Abstract
Study Objectives
The present study investigated the function of Hypocretin (Hcrt or Orexin/OX) receptor antagonists in sleep modulation and memory function with optical methods in transgenic mice.
Methods
We used Hcrt-IRES-Cre knock-in mice and AAV vectors expressing channelrhodopsin-2 (ChR2) to render Hcrt neurons sensitive to blue light stimulation. We optogenetically stimulated Hcrt neurons and measured latencies to wakefulness in the presence or absence of OX1/2R antagonists and Zolpidem. We also examined endogenous Hcrt neuronal activity with fiber photometry. Changes in memory after optogenetic sleep disruption were evaluated by the novel object recognition test (NOR) and compared for groups treated with vehicle, OX1/2R antagonists, or Zolpidem. We also analyzed electroencephalogram (EEG) power spectra of wakefulness, rapid eye movement (REM) sleep, and non-REM (NREM) sleep following the injections of vehicle, OX1/2R antagonists, and Zolpidem in young adult mice.
Results
Acute optogenetic stimulation of Hcrt neurons at different frequencies resulted in wakefulness. Treatment with dual OX1/2R antagonists (DORAs) DORA12 and MK6096, as well as selective OX2R antagonist MK1064 and Zolpidem, but not selective OX1R antagonist 1SORA1, significantly reduced the bout length of optogenetic stimulation-evoked wakefulness episode. Fiber photometry recordings of GCaMP6f signals showed that Hcrt neurons are active during wakefulness, even in the presence of OXR antagonists. Treatment with dual OX1/2R antagonists improved memory function despite optogenetic sleep fragmentation caused impaired memory function in a NOR test.
Conclusions
Our results show DORAs and selective OX2R antagonists stabilize sleep and improve sleep-dependent cognitive processes even when challenged by optogenetic stimulation mimicking highly arousing stimuli.
Keywords: EEG spectral analysis, orexin/hypocretin receptor antagonist, optogenetics, fiber photometry, sleep pattern, memory
Statement of Significance
Insomnia heavily impairs the daily functions of the brain, including cognition, learning, and memory. Our study shows dual antagonism of OX1/2Rs, and selective antagonism of OX2R, but not selective blockade of OX1R, increased the total amount of non-rapid eye movement (NREM) sleep. Importantly, dual OX1/2R and selective OX2R blockade reduced duration of wakefulness evoked by optogenetic stimulation of Hcrt neurons without affecting latencies to wakefulness. Furthermore, measurement of Ca2+ transients showed that Hcrt neurons are active during wakefulness even in the presence of DORAs or OX2R antagonist. Administration of DORAs or OX2R antagonist improved impairments in memory caused by optogenetically-induced sleep disruption. Thus, the hypnotic effect of DORAs is likely mediated by OX2R blockade. These data indicate that DORAs and OX2R selective antagonist, but not OX1R selective antagonist, can improve memory function following disrupted sleep.
Introduction
Since the discovery of Hcrt/OX two decades ago [1, 2], studies have demonstrated the functions of Hcrt in sleep regulation, anxiety, food intake, depression, addiction, and pain modulation [3–8]. In particular, the selective activation of Hcrt neurons via optogenetic stimulation established a causal role of Hcrt in the onset and maintenance of wakefulness [9]. Deficits of Hcrt neuronal function or mutations of OX2Rs cause narcolepsy characterized by excessive daytime sleepiness in rodents [10–12], canines [13, 14], and humans [15, 16]. Studies with Hcrtko/ko mice revealed lack of Hcrt impairs theta-dominated wakefulness and blunts delta power in slow-wave sleep which are relevant to narcoleptic excessive daytime sleepiness and poor sleep quality [17]. Interestingly, both OX1R-/- and OX2R-/- mice show attenuation of wakefulness compared to wild-type controls after intracerebroventricular administration of OX1/Hcrt1, with a stronger wakefulness attenuation observed in OX2R-/- mice [18].
Multiple lines of study have examined the effect of selective or dual OX1R and OX2R antagonism on sleep modulation [19–29]. ACT-078573 (Almorexant), a dual OX1/2R antagonist, has been shown to be effective in treatment of primary insomnia in humans [30], and has been shown to increase both non-rapid eye movement (NREM) and rapid eye movement (REM) sleep as measured by electroencephalogram (EEG) and electromyography (EMG) or behavioral changes, in rats, canines, and humans [19]. Suvorexant, a dual OX1/2R antagonist is effective in decreasing sleep latency, and has comparable EEG spectral profiles to normal sleep [20, 26, 27]. Interestingly, selective OX2R antagonists including C1 m [31] and IPSU [23, 32, 33] are sufficient to increase the amount of NREM sleep. However, a more detailed analysis of sleep patterns and architecture after OXR antagonism is necessary to understand the mechanisms of action by such agents for the treatment of insomnia. In addition, it was unclear which subtype of OXRs is essential for mediating the hypnotic effect in vivo. Here, we used optogenetic stimulation of Hcrt neurons to determine whether dual or selective antagonism of OXRs is sufficient to block optogenetically-evoked wakefulness or reduce wakefulness maintenance. We also used fiber photometry to characterize Hcrt neuronal activity in the presence of OX1/2R antagonists. Furthermore, we investigated whether memory deficits caused by optogenetic sleep fragmentation can be prevented by antagonism of OX1/2Rs. In addition, we provide a detailed comparison of power spectra upon drug treatment. We also compared the hypnotic effect by OXR antagonists with a typical hypnotic agent Zolpidem, an α1-GABAA receptor modulator [34]. Together, our findings provide a detailed analysis of the impact of OXR antagonists on sleep architecture and suggest blockade of OX2R is essential for promoting sleep. These findings have implications for sleep health and cognitive deficits related to sleep fragmentation, and hyperarousal/insomnia.
Methods
Animals
All experimental protocols were approved by the Stanford University Animal Care and Use Committee and in accordance to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Young adult wild-type C57BL6J (WT, 10–12 weeks) mice from the Jackson Laboratory (Sacremento, CA) were used for studying the effect of OX1/2R antagonists and Zolpidem on modulation of sleep patterns. For all the optogenetic and fiber photometry experiments, we used Hcrt-IRES-Cre knock-in heterozygote mice [35] backcrossed onto C57BL6J backgrounds. The mice were housed in a temperature-controlled facility with a 12/12-hour light-dark cycle (light phase: 09:00–21:00 (ZT0–ZT12), dark phase: 21:00–09:00 (ZT12–ZT24) for sleep pattern recording; light phase: 07:00–19:00 (ZT0–ZT12), dark phase: 07:00–19:00 (ZT0–ZT12) for novel object recognition [NOR] task). Mice were allowed to access food and water ad libitum. The facility was fully accredited by the American Association for the Accreditation of Laboratory Animal Care.
Chemicals
DORA12, MK6096, 1SORA1, and MK1064 were provided by Merck (One Merck Drive, Whitehouse Station, NJ) through the Merck Investigator Studies Program (MISP). Poly (ethylene glycol) average Mn 400 (PEG400) was obtained from Sigma-Aldrich (3050 Spruce Street, Saint Louis, MO). Zolpidem was purchased from Tocris. 0.9% Sodium Chloride (Saline) was purchased from Hospira (Lake Forest, IL). All the Merck compounds and Zolpidem were dissolved separately in a mixture of 50% Saline and 50% PEG400 to a concentration of 2 mg/ml. All injections were performed intraperitoneally (i.p.), and the compound solutions were administered to the mice at a dosage of 0.1 ml/10 g to reach a concentration of 20 mg/kg. All the injections were administered to the mice assigned through a balanced crossover design (Latin Square Design). At least 1 week was given between injections to prevent drug carryover effects.
Surgery
WT mice were anesthetized with a mixture of Ketamine (100 mg/kg) and Xylazine (20 mg/kg) and mounted onto an animal stereotaxic frame (David Kopf Instruments). Cortical EEG and EMG electrodes were implanted according to our previously published protocols [36]. Briefly, EEG mini-screws (US Micro Screw) connected with insulated wires were placed in the skull above the frontal (AP: −2 mm; ML: ±1 mm) and temporal (AP: 3 mm, ML: ±2.5 mm) cortices. Mini-rings connected to insulated wires were inserted into neck muscles for EMG recording. Electrode sockets were anchored with meta-bond and dental acrylic on skull for EEG/EMG signal sampling. After surgery, mice were singly housed and allowed to recover for at least 1 week prior recording.
