ABSTRACT
Background
Insulin response may be important in colorectal cancer development. Diet modulates insulin response and may be a modifiable factor in colorectal cancer prevention.
Objective
We examined associations between hyperinsulinemic diets and colorectal cancer risk with the use of an empirical dietary index for hyperinsulinemia (EDIH), a food-based index that characterizes dietary insulinemic potential on the basis of circulating C-peptide concentrations.
Design
Diet was assessed every 4 y with food-frequency questionnaires in 46,210 men (Health Professionals Follow-Up Study, 1986–2012) and 74,191 women (Nurses’ Health Study, 1984–2012) to calculate EDIH scores. Multivariable-adjusted Cox regression was used to calculate HRs and 95% CIs for colorectal, proximal/distal colon, and rectal cancer risk.
Results
During 26 y of follow-up, we documented 2683 incident colorectal cancer cases. Comparing participants in the highest with those in the lowest quintiles, higher EDIH scores were associated with 33% (men: HR: 1.33; 95% CI: 1.11, 1.61; P-trend = 0.0005), 22% (women: HR: 1.22; 95% CI: 1.03, 1.45; P-trend = 0.01), and 26% (men and women: pooled HR: 1.26; 95% CI: 1.12, 1.42; P-trend <0.0001) higher risk of developing colorectal cancer. The positive associations were limited to the distal colon and rectum in men and to the distal and proximal colon in women; however, combined risk estimates were significant for all anatomic locations except for the rectum. For example, comparing participants in extreme EDIH quintiles, there was no significant association for proximal colon cancer in men (HR: 1.15; 95% CI: 0.84, 1.57; P-trend = 0.32), but the risk was elevated for distal colon (HR: 1.63; 95% CI: 1.14, 2.32; P-trend = 0.002) and rectal (HR: 1.63; 95% CI: 1.09, 2.44; P-trend = 0.01) cancer. Among women, the risk was elevated for proximal (HR: 1.28; 95% CI: 1.00, 1.63; P-trend = 0.03) and distal (HR: 1.46; 95% CI: 1.05, 2.03; P-trend = 0.03) colon cancer but not for rectal cancer (HR: 0.88; 95% CI: 0.60, 1.29; P-trend = 0.61).
Conclusion
The findings suggest that the insulinemic potential of diet may partly underlie the influence of dietary intake on colorectal cancer development. This observational study was registered at www.clinicaltrials.gov as NCT03364582.
Keywords: hyperinsulinemia, C-peptide, dietary patterns, colorectal cancer, BMI, physical activity
INTRODUCTION
Colorectal cancer, a global public health problem, was the second most commonly diagnosed cancer in men and the third most commonly diagnosed cancer in women globally in 2012 (1). In the United States, it is the third most commonly diagnosed cancer in both men and women (2). Insulin resistance and hyperinsulinemia may play important roles in cancer development, including colorectal cancer (3, 4). Insulin may influence cancer risk through the regulation of energy metabolism and may directly influence colorectal cancer development via its mitogenic or antiapoptotic activities (5–9). Epidemiologic studies have shown positive associations between circulating C-peptide concentrations (considered a valid marker of chronic insulin secretion) and colorectal cancer risk (10–13). Given that dietary intake has been linked to insulin resistance and hyperinsulinemia independently of body weight and physical activity (PA) (14, 15), a dietary pattern that is associated with hyperinsulinemia may be more predictive of colorectal cancer risk than individual foods or nutrients analyzed separately.
The glycemic index (GI) classifies carbohydrate-containing foods by their ability to increase postprandial blood glucose concentration relative to glucose or white bread (16). The GI therefore indirectly assesses immediate insulin responses to food intake. As an improvement on the GI, a food insulin index was developed to directly quantify postprandial insulin response (17). However, although repeated bouts of postprandial insulin increases may contribute to cumulative insulin exposure, the GI does not account for additional dietary factors that may affect underlying insulin resistance, which could ultimately be the more important determinant of long-term insulin exposure. Interestingly, the GI and insulin index did not predict fasting C-peptide concentrations in our study populations (17, 18). Previously, we derived a dietary pattern associated with C-peptide concentrations in a relatively small sample of women and found this pattern to be significantly associated with colorectal cancer risk in the same cohort (19). We improved this pattern by developing and independently validating an empirical dietary index for hyperinsulinemia (EDIH), a weighted dietary pattern score to assess the insulinemic potential of usual diets to reflect long-term insulin exposure (18).
