Abstract
Background
Treatment options for soft tissue sarcoma (STS) patients aged ≥65 years (elderly) can be limited by concerns regarding the increased risk of toxicity associated with standard systemic therapies. Trabectedin has demonstrated improved disease control in a phase III trial (ET743-SAR-3007) of patients with advanced liposarcoma or leiomyosarcoma after failure of anthracycline-based chemotherapy. Since previous retrospective analyses have suggested that trabectedin has similar safety and efficacy outcomes regardless of patient age, we carried out a subgroup analysis of the safety and efficacy observed in elderly patients enrolled in this trial.
Patients and methods
Patients were randomized 2 : 1 to trabectedin (n = 384) or dacarbazine (n = 193) administered intravenously every-3-weeks. The primary end point was overall survival (OS); secondary end points were progression-free survival (PFS), time-to-progression, objective response rate (ORR), duration of response, symptom severity, and safety. A post hoc analysis was conducted in the elderly patient subgroup.
Results
Among 131 (trabectedin = 94; dacarbazine = 37) elderly patients, disease characteristics were well-balanced and consistent with those of the total study population. Treatment exposure was longer in patients treated with trabectedin versus dacarbazine (median four versus two cycles, respectively), with a significantly higher proportion receiving prolonged therapy (≥6 cycles) in the trabectedin arm (43% versus 23%, respectively; P = 0.04). Elderly patients treated with trabectedin showed significantly improved PFS [4.9 versus 1.5 months, respectively; hazard ratio (HR)=0.40; P = 0.0002] but no statistically significant improvement in OS (15.1 versus 8.0 months, respectively; HR = 0.72; P = 0.18) or ORR (9% versus 3%, respectively; P = 0.43). The safety profile for elderly trabectedin-treated patients was comparable to that of the overall trabectedin-treated study population.
Conclusions
This subgroup analysis of the elderly population of ET743-SAR-3007 suggests that elderly patients with STS and good performance status can expect clinical benefit from trabectedin similar to that observed in younger patients.
Trial registration
Keywords: elderly, soft tissue sarcomas, trabectedin
Key Message
This subgroup analysis of patients aged ≥65 years treated in a phase III trial of trabectedin versus dacarbazine in advanced liposarcoma and leiomyosarcoma demonstrates that trabectedin can be a reasonably safe option for elderly patients, and they can derive similar benefit to younger patients.
Introduction
Soft tissue sarcomas (STSs) are uncommon malignancies arising from tissues of mesenchymal origin [1] and comprise <1% of adult cancers [2]. Nearly, half of STS patients are aged >60 years at diagnosis [3]. Compared with younger STS patients, those aged ≥65 years (elderly) tend to have increased tumor size and grade at diagnosis and a worse prognosis [4] with higher local/distant recurrence rates [5]. Due to concerns regarding physiologic function and therapy tolerance, elderly patients often receive less intensive treatment [3, 4, 6]. Elderly sarcoma patients often present with significant comorbidities [7], including cardiovascular (e.g. hypertension, ischemic heart disease, venous thromboembolic events) [5, 8, 9], renal, and respiratory disease [10].
Elderly patients are also underrepresented in clinical trials, with limited data to guide management. Systemic therapy is the mainstay of treatment of advanced/metastatic disease, but the potential toxicities require careful consideration in the elderly population [8, 11, 12]. Given the specific issues faced by elderly STS patients, a clear need exists for prospectively collected data documenting the efficacy and tolerability of systemic therapies in this age group.
Trabectedin is an antineoplastic alkaloid that binds the DNA minor groove and bends it toward the major groove [13, 14], resulting in inhibition of DNA transcription and repair. It also impacts tumor-associated macrophages and pro-inflammatory factors, such as tumor-associated chemokines and cytokines [13, 15]. The phase II STS-201 trial lead to the approval of trabectedin in the European Union in 2007 for patients with advanced, unresectable STS following failure of anthracycline and ifosfamide or those considered unsuitable for those agents [16]. The US approval was based on a prospective, randomized phase III trial (ET743-SAR-3007), comparing trabectedin to dacarbazine in patients with metastatic or unresectable liposarcoma or leiomyosarcoma (LPS/LMS) following a prior anthracycline-containing regimen. Trabectedin was associated with a 45% reduction in risk of disease progression or death, with a median progression-free survival (PFS) of 4.2 months (versus 1.5 months in the dacarbazine arm) [17].
Previous retrospective studies [3, 18] and single case reports [8] have reported that trabectedin has similar efficacy and tolerability in elderly compared with younger STS patients. We report the elderly subgroup analysis of the safety and efficacy of trabectedin and dacarbazine in the ET743-SAR-3007 trial. The aim of this analysis was to provide oncologists with information and an evidence base for treatment selection for managing elderly STS patients.