Virus injection, glass fiber implantation and immunohistochemistry
Hcrt-IRES-Cre knock-in mice underwent stereotaxic surgery under anesthesia (Ketamine 100 mg/kg and Xylazine 20 mg/kg) and received injections of 0.3 μl AAV-DJ-EF1α-DIO-hChR2(H134R)-eYFP viruses or AAV-DJ-EF1α-DIO-eYFP (2.5 × 1012 genome copies per ml, Stanford Virus Core) to the right or left LH (AP: −1.35 mm, ML: ±0.95 mm, DV: −5.15 mm) with a 5 μl Hamilton microsyringe. A glass fiber (200 μm in diameter, Doric Lenses, Franquet, Québec, Canada) was implanted with the tip right above the injection site for optogenetic stimulations later on. For fiber photometry, 0.3 μl AAV vectors carrying genes encoding GCaMP6f (AAV-DJ-EF1α-DIO-GCaMP6f, 1.1 × 1013 genome copies per ml, Stanford Virus Core) were delivered to the right or left LH (AP: −1.35 mm, ML: ±0.95 mm, DV: −5.15 mm) with a 5 μl Hamilton micro-syringe, and a glass fiber (400 μm in diameter, Doric Lenses) was implanted with the tip at the injection site for GCaMP6f signal acquisition later on. AAV-DJ- EF1α-DIO-GFP was used as control. After fixing the glass fiber with meta-bond, the EEG and EMG electrodes were implanted similarly as for the WT mice.
Primary antibody against OX1/Hcrt1 (SC-8070, Lot#A2915, Goat polyclonal IgG) was purchased from Santa Cruz Biotechnology, INC. Secondary antibody Alexa Fluor 594 Donkey anti-Goat IgG (H+L) was purchased from Invitrogen (Manufacturer: Life Technologies). Upon experiment completion, animals were anesthetized with Ketamine and Xylazine (100 and 20 mg/kg, i.p.) and transcardially perfused with 5 ml 1× phosphate buffered saline (PBS), followed by 20 ml paraformaldehyde (PFA; 4%, in PBS). Brains were rapidly extracted, postfixed overnight in 4% PFA at 4°C, and cryoprotected for 2 days at 4°C in sucrose solution (30% sucrose in PBS containing 0.1% NaN3) until equilibrated with the solution. Brains were sliced into 35 μm coronal sections at −22°C on a cryostat (Leica Microsystems), collected consecutively in 24-well plates containing PBS with 0.1% NaN3, covered with aluminum foil, and stored at 4°C until immunostaining and imaging. Sections around the implantation site were washed in PBS for 5 minutes three times and then incubated for 1 hour in a blocking solution of PBS with 0.3% Triton X-100 (PBST) containing 4% bovine serum albumin (BSA). Then primary antibody against OX1/ Hcrt1 was applied (1:500) overnight in 4% BSA/PBST blocking buffer. On the following day, sections were washed in PBS for 5 minutes three times, and then incubated for an additional 2 hours in blocking buffer. After that, secondary antibody (donkey anti-goat Alexa 594, 1:500 dilution) was applied to the incubation solution for 2 hours. After three final 5-minute PBS washes, sections were mounted onto gelatin-coated glass slides (LabScientific, Inc.), and coverslipped with Fluoroshield containing DAPI Mounting Media (Sigma-Aldrich, F6057) for imaging with LSM 710 Confocal Microscope (Zeiss, Germany).
Optogenetic stimulation of Hcrt neurons
After connecting the fiber optic patch cord to the laser, the light intensity was calibrated with a light meter (Thorlabs). Then, fiber optic patch cord (MFP_200/240/900-0.22_3.0m_FC-MF2.5, Doric lenses) was connected to the glass fiber implant through a zirconia sleeve (SLEEVE_ZR_2.50, Doric lenses) together with the EEG/EMG lead for acclimation for at least 1 week. After habituation, optogenetic stimulation with varying frequencies was performed during EEG/EMG recording (light intensity at the fiber tip: 10 mW, light pulse width: 15 ms; 1, 5, 10, 20 Hz stimulation for 10 s were performed, different stimulation frequencies were randomly arranged, Figure 1). For testing the effect of OX1/2R antagonists and Zolpidem on optogenetically-evoked wakefulness, the light stimulations were delivered to the mice between 45 minutes and 90 minutes after the injections based on their effect on sleep architecture (Supplementary Figure S1). The latency to optogenetic stimulation-evoked wakefulness was defined as the time window between the start of optogenetic stimulation and onset of wakefulness. The bout length of optogenetic stimulation-evoked wakefulness was determined from the onset of wakefulness upon light stimulation to the end of the same wakefulness episode.
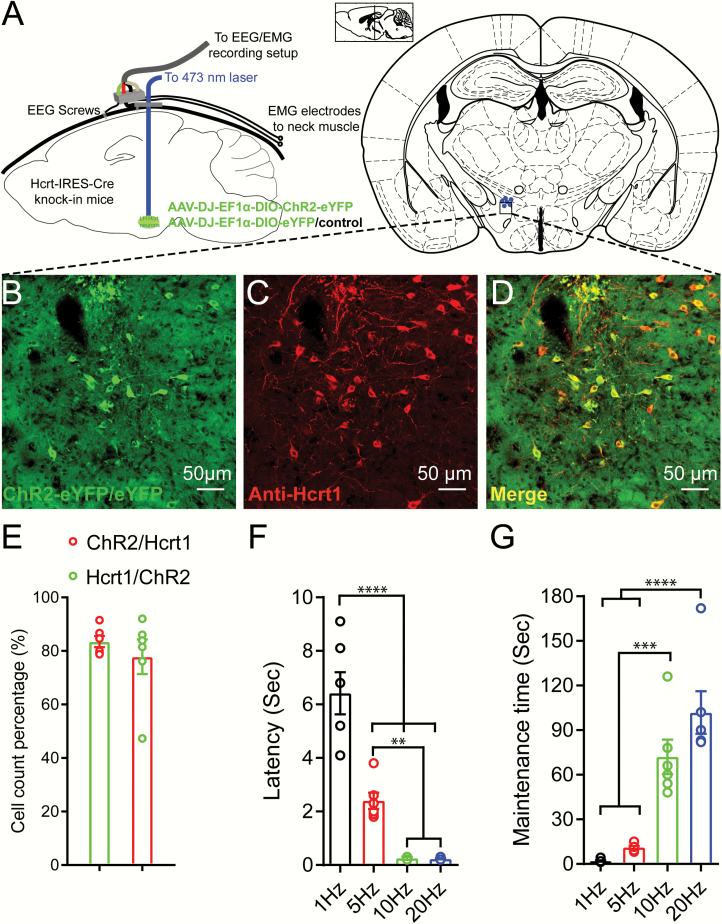
Figure 1.
Optogenetic stimulations triggered sleep-wake transitions in Hcrt-IRES-Cre knock-in mice injected with Cre-dependent AAV viruses expressing ChR2-eYFP. (A) Configuration of optogenetic stimulations and EEG/EMG recording in Hcrt-IRES-Cre knock-in mice. (B–D) Immunostaining against Hcrt1 confirmed the majority of the AAV viruses infected neurons are hypocretinergic. (E) More than 80% AAV viruses infected cells are positive for Hcrt1 antibody staining and about 78% Hcrt1 positive neurons are infected by AAV viruses. (F,G) 15 ms blue light pulses at 10 mW stimulation for 10 s with different frequencies trigger sleep-wake transitions with different latencies (F) and different post-stimulation wakefulness bout lengths (G) (linear mixed-effects model analysis followed by Tukey’s multiple comparisons, **p < 0.01, ***p < 0.005, ****p < 0.001, n = 6 for each group).