To elucidate the role of the insulinemic potential of diet in colorectal cancer risk, we investigated the association between the empirical food-based dietary index for hyperinsulinemia score with colorectal cancer risk in 2 prospective cohort studies, one in men and the other in women. We further examined the association in subgroups of factors that have been associated with colorectal cancer risk, particularly those involved in the insulin resistance pathway, including body weight and PA (20).
METHODS
Study population
We used data from 2 ongoing prospective cohorts: the Nurses' Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS). The NHS recruited 121,701 registered female nurses aged 30–55 y at baseline in 1976, and the HPFS enrolled 51,529 male health professionals aged 40–75 y at baseline in 1986 in the United States. Since the inception of both cohorts, participants have completed self-administered questionnaires at baseline and every 2 y thereafter, providing updated information on medical, lifestyle, and other health-related factors. Every 2 to 4 y, participants receive validated semi-quantitative food-frequency questionnaires (FFQs) for dietary assessments (21).
For the current study, we used the FFQ in 1984 as the NHS baseline FFQ because the FFQ in 1980 was shorter than all subsequent FFQs. As shown in Supplemental Figure 1, we excluded participants who did not complete FFQs or who had implausible energy intake values (<600 or >3500 kcal/d for women and <800 or >4200 kcal/d for men; n = 42,736), who reported any cancer except for nonmelanoma skin cancer (n = 6911), who died or were diagnosed with colorectal cancer at or before baseline (n = 2484), and participants who reported ulcerative colitis at baseline (n = 395). This resulted in the inclusion of 74,191women from the NHS and 46,210 men from the HPFS for a total of 120,401 participants (Supplemental Figure 1). The characteristics of excluded participants were generally similar to those retained in the final analysis (Supplemental Table 1). The institutional review boards at Brigham and Women's Hospital and at Harvard TH Chan School of Public Health approved this study. This observational study was registered at www.clinicaltrials.gov as NCT03364582.
Assessment of the EDIH score and other covariates
The EDIH score, described in detail elsewhere (18), was developed in a sample of 5812 women in the NHS to empirically create a score to measure the insulinemic potential of whole diets defined with the use of food groups. Thirty-nine predefined food groups (servings per day) (22) were entered into stepwise linear regression models to identify a dietary pattern most predictive of circulating C-peptide concentrations. The EDIH score is a weighted sum of 18 food groups, with higher (more positive) scores indicating higher insulinemic diets (hyperinsulinemia) and lower (more negative) scores indicating lower insulinemic diets. In the NHS, there was a 40% increase in C-peptide concentrations among the women in EDIH quintile 5 compared with those in quintile 1 (adjusted relative concentration: 1.40; 95% CI: 1.32, 1.46) (18). The EDIH score was further evaluated for validity in 2 independent samples of women (NHS-II; n = 1717) and men (HPFS; n = 4002) and found to significantly predict C-peptide concentrations. For example, the adjusted relative concentration of C-peptide in the highest EDIH quintile compared with the lowest was 1.29 (95% CI: 1.22, 1.39) in men and 1.32 (95% CI: 1.21, 145) in women (18).
The food groups contributing to higher EDIH scores are as follows: red meat, low-energy beverages (low-energy cola and other low-energy carbonated beverages), cream soups, processed meat, margarine, poultry, French fries, fish (other than dark-meat fish), high-energy beverages (cola and other carbonated beverages with sugar, fruit drinks), tomatoes, low-fat dairy, and eggs. The food groups contributing to lower EDIH scores are as follows: wine, coffee, whole fruit, high-fat dairy products, and green leafy vegetables (18). We calculated EDIH scores for each participant on the basis of self-administered FFQ data in 8 data cycles from 1984 to 2010 in the NHS and in 7 data cycles from 1986 to 2010 in the HPFS.