Patients and methods
Patients
Inclusion and exclusion criteria for eligibility in this trial have been reported [17]. Briefly, eligible patients were aged ≥15 years with histologically proven unresectable, locally advanced, or metastatic LPS (pleomorphic, dedifferentiated, or myxoid) or LMS who were previously treated with at least: (i) a regimen containing an anthracycline and ifosfamide, or (ii) an anthracycline and ≥1 additional cytotoxic chemotherapy regimen(s). Review boards at all participating institutions approved the trial, which was conducted according to the Helsinki Declaration of the World Medical Association. All patients provided written informed consent to participate.
Study design
ET743-SAR-3007 was a phase III, randomized, multicenter, open-label, parallel-group international trial conducted between 27 May 2011 and 5 January 2015. Patients were randomized 2 : 1 to either trabectedin or dacarbazine as previously reported [17]. Treatment setting (inpatient versus outpatient) was at the investigator’s discretion. Safety and efficacy assessments were conducted as previously reported and as described in the supplementary materials, available at Annals of Oncology online. The primary end point of the trial was overall survival (OS); secondary end points included PFS, objective response rate (ORR), time-to-progression, DOR, and safety. Clinical benefit rate (CBR) and duration of stable disease (SD) were analyzed to evaluate prolonged disease control. Time to initiation of subsequent anticancer therapy (including surgery and radiation) was carried out as an exploratory analysis (supplementary methods, available at Annals of Oncology online).
Statistical analyses
This exploratory analysis included all elderly patients who were randomized for the efficacy end points and all elderly patients who received ≥1 dose of study drug for the safety end points. The treatment effectiveness comparisons for OS and PFS were carried out using unstratified log-rank test. A Cox proportional hazard model was used to estimate hazard ratio for OS and PFS. The ORR and CBR were evaluated using Fisher’s exact test. Safety data were summarized descriptively. The secondary end points of PFS, ORR, DOR, and CBR were carried out using data collected at the time of the interim analysis (clinical cut-off: 16 September 2013), which was carried out after 189 death events had occurred in the overall study population. At that time, 115 elderly patients had been randomized to the trial, and 69 PFS events had occurred in elderly patients. The final OS and safety analyses were carried out using data collected after 381 death events had occurred in the overall study population (clinical cut-off: 5 January 2015), at which time, 86 death events had occurred in elderly patients.
Results
Patient disposition and baseline demographics
By the clinical cut off for final OS analysis (5 January 2015), 577 patients had been randomized (384 trabectedin, 193 dacarbazine) and 550 treated (378 trabectedin, 172 dacarbazine). Of those randomized, 131 (94 trabectedin, 37 dacarbazine) were elderly (23% of study population), with ages ranging from 65 to 81 years. Ninety-three and 35 elderly patients were treated with trabectedin and dacarbazine, respectively; all but one patient (trabectedin arm) discontinued treatment, with disease progression the most common reason for discontinuation in each arm (60% and 86%, respectively).
Demographics and disease characteristics were generally balanced across treatment arms and were consistent with those of the overall study population. Among elderly patients, median ages were 69 and 70 years for the trabectedin and dacarbazine arms, compared with 57 and 56 years, respectively, in the overall study population. Prior therapy was similar between elderly patients and the overall study population; 84% and 95% of elderly patients in the trabectedin and dacarbazine arms, respectively, received ≥2 prior chemotherapy lines, and the median time from last disease progression to randomization was <1 month in both treatment arms (0.85 and 0.82 months, respectively). Among elderly patients, however, the distributions of LMS subgroups were disparate between the two treatment groups, with more than twice as many non-uterine LMS (52%) versus uterine LMS (23%) patients randomized to the trabectedin arm. Additional demographics and baseline characteristics are provided in Table 1.
Table 1.
