Abstract
While average human life expectancy has increased dramatically in the last century, the maximum life span has only modestly increased. These observations prompted the notion that human life span might have reached its maximal natural limit of ~115 years. To evaluate this hypothesis, we conducted a systematic analysis of all-cause human mortality throughout the 20th century. Our analyses revealed that, once cause of death is accounted for, there is a proportional increase in both median age of death and maximum life span. To examine whether pathway targeted aging interventions affected both median and maximum life span, we analyzed hundreds of interventions performed in multiple organisms (yeast, worms, flies, and rodents). Three criteria: median, maximum, and last survivor life spans were all significantly extended, and to a similar extent. Altogether, these findings suggest that targeting the biological/genetic causes of aging can allow breaking the currently observed ceiling of human maximal life span.
Keywords: Life span, Longevity, Pathway Targeted Interventions
Questions surrounding maximum human life span and whether its upper limit is fixed or flexible have been topics of continuous debate (1–3). Are we restricted by the natural genetic makeup of our species, which limits us to a maximum potential life span of about 120 years? In search of an answer to this longstanding question, we look at life span variabilities seen across other animal species. For example, a laboratory mouse does not typically exceed the age of 3 years, while a tortoise may live upwards of 190 years. But, is it possible to challenge these genetic constraints?
In recent decades, medical advancements have resulted in dramatic increases in life expectancy (4). However, while this is translated into a substantial increase in average human life span (the average age of death), only a moderate increase in maximum life span [defined here as the age of the oldest 10% of the population (5)] and maximal life span (the maximum reported age of death) has been observed. For instance, the maximal age at death reported among the Swedish population increased only 7% between 1860 and 1990 [from 101 to 108 years, respectively (6)]. Similarly, Dong et al. (7) report that, in France between the years 1900 and 2014, there was an 82% increase in average life expectancy; climbing from the age of 45 in 1900 to the age of 82 in 2014. However, the age of the world’s oldest person has not changed since the 1990s, despite the dramatic growth in human life span during this time.
The compelling discrepancy between average and maximum life span imply that an upper limit to human life span may have been reached. Interestingly, Dong et al. (7), calculated the probability of exceeding the current maximum age at death and concluded that it was highly unlikely. Based on these findings, they determined that the maximal age barrier for humans is ~115 years (7).
Could the moderate increases in maximum life span mean that we have reached a plateau, or have approached the ceiling? Is it possible that we have already reached the upper possible limits of human life span? It seems that simple analysis of average life expectancy data might support this hypothesis (7). For a more thorough evaluation, we conducted a systematic analysis of the causes of human mortality throughout the 20th century and, once cause of death was controlled, discovered that there is a proportional increase in median age of death as well as maximum life span.
To date, most medical advancements have focused primarily on symptomatic medicine (e.g. insulin injections) or on primary and secondary preventative measures (e.g. immunizations). While such approaches have helped considerably in extending human life span, they do not prevent or delay the inevitable onset of aging or age-related diseases. However, it is possible that applying pathway-directed interventions, or those directly targeting the aging process, could further increase maximal human life span. Targeted aging interventions are established in various animal models. Yet, it is not clear if these increase maximum life span to a substantial equal degree as that of the median and whether they also contribute to further extend the age of death of the oldest individuals in the cohort. Here, we tested hundreds of interventions reported to increase longevity in multiple organisms and explore their effect on the above-mentioned parameters and whether these parameters are comparable in a consistent manner.
To this end, we examined the results of numerous genetic, pharmacological, and nutritional targeted interventions performed in multiple organisms (yeast, worms, flies, and rodents). These interventions consistently affected the treated populations, impacting mean and maximum life spans, as well as the longest-lived individuals. These life span targeted interventions have not yet been implemented in humans. However, data extrapolated from the abovementioned studies predicts a continual rise in the maximal life span of humans, potentially by as much as 30%.
Materials and Methods
Resources
Causes of death.
In 1900, 1950, and 2014 were extracted from Centers for Disease Control and Prevention (http://www.cdc.gov/nchs/data/hus/hus15.pdf#019; https://www.cdc.gov/nchs/data/dvs/lead1900_98.pdf, 2016) with their original disease categories. These diseases were later categorized as age-related, infectious disease or other as can be seen in Supplementary Table 1.
Human demographics data.
Of countries with reported data in the database of years 2010, 1950, or 1900 were all drawn from the Human Mortality Database (http://www.mortality.org, 2016).
Genetic interventions data.
Of studies showing increased life span and their respective % change in maximum and median (or mean—as a combined category) were drawn as reported from GenAge (http://genomics.senescence.info/genes/, 2016), in Mus musculus; C. elegance; S. cerevisia; and Drosophila melanogaster.
Pharmacologic interventions data.
Of studies showing increased life span were drawn from the Life span Observations Database (http://lifespandb.sageweb.org/, 2016) in Mus musculus; C. elegance; and Rattus norvegicus.
