ABSTRACT
Background
The carbohydrate-to-fiber ratio is a recommended measure of carbohydrate quality; however, its relation to incident coronary heart disease (CHD) is not currently known.
Objective
We aimed to assess the relation between various measures of carbohydrate quality and incident CHD.
Design
Data on diet and lifestyle behaviors were prospectively collected on 75,020 women and 42,865 men participating in the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS) starting in 1984 and 1986, respectively, and every 2–4 y thereafter until 2012. All participants were free of known diabetes mellitus, cancer, or cardiovascular disease at baseline. Cox proportional hazards regression models were used to assess the relation between dietary measures of carbohydrate quality and incident CHD.
Results
After 1,905,047 (NHS) and 921,975 (HPFS) person-years of follow-up, we identified 7,320 cases of incident CHD. In models adjusted for age, lifestyle behaviors, and dietary variables, the highest quintile of carbohydrate intake was not associated with incident CHD (pooled-RR = 1.04; 95% CI: 0.96, 1.14; P-trend = 0.31). Total fiber intake was not associated with risk of CHD (pooled-RR = 0.94; 95% CI: 0.85, 1.03; P-trend = 0.72), while cereal fiber was associated with a lower risk for incident CHD (pooled-RR = 0.80; 95% CI: 0.74, 0.87; P-trend < 0.0001). In fully adjusted models, the carbohydrate-to–total fiber ratio was not associated with incident CHD (pooled-RR = 1.04; 95% CI: 0.96, 1.13; P-trend = 0.46). However, the carbohydrate-to–cereal fiber ratio and the starch-to–cereal fiber ratio were associated with an increased risk for incident CHD (pooled-RR = 1.20; 95% CI: 1.11, 1.29; P-trend < 0.0001, and pooled-RR = 1.17; 95%CI: 1.09, 1.27; P-trend < 0.0001, respectively).
Conclusion
Dietary cereal fiber appears to be an important component of carbohydrate quality. The carbohydrate-to–cereal fiber ratio and the starch-to–cereal fiber ratio, but not the carbohydrate-to-fiber ratio, was associated with an increased risk for incident CHD. Future research should focus on how various measures of carbohydrate quality are associated with CHD prevention. This trial was registered at clinicaltrials.gov as NCT03214861.
Keywords: carbohydrates, carbohydrate quality, diet quality, whole grains, type 2 diabetes, starch, fiber
INTRODUCTION
Despite significant advances in cardiovascular medicine over the past 50 y, heart disease remains the leading cause of death for both men and women in the United States (1). While data from NHANES indicate a decline in the prevalence of major coronary heart disease (CHD) risk factors such as hypertension, dyslipidemia, and tobacco use (2), other factors such as overweight and obesity, diabetes, and the metabolic syndrome remain highly prevalent (3–5). Many of the chronic conditions which predispose to CHD are largely driven by unhealthy behaviors, such as poor dietary habits and physical inactivity. In fact, ≤80% of the risk for CHD events could be attributable to a lack of adherence to a healthy lifestyle (6, 7).
While diet has always been a major focus in the prevention of CHD, guidelines have historically emphasized a low-fat (and in particular low–saturated fat) diet (8, 9). This has had important clinical implications as many individuals have adopted low-fat, high-carbohydrate diets in an attempt to reduce their risk for CHD. Compared with total carbohydrates, saturated fat intake was not significantly associated with CHD or cardiovascular disease outcomes (10). However, saturated fat was associated with increased risk of CHD compared with high-quality carbohydrates such whole grains or unsaturated fats (11). Although the associations between total daily carbohydrate consumption and CHD have been inconsistent (12, 13), certain sources of carbohydrates such as refined grains and added sugars have been found to be associated with CHD (14, 15).
In 2010, the American Heart Association defined an ideal diet as part of its 2020 Impact Goals. One component of the ideal diet was fiber-rich whole grains, defined as having a carbohydrate-to-fiber ratio of <10:1 (16). Research suggests that this measure of carbohydrate quality appears to be superior to other consumer-oriented methods for identifying a restricted set of grain foods commonly consumed in Boston-area markets (17). In addition, a recent study indicated that this ratio can also be useful in evaluating the overall carbohydrate quality of the diet where it was positively associated with risk of type 2 diabetes among women (18). However, its relation to CHD outcomes has not been defined. Therefore, the purpose of the present study was to examine the association between the carbohydrate-to-fiber ratio and other measures of carbohydrate quality and quantity and incident CHD in a large cohort of US men and women.
MATERIALS AND METHODS
Study population
The Nurses’ Health Study (NHS) was initiated in 1976 as a prospective cohort study, where 121,701 female registered nurses between the ages of 30 and 55 y were recruited from 11 US states. The Health Professionals Follow-Up Study (HPFS) was initiated in 1986 and recruited 51,529 US men between the ages of 40 and 75 y. Every 2 y, participants updated their information on medical history, lifestyle, and incidence of chronic diseases using validated questionnaires. Follow-up rates are ≥90% of potential person-years of follow-up, and mortality follow-up is >98%. In the current investigation we excluded participants with a baseline history of diabetes, cardiovascular disease, or cancer because these diagnoses could result in important dietary changes (19). We also excluded participants who left ≥10 items blank on the food-frequency questionnaire (FFQ), or who had implausible energy intakes (<500 or >3500 kcal/d for women and <800 or >4200 kcal/d for men). The final analysis included 75,020 women and 42,865 men (Supplemental Figure 1). The study was approved by the Human Research Committee of Brigham and Women's Hospital and Harvard TH Chan School of Public Health in Boston, MA.
Assessment of diet
In the NHS and HPFS, an FFQ was administered at baseline in 1984 and 1986, respectively, and every 4 y thereafter, until 2010. Participants were asked how often on average (“never” to “six or more times per day”) they consumed a specified common portion size or serving size of specific foods. The validity and reproducibility of the FFQ in measuring food intake has been demonstrated previously (20–23). In a prior validation study involving a subsample of 173 nurses in the Boston area, FFQ assessment of total carbohydrate and total fiber were moderately correlated with the average of four 1-wk diet records (total carbohydrate, r = 0.64; total fiber, r = 0.56) (20, 24). Carbohydrate-rich food items had similar correlation coefficients (cold breakfast cereal, r = 0.79; white bread, r = 0.71; dark bread, r = 0.77; pasta and rice, r = 0.35; potatoes, r = 0.66) (21). The main exposure variables were carbohydrate, starch, total fiber, cereal fiber, fruit fiber, vegetable fiber, and ratios of carbohydrate-to–total fiber, carbohydrate-to–cereal fiber, starch-to–total fiber, and starch to cereal fiber. Nutrient intakes were calculated by multiplying the frequency of consumption by the nutrient content of the specified portion size of each food. Then, the nutrient content of all food items in a participant's diet were summed to estimate the individual nutrient intakes. The nutrient contents were determined using the USDA Food Composition tables and complemented with information from manufacturers (25). All dietary variables were adjusted for total energy intake using the residual method to control for confounding and removal of extraneous variation due to differences in body size, metabolic efficiency, and physical activity (26).
Assessment of coronary heart disease
The primary outcome of this analysis was incident CHD (defined as either fatal CHD event or nonfatal myocardial infarction) occurring between 1984 and 2012 in the NHS and 1986 and 2012 in the HPFS. Fatal CHD events were ascertained by next of kin or the National Death Index and confirmed by medical records, autopsy reports, or death certificates. CHD was confirmed as the etiology if it was listed as the cause of death, the most likely cause of death, or if a history of prior CHD was available. Nonfatal myocardial infarction was confirmed on the basis of medical records using criteria proposed by the WHO—characteristic symptoms plus typical electrocardiographic changes consistent with myocardial infarction or the presence of elevated cardiac biomarkers (27).
Assessment of covariates
On a biennial basis, questionnaires were distributed to study participants, who provided updated information on age, weight, and medical history (e.g., menopausal status, hormone use, vitamin use, and the presence or absence of hypertension and hypercholesterolemia). Physical activity was characterized as metabolic equivalent (MET) hours per week.
Statistical analysis
Person-years of follow-up were calculated from the time that the initial questionnaire was returned (1984 for NHS; 1986 for HPFS) to the end of the follow-up period (2012 for both), date of initial confirmed CHD event, or loss to follow-up, whichever was earliest. Associations between carbohydrate, starch, total fiber, cereal fiber, glycemic index, glycemic load, and the ratios of carbohydrate-to–total fiber, carbohydrate-to–cereal fiber, starch-to–total fiber, and starch to cereal fiber were assessed at baseline among study participants using Pearson correlation coefficients. Time-varying Cox proportional hazards regression was used to estimate the relative risk of CHD by quintiles of energy-adjusted intakes of various carbohydrate variables, stratified by 5-y age categories. A test for linear trend was performed using quintiles of the carbohydrate exposure variable as a continuous variable by assigning the median values of the quintile to the variable.
Cumulative averages of all available dietary data were used in order to reduce within-person variation, and to best characterize long-term dietary patterns (19). To minimize confounding due to change in diet after diagnosis of chronic diseases, dietary exposure variables were not updated after the diagnosis of diabetes mellitus, cancer, or stroke. To minimize the influence of outliers, study participants were grouped into quintiles of energy-adjusted carbohydrate exposure variables, with the reference group representing the lowest quintile.
