolk A.. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. Am J Clin Nutr 2012; 95: 362–366 [DOI] [PubMed] [Google Scholar]
- 14. An G, Du Z, Meng X. et al. Association between low serum magnesium level and major adverse cardiac events in patients treated with drug-eluting stents for acute myocardial infarction. PLoS One 2014; 9: e98971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kieboom BC, Niemeijer MN, Leening MJ. et al. Serum magnesium and the risk of death from coronary heart disease and sudden cardiac death. J Am Heart Assoc 2016; 5: e002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lutsey PL, Alonso A, Michos ED. et al. Serum magnesium, phosphorus, and calcium are associated with risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2014; 100: 756–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tin A, Grams ME, Maruthur NM. et al. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int 2015; 87: 820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Laecke S, Nagler EV, Verbeke F. et al. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am J Med 2013; 126: 825–831 [DOI] [PubMed] [Google Scholar]
- 19. Sakaguchi Y, Iwatani H, Hamano T. et al. Magnesium modifies the association between serum phosphate and the risk of progression to end-stage kidney disease in patients with non-diabetic chronic kidney disease. Kidney Int 2015; 88: 833–842 [DOI] [PubMed] [Google Scholar]
- 20. Abbott RD, Ando F, Masaki KH. et al. Dietary magnesium intake and the future risk of coronary heart disease (the Honolulu Heart Program). Am J Cardiol 2003; 92: 665–669 [DOI] [PubMed] [Google Scholar]
- 21. Ford ES. Serum magnesium and ischaemic heart disease: findings from a national sample of US adults. Int J Epidemiol 1999; 28: 645–651 [DOI] [PubMed] [Google Scholar]
- 22. Hashimoto T, Hara A, Ohkubo T. et al. Serum magnesium, ambulatory blood pressure, and carotid artery alteration: the Ohasama study. Am J Hypertens 2010; 23: 1292–1298 [DOI] [PubMed] [Google Scholar]
- 23. Ma J, Folsom AR, Melnick SL. et al. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. Atherosclerosis Risk in Communities Study. J Clin Epidemiol 1995; 48: 927–940 [DOI] [PubMed] [Google Scholar]
- 24. Kanbay M, Yilmaz MI, Apetrii M. et al. Relationship between serum magnesium levels and cardiovascular events in chronic kidney disease patients. Am J Nephrol 2012; 36: 228–237 [DOI] [PubMed] [Google Scholar]
- 25. Lacson E Jr, Wang W, Ma L, Passlick-Deetjen J.. Serum magnesium and mortality in hemodialysis patients in the United States: A cohort study. Am J Kidney Dis 2015; 66: 1056–1066 [DOI] [PubMed] [Google Scholar]
- 26. Sakaguchi Y, Fujii N, Shoji T. et al. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int 2014; 85: 174–181 [DOI] [PubMed] [Google Scholar]
- 27. Sakaguchi Y, Hamano T, Nakano C. et al. Association between density of coronary artery calcification and serum magnesium levels among patients with chronic kidney disease. PLoS One 2016; 11: e0163673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Roij van Zuijdewijn CL, Grooteman MP, Bots ML. et al. Serum magnesium and sudden death in European hemodialysis patients. PLoS One 2015; 10: e0143104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Victor RG, Haley RW, Willett DL. et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004; 93: 1473–1480 [DOI] [PubMed] [Google Scholar]
- 30. Levey AS, Bosch JP, Lewis JB. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470 [DOI] [PubMed] [Google Scholar]
- 31. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–266 [PubMed] [Google Scholar]
- 33. Stevens PE, Levin A.. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825–830 [DOI] [PubMed] [Google Scholar]
- 34. Mozaffarian D, Benjamin EJ, Go AS. et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 2015; 131: e29–e322 [DOI] [PubMed] [Google Scholar]
- 35. Jain T, Peshock R, McGuire DK. et al. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol 2004; 44: 1011–1017 [DOI] [PubMed] [Google Scholar]
- 36. Church TS, Levine BD, McGuire DK. et al. Coronary artery calcium score, risk factors, and incident coronary heart disease events. Atherosclerosis 2007; 190: 224–231 [DOI] [PubMed] [Google Scholar]
- 37. Arad Y, Goodman KJ, Roth M. et al. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol 2005; 46: 158–165 [DOI] [PubMed] [Google Scholar]
- 38. King KS, Chen KX, Hulsey KM. et al. White matter hyperintensities: use of aortic arch pulse wave velocity to predict volume independent of other cardiovascular risk factors. Radiology 2013; 267: 709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Drazner MH, Dries DL, Peshock RM. et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension 2005; 46: 124–129 [DOI] [PubMed] [Google Scholar]
- 40. Chandra A, Neeland IJ, Berry JD. et al. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J Am Coll Cardiol 2014; 64: 997–1002 [DOI] [PubMed] [Google Scholar]
- 41. Neeland IJ, Turer AT, Ayers CR. et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 2012; 308: 1150–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matthews DR, Hosker JP, Rudenski AS. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419 [DOI] [PubMed] [Google Scholar]
- 43. London GM. Arterial calcification: cardiovascular function and clinical outcome. Nefrologia 2011; 31: 644–647 [DOI] [PubMed] [Google Scholar]
- 44. Watanabe R, Lemos MM, Manfredi SR. et al. Impact of cardiovascular calcification in nondialyzed patients after 24 months of follow-up. Clin J Am Soc Nephrol 2010; 5: 189–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ferre S, Baldoli E, Leidi M. et al. Magnesium deficiency promotes a pro-atherogenic phenotype in cultured human endothelial cells via activation of NFkB. Biochim Biophys Acta 2010; 1802: 952–958 [DOI] [PubMed] [Google Scholar]
- 46. Montes de Oca A, Guerrero F, Martinez-Moreno JM. et al. Magnesium inhibits Wnt/beta-catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PLoS One 2014; 9: e89525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chacko SA, Song Y, Nathan L. et al. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care 2010; 33: 304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Turgut F, Kanbay M, Metin MR. et al. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int Urol Nephrol 2008; 40: 1075–1082 [DOI] [PubMed] [Google Scholar]
- 49. Spiegel DM, Farmer B.. Long-term effects of magnesium carbonate on coronary artery calcification and bone mineral density in hemodialysis patients: a pilot study. Hemodial Int 2009; 13: 453–459 [DOI] [PubMed] [Google Scholar]
- 50. Mortazavi M, Moeinzadeh F, Saadatnia M. et al. Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: a double-blind, randomized, placebo-controlled trial. Eur Neurol 2013; 69: 309–316 [DOI] [PubMed] [Google Scholar]
- 51. Meyer TE, Verwoert GC, Hwang SJ. et al. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six Loci influencing serum magnesium levels. PLoS Genet 2010; 6: e1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tin A, Kottgen A, Folsom AR. et al. Genetic loci for serum magnesium among African-Americans and gene-environment interaction at MUC1 and TRPM6 in European-Americans: the Atherosclerosis Risk in Communities (ARIC) study. BMC Genet 2015; 16: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ryschon TW, Rosenstein DL, Rubinow DR. et al. Relationship between skeletal muscle intracellular ionized magnesium and measurements of blood magnesium. J Lab Clin Med 1996; 127: 207–213 [DOI] [PubMed] [Google Scholar]
- 54. Williams GD, Mosher TJ, Smith MB.. Simultaneous determination of intracellular magnesium and pH from the three 31P NMR chemical shifts of ATP. Anal Biochem 1993; 214: 458–467 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.