Patient demographics and baseline disease characteristics
| Elderly (aged ≥65 years) patient subgroup (n = 131) |
All patients (n = 577) |
|||
|---|---|---|---|---|
| Dacarbazine (n = 37) | Trabectedin (n = 94) | Dacarbazine (n = 193) | Trabectedin (n = 384) | |
| Age, y | ||||
| Median (range) | 70 (65, 79) | 69 (65, 81) | 56 (17, 79) | 57 (18, 81) |
| Sex | ||||
| Women | 25 (68) | 50 (53) | 140 (73) | 262 (68) |
| Men | 12 (32) | 44 (47) | 53 (28) | 122 (32) |
| Race | ||||
| White | 28 (76) | 74 (79) | 144 (75) | 300 (78) |
| Black or African American | 2 (5) | 11 (12) | 22 (11) | 48 (13) |
| Asian | 3 (8) | 5 (5) | 11 (6) | 11 (3) |
| American Indian or Alaska Native | 1 (3) | 1 (1) | 4 (2) | 1 (0.3) |
| Baseline BMI, kg/m2 | ||||
| <30 | 27 (73) | 67 (71) | 124 (64) | 227 (59) |
| ≥30 | 10 (27) | 27 (29) | 69 (36) | 157 (41) |
| Median (range) | 28.1 (18.2, 39.7) | 26.4 (17.2, 44.4) | 27.5 (13.3, 66.7) | 28.1 (14.5, 78.1) |
| Baseline BSA, m² | 35 (95) | 93 (99) | 172 (89) | 378 (98) |
| Median (range) | 1.7 (1.4, 2.2) | 1.8 (1.3, 2.4) | 1.8 (1.3, 2.4) | 1.8 (1.3, 2.6) |
| Histology | ||||
| Leiomyosarcoma | 27 (73) | 71 (76) | 141 (73) | 282 (73) |
| Nonuterine | 14 (38) | 49 (52) | 53 (27) | 138 (36) |
| Uterine | 13 (35) | 22 (23) | 88 (46) | 144 (38) |
| Liposarcoma | 10 (27) | 23 (25) | 52 (27) | 102 (27) |
| Dedifferentiated | 8 (22) | 16 (17) | 28 (15) | 49 (13) |
| Myxoid±round cell | 1 (3) | 4 (4) | 19 (10) | 42 (11) |
| Pleomorphic | 1 (3) | 3 (3) | 5 (3) | 11 (3) |
| Baseline ECOG performance status score | ||||
| 0 | 18 (49) | 43 (46) | 93 (48) | 184 (48) |
| 1 | 19 (51) | 51 (54) | 100 (52) | 200 (52) |
| Previous therapies | ||||
| Chemotherapy | 37 (100) | 94 (100) | 193 (100) | 384 (100) |
| Surgery | 32 (86) | 85 (90) | 178 (92) | 363 (95) |
| Radiation | 17 (46) | 43 (46) | 89 (46) | 197 (51) |
| Lines of prior chemotherapy | ||||
| 1 | 2 (5) | 15 (16) | 26 (13) | 46 (12) |
| 2 | 21 (57) | 50 (53) | 83 (43) | 176 (46) |
| ≥3 | 14 (38) | 29 (31) | 84 (44) | 162 (42) |
| Time from initial diagnosis to randomization, months | ||||
| Median (range) | 37.3 (8.8, 267.1) | 32.5 (4.5, 300.6) | 27.3 (1.6, 267.1) | 32.8 (2.5, 318.5) |
| Time from last disease progression to randomization, months | ||||
| Median (range) | 0.8 (0.2, 8.7) | 0.9 (0.2, 13.7) | 0.9 (0.1, 9.8) | 0.9 (0.0, 13.7) |
Values are presented as n (%) unless otherwise specified.
BMI, body mass index; BSA, body surface area; ECOG, Eastern Cooperative Oncology Group.
Compared with the overall study population, chronic medical conditions were more frequently reported within the elderly subgroup, involving the musculoskeletal, cardiovascular, gastrointestinal, endocrine, and metabolic systems (supplementary Table S1, available at Annals of Oncology online). Elderly patients also reported more frequent use of analgesics, β blockers, antithrombotics, lipid-modifying drugs, diuretics, and renin–angiotensin system inhibitors before trial enrollment (supplementary Table S2, available at Annals of Oncology online).
Treatment exposure
Cumulative dose and dose intensities for both drugs were comparable between the elderly and overall study populations. In the elderly subgroup, the median number of treatment cycles in the trabectedin arm was twice that of the dacarbazine arm (four versus two cycles, respectively), consistent with the overall study population. The proportion of elderly patients achieving long-term disease control (defined as ≥6 cycles) was 43% in the trabectedin arm versus 23% in the dacarbazine arm, reflecting the overall study population. Elderly patients in the trabectedin arm received prolonged treatment exposures of 9 and 12 cycles (30% and 19%, respectively), similar to the overall study population. Cycle delays and dose reductions for elderly patients were more frequent for patients receiving trabectedin than dacarbazine. Dose reductions were reported in 53% of elderly patients in the trabectedin arm and 20% in the dacarbazine arm compared with 42% and 12%, respectively, in the overall study population (supplementary Table S3, available at Annals of Oncology online).