Several potential confounders were adjusted for in our Cox models. Model 1 was age-adjusted (<50, 50 to <55, 55 to <60, 60 to <65, or ≥65 y). Model 2 included various medical history and lifestyle-related covariates such as BMI (in kg/m2; <21, 21 to <23, 23 to <25, 25 to <27, 27 to <30, 30 to <33, 33 to <35, 35 to <40, or ≥40), race (Caucasian, African American, Native American, Asian, or Hawaiian), family history of myocardial infarction (yes or no), menopausal status and postmenopausal hormone use (premenopause, postmenopausal never user, postmenopausal current user, or postmenopausal past user), smoking (never, past, current 1–14 cigarettes/d, current 15–24 cigarettes/d, or current ≥25 cigarettes/d), alcohol use (0, 0.1 to <5, 5.0 to <10, 10 to <15, or ≥15 g/d), physical activity level (<3, 3 to <9, 9 to <18, 18 to <27, or ≥27 MET-h/wk), multivitamin use (yes or no), aspirin use (yes or no), vitamin E use (yes or no), and total energy intake (kilocalories per day, in quintiles), in addition to dietary variables such as polyunsaturated fat–to–saturated fat ratio and trans fat (percentage of total energy, in quintiles). Nondietary variables were updated every 2 y, whereas dietary records were updated every 4 y.
A priori defined stratified analyses and the potential for effect modification was tested for the associations between cereal fiber, carbohydrate-to–cereal fiber ratio, starch to cereal fiber ratio, and CHD by 1) age (<60 and ≥60 y); 2) BMI (<25 and ≥25); 3) physical activity (HPFS: <22 and ≥22 MET-h/wk; NHS: <10 and ≥10 MET-h/wk); 4) family history of myocardial infarction (yes or no); and 5) current smoking (yes or no).
Sensitivity analyses were also performed using dietary data which were updated throughout the follow-up period, even after the development of intermediate outcomes such as diabetes and cancer. Because individuals at higher risk of CHD may be motivated to improve their diet, mainly increasing their fruit and vegetable consumption, to alleviate their higher CHD risk, and therefore potentially causing reverse causation, we performed a 4-y “lagged” analysis between cereal, fruit, and vegetable fiber and risk of CHD among both men and women.
In addition, the potential for nonlinearity of the associations between cereal fiber, carbohydrate-to–cereal fiber ratio, and starch-to–cereal fiber ratio was also tested using restricted cubic splines with 5 knots, 5th, 25.5th, 50th, 72.5th and 95th percentiles of exposure (28), where we gave all values <Q1 – 3 × IQR the value of Q1 – 3 × IQR and all values >Q3 + 3 × IQR the value of Q3 + 3 × IQR. Due to differences in study design, all analyses were first performed separately in each cohort. To obtain overall estimates for both cohort studies, hazard ratios from the age and multivariate adjusted models were combined using a fixed-effects inverse-variance weighted meta-analysis. All statistical analyses were 2-sided with a P value <0.05 considered to be significant. SAS version 9.3 for UNIX (SAS Institute Inc.) was used for all statistical analyses.
RESULTS
During 1905,049 person-years of follow-up in the NHS there were a total of 3267 CHD events, and during 921,975 person-years of follow-up in the HPFS there were 4053 CHD events. The age-adjusted baseline characteristics of the study participants, according to quintiles of carbohydrate, starch, and cereal fiber intake, are presented in Table 1. In general, men and women with higher intakes of carbohydrate and starch had higher glycemic index and glycemic load values, were more likely to have hypercholesterolemia but were less likely to smoke, and had similar family histories of CHD to those with lower intakes of carbohydrate and starch. Men and women with higher intakes of cereal fiber were more likely to be physically active, and consumed less saturated fat and alcohol.
TABLE 1.
Age-adjusted baseline characters of the HPFS and NHS study populations by quintiles of carbohydrate, starch, and total and cereal fiber intake among participants at baseline1
| Carbohydrate | Starch | Cereal fiber | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| HPFS | |||||||||
| n | 8261 | 8693 | 8379 | 8526 | 8606 | 8460 | 9022 | 9045 | 8364 |
| Median, g | 182 | 234 | 288 | 44 | 66 | 94 | 2.5 | 5.0 | 10.1 |
| Age,2 y | 54 ± 9 | 53 ± 10 | 53 ± 10 | 55 ± 10 | 53 ± 10 | 52 ± 9 | 53 ± 9 | 52 ± 9 | 54 ± 10 |
| BMI, kg/m2 | 26.1 ± 3.4 | 25.5 ± 3.1 | 24.8 ± 3.2 | 25.8 ± 3.2 | 25.6 ± 3.3 | 25 ± 3.2 | 25.9 ± 3.4 | 25.5 ± 3.4 | 24.8 ± 2.9 |
| Caucasian, % | 96 | 96 | 93 | 96 | 96 | 92 | 94 | 96 | 96 |
| Family history of CHD, % | 32 | 32 | 33 | 31 | 32 | 32 | 31 | 31 | 33 |
| Hypertension, % | 22 | 18 | 19 | 21 | 19 | 18 | 23 | 19 | 18 |
| Hypercholesterolemia, % | 9 | 10 | 12 | 10 | 10 | 12 | 9 | 10 | 12 |
| Physical activity, MET-h/wk | 18 ± 24 | 21 ± 30 | 26 ± 33 | 22 ± 31 | 21 ± 29 | 22 ± 30 | 19 ± 29 | 21 ± 30 | 25 ± 33 |
| Current smoker, % | 17 | 8 | 5 | 13 | 10 | 7 | 17 | 8 | 4 |
| Alcohol intake, g/d | 22 ± 22 | 9 ± 12 | 5 ± 7 | 17.5 ± 21.2 | 10.6 ± 13.4 | 6.5 ± 9.3 | 17.1 ± 20.6 | 10.6 ± 13.9 | 7.7 ± 10.7 |
| Multivitamin supplement user, % | 40 | 41 | 45 | 43 | 41 | 42 | 37 | 40 | 48 |
| Aspirin use, % | 28 | 27 | 24 | 29 | 28 | 23 | 26 | 26 | 27 |
| Total energy intake, kcal/d | 1961 ± 632 | 2033 ± 623 | 1956 ± 625 | 1974 ± 645 | 2030 ± 613 | 1933 ± 608 | 1930 ± 639 | 2055 ± 627 | 1905 ± 568 |
| Glycemic load | 90 ± 14 | 124 ± 8 | 159 ± 18 | 109 ± 29 | 123 ± 21 | 142 ± 23 | 107 ± 27 | 124 ± 21 | 142 ± 24 |
| Glycemic index | 51 ± 4 | 53 ± 3 | 54 ± 4 | 51 ± 4 | 53 ± 3 | 56 ± 3 | 51 ± 4 | 54 ± 3 | 54 ± 3 |
| Polyunsaturated:saturated fat | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.3 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.2 |
| Saturated fat, % of total energy | 13 ± 2.9 | 11.3 ± 2 | 8.4 ± 2.1 | 11.7 ± 3.2 | 11.3 ± 2.4 | 9.7 ± 2.6 | 12.2 ± 3 | 11.2 ± 2.4 | 9.5 ± 2.6 |
| Polyunsaturated fat, % of total energy | 6.5 ± 1.9 | 6 ± 1.4 | 5.1 ± 1.3 | 5.8 ± 1.9 | 6 ± 1.5 | 5.9 ± 1.5 | 5.9 ± 1.8 | 6 ± 1.5 | 5.8 ± 1.5 |
| NHS | |||||||||
| n | 14,983 | 14,387 | 14,772 | 14,959 | 15,001 | 14,932 | 15,452 | 16,006 | 14,669 |
| Median, g | 146 | 185 | 224 | 39 | 58 | 79 | 2 | 3.6 | 7.1 |
| Age,2 y | 50 ± 7 | 50 ± 7 | 51 ± 7 | 51 ± 7 | 50 ± 7 | 50 ± 7 | 50 ± 7 | 50 ± 7 | 52 ± 7 |
| BMI, kg/m2 | 25.2 ± 4.6 | 25.1 ± 4.6 | 24.4 ± 4.3 | 24.9 ± 4.6 | 25.0 ± 4.5 | 24.8 ± 4.6 | 25.1 ± 4.7 | 25.1 ± 4.5 | 24.4 ± 4.1 |
| Caucasian, % | 99 | 98 | 96 | 97 | 98 | 97 | 97 | 98 | 98 |
| Postmenopausal, % | 46 | 46 | 47 | 47 | 46 | 45 | 47 | 45 | 47 |
| PMH use, past and current, % | 49 | 48 | 48 | 49 | 48 | 48 | 46 | 47 | 51 |
| Family history of CHD, % | 20 | 19 | 19 | 20 | 19 | 19 | 20 | 19 | 19 |
| Hypertension, % | 16 | 14 | 15 | 16 | 14 | 14 | 17 | 14 | 12 |
| Hypercholesterolemia, % | 5 | 6 | 7 | 6 | 6 | 7 | 5 | 5 | 7 |
| Physical activity, MET-h/wk | 13 ± 19 | 14 ± 19 | 16 ± 25 | 16 ± 23 | 14 ± 21 | 13 ± 21 | 14 ± 22 | 14 ± 20 | 16 ± 23 |
| Current smoker, % | 34 | 22 | 20 | 30 | 23 | 20 | 35 | 24 | 15 |
| Alcohol intake, g/d | 14.9 ± 17.5 | 5.5 ± 8.2 | 2.6 ± 4.9 | 11.5 ± 16.6 | 6.3 ± 9.5 | 3.8 ± 6.4 | 11.5 ± 16.4 | 6.2 ± 9.5 | 4.5 ± 7.5 |
| Multivitamin supplement user, % | 64 | 63 | 59 | 59 | 63 | 65 | 65 | 64 | 57 |
| Aspirin use, % | 72 | 72 | 68 | 70 | 73 | 69 | 70 | 72 | 71 |
| Total energy intake, kcal/d | 1698 ± 526 | 1795 ± 530 | 1686 ± 533 | 1719 ± 560 | 1789 ± 524 | 1651 ± 496 | 1703 ± 552 | 1775 ± 537 | 1690 ± 482 |
| Glycemic load | 74 ± 12 | 99 ± 7 | 125 ± 14 | 85 ± 23 | 99 ± 15 | 114 ± 16 | 88 ± 23 | 100 ± 16 | 110 ± 17 |
| Glycemic index | 52 ± 4 | 53 ± 3 | 55 ± 4 | 50 ± 5 | 54 ± 3 | 56 ± 3 | 52 ± 5 | 54 ± 3 | 54 ± 3 |
| Polyunsaturated:saturated fat | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.2 |
| Saturated fat, % of total energy | 14.4 ± 2.8 | 12.7 ± 1.9 | 10.1 ± 1.9 | 13.0 ± 3.2 | 12.6 ± 2.3 | 11.5 ± 2.3 | 13.2 ± 3.0 | 12.6 ± 2.3 | 11.3 ± 2.3 |
| Polyunsaturated fat, % of total energy | 7.3 ± 2.1 | 6.7 ± 1.5 | 5.7 ± 1.4 | 6.5 ± 2.2 | 6.7 ± 1.6 | 6.5 ± 1.6 | 6.4 ± 2.0 | 6.7 ± 1.6 | 6.5 ± 1.7 |
1Values are means ± SDs or percentages and are standardized to the age distribution of the study population. HPFS (1986), n = 42,865; NHS (1984), n = 75,020. CHD, coronary heart disease; HPFS, Health Professionals Follow-Up Study; MET-h, metabolic equivalent task-hours; NHS, Nurses' Health Study; Q, quintile; PMH, postmenopausal hormone.