Self-administered questionnaires were sent to participants every 2 y to assess medical and lifestyle factors other than diet (which was assessed every 4 y), including smoking habits, PA, alcohol intake, multivitamin use, endoscopy status, regular use of aspirin and other nonsteroidal anti-inflammatory drugs, family history of colorectal cancer, weight, and height, and menopausal status and postmenopausal hormone use (women only) in both cohorts, as previously described (22–24).
Colorectal cancer ascertainment
New colorectal cancer diagnoses were self-reported through biennial questionnaires, colorectal cancer–related deaths identified through family members, and the US National Death Index, and confirmed through medical records or cancer registry linkage. A physician reviewed medical and mortality records to confirm the diagnosis.
Statistical analysis
We calculated person-years of follow-up from the date of return of the first FFQ until the date of any cancer diagnosis (except for nonmelanoma skin cancer), death, or the end of follow-up (1 June 2012 for the NHS and 1 January 2012 for the HPFS), whichever came first. To reduce within-person variation and best represent habitual long-term dietary intake, we computed cumulative averages of EDIH scores from all previous questionnaires up to the start of each 2-y follow-up interval (dietary data were carried forward to nondiet 2-y data cycles). Then, EDIH scores were adjusted for total energy intake with the use of the residual method (25). Due to the high within-person correlations in EDIH scores between adjacent questionnaire cycles, we carried forward nonmissing dietary intake data from the previous questionnaire cycle to replace missing data in the next cycle. Covariate data (described below) were treated similarly.
We used Cox proportional hazards regression models with time-varying covariates to estimate HRs and 95% CIs for EDIH scores in relation to colorectal cancer risk, with the reference category being the lowest EDIH quintile. Early symptoms of undiagnosed colorectal cancer may alter habitual dietary intake. To address this potential bias, our main analytic approach utilized a 2-y lag between dietary assessment and incident colorectal cancer diagnosis. For example, in the HPFS, we used cumulative average EDIH scores from 1986 to 1990 as the exposure for the follow-up period from 1992 to 1994 and cumulative average score from 1986 to 1994 for follow-up from 1996 to 1998, etc. All of the analyses were stratified by age in months and calendar year of the current questionnaire. Multivariable models were additionally adjusted for risk factors for colorectal cancer, most of which were updated every 2 y. These included family history of colorectal cancer (yes or no), history of lower gastrointestinal endoscopy (yes or no), multivitamin use (yes or no), pack-years of smoking (continuous), alcohol intake (continuous; drinks per day), race (white or nonwhite), PA (continuous; metabolic equivalent task hours per week), regular nonsteroidal anti-inflammatory drug use (yes or no), and regular aspirin use (yes or no), and additionally for postmenopausal hormone use (yes or no) and menopausal status (pre- or postmenopausal) in women. These covariates were selected on the basis of previous studies of the association of dietary patterns, individual dietary and lifestyle factors, and colorectal cancer risk, and on literature reviews (e.g., meta-analyses, World Cancer Research Fund/American Institute for Cancer Research recommendations). Overall, we included known and suspected risk factors for colorectal cancer that may also be related to dietary intake. We checked each covariate for proportionality of hazards in the Cox models with the use of time × covariate interaction terms and found no violations (all P > 0.05).
Diabetes and BMI are possible intermediates in the association of hyperinsulinemia and colorectal cancer risk; therefore, we did not adjust for diabetes and BMI in the main analyses but additionally adjusted for these 2 covariates in sensitivity analyses. We used random-effects meta-analysis to pool HRs for men and women. For analyses of linear trend across EDIH quintiles, we used EDIH quintile medians as an ordinal variable. We used the likelihood ratio test in duplication-method cause-specific Cox models to test for heterogeneity in colorectal cancer risk by anatomic location (proximal colon, distal colon, and rectum) (26).