Dietary restriction data.
Of studies showing increased life span were drawn from Swindell, 2012 meta-analysis in Mus musculus; and Rattus norvegicus in numerous studies and strains.
Statistics
Normality and distribution measures.
Included coefficients of variation—cv; Kolmogorov–Smirnov test; Q–Q plots; and the F-test for equality of variance between two compared distributions.
Differences between median (or mean) and maximum life span.
Was performed using the student t-test, as can be applied given the typical normality distribution of these interventions (see Figure 2C; Supplementary Figures 2D–F and Figures 3A and C).
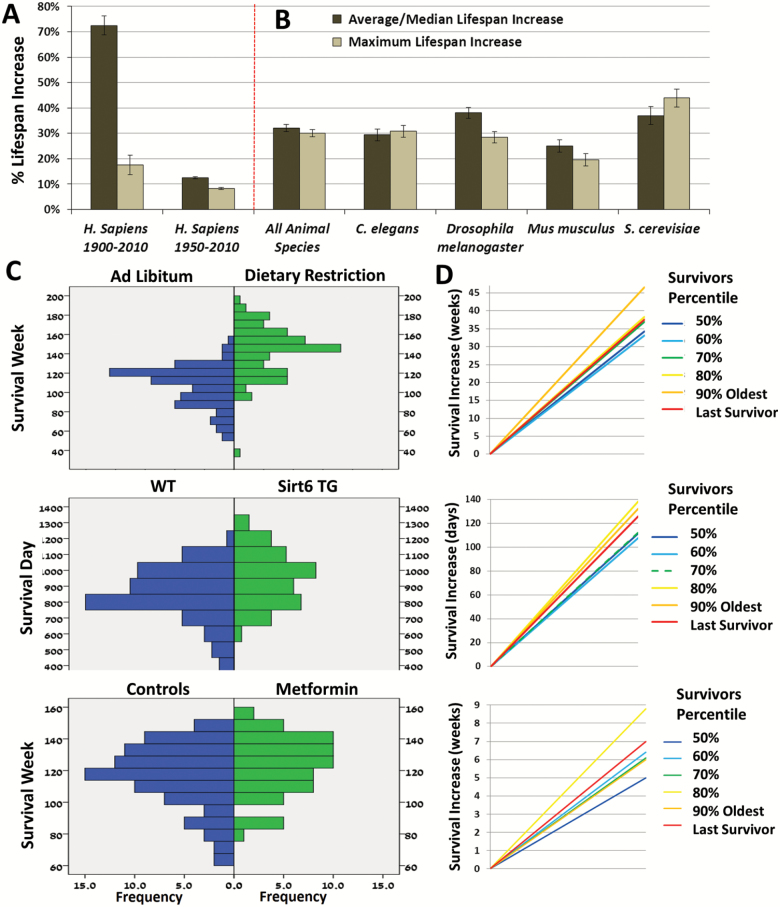
Figure 2.
Life span distribution increases across species. (A) The mean % increases in average life span and maximum life span in (from left to right): human 1900 and 2010 maximum 90% percentile verses standard average life span increase in all countries with data from 1900 in the Human Mortality Database (10 countries). The % increase was calculated as (2010–1900)/average of 1900; Human 1950 and 2010 maximum 90% percentile verses median > age 3 life span increase in all countries with data from 1950 in the Human Mortality Database (23 countries). The % increase was calculated as (2010–1950)/median > age 3 of 1950; Data are represented as mean of countries data ± SEM. (B) Across species showing increased life span in the GenAge database using the GenAge categories of average or median (combined) and maximum life span change as reported in the dataset based on original studies N = 127 genetic interventions; in C. elegance N = 57 interventions; in Drosophila melanogaster N = 35 interventions; in Mus musculus N = 19 interventions; and S. cerevisiae N = 16 interventions. Data are represented as mean of studies data ± SEM. (C) Mortality distribution histograms of nutritional, genetic, and pharmacological interventions: in mice undergoing 20% calorie restriction, (15); in Sirt6 transgenic male mice (16); and male mice supplemented with 0.1% metformin (17). (D) The corresponding % change in life span in different aging percentiles and last survivor individual mice in CR mice; Sirt6 TG mice; and metformin supplemented mice.