2Values were not age adjusted.
Significant correlations were noted in both study populations between baseline intakes of carbohydrate, starch, total fiber, cereal fiber, starch-to–total fiber ratio, starch-to–cereal fiber ratio, glycemic index, and glycemic load, with correlation coefficients ranging from –0.69 (total fiber and carbohydrate-to–total fiber ratio) to 0.95 (carbohydrates and glycemic load) (Supplemental Table 1).
In fully adjusted models, carbohydrate intake was not associated with an increased risk of CHD among men [quintile 5 (Q5) compared with Q1: RR = 1.07; 95% CI: 0.96, 1.20; P-trend = 0.33] or women (Q5 compared with Q1: RR = 1.01; 95% CI: 0.89, 1.14; P-trend = 0.74) (Table 2). Starch intake, however, was associated with a significantly reduced risk for CHD in men (Q5 compared with Q1: RR = 0.83; 95% CI: 0.74, 0.94; P-trend = 0.004), but not women (Q5 compared with Q1: RR = 1.11; 95% CI: 0.97, 1.27; P-trend = 0.13). Pooled associations for starch intake and risk of CHD were nonsignificant (Q5 compared with Q1: RR = 0.94; 95% CI: 0.86, 1.03; P-trend = 0.11).
TABLE 2.
Relative risks of CHD by quintiles of carbohydrate, starch and total, cereal, fruit and vegetable fiber intake among 42,865 men in the HPFS and 75,020 women in the NHS1
| Quintiles of energy-adjusted intake | ||||||
|---|---|---|---|---|---|---|
| Variable | 1 | 2 | 3 | 4 | 5 | P-trend2 |
| Carbohydrate | ||||||
| HPFS | ||||||
| Median, g/d | 191 | 221 | 241 | 261 | 290 | |
| Cases/person-years | 812/171,422 | 818/186,219 | 808/191,878 | 796/189,560 | 819/182,896 | |
| Model 1 | 1.00 (Ref) | 0.95 (0.87, 1.05) | 0.91 (0.82, 1.00) | 0.89 (0.81, 0.98) | 0.93 (0.84, 1.02) | 0.06 |
| Model 2 | 1.00 (Ref) | 1.02 (0.92, 1.12) | 0.98 (0.89, 1.09) | 0.99 (0.89, 1.11) | 1.07 (0.96, 1.20) | 0.33 |
| NHS | ||||||
| Median, g/d | 159 | 182 | 196 | 210 | 230 | |
| Cases/person-years | 681/367,365 | 652/397,606 | 664/397,590 | 655/391,329 | 615/351,157 | |
| Model 1 | 1.00 (Ref) | 0.88 (0.79, 0.98) | 0.87 (0.78, 0.97) | 0.85 (0.76, 0.94) | 0.83 (0.74, 0.92) | 0.0006 |
| Model 2 | 1.00 (Ref) | 0.95 (0.85, 1.06) | 0.99 (0.88, 1.11) | 1.00 (0.89, 1.13) | 1.01 (0.89, 1.14) | 0.74 |
| Pooled, MV adjusted | 1.00 (Ref) | 0.99 (0.92, 1.06) | 0.99 (0.91, 1.06) | 1.00 (0.92, 1.08) | 1.04 (0.96, 1.14) | 0.31 |
| Starch | ||||||
| HPFS | ||||||
| Median, g/d | 50 | 66 | 76 | 86 | 102 | |
| Cases/person-years | 888/148,458 | 846/179,393 | 823/193,650 | 783/200,715 | 713/199,759 | |
| Model 1 | 1.00 (Ref) | 0.83 (0.75, 0.91) | 0.78 (0.70, 0.85) | 0.74 (0.67, 0.81) | 0.70 (0.63, 0.77) | <0.0001 |
| Model 2 | 1.00 (Ref) | 0.89 (0.81, 0.98) | 0.88 (0.79, 0.97) | 0.86 (0.77, 0.96) | 0.83 (0.74, 0.94) | 0.004 |
| NHS | ||||||
| Median, g/d | 46 | 56 | 63 | 70 | 81 | |
| Cases/person-years | 757/352,229 | 698/391,095 | 645/402,791 | 619/400,356 | 548/358,577 | |
| Model 1 | 1.00 (Ref) | 0.87 (0.78, 0.96) | 0.81 (0.73, 0.90) | 0.81 (0.73, 0.90) | 0.83 (0.74, 0.92) | <0.0001 |
| Model 2 | 1.00 (Ref) | 1.00 (0.90, 1.11) | 1.00 (0.89, 1.12) | 1.05 (0.93, 1.18) | 1.11 (0.97, 1.27) | 0.13 |
| Pooled, MV adjusted | 1.00 (Ref) | 0.94 (0.87, 1.01) | 0.93 (0.86, 1.00) | 0.94 (0.86, 1.02) | 0.94 (0.86, 1.03) | 0.11 |
| Total Fiber | ||||||
| HPFS | ||||||
| Median, g/d | 14.4 | 18 | 20.9 | 24.2 | 30 | |
| Cases/person-years | 852/180,528 | 790/188,781 | 788/190,448 | 851/186,031 | 772/176,187 | |
| Model 1 | 1.00 (Ref) | 0.84 (0.76, 0.93) | 0.79 (0.71, 0.87) | 0.82 (0.74, 0.90) | 0.74 (0.67, 0.82) | <0.0001 |
| Model 2 | 1.00 (Ref) | 0.96 (0.87, 1.06) | 0.95 (0.86, 1.06) | 1.05 (0.94, 1.17) | 1.00 (0.88, 1.13) | 0.59 |
| NHS | ||||||
| Median, g/d | 12.3 | 15.0 | 17.2 | 19.6 | 23.6 | |
| Cases/person-years | 745/370,695 | 624/394,828 | 648/393,596 | 655/385,190 | 595/360,739 | |
| Model 1 | 1.00 (Ref) | 0.73 (0.66, 0.81) | 0.72 (0.64, 0.80) | 0.68 (0.61, 0.75) | 0.60 (0.54, 0.67) | <0.0001 |
| Model 2 | 1.00 (Ref) | 0.87 (0.78, 0.97) | 0.93 (0.83, 1.04) | 0.94 (0.83, 1.05) | 0.87 (0.76, 0.99) | 0.13 |
| Pooled, MV adjusted | 1.00 (Ref) | 0.92 (0.85, 0.99) | 0.94 (0.87, 1.02) | 0.99 (0.92, 1.08) | 0.94 (0.85, 1.03) | 0.72 |
| Cereal fiber | ||||||
| HPFS | ||||||
| Median, g/d | 3.0 | 4.4 | 5.8 | 7.4 | 10.3 | |
| Cases/person-years | 894/166,779 | 877/181,703 | 799/195,223 | 744/193,356 | 739/184,914 | |
| Model 1 | 1.00 (Ref) | 0.92 (0.84, 1.01) | 0.77 (0.70, 0.85) | 0.69 (0.63, 0.76) | 0.70 (0.63, 0.77) | <0.0001 |
| Model 2 | 1.00 (Ref) | 1.00 (0.91, 1.10) | 0.89 (0.80, 0.99) | 0.83 (0.75, 0.93) | 0.84 (0.75, 0.94) | 0.0003 |
| NHS | ||||||
| Median, g/d | 2.5 | 3.6 | 4.4 | 5.5 | 7.6 | |
| Cases/person-years | 777/353,171 | 735/388,528 | 617/401,767 | 590/395,086 | 548/366,497 | |
| Model 1 | 1.00 (Ref) | 0.87 (0.78, 0.96) | 0.70 (0.63, 0.77) | 0.64 (0.57, 0.71) | 0.58 (0.52, 0.65) | <0.0001 |
| Model 2 | 1.00 (Ref) | 0.98 (0.88, 1.08) | 0.84 (0.75, 0.94) | 0.80 (0.71, 0.90) | 0.76 (0.67, 0.85) | <0.0001 |
| Pooled, MV adjusted | 1.00 (Ref) | 0.99 (0.92, 1.06) | 0.86 (0.80, 0.93) | 0.82 (0.76, 0.89) | 0.80 (0.74, 0.87) | <0.0001 |
| Fruit fiber | ||||||
| HPFS | ||||||
| Median, g/d | 1.5 | 2.9 | 4.2 | 5.7 | 8.6 | |
| Cases/person-years | 790/184,613 | 811/188,427 | 751/188,865 | 838/185,656 | 863/174,414 | |
| Model 1 | 1.00 (Ref) | 0.92 (0.83, 1.01) | 0.79 (0.72, 0.87) | 0.84 (0.76, 0.92) | 0.87 (0.79, 0.96) | 0.0156 |