Sleep and GCaMP6f data acquisition and analysis
Once mice recovered, they were connected to flexible recording cables to habituate for at least 1 week. EEG/EMG signals were amplified through a multiple channel amplifier (Grass Instruments) and sampled with VitalRecorder (Kissei Comtec Co.) at 256 Hz sampling frequency filtered between 0 and 120 Hz for offline signal analysis. Raw EEG/EMG data were exported to Matlab and analyzed with custom scripts as well as built-in Matlab tools (The MathWorks). A power spectral density (PSD) function was used to plot power-frequency distributions (0–30 Hz). Band power of delta (1–4 Hz), theta (4–12 Hz), beta (12–30 Hz) was calculated in Matlab using custom scripts. For EEG/EMG recordings with optogenetic manipulations, real-time markers were tagged during recording when triggering the optogenetic stimulations for determining sleep-wake transition latency and bout length of optogenetic stimulation-evoked wakefulness. For mice expressing GCaMP6f, EEG/EMG and GCaMP6f signals were acquired simultaneously between second and fourth hour after the injections of vehicle, OX1/2R antagonists, or Zolpidem. EEG/EMG and GCaMP6f signals were staged offline in Matlab with custom scripts. To quantify the change of GCaMP6f signals, the values before state transitions were averaged as the baseline, and the area size (ΔF/F integral) between the baseline and GCaMP6f signal trace was determined and averaged for each individual mouse (Figure 3, Supplementary Figure S5).
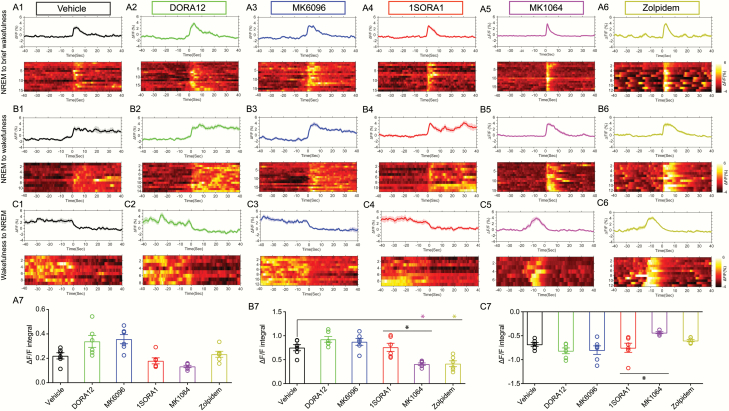
Figure 3.
Hcrt neural activities during sleep-wakefulness transitions in the presence of OXR antagonists and Zolpidem. (A1–A6) GCaMP6f signals for NREM sleep-to-brief wakefulness for Vehicle, DORA12, MK6096, 1SORA1, MK1064, Zolpidem treated group respectively. (B1–B6) GCaMP6f signals during NREM sleep-to-wakefulness transitions for Vehicle, DORA12, MK6096, 1SORA1, MK1064, Zolpidem treated group respectively. (C1–C6) GCaMP6f signals during wakefulness-to-NREM sleep transitions for Vehicle, DORA12, MK6096, 1SORA1, MK1064, Zolpidem treated group respectively. (A7–C7) Size of the area under the GCaMP signal trace (ΔF/F integral) after the transition of state for NREM-to-brief wakefulness (A7), NREM-to-wakefulness (B7), wakefulness-to-NREM (C7) (n = 6 mice per group, multiple trials may be staged from the same mouse, GCaMP6f ΔF/F integral has been averaged for each animal under different treatment, linear-mixed effects model followed by post hoc Tukey’s multiple comparisons test, *p < 0.05, n = 6 for each group).
NOR and optogenetic sleep fragmentation
The NOR task included a training session, delay period of 24 hours, and a test session (Figure 4). Previous studies have shown that learning in the NOR test is dependent on sleep during the light phase, and that disruption of sleep in the dark phase does not influence NOR performance. Thus, we conducted all NOR experiments during the light phase (stimulation starts at 09:30) [37, 38].
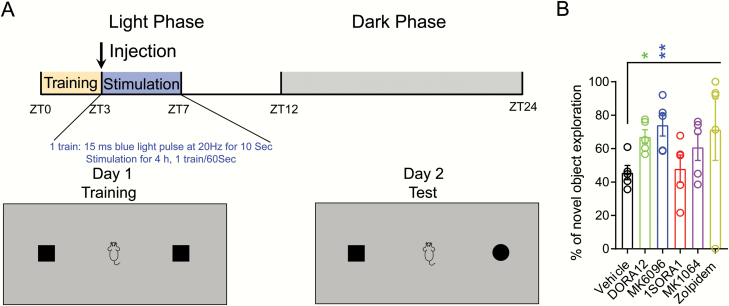
Figure 4.
Treatment with dual OX1/2R antagonists on memory function in mice that have undergone optogenetic sleep fragmentation. (A) Schematic representation of the behavioral paradigm. (B) Percentage of novel object exploration (out of the total time exploring) for each mouse (linear-mixed effects model followed by post hoc Tukey’s multiple comparisons test, *p < 0.05, **p < 0.01, n = 5 per group).
Training
After 1 hour of habituation to the experiment room and 10 minutes of habituation to the arena (black walled open field containing Sani-Chip pine bedding, 34 cm ×17 cm) the mice were given the opportunity to explore two identical objects for a total of 5 minutes. Each object was placed at the same distance from the walls and corners of the open field. No specific spatial or odor cues were provided within the field (bedding was changed and arena and objects were cleaned with 10% ethanol before each exposure).
Injection
Immediately after training, animals were injected with 20 mg/kg (i.p.) of DORA12, MK6096, 1SORA1, MK1064, Zolpidem or vehicle and returned to their home cages.
Optogenetic sleep disruption
Immediately after training, animals received 4 hours of optogenetic stimulation as described in earlier work from our lab [37].
Test
After a delay of 24 hours, animals were placed in the same arena with one of the objects from the training session replaced with a novel object. Real-time monitoring was videotaped during training and test sessions using a Panasonic WV-LZA61/2S videotaping system. The time spent around the objects (defined as a 7 cm radius around the objects) was scored with BORIS 5.1.0 software (University of Torino, Italy). Time spent climbing on object or facing away from object within the 7 cm radius was not counted as exploration. Mice that demonstrated over 65% preference for either object in the training session were excluded from the experiment (one mouse showed obvious bias toward an object on the training day was excluded from data analysis).
Statistical analysis
We used a linear mixed-effects model to analyze our data to rule out possible carryover effects by different compounds. Different treatments were used as fixed factors and position of individuals in the Latin Square Design were used as randomized factors. Post hoc Tukey’s multiple comparisons test was used to reveal whether there was a significant difference between different treatments. This method was used to analyze bout total length, bout counts, mean bout length, latency of NREM and REM sleep following injections in Supplementary Figure S1, latency to wakefulness and bout length of optogenetic stimulation-induced wakefulness for different frequencies in Figure 1, normalized band power of delta, theta, and beta following treatment of OX1/2R antagonists and Zolpidem in Supplementary Figure S2, optogenetic stimulation-evoked wakefulness latency and duration in Figure 2, comparison of GCaMP6f signals in Figure 3, and NOR task in Figure 4. Hourly based sleep comparisons were analyzed by two-way repeated-measures ANOVA followed by Tukey’s multiple comparisons to compare the difference by the treatments of vehicle and OXR antagonists, or Zolpidem. Significant difference levels were set at *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001, #p <0.0001. All statistical analyses were performed in IBM SPSS Statistics 21 software (Armonk, NY: IBM Corp).
Figure 2.