Results
Human Mortality Throughout the 20th Century
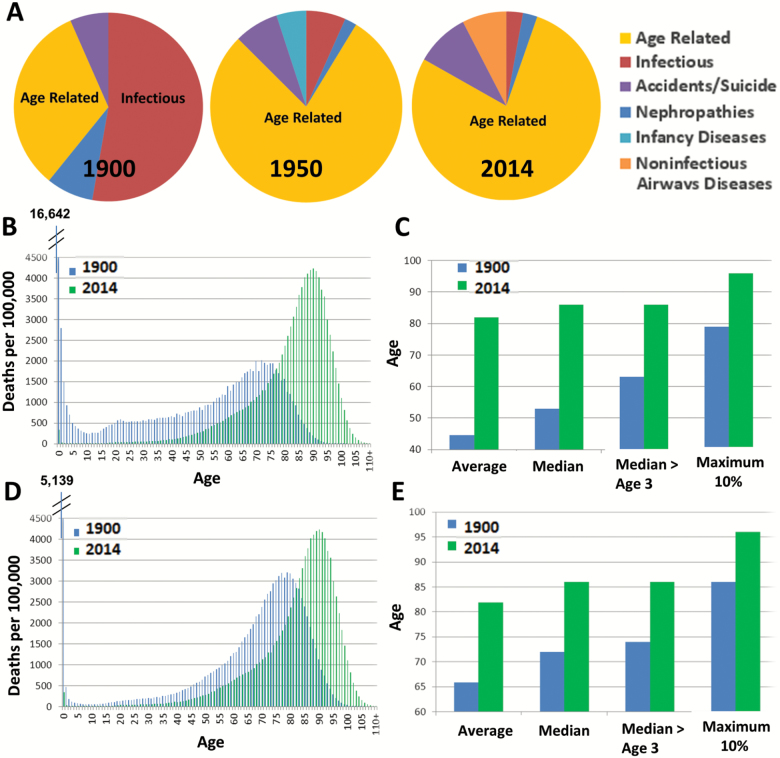
The dramatic increases in mean life span from 1900 till today, compared to the moderate increase in maximum life span, e.g. (7), have prompted our investigation into potential sources for this discrepancy. In order for such a comparison to be valid, it is crucial to verify that the proportions of deaths attributed to age-related diseases is comparable between these periods of analysis. Therefore, we examined the causes of human mortality during this time period. To this end, the top 10 causes of death for humans during the years 1900, 1950, and 2014 were evaluated and deaths were classified as relating to: (a) age, (b) infection, or (c) other (Figure 1A; Supplementary Table 1). Upon examination, the predominant cause of death in 1900 appears to be infection, with pneumonia, influenza, tuberculosis, and gastrointestinal infections being the most common. By contrast, in 1950 and 2014 deaths were most frequently age-related, sharing two primary contributing factors (i.e. heart diseases and cancers). The improvements in life span are likely due to the implementation of antibiotics and its widespread use following World War II, as well as extensive immunization programs. In 1900, the majority of the population were not dying from old age, indicating that the mean life expectancy was likely dramatically skewed and representative of a widely varying age at death.
Figure 1.
Human mortality distributions and causes of death throughout the 20th–21st Century. (A) frequencies of the top 10 causes of death in 1900, 1950, and 2014, data from the Centers for Disease Control and Prevention. The diseases categorized as infections were Pneumonia, influenza, tuberculosis, gastrointestinal infections, and diphtheria. The diseases categorized as age-related were Heart disease, Stroke, Cancer, Senility, Alzhemier’s diseases, Diabetes, and General Arteriosclerosis. (B, D) Calculated deaths per 100,000 individuals in France in 1900 and 2014 (B) and 1950 and 2014 (D) extracted from the Human Mortality Database. (C, E) Average; median; median > age of 3; and the maximum 90th percentiles (> age of 3) of the 1900 to 2014 (C) and 1950 to 2014 (E) mortality distributions.
In order to gain further insight into the distribution of deaths in 1900 France, we plotted the mortality distribution and compared it to France’s 2014 mortality tables (obtained from the “Human Mortality Database,” (8)). As seen in Figures 1B and Supplementary Figure 1a, the 1900 distribution is indeed greatly skewed to the left, and widely variable, as indicated by a high coefficient of variation (cv = 67.61%) in comparison to the 2014 distribution (cv = 18.09%). Yet, both deviate from a normal distribution (p < 10–256, Kolmogorov–Smirnov test; Supplementary Figures 2A and B), and exhibit unequal variance (p < 10–256, F-test). The highly variable mortality distribution biases both the average and median dramatically, most particularly in 1900 before the prevalent use of antibiotics. A major contributor to the skewed distribution is the rate of early childhood mortality, which was significantly reduced during the last century ((9), and Supplementary Table 1). In order to obtain a less biased life span measurement, we calculated the median life span for the portion of the population that survived past the age of 3 years, thus excluding early childhood mortality (Figure 1C, Supplementary Figure 1B). Once early childhood mortality is excluded, the increase in median life span becomes more comparable to the increase in maximum life span. Specifically, between the years 1900 and 2014, maximum life span increased by 17 years and median life span (for > 3 years of age) increased by 23 years (a difference of 6 years, as opposed to a 37-year difference between the standard averages).