Efficacy
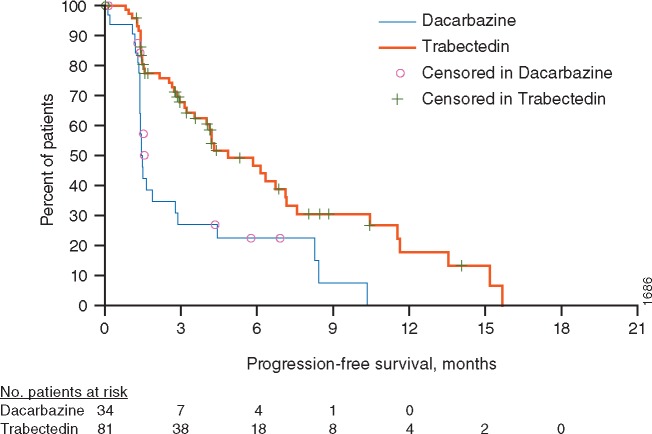
At the clinical cut-off for final PFS analysis (corresponding with interim OS analysis), 115 elderly patients were randomized (81 trabectedin, 34 dacarbazine). Among elderly patients, 54% and 74% reported progression-related events in the trabectedin and dacarbazine arms, respectively. Data were censored for 46% in the trabectedin arm and 26% in the dacarbazine arm to estimate PFS. Median PFS was 4.86 months (95% confidence interval [CI]: 3.55, 7.13) for trabectedin and 1.48 months (95% CI: 1.38, 2.79) for dacarbazine (Figure 1 and supplementary Table S4, available at Annals of Oncology online). The unstratified PFS analysis showed a significant improvement in the trabectedin arm, with an overall reduction in risk of disease progression or death by 60% compared with the dacarbazine arm [hazard ratio (HR) = 0.40; 95% CI: 0.24, 0.67; P = 0.0002], consistent with a significant improvement in PFS observed in the overall study population (HR = 0.55; 95% CI, 0.44–0.70; P < 0.0001). While no elderly patients achieved a complete response, 7 (9%) and 1 (3%) patients in the trabectedin and dacarbazine arms, respectively, achieved partial responses. Moreover, 72% and 44% of patients in the trabectedin and dacarbazine arms, respectively, achieved partial response or SD as best overall response; neither the increase in ORR [9% versus 3%; OR (95% CI) = 3.12 (0.37, 144.88; P = 0.433)] nor CBR [38% versus 21%; OR (95% CI)=2.39 (0.87, 7.26); P = 0.083] reached statistical significance in subgroup analyses (Table 2).
Figure 1.
Kaplan–Meier estimate of PFS in elderly (aged ≥65 years) patient subgroup. No., number.
Table 2.
Secondary efficacy end points
| Elderly (aged ≥65 years) patient subgroup (n = 115)a |
||
|---|---|---|
| Dacarbazine (n = 34) | Trabectedin (n = 81) | |
| Best overall response | ||
| CR | 0 | 0 |
| PR | 1 (3) | 7 (9) |
| SD | 14 (41) | 51 (63) |
| PD | 14 (41) | 15 (19) |
| Objective response rate (CR or PR) | 1 (3) | 7 (9) |
| Odds ratio (95% CI) | 3.12 (0.37, 144.88) | |
| P-value | 0.433 | |
| Clinical benefit rateb | 7 (21) | 31 (38) |
| Odds ratio (95% CI) | 2.39 (0.87, 7.26) | |
| P-value | 0.083 | |
For patients with a best overall response of SD, duration was defined as time from randomization to either date of initial documented PD or date of death, whichever occurred earlier.
Values are presented as n (%) unless otherwise specified.
Reflecting patients with baseline and ≥1 subsequent imaging timepoint.
CR, PR, or SD ≥18 weeks.
CI, confidence interval; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
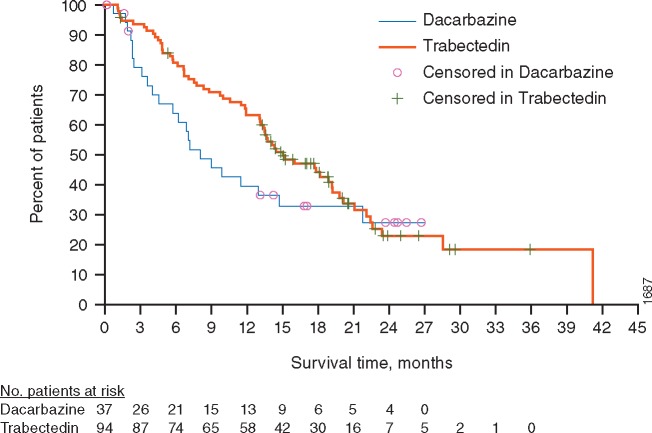
At the time of clinical cut-off for final OS analysis, 131 elderly patients had been randomized (94 trabectedin, 37 dacarbazine). A total of 86 elderly patient deaths occurred: 63 (67%) and 23 (62%) in the trabectedin and dacarbazine arms, respectively, with censoring of survival data for 31 (33%) patients in the trabectedin arm and 14 (38%) patients in the dacarbazine arm. Median OS was 15.1 months (95% CI: 13.14, 19.15) for the trabectedin arm and 8.1 months (95% CI: 4.57, 14.72) for the dacarbazine arm (Figure 2). The unstratified OS analysis, however, did not reach statistical significance, with an overall reduction in risk of death by 28% in the trabectedin arm compared with the dacarbazine arm (HR = 0.72; 95% CI: 0.45, 1.17; P = 0.181). Although the point estimate of HR was improved relative to the overall study population, the OS results of this smaller subgroup also failed to reach statistical significance, consistent with findings in the overall study population.