We tested whether any association between EDIH and colorectal cancer differed by categories of body weight and PA with the use of the likelihood ratio test, by comparing models with and without the interaction term of the EDIH score and potential effect modifier. Body weight [BMI (kg/m2)] was dichotomized at 25 (BMI: <25 and ≥25); and PA (metabolic equivalent task hours per week) was dichotomized at the study-specific median [<28.4 (men) or <13.4 (women) and ≥28.4 or ≥13.4]. We used sex-specific medians because of the differences in PA levels between men and women in these cohorts, with men reporting much higher levels than women. In a recent commentary (27), we proposed an integrative framework to describe how diet, PA, and body weight may act in an interactive manner to exert their influence on cancer risk. In additional multivariable-adjusted analyses, we assessed the association of the EDIH score and colorectal cancer risk in joint categories of BMI and PA as follows: high activity and lean (PA of median or higher and BMI <25), high activity and overweight or obese (PA of median or higher and BMI ≥25), low activity and lean (PA less than the median and BMI <25), and low activity and overweight or obese (PA less than the median and BMI ≥25). All of the analyses were performed by using SAS software, version 9.4 for Unix (SAS Institute) at a 2-sided P value of 0.05.
RESULTS
We documented 2683 incident colorectal cancer cases (1439 in women and 1244 in men) in >2,558,608 person-years of follow-up. Over the entire follow-up period in both cohorts (1986–2012 in the HPFS and 1984–2012 in the NHS), participants consuming the most-hyperinsulinemic diets (EDIH quintile 5) reported lower PA and higher BMI and were more likely to have diabetes. They also were less likely to be using multivitamins and reported lower intakes of dietary fiber, calcium, vitamin D, and whole grains than those consuming the most insulin-sensitive diets (quintile 1) (Table 1).
TABLE 1.
Distribution of participant characteristics (weighted by person-years) across the entire follow-up period, in quintiles of the EDIH score in the NHS (1984–2012) and the HPFS (1986–2012)1
| NHS (women) | HPFS (men) | |||||
|---|---|---|---|---|---|---|
| Characteristic | Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 |
| Median EDIH score | –1.15 | –0.03 | 1.18 | –1.18 | –0.02 | 1.19 |
| Age, y | 65.2 ± 9.72 | 63.9 ± 9.6 | 62.1 ± 9.6 | 64.0 ± 10.8 | 63.7 ± 10.8 | 60.3 ± 10.5 |
| Alcohol drinkers, % | 73.1 | 56.6 | 47.9 | 80.8 | 71.3 | 61.5 |
| Total alcohol among drinkers, drinks/wk | 7.3 ± 7.6 | 4.2 ± 5.4 | 3.9 ± 5.9 | 10.7 ± 10.1 | 7.1 ± 7.7 | 6.3 ± 7.5 |
| Current smoker, % | 11.8 | 11.7 | 15.5 | 4.0 | 4.6 | 6.4 |
| Regular aspirin use (yes), % | 61.6 | 60.5 | 59.8 | 45.9 | 45.7 | 40.4 |
| Family history of colorectal cancer (yes), % | 26.6 | 25.5 | 23.7 | 16.9 | 17.7 | 15.2 |
| History of endoscopy (yes), % | 23.6 | 21.8 | 16.4 | 24.6 | 24.8 | 20.2 |
| Multivitamin use (yes), % | 60.1 | 55.5 | 45.3 | 52.8 | 50.4 | 43.6 |
| Diabetes (yes), % | 2.5 | 5.3 | 8.7 | 3.8 | 6.5 | 9.3 |
| Total energy intake, kcal/d | 1850 ± 441 | 1667 ± 435 | 1821 ± 487 | 2107 ± 546 | 1879 ± 526 | 2086 ± 591 |
| Dietary fiber, g/d | 21.0 ± 6.5 | 19.2 ± 5.5 | 16.7 ± 4.9 | 26.2 ± 8.6 | 23.2 ± 7.0 | 19.7 ± 6.0 |