To assess changes in life span among populations with closer mortality distributions, we also examined the differences between 1950 and 2014 (Figure 1D, Supplementary Figure 1C). Consistent with our prediction, the 1950 distribution appears to be more homogeneous compared to 1900 (cv = 34.35%). Yet, as shown in Supplementary Figure 2C, it also deviates from a fully symmetric normal distribution (Kolmogorov–Smirnov test reveals p < 10–256), and still exhibits unequal variance with the 2014 distribution. In these more comparable populations, the maximum life span increase of 10 years was largely comparable to the median (>age of 3) increase of 12 years (Figure 1E, Supplementary Figure 1D). The equivalent increases between 1900, 1950, and 2014, across multiple countries, reiterated the result from the France data (see Figure 2A, left panel).
Though maximum life span did experience almost comparable gains during this lengthy time period (an increase of 10 years in France over ~60 years, or roughly 10% across countries), the gains are modest. Thus, the question whether we may be reaching the natural genetic limits of maximal human life span is still valid. To approach this question, we need to consider the fact that medical advancements that took place following the introduction of antibiotics have been primarily focused on symptomatic treatments (e.g. cardiac valve replacements, insulin injections, etc.). Although symptomatic medicine considerably extends lives, it does not prevent or delay the onset of disease. In fact, we argue that, in order for the increases in maximum life span to continue progressing, we should target the mechanisms underlying aging rather than the subsequent treatment of diseases and their symptoms (see also (10) and (11)).
Over the last 35 years, extensive molecular aging research has been conducted in multiple species, with remarkable success. Genetic, nutritional, and pharmacological interventions have all been deployed to target pathways involved in the basic biology of aging, with the goal of extending life span. In fact, these targeted-interventions extended life span by as much as 30% in organisms ranging from yeast cells, nematodes, flies, and mammals [for review see (12,13)]. Yet, to date, none of the interventions we investigated have been implemented in humans. Only recently preparations for an aging targeted clinical trial in humans have been made [see e.g. metformin TAME initiative; (14)]. Despite its potential to increase median human life span, it is unclear whether these interventions will have similar effects on maximum life span. Specifically, will these interventions also affect those who make it to extreme old age?
Aging Interventions Increase Median and Maximum Life span Across Species
To evaluate the efficacy of targeted approaches at extending all life span parameters, we examined the effect of various longevity interventions in multiple species. To this end, we first assessed whether these targeted interventions have a homogeneous contribution over the entire distribution. To do so, we examined the raw mortality distributions from dietary restriction (DR) studies performed using murine models. Dietary restriction is one of the most studied natural interventions. Once on a DR diet, mice were identified as having better metabolic measurements and increased life spans compared to ad libitum fed controls (15). Unlike the skewed human mortality distributions discussed earlier, the distribution of DR and control animal mortalities follow a normal bell curve shape (Supplementary Figure 2D; Figure 2C, top panel; cv = 18.04%; also, portraying equal variance with the controls distribution, p > .05, F-test). This may indicate that, while sporadic mortality still affects the human population, laboratory mice are a protected population that reaches old age almost in its entirety. Thus, differences in mean/median life span are less biased in this species and, as a result, fluctuations in mean/median and maximum life span can be reliably compared.
Indeed, an analysis of the increments across aging percentiles indicate that the longest-lived group exhibited a roughly comparable increase in life span (with an overall increase of 36% in the maximum oldest 90th percentiles and 31% in median, Figure 2D, top panel). Additionally, these results portray an analogously substantial increase, even in the longest lived, last surviving individual mouse. Almost identical patterns of results were observed in a plot of the mortality distribution for a long-lived genetic intervention overexpressing Sirt6 in mice ((16); Figures 2C and D middle panel; Supplementary Figure 2E). Moreover, these results were replicated with a pharmacological intervention applying the commonly used human diabetic drug metformin [(17); Figures 2C and D, bottom panel; Supplementary Figure 2F]. Finally, a genetic intervention overexpressing the stress resistance gene HCM1, comparably extends life span even among organisms evolutionarily distant from humans, such as S. cerevisiae [(18); Supplementary Figures 3A–C].
In order to verify that the effects we observed are consistent in multiple studies and interventions, we systematically compared these mean and maximum life span increases with published results from other similar interventions. To obtain relevant data, we first accessed a large database of aging interventions and their manipulated genes (19). Data was extracted from all interventions noted to have a positive effect on life span in various species. The analyses revealed comparable increases in mean and maximum life spans (Figure 2B, Supplementary Tables 2–5). The cumulative increase across species were 32.07% in mean/median life span and 30.14% in maximum life span (p > .05, student t-test). Interestingly, the 30% increase in life span observed in animal studies applying genetic interventions, is three times larger than the life span increases that occurred in humans over the last 60 years.