Figure 2.
Kaplan-Meier estimate of OS in elderly (aged ≥65 years) patient subgroup. No., number.
Among elderly patients, post-trial anticancer therapy use was common in both arms: 73% and 60% for the trabectedin and dacarbazine arms, respectively. The most common therapies (≥15% of patients) in the trabectedin arm were dacarbazine (30%), pazopanib (28%), and radiation (15%), while those in the dacarbazine arm were pazopanib (35%), gemcitabine (19%), and radiation (16%). Eribulin use as a subsequent therapy was not frequently reported in either arm (4% and 0% for the trabectedin and dacarbazine arms, respectively) (supplementary Table S5, available at Annals of Oncology online). Consistent with an increase in time to initiation of subsequent anticancer therapy observed in the overall study population [17, 19], a similar increase was observed in this smaller subgroup of elderly patients but did not reach statistical significance: median 6.9 versus 4.7 months for the trabectedin and dacarbazine arms, respectively [HR (95% CI)=0.62 (0.38, 1.01); P = 0.053].
Safety
Of the elderly patients, 97% and 94% reported a treatment-related AE in the trabectedin and dacarbazine arms, respectively. Incidence of grade 3–4 AEs were similar to those of the overall study population, reported in 79% and 66% of elderly patients in the trabectedin and dacarbazine arms, respectively, versus 81% and 57% percent of patients in the overall study population. Of grade 3–4 AEs observed, 70% and 51% were considered treatment-related for the trabectedin and dacarbazine arms, respectively, versus 69% and 40% among the overall study population. Serious AE (SAE) rates were reported in 45% and 31% of patients in the trabectedin and dacarbazine arms, respectively, versus 41% and 30% of patients in the overall study population. Of those, 34% and 14% were considered treatment-related in elderly patients in the trabectedin and dacarbazine arms, respectively, versus 21% and 11% among the overall study population. Of note, AEs leading to discontinuation that were considered treatment-related were increased (28% versus 9% for trabectedin versus dacarbazine, respectively) in the elderly compared with the overall study population (17% versus 8%) (supplementary Table S6, available at Annals of Oncology online).
No unique or unexpected toxicities were noted in this elderly subgroup. The most frequently experienced AEs in both arms were fatigue (76% and 57%) and nausea (74% and 49%). The majority of grade 3–4 toxicities were myelosuppression-related laboratory abnormalities and AEs for patients in either treatment arm as well as liver-related AEs in the trabectedin arm (Table 3). While neutropenia was reported more frequently in the trabectedin arm (50% and 31%, respectively), thrombocytopenia was more prevalent in the dacarbazine arm (37% and 51%, respectively), and anemia was similar in both arms (43% and 37%, respectively). Febrile neutropenia was infrequent (2% and 0% of trabectedin and dacarbazine patients, respectively).
Table 3.