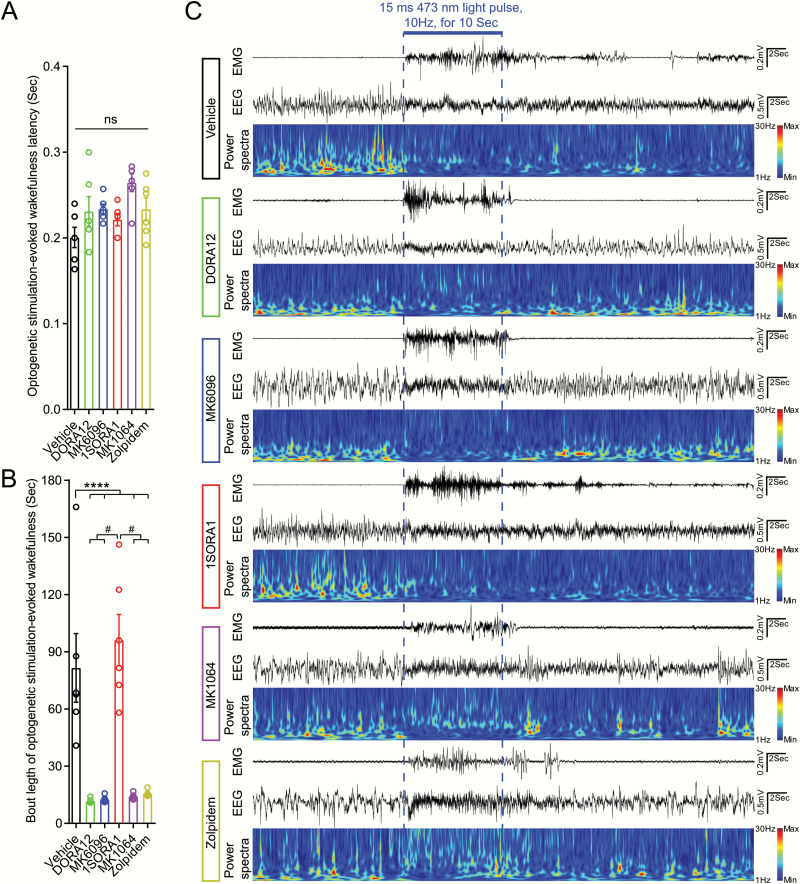
DORA12 (20 mg/kg), MK6096 (20 mg/kg), 1SOARA1 (20 mg/kg), MK1064 (20 mg/kg), and Zolpidem (20 mg/kg) reduced bout length of optogenetic stimulation-evoked wakefulness in Hcrt-IRES-Cre knock-in mice injected with AAV viruses expressing ChR2 in Hcrt neurons. (A) Optogenetic stimulation triggered immediate sleep-wake transition even in the presence of DORA12, MK6096, 1SORA1, MK1064, Zolpidem with no difference with vehicle-treated group. (B) DORA12, MK6096, MK1064 and Zolpidem significantly reduced the mean bout length of optogenetic stimulation-evoked wakefulness in Hcrt-IRES-Cre knock-in mice expressing ChR2. (C) Raw traces of EMG, EEG, and power spectra of EEG for each group (n = 6, light stimulation were delivered to the animals at 45–90 minutes following the injections. Experiments were performed during ZT5-ZT9 (light phase: ZT0-ZT12; dark phase: ZT12-ZT24), Vehicle (solvent): 50% 0.9% Sodium Chloride, 50% PEG400; DORA12: 20 mg/kg; 1SORA1: 20 mg/kg; MK6096: 20 mg/kg; MK1064: 20 mg/kg; Zolpidem: 20 mg/kg; linear-mixed effects model followed by post hoc Tukey’s multiple comparisons test, ****p < 0.001, #p < 0.00001, n = 6 for each group).
Results
DORAs, OX2R selective antagonist MK1064, Zolpidem significantly increased NREM sleep and altered EEG band power distribution
We first examined the effect of OXR antagonists and Zolpidem on sleep architecture modulation in young adult mice (Supplementary Figure S1, Table 1). All the drugs were administered at the beginning of dark (active) phase when the mice have strong drive for wakefulness. Dual blockade of OX1/2Rs by DORA12 and MK6096, selective antagonism of OX2R by MK1064, and treatment with α1-GABAA receptor modulator Zolpidem significantly decreased the amount of wakefulness and increased the amount of NREM sleep from the first hour until the sixth hour post-injection (Supplementary Figure S1). Interestingly, selective antagonism of OX1R by 1SORA1 had no obvious effect of sleep patterns compared to the vehicle-treated group (Supplementary Figure S1, G–I; Table 1). In contrast to OX1R antagonist 1SORA1, selective antagonism of OX2R had pronounced effect in promoting NREM sleep (Supplementary Figure S1, J–L; Table 1). These data are consistent with previous reports showing that dual OXR antagonist or OX2R selective antagonist increases the amount of NREM sleep [19, 20, 31, 39–43].
Table 1.
DORAs, selective OX2R antagonist MK1064, and typical hypnotic Zolpidem significantly increased the amount of NREM sleep within 6 hours following the injections (unit: minute)
| Test | Sleep/wake state | Optogenetic stimulation-evoked wakefulness | NOR | |||
|---|---|---|---|---|---|---|
| Group | Wakefulness (minute) | NREM sleep (minute) | REM sleep (minute) | Latency (s) | Bout length (s) | Novel time (%) |
| Vehicle | 195.997 ± 9.915 | 155.621 ± 8.939 | 8.198 ± 1.454 | 0.201 ± 0.012 | 81.53 ± 18.00 | 45.660 ± 4.252 |
| DORA12 | 135.444 ± 21.109* | 217.724 ± 21.098** | 9.487 ± 0.572 | 0.231 ± 0.017 | 11.76 ± 0.4826**** | 67.140 ± 4.153* |
| MK6096 | 87.079 ± 7.458**** | 270.212 ± 8.000**** | 3.290 ± 1.426* | 0.234 ± 0.006 | 12.69 ± 0.6572**** | 74.160 ± 6.643** |
| 1SORA1 | 222.313 ± 9.603 | 130.188 ± 8.908 | 7.420 ± 1.258 | 0.221 ± 0.007 | 96.18 ± 13.44 | 48.033 ± 8.124 |
| MK1064 | 56.152 ± 3.316**** | 303.848 ± 3.316**** | 0# | 0.264 ± 0.010 | 14.272 ± 0.596**** | 60.834 ± 7.091 |
| Zolpidem | 95.708 ± 4.904**** | 263.082 ± 4.714**** | 0.866 ± 0.532**** | 0.234 ± 0.013 | 15.680 ± 0.639**** | 71.352 ± 18.468 |
Dual antagonism of OX1/2Rs and selective antagonism of OX2R as well as Zolpidem decreased the duration of optogenetic stimulation-evoked wakefulness (unit: s). DORAs ameliorated the memory deficits by optogenetic stimulation-induced sleep fragmentation (optogenetic stimulations: 15 ms blue light pulse, at 10 Hz for 10 s; linear-mixed effects model followed by Tukey’s multiple comparisons, *p < 0.05, **p < 0.01, ****p < 0.001, #p < 0.0001; n = 6 for the panels sleep/wakefulness state and optogenetic stimulation-evoked wakefulness, n = 5 for NOR; Vehicle (solvent): 50% 0.9% Sodium Chloride, 50% PEG400; DORA12: 20 mg/kg; MK6096: 20 mg/kg; 1SORA1: 20 mg/kg; MK1064: 20 mg/kg; Zolpidem: 20 mg/kg).
To assess whether blockade of OXRs affects the distribution of specific EEG power bands, we pooled the wakefulness episodes from hours 2–6 after injection for each individual animal and compared the EEG power spectra. Surprisingly, DORAs, OX2R antagonist MK1064, and Zolpidem suppressed the typical theta peak during wakefulness and produced a delta peak during wakefulness, whereas 1SORA1 did not change power spectral distribution (Supplementary Figure S2, A). Neither selective OX1R antagonist, nor DORAs, changed the power spectral distribution during NREM sleep following injection. However, OX2R selective antagonist MK1064 as well as Zolpidem increased delta band power and decreased beta band power (Supplementary Figure S2, E–H). As all the drugs we have tested in our study postponed REM sleep onset, we analyzed the initial 60-s REM sleep episode after the injection of the drugs. Interestingly, during the first 60 s of REM sleep after injection, there was a small delta peak in the DORA and OX2R antagonist MK1064 treated groups and an obvious delta peak in the Zolpidem treated group (Supplementary Figure S2, I).
Reduced maintenance of optogenetic stimulation-evoked wakefulness episode by DORAs and OX2R selective antagonist MK1064, α1-GABAA receptor modulator Zolpidem
Our lab previously showed that optogenetic stimulation of Hcrt neurons in the lateral hypothalamus (LH) increased the probability of sleep-to-wake transitions [9]. However, whether OX1/2R antagonism could reduce the latency and bout length of wakefulness upon optogenetic stimulation of Hcrt/OX neurons was unknown. Thus, we tested whether optical stimulation of Hcrt neurons expressing ChR2 could reliably trigger sleep-to-wake transitions and maintain wakefulness in a new line of Hcrt-IRES-Cre knock-in mice [35] in the presence or absence of OXR antagonists and Zolpidem (Figure 2).