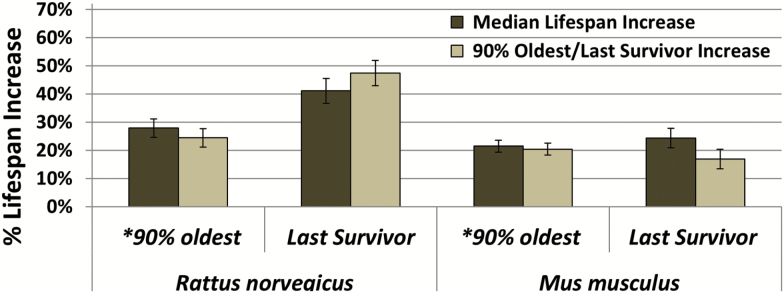
Apart from genetic manipulations, DR is one of the most studied interventions with robust life span and health span extending capabilities across species (20,21). Therefore, data was extracted from a large meta-analysis that includes over 150 DR studies performed in a variety of rodent strains (22). Analysis included data from all studies showing any increase in life span (Supplementary Tables 6–7). Once again, there was a comparable increase in median and maximum life span (90th percentile) in rats and mice (p > .05, t-test and p > .05, t-test, respectively) (see Figure 3 for percent changes in life span). Importantly, comparable increases were also evident between the oldest surviving individuals in each of the cohorts.
Figure 3.
Effects of nutritional dietary restriction on median and maximum life span. The mean % increases in median life span and maximum life span of all studies showing increased life span in dietary restriction as published in the meta-analysis of (22) in Rattus norvegicus (left panel) compared to the *maximum 90% percentile (21 studies), 75% percentile (1 study) or average of last 10% survivors (5 studies); and in studies comparing to the last individual survivors in the cohort (23 studies; second panel); and in Mus musculus (right panels) compared to the *maximum 90% percentile (12 studies), average of last 10% survivors (15 studies), or last 20% survivors (3 studies); and in studies comparing to the last individual survivors in the cohort (79 studies; last panel). The % increase was calculated as (dietary restriction – control)/average of control. Data are represented as mean of studies data ± SEM.
To conclude our analysis, differences in median and maximum life span resulting from pharmacological interventions were compared as well. It is worth noting that such interventions are almost certainly the most applicable to humans in the near future. For this reason, we extracted all pharmacological interventions that appeared in the Life span Observation Database (23), that had annotations for such interventions in various species. In published pharmacological intervention studies, maximum life span increased by 22.4% and mean life span by 19.1% (p > .05, t-test; see Table1). Overall, nutritional, genetic, and pharmacological interventions affect median and maximum life span in a comparable manner.
Discussion
In the last century, we have experienced a dramatic increase in human life span. However, this increase was mainly attributed to changes in median life expectancy, with minimal gains in maximum life span. This suggests that humanity might have reached its maximum life span potential due to inherent constraints of our species (e.g. 7). In order to explore the possibility of breaking the ceiling of human life span, we systematically analyzed human mortality data and results from numerous aging experiments applied across multiple species. Our investigation revealed the following: (1) only 30% of the frequent deaths in 1900 were age-related, but consistently accounted for 80% following 1950; (2) between 1900 and 2010, the increase in median life span (72%) was fourfold higher than the increase in maximum life span (18%); (3) yet, between 1950 and 2010 the increase in median life span (13%) was approximately comparable to the increase in maximum life span (8%); (4) in yeast, nematode, flies, mice, and rats introduction of genetic, nutritional, or pharmacological interventions increased median, maximum, and last survivor life span comparably; (5) in the last 60 years, the gain in human life span was only ~10%, but was ~30% in model organisms after applying the abovementioned targeted interventions. These findings strongly suggest that employing similar interventions in humans has the potential to significantly extend median and maximum life span.
The significant differences in median and maximum life span gains observed from 1900 to today, and their relative absence from 1950 to today, suggests that these differences probably do not stem from constraints on the maximal human life span. Rather, a simpler explanation is to implicate the shift in cause of death after modern medical advancements, which have enabled the majority of the population to reach old-age. To date, the modern medical approach toward aging has two distinct characteristics: (a) it focuses on symptomatic medicine in reaction to a medical condition, and (b) it is typically initiated following the onset of symptoms (i.e. it is reactive rather than proactive). Yet, targeted interventions, whether genetic, nutritional, or pharmacological, directly treat the mechanisms underlying the aging process. Given the improved health parameters associated with such interventions (24–27), it is likely that they affect health span in addition to life span. Moreover, the finding that such interventions do not predominantly affect only a specific portion of the distribution, or merely those who die early and are less resilient, imply that the entire distribution may actually become “younger.”