Most commonly occurring adverse events among elderly (aged ≥65 years) patientsa
| Dacarbazine (n = 35) |
Trabectedin (n = 93) |
|||||
|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Total | Grade 3 | Grade 4 | Total | |
| Patients with treatment-emergent adverse events | 15 (43) | 8 (23) | 35 (100) | 44 (47) | 29 (31) | 93 (100) |
| Gastrointestinal disorders | ||||||
| Nausea | 1 (3) | 0 | 17 (49) | 9 (10) | 0 | 69 (74) |
| Constipation | 0 | 0 | 9 (26) | 0 | 0 | 39 (42) |
| Diarrhea | 0 | 0 | 9 (26) | 0 | 0 | 35 (38) |
| Vomiting | 0 | 0 | 8 (23) | 0 | 0 | 35 (38) |
| General disorders and administration site conditions | ||||||
| Fatigue | 2 (6) | 1 (3) | 20 (57) | 11 (12) | 0 | 71 (76) |
| Peripheral edema | 0 | 0 | 8 (23) | 0 | 0 | 26 (28) |
| Pyrexia | 0 | 0 | 6 (17) | 0 | 0 | 19 (20) |
| Investigations | ||||||
| Alanine aminotransferase increased | 0 | 0 | 0 | 20 (22) | 2 (2) | 38 (41) |
| Aspartate aminotransferase increased | 0 | 0 | 0 | 13 (14) | 1 (1) | 37 (40) |
| Blood alkaline phosphatase increased | 0 | 0 | 2 (6) | 0 | 0 | 22 (24) |
| Blood and lymphatic system disorders | ||||||
| Neutropenia | 6 (17) | 2 (6) | 11 (31) | 20 (22) | 17 (18) | 46 (50) |
| Anemia | 7 (20) | 1 (3) | 13 (37) | 17 (18) | 0 | 40 (43) |
| Thrombocytopenia | 6 (17) | 6 (17) | 18 (51) | 8 (9) | 11 (12) | 34 (37) |
| Leukopenia | 2 (6) | 2 (6) | 5 (14) | 19 (20) | 7 (8) | 34 (37) |
| Metabolism and nutrition disorders | ||||||
| Decreased appetite | 0 | 0 | 11 (31) | 0 | 0 | 45 (48) |
| Respiratory, thoracic, and mediastinal disorders | ||||||
| Dyspnea | 0 | 0 | 8 (23) | 0 | 0 | 24 (26) |
| Cough | 0 | 0 | 11 (31) | 0 | 0 | 20 (22) |
| Musculoskeletal and connective tissue disorders | ||||||
| Back pain | 2 (6) | 0 | 9 (26) | 1 (1) | 0 | 15 (16) |
Values are presented as n (%) unless otherwise specified. Adverse events reported any time from the first treatment dose to within 30 days of last treatment dose are included.
Occurring in ≥20% of patients.
Discussion
Case studies [8] and retrospective analyses [3, 18] have suggested that trabectedin may be an effective and tolerable option for elderly STS patients. Of the 577 patients randomized in the ET743-SAR-3007 trial, 131 were aged ≥65 years. The results of this subgroup analysis suggest similar outcomes between elderly patients and the overall population.
With a median PFS of 4.86 months versus 1.48 months (HR = 0.400; P = 0.0002) for patients in the trabectedin and dacarbazine arms, respectively, trabectedin significantly improved disease control in elderly patients with advanced LPS/LMS compared with dacarbazine, consistent with the overall trial population and other end points of disease control: ORR, DOR, and CBR.
At the time of the final OS analysis, prolonged study drug administration (≥6 cycles) among elderly patients in the trabectedin arm was observed almost twice as frequently as in the dacarbazine arm (43% versus 23%, respectively). Elderly patients were just as likely to achieve disease control of ≥12 cycles as the overall study population (19% versus 11% in the trabectedin versus dacarbazine arms), with the maximum treatment exposure of 36 cycles in the elderly subpopulation.
As with the overall study population, improved disease control did not result in a statistically significant improvement in OS. Use of post-trial anticancer therapy may have confounded the OS end point, as OS is a composite end point of all therapy administered [20, 21]. While findings from ad hoc analyses of OS in the overall study population were consistent with this hypothesis [19], the smaller size of the elderly subgroup prevents similar sensitivity analyses.
Toxicities observed in elderly patients treated with trabectedin were comparable in type and incidence to the overall study population, including grade 3–4 AEs reported in 79% and 66% of elderly patients in the trabectedin and dacarbazine arms, respectively; most toxicities were hepatic or hematologic. In light of the similar safety profile of trabectedin in the elderly subgroup, the increased rate of discontinuation due to drug-related AEs (28% versus 9% for trabectedin versus dacarbazine, respectively) compared with the overall study population (17% versus 8%) may reflect a more conservative approach by investigators in treating elderly patients. The greatest differences in rates of drug-related AEs leading to discontinuation, by system-organ class, were attributed to investigation-related AEs (13% versus 0% for trabectedin versus dacarbazine), primarily liver and bone marrow toxicities, and to musculoskeletal/connective tissue disorders (4% versus 0% for trabectedin versus dacarbazine), primarily muscular weakness, arthralgia, and myalgia. The higher rate of discontinuation, however, did not impair the ability to demonstrate an improvement in disease control with trabectedin.
In the overall trial population, an unexpected increased rate of cardiac-related AEs was observed among trabectedin-treated patients; however, objective declines in left ventricular ejection fraction were observed at similar rates in both arms (14% and 11% of assessed patients in the trabectedin and dacarbazine arms, respectively), though incomplete compliance and increased treatment exposure in trabectedin-treated patients may have confounded the findings. A post hoc multivariate analysis suggested that while treatment with trabectedin was not an independent risk factor, advanced age was suggested to present an increased risk in cardiac-related decline [22]. Therefore, monitoring for cardiac function is important in elderly trabectedin-treated patients who have been previously treated with anthracyclines.