Here we used a new Hcrt-IRES-Cre mouse line that achieved ChR2 expression eutopically in Hcrt neurons by the endogenous Hcrt gene locus, combined with the powerful Cre-dependent EF1α promoter. This combination produces many more ChR2 molecules per neuron than the original 1.2 Kb mouse promoter packaged into lentiviral vectors [9]. Therefore, we observed short latencies and longer bout lengths of wakefulness upon optogenetic stimulations. A mean of 83% AAV virus-infected neurons were positive for anti-Hcrt1 staining and a mean of 78% Hcrt1 positive neurons were infected by AAV viruses (Figure 1, B–E). Blue light (473 nm, 15 ms pulse width, 10 mW light intensity at the tip of the glass fiber) stimulation for 10 s could reliably trigger sleep-to-wake transitions to varying degrees based on stimulation frequencies (Figure 1, F and G). Light stimulation at 10 Hz and 20 Hz triggered immediate sleep-to-wake transitions in contrast to 1 Hz and 5 Hz (Figure 1F). Importantly, the bout length of optogenetic stimulation-evoked wakefulness correlated with the stimulation frequency (Figure 1G, Supplementary Figure S3).
We then performed optogenetic stimulation of Hcrt neurons in the presence of dual OX1/2R antagonists DORA12 and MK6096, selective OX1R antagonist 1SORA1, selective OX2R antagonist MK1064, and a typical hypnotic Zolpidem. As DORAs, OX2R antagonist MK1064, and Zolpidem significantly postponed the onset of REM sleep (Supplementary Figure S1, S), optogenetic stimulations (15 ms pulse width, 10 mW, 10 Hz for 10 s) were delivered to the mice during NREM sleep between 45 minutes and 90 minutes after injections according to the alteration in sleep patterns by the treatment of different drugs (Supplementary Figure S1). As the bout length of NREM sleep episode may vary, we delivered the optogenetic stimulations 15 s after the onset of NREM sleep to avoid a short-lasting NREM episode [9]. Neither selective antagonism of OX1/2Rs, nor dual blockade of OX1/2Rs by DORAs or treatment with α1-GABAA receptor modulator Zolpidem blocked the changes in NREM sleep-to-wake as characterized by immediate increases of EMG activities and EEG changes from irregular delta patterns to theta patterns (Figure 2, A and C). However, compared to persisted muscle activities and changed EEG patterns in vehicle and 1SORA1 treated groups, wakefulness-like activities in EEG and EMG switched back to NREM sleep pattern shortly after termination of optogenetic stimulations in the presence of Zolpidem, DORAs, and OX2R selective antagonist MK1064 (Figure 2, B and C; Table 1). Importantly, OX2R antagonist MK1064 significantly reduced the length of wakefulness elicited by optogenetic stimulations compared to OX1R antagonist 1SORA1, which has a similar effect to vehicle (Figure 2, B and C; Table 1).
Endogenous Hcrt neural activity during sleep-to-wakefulness transitions in the presence of OX1/2R antagonists
Single unit recording in combination with neurobiotin juxtacellualr labeling and anti-Hcrt immunostaining revealed that activity of Hcrt/OX neurons correlate with wakefulness in head-retrained mice [44] and rats [45, 46]. Although Zolpidem, DORAs and selective OX2R antagonist MK1064 reduced the bout length of optogenetic stimulation-evoked wakefulness (Figure 2, Table 1), whether Hcrt neurons are still active in the presence of Zolpidem or OXR antagonism remained unexplored. Thus, we transduced Hcrt neurons in Hcrt-IRES-Cre knock-in mice with AAV vectors encoding GCaMP6f and used in vivo fiber photometry Ca2+ recording to study the endogenous activity of Hcrt neurons in the presence of OX1/2R antagonists or Zolpidem (Figure 3, Supplementary Figure S4). To quantify whether there is difference among different treatments, we calculated size of the area (SOA) between the GCaMP signal trace and the mean value line based on the GCaMP signal prior to the state transition (Time 0, Supplementary Figure S5). Our data showed that both DORAs (Figure 3, A2–C2 and A3–C3) or OX1R selective antagonist (Figure 3, A4–C4) had a marginal effect compared to vehicle-treated group (p > 0.05) on Hcrt neuronal activities during brief wakefulness and sustained wakefulness (Figure 3, A7 and B7). However, Zolpidem and OX2R selective antagonist MK1064 significantly reduced the SOA when mice switched from NREM to wakefulness compared to vehicle and DORA treated groups, indicating a shorter lasting episode of wakefulness (Figure 3, B5–B7). Opposite to this state transition, when mice switched from wakefulness to NREM sleep, the change of SOA was significantly smaller in OX2R antagonist (MK1064) treated group compared to the groups treated by OX1R antagonist 1SORA1 (Figure 3, C7). Importantly, once the mice switched from NREM sleep to brief wakefulness, there was an abrupt increase in GCaMP6f signals and a rapid decrease once back to NREM sleep, suggesting a causal relationship between Hcrt neuronal activity and wakefulness (Figure 3, A1–A6). When the mice switched from NREM sleep to sustained wakefulness, an increase in GCaMP6f signal emerged and remained at high level (Figure 3, B1–B6). Opposite to this, sustained GCaMP6f signal at high level rapidly declined to baseline when the mice switched from wakefulness to NREM sleep (Figure 3, C1–C6). Our results show that Hcrt neurons are active during wakefulness even in the presence of Zolpidem and OX1/2R antagonists. As REM sleep was suppressed after administration of Zolpidem and OX1/2R antagonists (Supplementary Figure S1), GCaMP6f signal examples during REM episode following NREM sleep or REM sleep to brief/sustained wakefulness were shown for the vehicle-treated group (Supplementary Figure S6). In the mice injected with control viruses, there was no Ca2+-dependent fluorescent signals.
DORAs ameliorate memory deficits caused by sleep fragmentation
To assess the effect of OX1/2R antagonists on memory, we used the NOR test which our lab has previously used to test the effect of sleep continuity on memory consolidation [37]. Briefly, mice were allowed to explore two identical objects placed in an arena for 5 minutes after a 10-minute adaptation period. Mice were then injected with either vehicle, OX1/2R antagonists or Zolpidem and connected to glass fiber patch cords for optogenetic stimulations for a total of 4 hours (15 mW light intensity, 20 Hz for 10 s, every 60 s) (Figure 4A). Twenty-four hours after training, the mice were subjected to the NOR test in which one familiar object was replaced by a novel object. As we observed reduction of wakefulness following optogenetic stimulation with DORAs (Figure 2), we found both DORAs significantly improved memory for the familiar object compared to the vehicle-treated group (Figure 4B, Table 1). Interestingly, even though Zolpidem and the OX2R antagonist MK1064 showed significant hypnotic effect on sleep architecture (Supplementary Figure S1) and reduced the bout length of optogenetic stimulation-evoked wakefulness (Figure 2, B and C), the improvement by Zolpidem and MK1064 on memory function was not significantly different from the vehicle-treated group.
Discussion
Our investigation of OX1/2R antagonism demonstrates that DORAs and OX2R antagonist MK1064 significantly increased the total amount of NREM sleep and reduced wakefulness within 6 hours following treatment, whereas selective antagonism of OX1R by 1SORA1 showed no obvious effect on sleep architecture (Supplementary Figure S1, Table 1). In addition to the alteration of sleep architecture by DORAs and OX2R selective antagonist MK1064, the band power distribution was shifted to lower frequencies, i.e. delta band with more weight during wakefulness and REM sleep onset (Supplementary Figure S2). Importantly, the duration of wakefulness following optogenetic stimulations of Hcrt/OX neurons was significantly reduced in the presence of DORAs and OX2R selective antagonist MK1064 (Figure 2). Furthermore, Hcrt neurons were observed to remain active during wakefulness in the presence of DORAs and OX2R selective antagonist as revealed by monitoring Ca2+ signals (Figure 3). In a cognitive paradigm for memory function (NOR), DORAs and OX2R selective antagonist MK1064 improved the performance of mice that had experienced optogenetic sleep disruption (Figure 4). Importantly, these findings have strong implications for various sleep-related conditions such as insomnia characterized by bouts of wakefulness after sleep onset (WASO) [47]. Indeed, Suvorexant and Almorexant are effective to decrease bouts of wakefulness in patients with insomnia characterized by WASO [30, 43] and the data presented here suggest that this may be a result of its effects on specific sleep features such as increasing NREM sleep and the intrusion of NREM sleep into wakefulness. These results indicate that DORAs and OX2R selective antagonist increase the amount of sleep, and have the potential to overcome memory deficits caused by sleep fragmentation. As DORAs and OX2R selective antagonist have similar effect in increasing the amount of sleep, decreasing the length of wakefulness elicited by optogenetic stimulations of Hcrt neurons, as well as in the improvement of memory function, our results strongly suggest selective antagonism of OX2R may be sufficient to improve sleep quality and associated cognitive function.