| Dietary calcium, mg/d | 821 ± 302 | 793 ± 302 | 697 ± 269 | 871 ± 319 | 862 ± 330 | 785 ± 314 |
| Vitamin D, IU/d | 211 ± 118 | 212 ± 118 | 188 ± 107 | 268 ± 161 | 264 ± 150 | 241 ± 136 |
| Whole grains, g/d | 27.8 ± 19.1 | 25.2 ± 18.4 | 17.4 ± 15.2 | 35.1 ± 24.6 | 31.5 ± 21.8 | 23.3 ± 18.3 |
| Physical activity, MET-h/wk | 22.5 ± 25.4 | 17.0 ± 20.2 | 14.2 ± 17.7 | 35.4 ± 29.6 | 31.3 ± 26.7 | 29.0 ± 25.4 |
| Median or higher physical activity,3 % | 58.7 | 48.3 | 43.0 | 55.1 | 49.5 | 47.1 |
| BMI, kg/m2 | 24.7 ± 4.0 | 26.3 ± 4.6 | 27.9 ± 5.8 | 24.4 ± 5.8 | 24.9 ± 6.3 | 25.8 ± 7.1 |
| Overweight or obese (BMI ≥25), % | 43.9 | 58.7 | 69.4 | 42.2 | 49.1 | 57.5 |
| Postmenopausal, % | 89.2 | 86.8 | 74.3 | NA | NA | NA |
| Hormone therapy use ever,4 % | 68.8 | 68.0 | 62.7 | NA | NA | NA |
1Weighted by follow-up time (person-years) accrued by each participant. EDIH scores were adjusted for energy intake using the residual method. Lower EDIH scores indicate insulin sensitive diets, and higher scores indicate hyperinsulinemic diets. EDIH, empirical dietary index for hyperinsulinemia; HPFS, Health Professionals Follow-Up Study; MET-h, metabolic equivalent task hours; NA, not applicable; NHS, Nurses’ Health Study.
2Mean ± SD (all such values)
3Median physical activity was 28.4 MET-h/wk in men and 13.4 MET-h/wk in women.
4Among postmenopausal women.
Results from the minimally adjusted model that included only age, alcohol intake, and calendar year of current questionnaire were generally similar to the multivariable-adjusted results (Supplemental Table 2). Comparing participants in the highest with the lowest EDIH quintile in multivariable-adjusted analyses, dietary insulinemic potential was associated with a 33% higher risk of colorectal cancer in men (HR: 1.33; 95% CI: 1.11, 1.61; P-trend = 0.0005), a 22% higher risk in women (HR: 1.22; 95% CI: 1.03, 1.45; P-trend = 0.01), and a 26% higher risk in men and women combined (pooled HR: 1.26; 95% CI: 1.12, 1.42; P-trend < 0.0001) (Table 2). Although there was no statistical evidence of heterogeneity in risk by anatomic location of cancer in both men (P-heterogeneity = 0.53) and women (P-heterogeneity = 0.48), HRs appeared to be different across anatomic subsites. For example, comparing participants in extreme EDIH quintiles, there were no significant findings for cancers located in the proximal colon in men (HR: 1.15; 95% CI: 0.84, 1.57; P-trend = 0.32), but the risk was much elevated for cancers in the distal colon (HR: 1.63; 95% CI: 1.14, 2.32; P-trend = 0.002) and rectum (HR: 1.63; 95% CI: 1.09, 2.44; P-trend = 0.01). Among women, the risk was elevated for cancers in the proximal (HR: 1.28; 95% CI: 1.00, 1.63; P-trend = 0.03) and distal (HR: 1.46; 95% CI: 1.05, 2.03; P-trend = 0.03) colon but not in the rectum (HR: 0.88; 95% CI: 0.60, 1.29; P-trend = 0.61). In pooling the HRs, there was no significant heterogeneity in risk between men and women for colorectal cancer overall and by subsite, except for the rectum (P-difference in rectal cancer risk by sex = 0.03) (Table 2). Sensitivity analyses additionally adjusting for BMI and type 2 diabetes—covariates potentially in the causal pathway between hyperinsulinemic diets and colorectal cancer risk—showed similar results (Supplemental Table 3).