Animal studies, therefore, offer an optimistic assessment for the future of the human species. Yet, the optimal age at initiation and duration of such interventions remain open questions. Many aging theories, such as the antagonism pleotropic (28) and the hyper-function (29) theories, suggest that pathways that are important for youthful development are continuously active later in life, resulting in aging. In particular, anabolic pathways, such as mTOR and IGF1, would play a crucial role in the translation of nutrients to growth at an early age, but their high activity during adulthood results in age-related pathologies. Thus, such interventions should most likely be initiated after reaching adulthood to avoid harm during early development. Interestingly, the effect of DR was stronger when started in young adults compared to an old-onset (30). However, Miller et al. (31) showed that the effect of rapamycin, an mTOR inhibitor, had similar results whether started at 270 or 600 days, both are after adulthood in mice (Table 1). It is clear, that a suitable age for beginning such interventions has yet to be determined. Hence, though animal studies offer great potential for applying such interventions in humans, substantial research is still required in order to evaluate the timing, dosage, efficacy, and safety of these treatments in humans.
Table 1.
Effects of Pharmacological Interventions on Mean and Maximum Life Span
| Species | Pharmacological Intervention | Condition | Quantity | Average Life Span Increase | Maximum Life Span Increase | Ref. |
|---|---|---|---|---|---|---|
| Mus musculus | Rapamycin | Females starting 600 days | 2.24 mg/kg/day | 13% | 14% | (32) |
| Mus musculus | Rapamycin | Males starting 600 days | 2.24 mg/kg/day | 9% | 9% | (32) |
| Mus musculus | Rapamycin | Females starting 9 months | 2.24 mg/kg/day | 18% | 13% | (31) |
| Mus musculus | Rapamycin | Males starting 9 months | 2.24 mg/kg/day | 10% | 16% | (31) |
| Mus musculus | Phenformin | Starting 3.5 months | 5 mg/mouse 5 doses/week | 21% | 28% | (33) |
| Rattus norvegicus | Phenformin | Starting 3.5 months | 5 mg/rat/day | 0% | 10% | (33) |
| Rattus norvegicus | Buformin | Starting 3.5 months | 5 mg/rat/day | 7% (NS) | 12% | (33) |
| Mus musculus | Metformin | Female HER-2/neu mice | 100 mg/kg 5 doses/week | 8% | 13% | (34) |
| Mus musculus | Metformin | Starting 3 months | 100 mg/kg | 14% | 3% | (35) |
| Rattus norvegicus | Marine collagen peptides | Male + female | 2.25% | 10% | 11% | (36) |
| Rattus norvegicus | Marine collagen peptides | Male + female | 4.50% | 8% | 10% | (36) |
| Rattus norvegicus | Marine collagen peptides | Male + female | 9% | 11% | 11% | (36) |
| C.elegans | Fibril-binding flavonoid ThT | N2 worms | 50 µM | 43% | 58% | (37) |
| C.elegans | N-acetyl-cysteine | N2 worms | 10 mM | 10% (NS) | 3% (NS) | (38) |
| C.elegans | Paraquat (Hormesis) | N2 worms | 0.1 mM | 58% | 48% | (38) |
| C.elegans | Glaucarubinone | N2 worms | 100 nM | 9% | 9% | (39) |
| C.elegans | Ethosuximide | N2 worms | 2mg/ml | 13% | 24% | (40) |
| C.elegans | Ethosuximide | N2 worms | 4mg/ml | 17% | 22% | (40) |
| C.elegans | Trimethadione | N2 worms | 4mg/ml | 47% | 57% | (40) |
| C.elegans | Trimethadione | N2, starting from L4 | 24% | 29% | (40) | |
| C.elegans | 3,3-diethyl-2- pyrrolidinone | N2 worms | 2mg/ml | 31% | 49% | (40) |
| Across species | Average | Average | ||||
| 19.1% | 22.4% | |||||
| Mus musculus | 13% | 14% | ||||
| Rattus norvegicus | 7.2% | 10.8% | ||||
| C. elegans | 30% | 37% |
Note: Pharmacological interventions that increased life span as appearing in the Life span Observation Database and which also had reference to maximum life span data. The % increase was calculated as (dietary restriction – control)/ median of control or if available extracted as published from the original paper.
Regarding the length of the intervention, Fontana and his colleagues (41), investigated the dynamics of C. elegance nematodes life span in response to multiple laboratory interventions and reported a temporal scaling of mortality. This suggests that if such treatments are applied before the onset of mortality, even a transient intervention may cause a shift in the entire life span distribution. Importantly, a natural example of calorie restriction exists in human old Okinawans (aged over 65) which consumed ~11% less calories in their natural diets for almost half their adult lives (before western diets became more prevalent). This natural calorie restriction resulted in reports of fewer health pathologies in old Okinawans, and longer life spans compared to other Japanese or Americans. More importantly, this CR like diet in humans extends both average and maximum life spans with greater differences in maximum life span (42,43). While, the ~30% increase observed in numerous interventions are likely a representation of the more robust interventions in animals with larger effect sizes; additional interventions with lower effect sizes may potentially be discovered with larger sample size experiments in humans. It is important to note that targeted interventions in animals have unveiled multiple pathways, and some have distinct and separate contributions. Hence, applying multiple interventions on parallel routes could potentially have an additive effect on life span (44,45). Taken together, in addition to the persistence of advancements in medicine, this evidence suggests that application of aging-focused interventions will result in a continued increase in the median, maximum, and maximal life span in humans. Furthermore, it seems reasonable to posit that, in the foreseeable future, humans will likely breakout from the 115-year life span maximum.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by the Israel Science Foundation (#621/13 and 777/16), I-Core Foundation (#41/11), Israeli Ministry of Health (#3.9194), TEVA NNE Program (#PO 1237680), ESFD, D-Cure, Israel Cancer Association (#2016-0103), ICRF and the BSF. Moshe Shay Ben-Haim is supported by the Herczeg Institute on Aging.