| Model 2 | 1.00 (Ref) | 1.06 (0.96, 1.18) | 0.98 (0.88, 1.09) | 1.08 (0.97, 1.20) | 1.15 (1.02, 1.29) | 0.0147 |
| NHS | ||||||
| Median, g/d | 1.5 | 2.6 | 3.6 | 4.8 | 6.8 | |
| Cases/person-years | 689/380,953 | 621/400,867 | 650/392,111 | 648/380,614 | 659/350,504 | |
| Model 1 | 1.00 (Ref) | 0.77 (0.69, 0.86) | 0.74 (0.67, 0.83) | 0.70 (0.63, 0.78) | 0.69 (0.62, 0.77) | <0.0001 |
| Model 2 | 1.00 (Ref) | 0.94 (0.84, 1.05) | 1.01 (0.90, 1.13) | 1.00 (0.88, 1.12) | 0.99 (0.87, 1.13) | 0.82 |
| Pooled, MV adjusted | 1.00 (Ref) | 1.01 (0.93, 1.09) | 0.99 (0.92, 1.07) | 1.04 (0.96, 1.13) | 1.08 (0.99, 1.17) | 0.0305 |
| Vegetable fiber | ||||||
| HPFS | ||||||
| Median, g/d | 3.8 | 5.3 | 6.6 | 8.2 | 11.2 | |
| Cases/person-years | 820/175,937 | 797/188,927 | 808/192,035 | 841/186,633 | 787/178,444 | |
| Model 1 | 1.00 (Ref) | 0.90 (0.81, 0.99) | 0.88 (0.79, 0.96) | 0.92 (0.83, 1.01) | 0.87 (0.79, 0.96) | 0.0278 |
| Model 2 | 1.00 (Ref) | 0.98 (0.89, 1.08) | 0.99 (0.90, 1.10) | 1.07 (0.97, 1.19) | 1.02 (0.91, 1.13) | 0.42 |
| NHS | ||||||
| Median, g/d | 3.7 | 4.9 | 5.9 | 7.2 | 9.3 | |
| Cases/person-years | 681/362,502 | 630/389,829 | 646/399,069 | 657/389,815 | 653/363,833 | |
| Model 1 | 1.00 (Ref) | 0.83 (0.75, 0.93) | 0.82 (0.73, 0.91) | 0.82 (0.74, 0.92) | 0.84 (0.75, 0.93) | 0.009 |
| Model 2 | 1.00 (Ref) | 0.94 (0.84, 1.04) | 0.97 (0.87, 1.09) | 1.01 (0.90, 1.13) | 1.03 (0.91, 1.16) | 0.36 |
| Pooled, MV adjusted | 1.00 (Ref) | 0.96 (0.89, 1.03) | 0.98 (0.91, 1.06) | 1.04 (0.97, 1.12) | 1.02 (0.94, 1.11) | 0.24 |
1RRs and 95% CIs were calculated with the use of the Cox proportional hazards regression model. Model 1: age adjusted. Model 2: adjusted for age, BMI, family history of CHD, smoking status, alcohol intake, physical activity level, multivitamin use, aspirin use, vitamin E use, race, total energy, polyunsaturated fat–to–saturated fat ratio and trans fat (dietary variables all in quintiles). Model for starch was additionally adjusted for cereal fiber, sugar-sweetened beverages, and fruits and vegetables. Model for total fiber was additionally adjusted for glycemic load. Models for cereal, fruit, and vegetable fibers were additionally adjusted for glycemic load and the other two subtypes of fiber. CHD, coronary heart disease; HPFS, Health Professionals Follow-Up Study; NHS, Nurses' Health Study; Ref, reference.
2Test for trend based on variable containing median value for each quintile.
In age-adjusted models, there were significant inverse associations between both total and cereal fiber and incident CHD. However, after adjusting for all potential confounders in model 2, the association was nonsignificant (pooled association Q5 compared with Q1: RR = 0.94; 95% CI: 0.85, 1.03; P-trend = 0.72). Cereal fiber, however, was consistently and significantly associated with a reduced risk of CHD in both populations (pooled association Q5 compared with Q1: RR = 0.80; 95% CI: 0.74, 0.87; P-trend < 0.0001). Fruit fiber was associated with an increased risk for CHD in men (Q5 compared with Q1: RR = 1.15; 95% CI: 1.02, 1.29; P-trend = 0.0147) but not women (Q5 compared with Q1: RR = 0.99; 95% CI: 0.87, 1.13; P-trend = 0.82). However, in the 4-y lagged sensitivity analysis (Supplemental Table 2), the positive association between fruit fiber and risk of CHD among men was diminished (Q5 compared with Q1: RR = 1.11; 95% CI: 0.98, 1.26; P-trend = 0.10) and remained insignificant among women. Vegetable fiber was not significantly associated with CHD risk in either men or women (pooled association Q5 compared with Q1: RR = 1.02; 95% CI: 0.94, 1.11; P-trend 0.24). In addition, in the 4-y lagged analysis the association between cereal fiber and risk of CHD was only slightly attenuated, and remained insignificant between vegetable fiber and risk of CHD.
In age-adjusted models, the carbohydrate-to–total fiber ratio was associated with an elevated risk of CHD in both the men and the women (Table 3). However, in fully adjusted models this relation became nonsignificant, as was the pooled association (Q5 compared with Q1: RR = 1.04; 95% CI: 0.96, 1.13; P-trend = 0.46). The carbohydrate-to–cereal fiber ratio and the starch-to–cereal fiber ratio were both consistently and significantly positively associated with incident CHD in both age-adjusted and fully adjusted models (pooled association for carbohydrate-to–cereal fiber ratio Q5 compared with Q1: RR = 1.20; 95% CI: 1.11, 1.29; P-trend < 0.0001; pooled association for the starch-to–cereal fiber ratio Q5 compared with Q1: RR = 1.17; 95% CI: 1.09, 1.27; P-trend < 0.0001). The starch-to–total fiber ratio was inversely associated with risk of CHD in fully adjusted models (pooled association Q5 compared with Q1: RR = 0.88; 95% CI: 0.81, 0.96; P-trend = 0.0095). Overall similar associations were noted after additional adjustment for baseline hypertension and hypercholesterolemia (Supplemental Table 3), and after continuously updating dietary variables throughout the follow-up period (Supplemental Table 4), with the exception of total carbohydrate intake becoming associated with an increased risk of CHD (Q5 compared with Q1: RR = 1.14; 95% CI: 1.04, 1.24; P-trend = 0.0008), and the marginal inverse association between the starch-to–total fiber ratio and risk of CHD, which became nonsignificant (Q5 compared with Q1: RR = 0.94; 95% CI: 0.87, 1.03; P-trend = 0.22).
TABLE 3.