Selective antagonism of OX1R has minimal effect on sleep
Selective blockade of OX1R had no significant effect on the sleep architecture (Supplementary Figure S1, G–I). This observation is consistent with Dugovic’s work showing that OX1R blockade with SB-408124 has no effect on sleep patterns [48]. Similarly, other OX1R antagonists, JNJ-54717793 and compound 56, also exhibits minimal hypnotic effect on spontaneous sleep in rodents [49–51]. Our data showed slight but not significant increase of wakefulness particularly during the first 3 hours following the injection of 1SORA1 (Supplementary Figure S1, G and P). This minimal increase of wakefulness upon antagonism of OX1R is likely due to the increased DA release in the prefrontal cortex (PFC) [48]. Interestingly, the time course of DA increase in PFC upon OX1R antagonist, SB-408124 treatment [48], has a similar temporal pattern with our observed trend toward wakefulness following administration of 1SORA1 (Supplementary Figure S1, G). The power distribution of NREM following 1SORA1 treatment has a lower delta peak compared to the vehicle-treated group (Supplementary Figure S2, E and F) suggesting that blocking of OX1R may counteract NREM sleep [42].
DORAs, OX2R selective antagonist, zolpidem promote sleep
Our data showed DORAs (DORA12 and MK6096) and OX2R selective antagonist MK1064 increase the total amount of NREM sleep and reduces the total time in wakefulness (Supplementary Figure S1, P) with stronger effect by MK6096 and MK1064. MK6096 (Filorexant) has been characterized to promote sleep in humans in a phase II dose-ranging study to treat insomnia [28, 41]. Our results are in line with the reported data regarding increased NREM sleep and reduction of NREM sleep latency in mice [28]. In particular, our data showing selective antagonism of OX2R is sufficient to promote NREM sleep is consistent with NREM promoting effect by OX2R antagonists [23, 31–33]. Moreover, pharmacological studies in OXR-deficient mice also showed sleep-promoting effect by Almorexant is absent in OX2R-/- mice [52]. However, our observation of significant increase of REM sleep latency (Supplementary Figure S1, S) is different from the REM sleep latency reduction caused by MK6096 in rats reported by Winrow and colleagues [28]. There might be a difference of OXR antagonist effect between mice and rats as different rat strains may have different sleep patterns. For example, brown Norway rats have more paradoxical sleep (REM sleep) than Lewis rats [53]. Another possible reason is the difference of drug administration time as we administered treatments intraperitoneally at the beginning of the dark (active) phase where mice have strong drive for wakefulness whereas Winrow and colleagues administered the drug through oral gavage 4 hours prior to the inactive phase [28] when rodents have a strong drive for REM sleep in rats [54] and mice [55]. Indeed, cerebrospinal fluid Hcrt1/OX1 levels fluctuate throughout the light-dark cycle [56], season, and with day length [57] which may explain the differences in REM sleep latency described here.
We also compared the power spectra among different groups (Supplementary Figure S2). It has been reported that DORA22 promotes somnolence without affecting network EEG [58] and dual OX1/2R antagonist Suvorexant exhibits limited effects on PSD of EEG [59]. Our data from mice showed that, in the presence DORAs and OX2R selective antagonist MK1064, the typical theta peak during wakefulness [12, 60, 61] is abolished and significant increase of delta band power (Supplementary Figure S2, A–C), a typical feature of NREM sleep [12, 60, 61] appeared similar to reported effect of Zolpidem on wakefulness in reducing theta band frequencies [62]. Additionally, band power in the delta range during REM onset in DORA and OX2R selective antagonist-treated groups was significantly higher than the vehicle group (Supplementary Figure S2, I and J). There is a significant increase of delta band power during NREM sleep following OX2R selective antagonism, but a minor reduction of delta band power in the 1SORA1 treated group (Supplementary Figure S2, E and F) consistent with previous work in rats and humans [58, 59]. Treatment with DORAs and OX2R selective antagonist mimics natural NREM sleep spectral profiles. The peak shift from theta to delta range and appearance of delta peak during REM sleep onset following DORAs and OX2R selective antagonist treatment strongly suggest that OX1/2R antagonists exert their therapeutic effects via coordinating brain network activities underlying NREM sleep.
Optogenetic interrogation of OXR antagonists
Hcrt neurons can co-release multiple excitatory transmitters, including glutamate in addition to Hcrt peptides [63, 64]. In an in vitro brain microcircuit comprised of presynaptic Hcrt and postsynaptic histamine neurons, optical stimulation of Hcrt neurons produced both rapid excitation and late excitation on postsynaptic histaminergic sites [63]. The rapid excitation, but not late excitation, during the stimulation was blocked by glutamate AMPA receptor (AMPAR) antagonist CNQX. Interestingly, the postsynaptic spike patterns are temporarily and pharmacologically distinct. Postsynaptic histamine neurons upon excitation by Hcrt generated about fivefold more spikes than by glutamate [63]. Consistent with these results in vitro, our in vivo observations revealed that upon optogenetic stimulation of Hcrt neurons in the presence of DORAs and OX2R selective antagonist MK1064, wakefulness-like EEG patterns quickly switched back to those characterizing NREM sleep (Figure 2C) suggesting that glutamatergic transmission from Hcrt neurons is sufficient to induce wakefulness, but Hcrt signaling is necessary to maintain wakefulness after stimulation. Furthermore, by monitoring Ca2+ transients, we found Hcrt neurons are active during wakefulness even in the presence of OX1/2R antagonists suggesting that their sleep-promoting effect is mediated through downstream of Hcrt neurons. Our data from the control group showing Hcrt neuronal activities correlate with wakefulness independent of the bout length (brief wakefulness and persisted wakefulness) is consistent with juxtacellular recording from Hcrt neurons [44, 45]. Whether the antagonism of either or both OXRs is more hypnotic is still under debate. Hcrt-1 has a significantly stronger wake-promoting effect in OX1R-/- mice than in OX2R-/- mice suggesting a pivotal role of OX2R in promotion of wakefulness [18]. It has been reported selective antagonism of OX2R (JNJ-10397049) significantly reduces the latency for persistent sleep and increases the amount of NREM and REM, whereas OX1R blockade by SB-408124 has no effect [48]. Additionally, Gotter and colleagues showed that selective blockade of OX2R is sufficient to promote NREM and REM in a dose-dependent manner similar to dual antagonism of OX1R and OX2R [21]. In contrast to OX2R antagonist, OX1R antagonist blocks OX1 microinjection mediated liquid food ingestion and water intake [65]. Another OX1R antagonist compound 56 attenuates lactate-induced panic-like behavior but without alteration of spontaneous sleep [50]. OX1R antagonist counteracts OX1-induced reduction of amount and increased latency to paradoxical sleep (REM sleep) [66]. Interestingly, co-administration of OX1R with OX2R antagonists greatly attenuates the sleep-promoting effect by OX2R by increasing dopamine (DA) release caused by OX1R antagonism [48]. Furthermore, in vitro electrophysiology data indicate OX2 plays a more important role in mediating OX1-triggered slow VTA dopaminergic firing [67]. However, there is study showing dual antagonism of OX1/2Rs is more effective than either alone in promoting sleep [68]. Nonetheless, given a clear hypnotic effect by OX2R antagonist, the effect of OX1R antagonist in sleep promotion is unlikely. Our results indicate the hypnotic effect of dual OX1/2Rs may be mainly through the blockade of OX2R as selective antagonism of OX2R significantly increased the amount of NREM sleep (Supplementary Figure S1, K and P), reduced the bout length of optogenetic stimulation-evoked wakefulness (Figure 2, B and C), which were similar to the effect by DORA12 and MK6096 (Supplementary Figure S1, B, E, P; Figure 2, B and C). Our findings demonstrated by optogenetic stimulations of Hcrt neurons in the presence of either dual OX1/2R antagonist, or OX1R antagonist, OX2R antagonist strongly suggest that OX2R antagonism underlies the mixed effect of dual OX1/2R antagonism in sleep promotion.