Acknowledgments
We thank the members of the Cohen and Rechavi labs and especially to Shoshana Naiman for helpful comments on the manuscript. MSBH and HYC conceived the idea and designed the analyses with help from GR and RdC. MSBH conducted the analyses. YK, NM, and SJM performed life span experiments. MSBH and HYC wrote the paper with help from GR, BL, NA, and R.dC.
Conflict of Interest Statement
None declared.
References
- 1. Fries JF. Aging, natural death, and the compression of morbidity. 1980. Bull World Health Organ. 2002;80:245–250. [PMC free article] [PubMed] [Google Scholar]
- 2. Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296:1029–1031. doi:10.1126/science.1069675 [DOI] [PubMed] [Google Scholar]
- 3. Olshansky SJ, Carnes BA, Cassel C. In search of Methuselah: estimating the upper limits to human longevity. Science. 1990;250:634–640. [DOI] [PubMed] [Google Scholar]
- 4. Vaupel JW. Biodemography of human ageing. Nature. 2010;464:536–542. doi:10.1038/nature08984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang C, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on “maximum lifespan”. Mech Ageing Dev. 2004;125:629–632. doi:S0047-6374(04)00149-6 [pii] [DOI] [PubMed] [Google Scholar]
- 6. Wilmoth JR, Deegan LJ, Lundstrom H, Horiuchi S. Increase of maximum life-span in sweden, 1861–1999. Science. 2000;289:2366–2368. [DOI] [PubMed] [Google Scholar]
- 7. Dong X, Milholland B, Vijg J. Evidence for a limit to human lifespan. Nature. 2016;538:257–259. doi:10.1038/nature19793 [DOI] [PubMed] [Google Scholar]
- 8. Human mortality database. University of California, Berkeley (USA), and Max Planck Institute For Demographic Research (Germany) Available at www.mortality.org or www.humanmortality.de.
- 9. CDC - National Center for Health Statistics, Centers for Disease Control and Prevention Retrived from http://www.cdc.gov/nchs/data/hus/hus15.pdf#019;https://www.cdc.gov/nchs/data/dvs/lead1900_98.pdf.
- 10. Kennedy BK, Berger SL, Brunet A, et al. . Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi:10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sierra F. The emergence of geroscience as an interdisciplinary approach to the enhancement of health span and life span. Cold Spring Harb Perspect Med. 2016;6:a025163. doi:10.1101/cshperspect.a025163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD(+) and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17:679–690. doi:10.1038/nrm.2016.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Longo VD, Antebi A, Bartke A, et al. . Interventions to slow aging in humans: are we ready?Aging Cell. 2015;14:497–510. doi:10.1111/acel.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23:1060–1065. doi:10.1016/j.cmet.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, et al. . Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 2016;23:1093–1112. doi:10.1016/j.cmet.2016.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanfi Y, Naiman S, Amir G, et al. . The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi:10.1038/nature10815 [DOI] [PubMed] [Google Scholar]
- 17. Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. . Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi:10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maoz N, Gabay O, Waldman Ben-Asher H, Cohen HY. The yeast forkhead HCM1 controls life span independent of calorie restriction. J Gerontol A Biol Sci Med Sci. 2015;70:444–453. doi:10.1093/gerona/glu059 [DOI] [PubMed] [Google Scholar]
- 19. Tacutu R, Craig T, Budovsky A, et al. . Human ageing genomic resources: integrated databases and tools for the biology and genetics of ageing. Nucleic Acids Res. 2013;41(Database issue):D1027–D1033. doi:10.1093/nar/gks1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masoro EJ. Caloric restriction-induced life extension of rats and mice: a critique of proposed mechanisms. Biochim Biophys Acta. 2009;1790:1040–1048. doi:10.1016/j.bbagen.2009.02.011 [DOI] [PubMed] [Google Scholar]
- 21. Spindler SR. Rapid and reversible induction of the longevity, anticancer and genomic effects of caloric restriction. Mech Ageing Dev. 2005;126:960–966. doi:10.1016/j.mad.2005.03.016 [DOI] [PubMed] [Google Scholar]
- 22. Swindell WR. Dietary restriction in rats and mice: a meta-analysis and review of the evidence for genotype-dependent effects on lifespan. Ageing Res Rev. 2012;11:254–270. doi:10.1016/j.arr.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olsen B, Kaeberlein M. 2017. Lifespan observation database http://sageweb.org/lifespandb.