Relative risks of CHD by quintiles of the ratios of carbohydrate-to–total fiber, carbohydrate-to–cereal fiber, starch-to–total fiber and starch to cereal fiber intake among 42,865 men in the HPFS and 75,020 women in the NHS1
| Quintiles of energy-adjusted intake | ||||||
|---|---|---|---|---|---|---|
| Variable | 1 | 2 | 3 | 4 | 5 | P-trend2 |
| Carbohydrate:total fiber | ||||||
| HPFS | ||||||
| Median, g/d | 8.6 | 10.1 | 11.3 | 12.8 | 15.6 | |
| Cases/person-years | 773/167,974 | 823/183,710 | 814/188,953 | 771/192,416 | 872/188,922 | |
| Model 1 | 1.00 (Ref) | 1.04 (0.94, 1.14) | 1.05 (0.95, 1.16) | 1.05 (0.95, 1.16) | 1.31 (1.18, 1.44) | <0.0001 |
| Model 2 | 1.00 (Ref) | 1.00 (0.90, 1.11) | 0.97 (0.87, 1.07) | 0.91 (0.82, 1.02) | 1.02 (0.91, 1.13) | 0.94 |
| NHS | ||||||
| Median, g/d | 8.8 | 10.2 | 11.3 | 12.7 | 15.3 | |
| Cases/person-years | 592/358,380 | 630/384,143 | 678/391,609 | 658/393,558 | 709/377,359 | |
| Model 1 | 1.00 (Ref) | 1.07 (0.95, 1.20) | 1.20 (1.07, 1.34) | 1.23 (1.10, 1.38) | 1.49 (1.34, 1.66) | <0.0001 |
| Model 2 | 1.00 (Ref) | 1.03 (0.92, 1.16) | 1.09 (0.97, 1.23) | 1.05 (0.93, 1.18) | 1.08 (0.96, 1.22) | 0.27 |
| Pooled, MV adjusted | 1.00 (Ref) | 1.01 (0.94, 1.09) | 1.02 (0.94, 1.10) | 0.97 (0.90, 1.05) | 1.04 (0.96, 1.13) | 0.46 |
| Carbohydrate:cereal fiber | ||||||
| HPFS | ||||||
| Median, g/d | 26.3 | 35.0 | 43.2 | 54.1 | 77.7 | |
| Cases/person-years | 713/177,955 | 760/192,056 | 772/192,574 | 871/186,435 | 937/172,954 | |
| Model 1 | 1.00 (Ref) | 1.03 (0.93, 1.14) | 1.08 (0.98, 1.20) | 1.27 (1.15, 1.40) | 1.46 (1.32, 1.60) | <0.0001 |
| Model 2 | 1.00 (Ref) | 1.02 (0.92, 1.13) | 1.02 (0.92, 1.13) | 1.13 (1.02, 1.25) | 1.21 (1.09, 1.33) | <0.0001 |
| NHS | ||||||
| Median, g/d | 29.7 | 38.9 | 47.0 | 57.1 | 78.2 | |
| Cases/person-years | 520/350,789 | 579/390,030 | 676/398,420 | 714/396,020 | 778/369,790 | |
| Model 1 | 1.00 (Ref) | 1.09 (0.96, 1.22) | 1.29 (1.15, 1.45) | 1.37 (1.22, 1.53) | 1.56 (1.40, 1.75) | <0.0001 |
| Model 2 | 1.00 (Ref) | 1.03 (0.92, 1.16) | 1.17 (1.04, 1.31) | 1.17 (1.04, 1.31) | 1.19 (1.06, 1.33) | 0.0018 |
| Pooled, MV adjusted | 1.00 (Ref) | 1.02 (0.95, 1.11) | 1.08 (1.00, 1.17) | 1.15 (1.06, 1.24) | 1.20 (1.11, 1.29) | <0.0001 |
| Starch:total fiber | ||||||
| HPFS | ||||||
| Median, g/d | 2.31 | 3.07 | 3.64 | 4.26 | 5.32 | |
| Cases/person-years | 849/150,090 | 834/181,544 | 774/191,754 | 796/198,117 | 800/200,470 | |
| Model 1 | 1.00 (Ref) | 0.88 (0.80, 0.97) | 0.84 (0.76, 0.93) | 0.91 (0.83, 1.00) | 0.99 (0.90, 1.09) | 0.65 |
| Model 2 | 1.00 (Ref) | 0.89 (0.80, 0.98) | 0.82 (0.74, 0.91) | 0.84 (0.76, 0.93) | 0.83 (0.75, 0.93) | 0.0022 |
| NHS | ||||||
| Median, g/d | 2.56 | 3.23 | 3.74 | 4.31 | 5.25 | |
| Cases/person-years | 674/369,624 | 663/394,132 | 613/389,625 | 706/387,349 | 611/364,319 | |
| Model 1 | 1.00 (Ref) | 1.00 (0.90, 1.12) | 0.99 (0.89, 1.11) | 1.26 (1.13, 1.40) | 1.27 (1.13, 1.42) | <0.0001 |
| Model 2 | 1.00 (Ref) | 1.00 (0.90, 1.12) | 0.92 (0.82, 1.03) | 1.08 (0.96, 1.21) | 0.95 (0.84, 1.08) | 0.80 |
| Pooled, MV adjusted | 1.00 (Ref) | 0.93 (0.87, 1.01) | 0.86 (0.80, 0.93) | 0.94 (0.87, 1.02) | 0.88 (0.81, 0.96) | 0.0095 |
| Starch:cereal fiber | ||||||
| HPFS | ||||||
| Median, g/d | 8.0 | 11.2 | 13.8 | 16.8 | 22.0 | |
| Cases/person-years | 729/165,230 | 823/191,098 | 776/194,543 | 830/190,843 | 895/180,260 | |
| Model 1 | 1.00 (Ref) | 1.04 (0.94, 1.15) | 1.03 (0.93, 1.14) | 1.17 (1.06, 1.29) | 1.35 (1.22, 1.49) | <0.0001 |
| Model 2 | 1.00 (Ref) | 1.05 (0.95, 1.16) | 0.99 (0.89, 1.10) | 1.06 (0.95, 1.17) | 1.13 (1.02, 1.26) | 0.0244 |
| NHS | ||||||
| Median, g/d | 9.7 | 12.8 | 15.3 | 18.0 | 22.5 | |
| Cases/person-years | 576/359,986 | 585/385,587 | 643/395,006 | 685/395,135 | 778/369,334 | |
| Model 1 | 1.00 (Ref) | 1.07 (0.95, 1.20) | 1.24 (1.11, 1.39) | 1.40 (1.25, 1.57) | 1.70 (1.53, 1.90) | <0.0001 |
| Model 2 | 1.00 (Ref) | 1.01 (0.91, 1.14) | 1.10 (0.98, 1.23) | 1.14 (1.01, 1.28) | 1.23 (1.10, 1.39) | <0.0001 |
| Pooled, MV adjusted | 1.00 (Ref) | 1.04 (0.96, 1.12) | 1.04 (0.96, 1.12) | 1.09 (1.01, 1.18) | 1.17 (1.09, 1.27) | <0.0001 |
1RRs and 95% CIs were calculated with the use of the Cox proportional hazards regression model. Model 1: Age adjusted. Model 2: Adjusted for age, BMI, family history of CHD, smoking status, alcohol intake, physical activity level, multivitamin use, aspirin use, vitamin E use, race, total energy, polyunsaturated fat–to–saturated fat ratio, and trans fat. The starch:total fiber model was additionally adjusted for sugar-sweetened beverages. The starch:cereal fiber model was additionally adjusted for sugar-sweetened beverages, and fruit and vegetable fiber. CHD, coronary heart disease; HPFS, Health Professionals Follow-Up Study; NHS, Nurses' Health Study; Ref, reference.
2Test for trend based on variable containing median value for each quintile.
There was no evidence of effect modification of the associations between cereal fiber, carbohydrate-to–cereal fiber ratio, or starch-to–cereal fiber ratio and CHD by physical activity (HPFS: <22 or ≥22 MET-h/wk, NHS: <9 or ≥9 MET-h/wk), family history of myocardial infarction (yes or no), or current smoking status (yes or no) for both men and women (all P-interaction ≥ 0.05). However, there was significant effect modification of the associations between cereal fiber, carbohydrate-to–cereal fiber, and starch to cereal fiber and risk of CHD by age (<60, ≥60 y) among women. In an a priori defined stratified analysis, the associations were stronger among women <60 y old, compared with those ≥60 y old. When comparing extreme quintiles of intake, women <60 y old and at the highest quintile of cereal fiber, carbohydrate-to–cereal fiber, and starch to cereal fiber intake had a 45% lower risk, and a 68% and 76% higher risk of developing CHD, respectively, than did those at the lowest quintile of intake, whereas women aged ≥60 y had a 23% lower risk, and a 12% and 22% higher risk of developing CHD, respectively, than did those at the lowest quintile of intake (all P-interaction ≤ 0.0004) (Table 4). In addition, cereal fiber and the 2 ratios were significantly associated with CHD among both age groups (<60 and ≥60 y) in men (all P-trend ≤ 0.0125).
TABLE 4.