We acknowledge the limitations of this study. Specifically, these findings reflect a retrospective analysis of data for a subgroup of elderly patients who were enrolled in the ET743-SAR-3007 trial rather than a prospective analysis designed to evaluate trabectedin in elderly patients (e.g. by incorporating a geriatric assessment tool). Consequently, all elderly patients were subjected to the same inclusion and exclusion criteria as the overall study population, thus limiting enrolled elderly patients to those who were sufficiently fit to meet those criteria and potentially excluding more fragile elderly STS patients. To compensate, we used descriptive statistics to assess for differences between elderly patients and their younger counterparts. This study demonstrated that elderly patients had increased rates of concomitant medication use and chronic medical conditions compared with the overall study population. Despite the limitations, these findings may serve as a basis for future prospective studies for data collection in older STS patients.
The clinical benefit observed with trabectedin in the elderly subgroup was consistent with the overall study population and with findings from previous retrospective analyses of trabectedin in patients aged >60 years [3, 18]. The higher rates of prolonged trabectedin exposure observed in the elderly subgroup and in the overall study population of ET743-SAR-3007 illustrate the tolerability of trabectedin. In conclusion, trabectedin is a reasonably tolerated and effective treatment option for elderly STS patients. These data provide practicing oncologists evidence regarding the safety/efficacy of trabectedin in elderly advanced sarcoma patients and indicate that participation of elderly STS patients in clinical trials should be encouraged.
Supplementary Material
Acknowledgements
This study was supported by Janssen Research & Development, LLC. In addition to those who participated as authors, we thank all site investigators for their involvements in the ET743-SAR-3007 study as well as the patients who participated. The authors also thank Gianna Paone, MS, of Janssen Scientific Affairs, LLC, for providing medical writing, editorial, and submission support.
Funding
This work was supported by Janssen Research & Development, LLC (grant number P30 CA006927).
Disclosure
RLJ has been a consultant for Janssen Oncology and PharmaMar. GD has received research support from Bayer, Pfizer, Novartis, and Janssen Oncology to Dana-Farber for his role as PI in clinical trial agreements in the Dana-Farber Cancer Institute sarcoma unit and has received consulting fees from Novartis, Pfizer, EMD-Serono, Sanofi Oncology, Janssen Oncology, PharmaMar, Daiichi-Sankyo, Adaptimmune, Eisai, and Epizyme. GD also reports a patent licensed to Novartis from Dana-Farber with royalty paid to Dana-Farber; membership on the Board of Directors for Blueprint Medicines; and scientific advisory board membership as well as consulting fees and minor equity for Blueprint Medicines, GI Therapeutics, and Caris Life Sciences. SMS served as a paid consultant to Janssen Oncology and received research funding from Janssen Oncology, Lilly, Plexicon, Daiichi Sankyo, AB Science, and Amgen. MM has served on advisory boards for Eisai, BMS, Genentech, Novartis, and EMD-Serono. AE reports having stocks <$10 000 for Johnson & Johnson, Merck, Lilly, Gilead, Pfizer, and Abbvie. BVT has served as an advisor and speaker for and paid consultant to Johnson & Johnson. John Hamm serves on a speakers’ board for Janssen and has received research funding from Janssen. SM, GW, TP, and RK are employees of Janssen and own stock in Johnson & Johnson. MH has been paid for consulting/advisory roles for Lilly, EMD Serono, and Janssen Oncology and has received research funding from Janssen Oncology. Additionally, MH is supported in part by the MSK Cancer Center Support Grant P30 CA008748. RM has received consulting fees (personal) and research support (institution) from Janssen and PharmaMar. SP has received grants from Janssen, Eisai, and Morphotek and has been a consultant for Janssen, Eisai, Morphotek, Bayer, EMD-Serono, Eli Lilly, Bayer, Epizyme, and Novartis. MvM served on the scientific leadership committee of the ET743-SAR-3007 study and has served as a paid consultant to Esai, CytRx, and Janssen.