- 24. Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi:10.1038/nrm1616 [DOI] [PubMed] [Google Scholar]
- 25. Roichman A, Kanfi Y, Glazz R, et al. . SIRT6 overexpression improves various aspects of mouse healthspan. J Gerontol A Biol Sci Med Sci. 2017;72:603–615. doi:10.1093/gerona/glw152 [DOI] [PubMed] [Google Scholar]
- 26. Swindell WR. Meta-analysis of 29 experiments evaluating the effects of rapamycin on life span in the laboratory mouse. J Gerontol A Biol Sci Med Sci. 2017;72:1024–1032. doi:10.1093/gerona/glw153 [DOI] [PubMed] [Google Scholar]
- 27. Wu Q, Lian T, Fan X, et al. . 2,5-dimethyl-celecoxib extends drosophila life span via a mechanism that requires insulin and target of rapamycin signaling. J Gerontol A Biol Sci Med Sci. 2017;72:1334–1341. doi:10.1093/gerona/glw244 [DOI] [PubMed] [Google Scholar]
- 28. Kirkwood TB, Austad SN. Why do we age?Nature. 2000;408:233–238. doi:10.1038/35041682 [DOI] [PubMed] [Google Scholar]
- 29. Zimniak P. What is the proximal cause of aging?Front Genet. 2012;3:189. doi:10.3389/fgene.2012.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. [DOI] [PubMed] [Google Scholar]
- 31. Miller RA, Harrison DE, Astle CM, et al. . Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi:10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harrison DE, Strong R, Sharp ZD, et al. . Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi:10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anisimov VN, Khavinson VKh, Popovich IG, et al. . Effect of Epitalon on biomarkers of aging, life span and spontaneous tumor incidence in female Swiss-derived SHR mice. Biogerontology. 2003;4:193–202. [DOI] [PubMed] [Google Scholar]
- 34. Anisimov VN, Berstein LM, Egormin PA, et al. . Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–693. doi:10.1016/j.exger.2005.07.007 [DOI] [PubMed] [Google Scholar]
- 35. Anisimov VN, Berstein LM, Popovich IG, et al. . If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY). 2011;3:148–157. doi:10.18632/aging.100273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liang J, Pei XR, Wang N, Zhang ZF, Wang JB, Li Y. Marine collagen peptides prepared from chum salmon (Oncorhynchus keta) skin extend the life span and inhibit spontaneous tumor incidence in Sprague-Dawley rats. J Med Food. 2010;13:757–770. doi:10.1089/jmf.2009.1279 [DOI] [PubMed] [Google Scholar]
- 37. Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans PLoS Biol. 2010;8:e1000556. doi:10.1371/journal.pbio.1000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmeisser S, Zarse K, Ristow M. Lonidamine extends lifespan of adult Caenorhabditis elegans by increasing the formation of mitochondrial reactive oxygen species. Horm Metab Res. 2011;43:687–692. doi:10.1055/s-0031-1286308 [DOI] [PubMed] [Google Scholar]
- 39. Alavez S, Vantipalli MC, Zucker DJ, Klang IM, Lithgow GJ. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature. 2011;472:226–229. doi:10.1038/nature09873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Evason K, Huang C, Yamben I, Covey DF, Kornfeld K. Anticonvulsant medications extend worm life-span. Science. 2005;307:258–262. doi:10.1126/science.1105299 [DOI] [PubMed] [Google Scholar]
- 41. Stroustrup N, Anthony WE, Nash ZM, et al. . The temporal scaling of Caenorhabditis elegans ageing. Nature. 2016;530:103–107. doi:10.1038/nature16550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Willcox BJ, Willcox DC, Todoriki H, et al. . Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci. 2007;1114:434–455. doi:10.1196/annals.1396.037 [DOI] [PubMed] [Google Scholar]
- 43. Willcox DC, Willcox BJ, Todoriki H, Curb JD, Suzuki M. Caloric restriction and human longevity: what can we learn from the Okinawans?Biogerontology. 2006;7:173–177. doi:10.1007/s10522-006-9008-z [DOI] [PubMed] [Google Scholar]
- 44. Iser WB, Wolkow CA. DAF-2/insulin-like signaling in C. elegans modifies effects of dietary restriction and nutrient stress on aging, stress and growth. PLoS One. 2007;2:e1240. doi:10.1371/journal.pone.0001240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Raamsdonk JM, Meng Y, Camp D, et al. . Decreased energy metabolism extends life span in Caenorhabditis elegans without reducing oxidative damage. Genetics. 2010;185:559–571. doi:10.1534/genetics.110.115378 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.