Relative risks of CHD by quintiles of cereal fiber, carbohydrate-to–cereal fiber, and starch to cereal fiber intake, stratified by age, physical activity, and BMI among 42,865 men in the HPFS and 75,020 women in the NHS1
| Quintiles of energy-adjusted intake | |||||||
|---|---|---|---|---|---|---|---|
| Variable | 1 | 2 | 3 | 4 | 5 | P-trend2 | P-interaction |
| Cereal fiber | |||||||
| HPFS | |||||||
| Age <60 y | 1.00 (Ref) | 0.89 (0.72, 1.10) | 0.85 (0.68, 1.06) | 0.67 (0.52, 0.86) | 0.65 (0.49, 0.85) | 0.0004 | 0.0317 |
| Age ≥60 y | 1.00 (Ref) | 1.02 (0.91, 1.13) | 0.88 (0.78, 0.98) | 0.85 (0.75, 0.96) | 0.85 (0.75, 0.96) | 0.0024 | |
| Physical activity <22 MET-h/wk | 1.00 (Ref) | 1.02 (0.90, 1.15) | 0.86 (0.75, 0.98) | 0.82 (0.71, 0.94) | 0.87 (0.75, 1.00) | 0.0123 | 0.37 |
| Physical activity ≥22 MET-h/wk | 1.00 (Ref) | 0.96 (0.81, 1.12) | 0.92 (0.78, 1.08) | 0.84 (0.71, 1.00) | 0.80 (0.67, 0.97) | 0.0100 | |
| BMI <25 | 1.00 (Ref) | 1.00 (0.85, 1.18) | 0.91 (0.77, 1.08) | 0.79 (0.66, 0.94) | 0.77 (0.64, 0.92) | 0.0006 | 0.15 |
| BMI ≥25 | 1.00 (Ref) | 1.00 (0.89, 1.12) | 0.87 (0.76, 0.99) | 0.86 (0.75, 0.98) | 0.90 (0.78, 1.04) | 0.07 | |
| NHS | |||||||
| Age <60 y | 1.00 (Ref) | 0.98 (0.79, 1.20) | 0.71 (0.56, 0.90) | 0.73 (0.56, 0.94) | 0.55 (0.40, 0.74) | <0.0001 | 0.0004 |
| Age ≥60 y | 1.00 (Ref) | 0.97 (0.86, 1.10) | 0.86 (0.76, 0.98) | 0.81 (0.71, 0.92) | 0.77 (0.67, 0.8) | <0.0001 | |
| Physical activity <10 MET-h/wk | 1.00 (Ref) | 0.98 (0.86, 1.12) | 0.83 (0.71, 0.96) | 0.78 (0.67, 0.91) | 0.80 (0.68, 0.94) | 0.0008 | 0.38 |
| Physical activity ≥10 MET-h/wk | 1.00 (Ref) | 0.89 (0.75, 1.07) | 0.82 (0.68, 0.98) | 0.78 (0.64, 0.94) | 0.69 (0.56, 0.84) | 0.0002 | |
| BMI <25 | 1.00 (Ref) | 0.92 (0.78, 1.08) | 0.83 (0.69, 0.98) | 0.76 (0.63, 0.90) | 0.68 (0.57, 0.82) | <0.0001 | 0.20 |
| BMI ≥25 | 1.00 (Ref) | 1.02 (0.89, 1.17) | 0.84 (0.73, 0.98) | 0.84 (0.72, 0.98) | 0.82 (0.70, 0.97) | 0.0032 | |
| Carbohydrate to cereal fiber | |||||||
| HPFS | |||||||
| Age <60 y | 1.00 (Ref) | 1.08 (0.83, 1.40) | 1.12 (0.86, 1.44) | 1.45 (1.13, 1.85) | 1.39 (1.08, 1.79) | 0.0015 | 0.21 |
| Age ≥60 y | 1.00 (Ref) | 1.01 (0.91, 1.13) | 1.02 (0.91, 1.14) | 1.08 (0.97, 1.21) | 1.20 (1.07, 1.34) | 0.0002 | |
| Physical activity <22 MET-h/wk | 1.00 (Ref) | 1.00 (0.87, 1.14) | 1.00 (0.87, 1.15) | 1.13 (0.99, 1.28) | 1.21 (1.07, 1.38) | 0.0002 | 0.99 |
| Physical activity ≥22 MET-h/wk | 1.00 (Ref) | 1.05 (0.89, 1.22) | 1.04 (0.89, 1.22) | 1.12 (0.95, 1.31) | 1.20 (1.02, 1.42) | 0.0155 | |
| BMI <25 | 1.00 (Ref) | 1.03 (0.88, 1.21) | 1.16 (0.98, 1.36) | 1.24 (1.05, 1.46) | 1.33 (1.13, 1.56) | 0.0001 | 0.08 |
| BMI ≥25 | 1.00 (Ref) | 1.00 (0.87, 1.14) | 0.93 (0.81, 1.06) | 1.05 (0.93, 1.20) | 1.12 (0.98, 1.27) | 0.0185 | |
| NHS | |||||||
| Age <60 y | 1.00 (Ref) | 1.20 (0.88, 1.64) | 1.41 (1.05, 1.89) | 1.37 (1.02, 1.84) | 1.68 (1.26, 2.22) | 0.0002 | 0.0003 |
| Age ≥60 y | 1.00 (Ref) | 1.02 (0.90, 1.16) | 1.16 (1.02, 1.32) | 1.16 (1.03, 1.32) | 1.12 (0.99, 1.28) | 0.0471 | |
| Physical activity <10 MET-h/wk | 1.00 (Ref) | 1.06 (0.90, 1.25) | 1.17 (1.00, 1.37) | 1.19 (1.02, 1.39) | 1.16 (1.00, 1.36) | 0.06 | 0.70 |
| Physical activity ≥10 MET-h/wk | 1.00 (Ref) | 0.99 (0.82, 1.18) | 1.13 (0.94, 1.35) | 1.08 (0.90, 1.30) | 1.22 (1.01, 1.46) | 0.019 | |
| BMI <25 | 1.00 (Ref) | 1.07 (0.89, 1.28) | 1.16 (0.97, 1.39) | 1.16 (0.97, 1.39) | 1.31 (1.10, 1.55) | 0.0014 | 0.0422 |
| BMI ≥25 | 1.00 (Ref) | 1.00 (0.86, 1.17) | 1.16 (1.00, 1.36) | 1.15 (0.98, 1.34) | 1.07 (0.92, 1.25) | 0.31 | |
| Starch to cereal fiber | |||||||
| HPFS | |||||||
| Age <60 y | 1.00 (Ref) | 1.20 (0.90, 1.58) | 1.21 (0.92, 1.59) | 1.41 (1.08, 1.84) | 1.39 (1.06, 1.82) | 0.0125 | 0.054 |
| Age ≥60y | 1.00 (Ref) | 1.09 (0.98, 1.22) | 1.01 (0.90, 1.13) | 1.07 (0.96, 1.20) | 1.19 (1.06, 1.34) | 0.0062 | |
| Physical activity <22 MET-h/wk | 1.00 (Ref) | 1.06 (0.93, 1.21) | 0.94 (0.82, 1.08) | 1.06 (0.93, 1.21) | 1.14 (1.00, 1.30) | 0.0438 | 0.69 |
| Physical activity ≥22 MET-h/wk | 1.00 (Ref) | 1.05 (0.90, 1.23) | 1.06 (0.90, 1.23) | 1.05 (0.89, 1.23) | 1.12 (0.94, 1.32) | 0.27 | |
| BMI <25 | 1.00 (Ref) | 1.15 (0.99, 1.34) | 1.13 (0.96, 1.33) | 1.08 (0.91, 1.28) | 1.16 (0.98, 1.37) | 0.21 | 0.62 |
| BMI ≥25 | 1.00 (Ref) | 0.99 (0.87, 1.13) | 0.91 (0.79, 1.03) | 1.03 (0.90, 1.17) | 1.10 (0.97, 1.26) | 0.06 | |
| NHS | |||||||
| Age <60 y | 1.00 (Ref) | 1.15 (0.83, 1.59) | 1.34 (0.98, 1.82) | 1.37 (1.00, 1.86) | 1.76 (1.31, 2.38) | <0.0001 | <0.0001 |
| Age ≥60 y | 1.00 (Ref) | 1.03 (0.91, 1.17) | 1.12 (0.99, 1.27) | 1.20 (1.05, 1.36) | 1.22 (1.07, 1.38) | 0.0007 | |
| Physical activity <10 MET-h/wk | 1.00 (Ref) | 1.04 (0.89, 1.22) | 1.07 (0.91, 1.26) | 1.20 (1.03, 1.41) | 1.19 (1.01, 1.39) | 0.0107 | 0.45 |
| Physical activity ≥10 MET-h/wk | 1.00 (Ref) | 0.94 (0.79, 1.13) | 1.10 (0.92, 1.31) | 1.03 (0.85, 1.24) | 1.34 (1.11, 1.62) | 0.0014 | |
| BMI <25 | 1.00 (Ref) | 1.01 (0.84, 1.20) | 1.06 (0.88, 1.26) | 1.29 (1.08, 1.54) | 1.37 (1.15, 1.63) | <0.0001 | 0.0121 |
| BMI ≥25 | 1.00 (Ref) | 1.01 (0.86, 1.18) | 1.11 (0.95, 1.29) | 1.04 (0.89, 1.21) | 1.13 (0.97, 1.32) | 0.12 | |
1RRs and 95% CIs were calculated with the use of the Cox proportional hazards regression model. The associations were adjusted for age, BMI (in kg/m2), family history of CHD, menopausal status and postmenopausal hormone use (among NHS participants), smoking status, alcohol intake, physical activity level, multivitamin use, aspirin use, vitamin E use, race, total energy, polyunsaturated fat–to–saturated fat ratio, and trans fat intake (in quintiles). The model for cereal fiber was additionally adjusted for glycemic load and fruit and vegetable fiber. The starch:cereal fiber model was additionally adjusted for sugar-sweetened beverages, and fruit and vegetable fiber. CHD, coronary heart disease; HPFS, Heath Professionals Follow-Up Study; MET-h, metabolic equivalent task-hours; NHS, Nurses' Health Study; Ref, reference.