References
- 1. Grimer R, Judson I, Peake D, Seddon B.. Guidelines for the management of soft tissue sarcomas. Sarcoma 2010; 2010: 506182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones NB, Iwenofu H, Scharschmidt T, Kraybill W.. Prognostic factors and staging for soft tissue sarcomas: an update. Surg Oncol Clin N Am 2012; 21(2): 187–200. [DOI] [PubMed] [Google Scholar]
- 3. Cesne AL, Judson I, Maki R. et al. Trabectedin is a feasible treatment for soft tissue sarcoma patients regardless of patient age: a retrospective pooled analysis of five phase II trials. Br J Cancer 2013; 109(7): 1717–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garbay D, Maki RG, Blay JY. et al. Advanced soft-tissue sarcoma in elderly patients: patterns of care and survival. Ann Oncol 2013; 24(7): 1924–1930. [DOI] [PubMed] [Google Scholar]
- 5. Lahat G, Dhuka AR, Lahat S. et al. Complete soft tissue sarcoma resection is a viable treatment option for select elderly patients. Ann Surg Oncol 2009; 16(9): 2579–2586. [DOI] [PubMed] [Google Scholar]
- 6. Biau DJ, Ferguson PC, Turcotte RE. et al. Adverse effect of older age on the recurrence of soft tissue sarcoma of the extremities and trunk. J Clin Oncol 2011; 29(30): 4029–4035. [DOI] [PubMed] [Google Scholar]
- 7. Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A. et al. Prevalence and prognostic impact of comorbidity in soft tissue sarcoma: a population-based cohort study. Acta Oncol 2014; 53(9): 1188–1196. [DOI] [PubMed] [Google Scholar]
- 8. Maruzzo M, Brunello A, Diminutto A. et al. Long-term response to first-line trabectedin in an elderly female patient with a metastatic leiomyosarcoma unfit for anthracycline. Anticancer Drugs 2016; 27(3): 264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shantakumar S, Connelly-Frost A, Kobayashi MG. et al. Older soft tissue sarcoma patients experience increased rates of venous thromboembolic events: a retrospective cohort study of SEER-Medicare data. Clin Sarcoma Res 2015; 5(1): 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mir O, Domont J, Cioffi A. et al. Feasibility of metronomic oral cyclophosphamide plus prednisolone in elderly patients with inoperable or metastatic soft tissue sarcoma. Eur J Cancer 2011; 47(4): 515–519. [DOI] [PubMed] [Google Scholar]
- 11. Lorigan P, Verweij J, Papai Z. et al. Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 2007; 25(21): 3144–3150. [DOI] [PubMed] [Google Scholar]
- 12. Judson I, Verweij J, Gelderblom H. et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 2014; 15(4): 415–423. [DOI] [PubMed] [Google Scholar]
- 13. D'Incalci M, Galmarini CM.. A review of trabectedin (ET-743): a unique mechanism of action. Mol Cancer Ther 2010; 9(8): 2157–2163. [DOI] [PubMed] [Google Scholar]
- 14. Zewail-Foote M, Hurley LH.. Ecteinascidin 743: a minor groove alkylator that bends DNA toward the major groove. J Med Chem 1999; 42(14): 2493–2497. [DOI] [PubMed] [Google Scholar]
- 15. Di Giandomenico S, Frapolli R, Bello E. et al. Mode of action of trabectedin in myxoid liposarcomas. Oncogene 2014; 33(44): 5201–5210. [DOI] [PubMed] [Google Scholar]
- 16. Demetri GD, Chawla SP, von Mehren M. et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol 2009; 27(25): 4188–4196. [DOI] [PubMed] [Google Scholar]
- 17. Demetri GD, von Mehren M, Jones RL. et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016; 34(8): 786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoiczyk M, Grabellus F, Podleska L. et al. Trabectedin in metastatic soft tissue sarcomas: role of pretreatment and age. Int J Oncol 2013; 43(1): 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Demetri GD, Patel SR, Thomas S. et al. Efficacy and safety of trabectedin or dacarbazine for treatment of patients with advanced leiomyosarcoma or liposarcoma after prior chemotherapy. Poster Presented at European Cancer Congress 2015; 25–29 September 2015, Vienna, Austria.
- 20. Zer A, Prince RM, Amir E, Abdul Razak A.. Evolution of randomized trials in advanced/metastatic soft tissue sarcoma: end point selection, surrogacy, and quality of reporting. J Clin Oncol 2016; 34(13): 1469–1475. [DOI] [PubMed] [Google Scholar]
- 21. Peugniez C, Cousin S, Penel N.. Trabectedin is an effective second-line treatment in soft tissue sarcoma patients. Ann Oncol 2016; 27(3): 551–552. [DOI] [PubMed] [Google Scholar]
- 22. Schuetze SM, Patel SR, Von Mehren M. et al. Cardiac safety analysis of trabectedin vs. dacarbazine in patients with advanced leiomyosarcoma or liposarcoma after prior anthracycline chemotherapy. Poster Presented at American Society of Clinical Oncology Annual Meeting 2016; 3–7 June 2016, Chicago, IL.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.