OXR antagonists and endogenous Hcrt activities
Mileykovskiy and colleagues have identified Hcrt neuronal activities with broad action potential with elongated later positive deflections by micropipette recording with juxtacellular Neurobiotin ejection in Urethane-anesthetized rats [46]. They further demonstrated Hcrt neurons are silent during slow wave sleep and tonic periods of REM sleep, but active during voluntary behaviors including grooming, eating and exploratory behavior [46]. Interestingly, they also observed occasional burst discharge of Hcrt neurons in phasic REM [46]. Similarly, juxtacelullar recordings revealed that activity of Hcrt/OX neurons trigger and correlate with wakefulness in head-retrained mice [44] and rats [45] and transiently appear during paradoxical sleep (REM sleep). Our recordings showed the Hcrt neuronal activities correlate with brief or sustained wakefulness even in the presence of OXR antagonists (Figure 3) consist with the aforementioned Hcrt neuronal activities by juxtacellular recordings. As the drugs significantly delayed the onset of REM sleep (Supplementary Figure S1), we have not recorded GCaMP6f signals from the Hcrt in the presence of OXR antagonists for REM sleep relevant state transitions (NREM sleep to REM sleep, REM sleep to brief wakefulness, REM sleep to wakefulness). However, we have recorded GCaMP6f signals from Hcrt neurons in the vehicle-treated animals between second and fourth hour after the injections (Supplementary Figure S6). The Hcrt neuronal activities are correlated with wakefulness independent of the bout length. We did not observe an obvious phasic discharge of Hcrt neurons during REM sleep as revealed by fiber photometry recording of Ca2+ transients (Supplementary Figure S6). Since Ca2+ recordings have less temporal resolution than unit recordings we cannot rule out that a subset of Hcrt neurons are active in anticipation to waking.
Effect of OX1/2R antagonists on memory consolidation
Sleep is vital for sustaining normal functioning at multiple levels, such as basic alertness, emotion, and cognitive function [69–73]. It has been reported that sleep fragmentation leads to impaired motor memory consolidation in humans [74]. Earlier work from our lab has shown that fragmenting sleep following the learning phase of the NOR paradigm significantly disturbed the performance of the mice on test day [37]. We used the same experimental paradigm to test whether dual blockade of OX1/2Rs or selective blockade of OX1R or OX2R can prevent the memory deficit caused by optogenetic sleep fragmentation. Interestingly, compared to the mice treated by vehicle, DORAs significantly improved the memory function as indicated by spending more time investigating the novel object on test day (Figure 4B). OX2R antagonist MK1064 slightly increased the time of novel object exploration but not reaching a significant level likely due to a strong and long lasting hypnotic effect (Supplementary Figure S1, K and P), which may affect food and water consumption. Zolpidem can decrease the frequency of calcium transients in hippocampal CA1 [75], a brain region that is important for memory [76, 77]. Indeed, Zolpidem negatively impacts psychomotor speed, speed of processing, and verbal memory in a dose-dependent manner [78, 79]. Interestingly, there is a study showing Zolpidem significantly impairs memory for material presented after drug administration other than before the drug administration or previously acquired knowledge [80]. Similar to MK1064, even though Zolpidem significantly increased the amount of NREM sleep (Supplementary Figure S1), it has a limited effect on preventing memory deficits due to optogenetic sleep fragmentation.
In conclusion, our data showed that both DORAs and OX2R selective antagonist are effective in suppressing wakefulness and promoting NREM sleep. Power spectral analysis of EEG during wakefulness revealed intrusion of delta band in wakefulness and a strong effect of DORAs and OX2R selective antagonist in promoting NREM sleep-like brain activity. These effects are mediated by antagonism of OX1/2Rs as optogenetic stimulation of Hcrt neurons did not affect the rapid excitation effect by glutamate release during light stimulations but hampered late excitation (persisted wakefulness) seen under normal conditions. Importantly, OX2R selective antagonism, but not OX1R antagonism, has a similar effect to dual blockade of OX1/2Rs in sleep architecture modulation, decreasing wakefulness following optogenetic stimulation of Hcrt neurons. Thus, our data suggest that the antagonism of OX2R plays the major role in promoting sleep. Our observations also highlight the potential of DORAs and OX2R selective antagonist in treating memory deficits caused by sleep fragmentation.
Supplementary material
Supplementary material is available at SLEEP online.
Acknowledgments
We thank Prof. Lihong Qi, Quantitative Methods, Biostatistics, Public Health Sciences, UC Davis School of Medicine for advising us on appropriate statistical method for data analysis. We also thank Dr. Andrew Olson and Stanford Neuroscience Microscopy Service, NIH NS069375 for imaging support.
Funding
The present study was funded by Merck (MISP VT ID 543219-0), NIH R01 AG047671 (L.d.L.), and R01 MH102638 (L.d.L.).
Conflict of interest statement. None declared.
References
- 1. de Lecea L, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95(1):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakurai T, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. [DOI] [PubMed] [Google Scholar]
- 3. de Lecea L. Hypocretins and the neurobiology of sleep-wake mechanisms. Prog Brain Res. 2012;198:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li SB, et al. Hypocretins and arousal. Curr Top Behav Neurosci. 2017;33:93–104. [DOI] [PubMed] [Google Scholar]
- 5. Li SB, et al. Hypocretins, neural systems, physiology, and psychiatric disorders. Curr Psychiatry Rep. 2016;18(1):7. [DOI] [PubMed] [Google Scholar]
- 6. Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–181. [DOI] [PubMed] [Google Scholar]
- 7. Sakurai T. The role of orexin in motivated behaviours. Nat Rev Neurosci. 2014;15(11):719–731. [DOI] [PubMed] [Google Scholar]
- 8. Sutcliffe JG, et al. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3(5):339–349. [DOI] [PubMed] [Google Scholar]
- 9. Adamantidis AR, et al. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hara J, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–354. [DOI] [PubMed] [Google Scholar]
- 11. Kornum BR, et al. Narcolepsy. Nat Rev Dis Primers. 2017;3:16100. [DOI] [PubMed] [Google Scholar]
- 12. Tabuchi S, et al. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci. 2014;34(19):6495–6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin L, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98(3):365–376. [DOI] [PubMed] [Google Scholar]
- 14. Wu MF, et al. Role of the hypocretin (orexin) receptor 2 (Hcrt-r2) in the regulation of hypocretin level and cataplexy. J Neurosci. 2011;31(17):6305–6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peyron C, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6(9):991–997. [DOI] [PubMed] [Google Scholar]
- 16. Thannickal TC, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vassalli A, et al. Hypocretin (orexin) is critical in sustaining theta/gamma-rich waking behaviors that drive sleep need. Proc Natl Acad Sci USA. 2017;114(27):E5464–E5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mieda M, et al. Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J Neurosci. 2011;31(17):6518–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brisbare-Roch C, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13(2):150–155. [DOI] [PubMed] [Google Scholar]
- 20. Coleman PJ, et al. The discovery of suvorexant, the first orexin receptor drug for insomnia. Annu Rev Pharmacol Toxicol. 2017;57:509–533. [DOI] [PubMed] [Google Scholar]
- 21. Gotter AL, et al. Orexin 2 receptor antagonism is sufficient to promote NREM and REM sleep from mouse to man. Sci Rep. 2016;6:27147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gotter AL, et al. Differential sleep-promoting effects of dual orexin receptor antagonists and GABAA receptor modulators. BMC Neurosci. 2014;15:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoyer D, et al. Distinct effects of IPSU and suvorexant on mouse sleep architecture. Front Neurosci. 2013;7:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoyer D, et al. Orexin in sleep, addiction and more: is the perfect insomnia drug at hand?Neuropeptides. 2013;47(6):477–488. [DOI] [PubMed] [Google Scholar]
- 25. Mercer SP, et al. Discovery of 2,5-diarylnicotinamides as selective orexin-2 receptor antagonists (2-SORAs). Bioorg Med Chem Lett. 2013;23(24):6620–6624. [DOI] [PubMed] [Google Scholar]
- 26. Rhyne DN, et al. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6(5):189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Winrow CJ, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1–2):52–61. [DOI] [PubMed] [Google Scholar]
- 28. Winrow CJ, et al. Pharmacological characterization of MK-6096 - a dual orexin receptor antagonist for insomnia. Neuropharmacology. 2012;62(2):978–987. [DOI] [PubMed] [Google Scholar]