2Test for trend based on variable containing median value for each quintile.
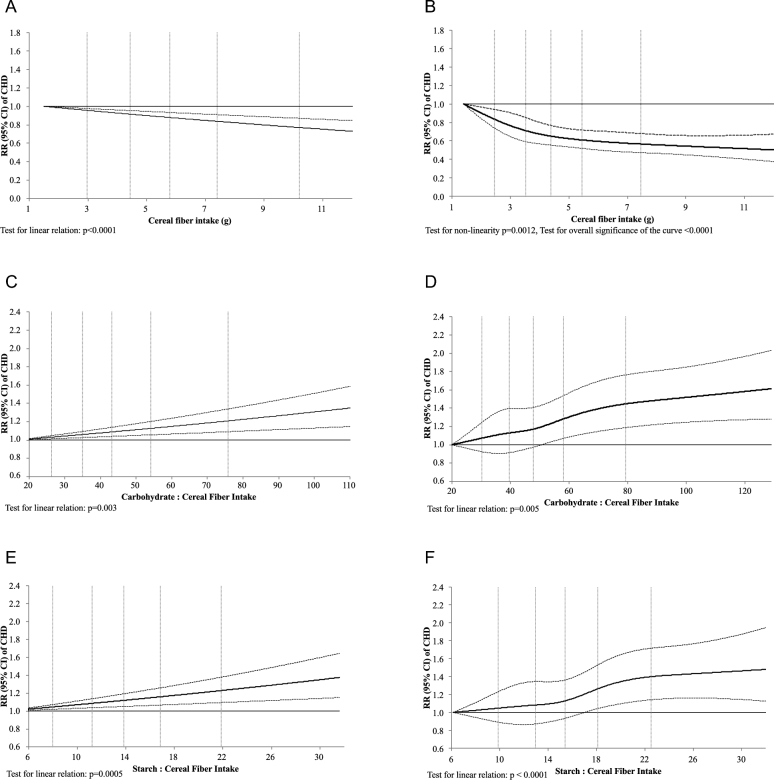
The associations between cereal fiber, carbohydrate-to–cereal fiber ratio, and starch-to–cereal fiber ratio and the risk of incident CHD were also examined in both the HPFS and NHS using spline regression (Figure 1A–F). All associations were linear in both men and women (all P-linear relation ≤ 0.005), except for the association between cereal fiber and incident CHD among women where there was a plateau around 7 g of cereal fiber/d (P-nonlinear relation = 0.0012, and P-overall significance of the curve < 0.0001).
FIGURE 1.
Relative risks (and 95% CI) of CHD by cereal fiber (A, B), carbohydrate to cereal fiber (C, D) and starch-to–cereal fiber (E, F) intake, using restricted cubic spline regression in 42,865 men in the HPFS and 75,020 women in the NHS, respectively. The associations were adjusted for age, BMI, family history of CHD, menopausal status and postmenopausal hormone use (among NHS participants), smoking status, alcohol intake, physical activity level, multivitamin use, aspirin use, vitamin E use, race, total energy, polyunsaturated fat–to–saturated fat ratio, and trans fat intake (in quintiles). The model for cereal fiber was additionally adjusted for glycemic load and fruit and vegetable fiber. The starch:cereal fiber model was additionally adjusted for sugar-sweetened beverages, and fruit and vegetable fiber. CHD, coronary heart disease; HPFS, Health Professionals Follow-Up Study; NHS, Nurses' Health Study.
DISCUSSION
A growing body of literature relates various measures of carbohydrate quality to important health outcomes, including diabetes, CHD, and mortality. In our prospective cohort study, we found the carbohydrate-to–cereal fiber ratio and the starch-to–cereal fiber ratio, but not the carbohydrate-to-fiber ratio, to be important measures of carbohydrate quality that were associated with a 20% and 17% increased risk for incident CHD, respectively. These findings were largely driven by the strong and consistent inverse association between cereal fiber intake and CHD outcomes.
We did not find evidence that total daily carbohydrate or starch intake was associated with an increased risk for incident CHD. Data from the Framingham Offspring Study, however, did show evidence of an association between the highest quintile of daily carbohydrate consumption and low HDL and high triglycerides, known risk factors for CHD (29). Similarly, data from the Shanghai Men's and Women's Health Study showed that the highest quartile of daily carbohydrate intake was associated with a nearly 3-fold increase in risk for incident CHD (12). Conversely, and consistent with our findings, the Singapore Chinese Health Study showed no evidence of increased risk for CHD according to total daily carbohydrate and starch intake (13). The Prospective Urban Rural Epidemiology (PURE) study, a prospective cohort study with 135,335 participants from 18 countries, has found that participants with the highest carbohydrate intake (77% of daily energy intake) did not have a higher risk of cardiovascular disease than did those at the lowest quintile of intake (46% of daily energy intake) (30). However, this study and the PURE study cannot be compared because most participants of the PURE study were in low-income countries where the diet was heavily composed of carbohydrates from refined sources such as white rice—a characteristic of poverty diets, which are also high in sodium and low in animal fat and vegetable oils, and therefore the individual effects of diet and poverty cannot be separated. The inconsistent findings between carbohydrate intake and CHD risk may reflect the dietary fiber and whole-grain content of the diet in the aforementioned study populations.
In the NHS, we found that total daily fiber and cereal fiber were associated with an 18% and 33% reduced risk for CHD, respectively. In the HPFS, only cereal fiber was significantly and inversely associated with CHD risk. These findings are consistent with a recent meta-analysis of 17 cohort studies involving >900,000 participants where the highest tertile of dietary fiber intake was associated with a 16% reduced risk of all-cause mortality (31). Fruit and vegetable fiber, however, were not associated with a significantly reduced risk for CHD in our combined populations. These findings are similar to prior published data from the HPFS, where fruit and vegetable fiber were not associated with a reduced risk of CHD (32). The lack of association between fruit and vegetable fiber and risk of CHD could be because fiber in fruits and vegetables works synergistically with the other components of the whole foods, which could explain the limited benefit of isolated or synthetic fiber compared with whole foods such as fruits and vegetables.
In addition to the inverse association of daily fiber intake and CHD, and in particular cereal fiber, we found the carbohydrate-to–cereal fiber ratio and the starch-to–cereal fiber ratio to be important measures of carbohydrate quality as it relates to CHD. These ratios likely reflect the extent to which fiber and whole grains are present in the diet. This is consistent with a large body of evidence linking whole-grain consumption with important health outcomes (33). Recently, the Scandinavian HELGA cohort observed a 32% reduction in all-cause mortality for women and a 25% reduction in all-cause mortality for men in the highest compared with the lowest quartile of whole-grain product consumption (34). Similar associations between whole-grain intake and all-cause mortality were noted in prior publications involving the HPFS (35), and with CHD events in the NHS (36). A recent meta-analysis of 18 studies involving >14,000 patients also found whole-grain consumption to be associated with a 21% reduced risk for CHD (37). Much of the benefits from some whole grains could be completely attributable to the cereal content, but more detailed feeding studies with exact measures of intake may be necessary to tease out the factors most responsible for the beneficial effect of cereal whole grains.
Other measures of carbohydrate quality also have strong associations with CHD risk. Prior data from the NHS suggest that diets with a high glycemic load are associated with a nearly 2-fold increased risk for incident CHD (14). Similarly, in a meta-analysis of 37 prospective cohort studies, diets with a high glycemic index and glycemic load were both associated with an increased risk for a number of chronic diseases including diabetes and CHD (38). Taken together, these findings suggest a robust relation between measures of carbohydrate quality and CHD.
A number of potential mechanisms explain how carbohydrate quality measures, such as the carbohydrate-to–cereal fiber ratio and the starch-to–cereal fiber ratio, are associated with CHD risk. First, low ratios are consistent with a diet rich in fiber and whole grains. Such diets have been shown to improve postprandial glucose and insulin responses, enhance satiety, and reduce overall energy intake (39, 40). In related work, data from the Multi-Ethnic Study of Atherosclerosis (MESA) study showed that diets rich in whole grains were inversely associated with obesity, insulin resistance, inflammatory markers, elevated fasting glucose, and incident diabetes (41). Conversely, when diets are lacking adequate fiber and whole grains, there is an increased risk for diabetes. Recent work involving >70,000 participants in the NHS found that diets high in refined grains (defined by high carbohydrate-to–cereal fiber and starch to cereal fiber ratios) were associated with a 28% and 39% increased risk of incident type 2 diabetes mellitus, respectively (18). Cardiometabolic risk related to a diet high in refined grains has also been associated with other potent risk factors for CHD, including hypertension, dyslipidemia, and impaired fibrinolysis (42–44).