TABLE 2.
Multivariable-adjusted HRs (95% CIs) for colorectal cancer risk by quintile of EDIH scores among men and women1
| Quintile 1 (reference) | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend2 | |
|---|---|---|---|---|---|---|
| Colorectal cancer | ||||||
| Men | ||||||
| Cases/person-years | 254/189664 | 234/190395 | 272/190593 | 249/190635 | 235/189983 | |
| HR (95% CI) | 1.00 | 0.94 (0.79, 1.13) | 1.15 (0.97, 1.37) | 1.12 (0.94, 1.34) | 1.33 (1.11, 1.61) | 0.0005 |
| Women | ||||||
| Cases/person-years | 298/320941 | 291/321026 | 294/321112 | 280/321696 | 276/322426 | |
| HR (95% CI) | 1.00 | 0.99 (0.84, 1.17) | 1.05 (0.89, 1.24) | 1.08 (0.92, 1.28) | 1.22 (1.03, 1.45) | 0.01 |
| Cases, n | 552 | 525 | 566 | 529 | 511 | |
| Pooled HR (95% CI)3 | 1.00 | 0.97 (0.86, 1.10) | 1.10 (0.97, 1.24) | 1.10 (0.97, 1.25) | 1.26 (1.12, 1.42) | <0.0001 |
| Colon cancer | ||||||
| Men | ||||||
| Cases (n = 984), n | 207 | 187 | 213 | 198 | 179 | |
| HR (95% CI) | 1.00 | 0.93 (0.76, 1.13) | 1.11 (0.91, 1.35) | 1.11 (0.91, 1.36) | 1.26 (1.03, 1.56) | 0.009 |
| Women | ||||||
| Cases (n = 1129), n | 229 | 226 | 227 | 219 | 228 | |
| HR (95% CI) | 1.00 | 0.99 (0.82, 1.19) | 1.04 (0.86, 1.25) | 1.10 (0.91, 1.33) | 1.32 (1.09, 1.60) | 0.003 |
| Cases, n | 436 | 413 | 440 | 417 | 407 | |
| Pooled HR (95% CI)3 | 1.00 | 0.96 (0.84, 1.10) | 1.07 (0.93, 1.23) | 1.10 (0.96, 1.27) | 1.29 (1.12, 1.49) | <0.0001 |
| Proximal colon cancer | ||||||
| Men | ||||||
| Cases (n = 424), n | 94 | 86 | 85 | 83 | 76 | |
| HR (95% CI) | 1.00 | 0.91 (0.67, 1.22) | 0.95 (0.70, 1.28) | 1.01 (0.74, 1.37) | 1.15 (0.84, 1.57) | 0.32 |
| Women | ||||||
| Cases (n = 714), n | 148 | 146 | 144 | 144 | 132 | |
| HR (95% CI) | 1.00 | 1.00 (0.79, 1.26) | 1.05 (0.83, 1.32) | 1.17 (0.92, 1.48) | 1.28 (1.00, 1.64) | 0.03 |
| Cases, n | 242 | 232 | 229 | 227 | 208 | |
| Pooled HR (95% CI)3 | 1.00 | 0.96 (0.80, 1.16) | 1.01 (0.84, 1.21) | 1.10 (0.91, 1.33) | 1.23 (1.01, 1.49) | 0.02 |
| Distal colon cancer | ||||||
| Men | ||||||
| Cases (n = 354), n | 66 | 60 | 90 | 71 | 67 | |
| HR (95% CI) | 1.00 | 0.98 (0.68, 1.40) | 1.56 (1.13, 2.17) | 1.28 (0.91, 1.82) | 1.63 (1.14, 2.32) | 0.002 |
| Women | ||||||
| Cases (n = 387), n | 71 | 75 | 81 | 70 | 90 | |
| HR (95% CI) | 1.00 | 1.03 (0.74, 1.43) | 1.14 (0.82, 1.58) | 1.04 (0.74, 1.46) | 1.46 (1.05, 2.03) | 0.03 |
| Cases, n | 137 | 135 | 171 | 141 | 157 | |
| Pooled HR (95% CI)3 | 1.00 | 1.00 (0.79, 1.28) | 1.33 (0.98, 1.82) | 1.15 (0.91, 1.47) | 1.54 (1.21, 1.96) | 0.0002 |
| Rectal cancer | ||||||
| Men | ||||||
| Cases (n = 260) n | 47 | 47 | 59 | 51 | 56 | |