- 29. Winrow CJ, et al. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. 2014;171(2):283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roth T, et al. Dual orexin receptor antagonist, almorexant, in elderly patients with primary insomnia: a randomized, controlled study. Sleep. 2017;40(2). [DOI] [PubMed] [Google Scholar]
- 31. Etori K, et al. Effects of a newly developed potent orexin-2 receptor-selective antagonist, compound 1 m, on sleep/wakefulness states in mice. Front Neurosci. 2014;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Betschart C, et al. Identification of a novel series of orexin receptor antagonists with a distinct effect on sleep architecture for the treatment of insomnia. J Med Chem. 2013;56(19):7590–7607. [DOI] [PubMed] [Google Scholar]
- 33. Eban-Rothschild A, et al. VTA dopaminergic neurons regulate ethologically relevant sleep–wake behaviors. Nat. Neurosci. 2016;19:1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crestani F, et al. Mechanism of action of the hypnotic zolpidem in vivo. Br J Pharmacol. 2000;131(7):1251–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giardino WJ, et al. Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat Neurosci. doi:10.1038/s41593-018-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Castellano JM, et al. In vivo assessment of behavioral recovery and circulatory exchange in the peritoneal parabiosis model. Sci Rep. 2016;6:29015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rolls A, et al. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci USA. 2011;108(32):13305–13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palchykova S, et al. Sleep deprivation in the dark period does not impair memory in OF1 mice. Chronobiol Int. 2009;26(4):682–696. [DOI] [PubMed] [Google Scholar]
- 39. Bennett T, et al. Suvorexant, a dual orexin receptor antagonist for the management of insomnia. P T. 2014;39(4):264–266. [PMC free article] [PubMed] [Google Scholar]
- 40. Coleman PJ, et al. Discovery of [(2R,5R)-5-{[(5-fluoropyridin-2-yl)oxy]methyl}-2-methylpiperidin-1-yl][5-methyl-2 -(pyrimidin-2-yl)phenyl]methanone (MK-6096): a dual orexin receptor antagonist with potent sleep-promoting properties. ChemMedChem. 2012;7(3):415–424, 337. [DOI] [PubMed] [Google Scholar]
- 41. Connor KM, et al. A phase ii dose-ranging study evaluating the efficacy and safety of the orexin receptor antagonist filorexant (MK-6096) in patients with primary insomnia. Int J Neuropsychoph. 2016;19(8):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dugovic C, et al. Orexin-1 receptor blockade dysregulates REM sleep in the presence of orexin-2 receptor antagonism. Front Neurosci. 2014;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Svetnik V, et al. Insight into reduction of wakefulness by suvorexant in patients with insomnia: analysis of wake bouts. Sleep. 2018;41(1). [DOI] [PubMed] [Google Scholar]
- 44. Takahashi K, et al. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153(3):860–870. [DOI] [PubMed] [Google Scholar]
- 45. Lee MG, et al. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25(28):6716–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mileykovskiy BY, et al. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46(5):787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Naismith SL, et al. Sleep well, think well: sleep-wake disturbance in mild cognitive impairment. J Geriatr Psychiatry Neurol. 2010;23(2):123–130. [DOI] [PubMed] [Google Scholar]
- 48. Dugovic C, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330(1):142–151. [DOI] [PubMed] [Google Scholar]
- 49. Bonaventure P, et al. Evaluation of JNJ-54717793 a novel brain penetrant selective orexin 1 receptor antagonist in two rat models of panic attack provocation. Front Pharmacol. 2017;8:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bonaventure P, et al. A selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects. J Pharmacol Exp Ther. 2015;352(3):590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Merlo Pich E, et al. Orexin 1 receptor antagonists in compulsive behavior and anxiety: possible therapeutic use. Front Neurosci. 2014;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mang GM, et al. The dual orexin receptor antagonist almorexant induces sleep and decreases orexin-induced locomotion by blocking orexin 2 receptors. Sleep. 2012;35(12):1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rosenberg RS, et al. Strain differences in the sleep of rats. Sleep. 1987;10(6):537–541. [PubMed] [Google Scholar]
- 54. Oishi Y, et al. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc Natl Acad Sci USA. 2008;105(50):19992–19997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wimmer ME, et al. Aging in mice reduces the ability to sustain sleep/wake states. PLoS One. 2013;8(12):e81880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yoshida Y, et al. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14(7):1075–1081. [DOI] [PubMed] [Google Scholar]
- 57. Boddum K, et al. Cerebrospinal fluid hypocretin-1 (Orexin-A) level fluctuates with season and correlates with day length. PLoS One. 2016;11(3):e0151288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fox SV, et al. Quantitative electroencephalography within sleep/wake states differentiates GABAA modulators eszopiclone and zolpidem from dual orexin receptor antagonists in rats. Neuropsychopharmacology. 2013;38(12):2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ma J, et al. Electroencephalographic power spectral density profile of the orexin receptor antagonist suvorexant in patients with primary insomnia and healthy subjects. Sleep. 2014;37(10):1609–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Panagiotou M, et al. Differences in electroencephalographic non-rapid-eye movement sleep slow-wave characteristics between young and old mice. Sci Rep. 2017;7:43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Scheffzük C, et al. Selective coupling between theta phase and neocortical fast gamma oscillations during REM-sleep in mice. PLoS One. 2011;6(12):e28489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Huang MP, et al. Effects of eszopiclone and zolpidem on sleep-wake behavior, anxiety-like behavior and contextual memory in rats. Behav Brain Res. 2010;210(1):54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schöne C, et al. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep. 2014;7(3):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schöne C, et al. Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ. J Neurosci. 2012;32(36):12437–12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hangodi OU, et al. Orexin-A microinjection mediated food and water intake are antagonized by selective orexin-1 receptor antagonist in the bed nucleus of stria terminalis. Int Congr Ser. 2006;1291:141–144. [Google Scholar]
- 66. Smith MI, et al. Evidence implicating a role for orexin-1 receptor modulation of paradoxical sleep in the rat. Neurosci Lett. 2003;341(3):256–258. [DOI] [PubMed] [Google Scholar]
- 67. Malherbe P, et al. Biochemical and electrophysiological characterization of almorexant, a dual orexin 1 receptor (OX1)/orexin 2 receptor (OX2) antagonist: comparison with selective OX1 and OX2 antagonists. Mol Pharmacol. 2009;76(3):618–631. [DOI] [PubMed] [Google Scholar]
- 68. Morairty SR, et al. Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS One. 2012;7(7):e39131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Inostroza M, et al. Sleep for preserving and transforming episodic memory. Annu Rev Neurosci. 2013;36:79–102. [DOI] [PubMed] [Google Scholar]
- 70. Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. [DOI] [PubMed] [Google Scholar]
- 71. Rasch B, et al. About sleep’s role in memory. Physiol Rev. 2013;93(2):681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. [DOI] [PubMed] [Google Scholar]
- 73. Walker MP, et al. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. [DOI] [PubMed] [Google Scholar]
- 74. Djonlagic I, et al. Increased sleep fragmentation leads to impaired off-line consolidation of motor memories in humans. PLoS One. 2012;7(3):e34106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Berdyyeva T, et al. Zolpidem reduces hippocampal neuronal activity in freely behaving mice: a large scale calcium imaging study with miniaturized fluorescence microscope. PLoS One. 2014;9(11):e112068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44(1):109–120. [DOI] [PubMed] [Google Scholar]
- 77. van Strien NM, et al. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10(4):272–282. [DOI] [PubMed] [Google Scholar]
- 78. Roehrs T, et al. Sedative, memory, and performance effects of hypnotics. Psychopharmacology (Berl). 1994;116(2):130–134. [DOI] [PubMed] [Google Scholar]
- 79. Stranks EK, et al. The acute cognitive effects of zopiclone, zolpidem, zaleplon, and eszopiclone: a systematic review and meta-analysis. J Clin Exp Neuropsychol. 2014;36(7):691–700. [DOI] [PubMed] [Google Scholar]
- 80. Mintzer MZ, et al. Selective effects of zolpidem on human memory functions. J Psychopharmacol. 1999;13(1):18–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.