Our study has a number of strengths including the large sample size with a large number of CHD events, long follow-up period of ≤28 y, and a minimal number of participants with missing exposure data. Furthermore, in order to best represent long-term dietary patterns, cumulative averages from all available FFQs were used as the exposure variables. Finally, to reduce the risk of bias due to the presence of newly diagnosed chronic disease states, dietary records were not updated during the follow-up period after such a disease state was diagnosed. Our study also has a few important limitations. First, study participants were predominantly Caucasian health professionals, which may limit the generalizability of our findings to other more ethnically and socioeconomically diverse populations. Second, exposure variables were measured using an FFQ. Although previously validated (20), FFQs are subject to misclassification. Finally, as is the case with all prospective cohort studies, there is the chance for bias due to residual and/or unmeasured confounding factors.
The results of our study confirm the consistent association between dietary fiber, especially cereal fiber and risk for CHD. Furthermore, although the positive association between the carbohydrate-to–cereal fiber ratio and the starch-to–cereal fiber ratio and risk of CHD was mainly driven by cereal fiber, the ratios were predictive of risk of type 2 diabetes in a previous study and warrant further research in relation to health endpoints in diverse populations. Given these associations, future work should focus on how these global measures of carbohydrate quality are related to the primary and secondary prevention of CHD.
Supplementary Material
Acknowledgements
We thank all the participants in the Nurses’ Health Study for their tremendous contributions.
The authors’ responsibilities were as follows—HBA and RC: conceived of the project, wrote the paper, and had primary responsibility for all parts of the manuscript; HBA and SNA: performed the statistical analysis; and all authors: reviewed and interpreted the data, and read and approved the final manuscript. The authors had no conflicts of interest to declare related to the study.
Notes
Supported by NIH grants UM1 CA186107, UM1 CA167552, HL34594, HL35464, and DK58845, The Kuwait Program at the Harvard Kennedy School, and a postdoctoral fellowship, 01/2016, awarded to HBA by the Kuwait Foundation for the Advancement of Sciences.
Supplemental Tables 1–4 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used:
- CHD
coronary heart disease
- FFQ
food-frequency questionnaire
- HPFS
Health Professionals Follow-Up Study
- MET
metabolic equivalent
- NHS
Nurses’ Health Study.
REFERENCES
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres J-P, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics 2015 update: a report from the American Heart Association. Circulation 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd-Jones DM. Cardiovascular health behavior and health factor changes (1988-2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation 2012;125:2595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007–2012. JAMA Intern Med 2015;175:1412–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Long-Term Trends in Diagnosed Diabetes CDC's Division of Diabetes Translation. National Diabetes Surveillance System. 2011. http://www.cdc.gov/diabetes/statistics [Google Scholar]
- 5. Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999–2006. Diabetes Care 2011;34:216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000;343:16–22. [DOI] [PubMed] [Google Scholar]
- 7. Akesson A, Larsson SC, Discacciati A, Wolk A. Low-risk diet and lifestyle habits in the primary prevention of myocardial infarction in men: a population-based prospective cohort study. J Am Coll Cardiol 2014;64:1299–306. [DOI] [PubMed] [Google Scholar]
- 8. US Department of Agriculture and the US Department of Health and Human Services Nutrition and your health. Dietary guidelines for Americans. 1980. [Google Scholar]
- 9. US Department of Agriculture and the US Department of Health and Human Services Dietary Guidelines for Americans. 7th ed, Washington (DC): US Government Printing Office; 2010. [Google Scholar]
- 10. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr 2010;91:535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y, Hruby A, Bernstein AM, Ley SH, Wang DD, Chiuve SE, Sampson L, Rexrode KM, Rimm EB, Willett WC, et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease. J Am Coll Cardiol 2015;66:1538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu D, Shu XO, Li H, Xiang YB, Yang G, Gao YT, Zheng W, Zhang X. Dietary carbohydrates, refined grains, glycemic load, and risk of coronary heart disease in Chinese adults. Am J Epidemiol 2013;178:1542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rebello SA, Koh H, Chen C, Naidoo N, Odegaard AO, Koh WP, Butler LM, Yuan JM, van Dam RM. Amount, type, and sources of carbohydrates in relation to ischemic heart disease mortality in a Chinese population: a prospective cohort study. Am J Clin Nutr 2014;100:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, Hennekens CH, Manson JE. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr 2000;71:1455–61. [DOI] [PubMed] [Google Scholar]
- 15. Yang Q, Zhang Z, Gregg EW, Flanders W, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among us adults. JAMA Intern Med 2014;174:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. on behalf of the American Heart Association Strategic Planning Task F, Statistics C Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 17. Mozaffarian RS, Lee RM, Kennedy MA, Ludwig DS, Mozaffarian D, Gortmaker SL. Identifying whole grain foods: a comparison of different approaches for selecting more healthful whole grain products. Public Health Nutr 2013;16:2255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. AlEssa H, Bhupathiraju S, Malik V, Wedick N, Campos H, Rosner B, Willett W, Hu FB. Carbohydrate quality and quantity and risk of type 2 diabetes in US women. Am J Clin Nutr 2015;102:1543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–40. [DOI] [PubMed] [Google Scholar]
- 20. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 21. Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 22. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26; discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 23. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 24. Willett WC. Nutritional epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 25. Nutrient Data Laboratory (US), Consumer and Food Economics Institute (US), USDA nutrient database for standard reference Riverdale (MD): USDA Nutrient Data Laboratory, Agricultural Research Service; 1999:CD-ROMs. [Google Scholar]
- 26. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:S1220–8; discussion S1229–31. [DOI] [PubMed] [Google Scholar]
- 27. Rose GA, Blackburn H. Cardiovascular survey methods. World Health Organ Monogr Ser 1982;58:1–188. [PubMed] [Google Scholar]
- 28. Jr. FEH Regression modeling strategies: applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 29. McKeown NM, Meigs JB, Liu S, Rogers G, Yoshida M, Saltzman E, Jacques PF. Dietary carbohydrates and cardiovascular disease risk factors in the Framingham offspring cohort. J Am Coll Nutr 2009;28:150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, Iqbal R, Kumar R, Wentzel-Viljoen E, Rosengren A, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017. doi: 10.1016/S0140-6736(17)32252-3. [DOI] [PubMed] [Google Scholar]
- 31. Yang Y, Zhao L-G, Wu Q-J, Ma X, Xiang Y-B. Association between dietary fiber and lower risk of all-cause mortality: a meta-analysis of cohort studies. Am J Epidemiol 2015;181:83–91. [DOI] [PubMed] [Google Scholar]
- 32. Rimm EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer MJ, Willett WC. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA 1996;275:447–51. [DOI] [PubMed] [Google Scholar]
- 33. Zong G, Gao A, Hu FB, Sun QS. Whole grain intake and mortality from all causes, cardiovascular disease, and cancer: a meta-analysis of prospective cohort studies. Circulation 2016;133:2370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnsen NF, Frederiksen K, Christensen J, Skeie G, Lund E, Landberg R, Johansson I, Nilsson LM, Halkjaer J, Olsen A, et al. Whole-grain products and whole-grain types are associated with lower all-cause and cause-specific mortality in the Scandinavian HELGA cohort. Br J Nutr 2015:1–16. [DOI] [PubMed] [Google Scholar]
- 35. Wu H, Flint AJ, Qi Q, van Dam RM, Sampson LA, Rimm EB, Holmes MD, Willett WC, Hu FB, Sun Q. Association between dietary whole grain intake and risk of mortality: two large prospective studies in us men and women. JAMA Intern Med 2015;175:373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu S, Stampfer MJ, Hu FB, Giovannucci E, Rimm E, Manson JE, Hennekens CH, Willett WC. Whole-grain consumption and risk of coronary heart disease: results from the Nurses' Health Study. Am J Clin Nutr 1999;70:412–9. [DOI] [PubMed] [Google Scholar]
- 37. Tang G, Wang D, Long J, Yang F, Si L. Meta-analysis of the association between whole grain intake and coronary heart disease risk. Am J Cardiol 2015;115:625–9. [DOI] [PubMed] [Google Scholar]
- 38. Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk - a meta-analysis of observational studies. Am J Clin Nutr 2008;87:627–37. [DOI] [PubMed] [Google Scholar]
- 39. Jenkins DJ, Wolever TM, Leeds AR, Gassull MA, Haisman P, Dilawari J, Goff DV, Metz GL, Alberti KG. Dietary fibres, fibre analogues, and glucose tolerance: importance of viscosity. BMJ 1978;1:1392–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev 2001;59:129–39. [DOI] [PubMed] [Google Scholar]
- 41. Lutsey PL, Jacobs DR Jr., Kori S, Mayer-Davis E, Shea S, Steffen LM, Szklo M, Tracy R. Whole grain intake and its cross-sectional association with obesity, insulin resistance, inflammation, diabetes and subclinical CVD: the MESA Study. Br J Nutr 2007;98:397–405. [DOI] [PubMed] [Google Scholar]
- 42. Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S. Insulin resistance in essential hypertension. N Engl J Med 1987;317:350–7. [DOI] [PubMed] [Google Scholar]
- 43. Laws A, Reaven GM. Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, triglyceride and insulin concentrations. J Intern Med 1992;231:25–30. [DOI] [PubMed] [Google Scholar]
- 44. Juhan-Vague I, Alessi MC, Joly P, Thirion X, Vague P, Declerck PJ, Serradimigni A, Collen D. Plasma plasminogen activator inhibitor-1 in angina pectoris. Influence of plasma insulin and acute-phase response. Arteriosclerosis 1989;9:362–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.