| HR (95% CI) | 1.00 | 1.00 (0.67, 1.52) | 1.35 (0.91, 2.01) | 1.18 (0.79, 1.78) | 1.63 (1.09, 2.44) | 0.01 |
| Women | ||||||
| Cases (n = 310), n | 69 | 65 | 67 | 61 | 48 | |
| HR (95% CI) | 1.00 | 1.00 (0.71, 1.41) | 1.10 (0.78, 1.55) | 1.03 (0.72, 1.48) | 0.88 (0.60, 1.29) | 0.61 |
| Cases, n | 116 | 112 | 126 | 112 | 104 | |
| Pooled HR (95% CI)3 | 1.00 | 1.00 (0.77, 1.31) | 1.20 (0.93, 1.56) | 1.10 (0.84, 1.43) | 1.19 (0.65, 2.19)3 | 0.513 |
1EDIH scores were adjusted for total energy intake with the use of the residual method. Lower scores indicate insulin-sensitive diets and higher scores indicate hyperinsulinemic diets. Heterogeneity for risk by anatomic subsite was tested by using duplication-method, cause-specific Cox regression analyses (P-heterogeneity = 0.53 among men and 0.48 among women). All analyses were conducted by using Cox models and were adjusted for the following potential confounding variables: age, calendar year of current questionnaire, race, family history of cancer, history of endoscopy, multivitamin use, total alcohol intake, physical activity, pack-years of smoking, regular aspirin use, and regular NSAID use, and additionally for menopausal status and postmenopausal hormone use in women. EDIH, empirical dietary index for hyperinsulinemia; NSAID, nonsteroidal anti-inflammatory drug.
2The P value for linear trend across EDIH quintiles was the P value of the ordinal variable constructed by assigning the EDIH score quintile medians to all of the participants in the quintile. Cox models for linear trend were adjusted for all covariates listed in footnote 1.
3HRs for men and women were pooled by using random-effects meta-analyses and the likelihood ratio test was used to test for heterogeneity in risk between men and women for each anatomic subsite. The difference in risk by sex for the rectum was significant, P-heterogeneity = 0.03.
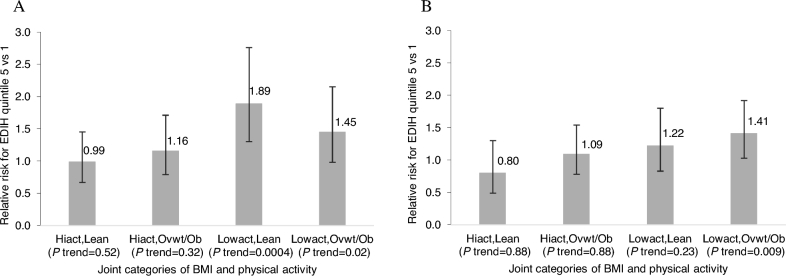
Although interactions were not significant, HRs for the association between dietary insulinemic potential and colorectal cancer risk were suggestively different for the PA subgroups. Results showed stronger associations among men (HR: 1.62; 95% CI: 1.24, 2.12; P-trend = 0.0003) and women (HR: 1.30; 95% CI: 1.03, 1.65; P-trend = 0.01) who reported low levels of PA, than among those who reported high levels of PA (Table 3). In the joint categories of body weight and PA, EDIH score was not associated with colorectal cancer risk among either men or women who were physically active and lean, whereas significant positive associations were stronger among men who had low PA levels and were also lean (HR: 1.89; 95% CI: 1.30, 2.76; P-trend = 0.002) or also overweight or obese (HR: 1.45; 95% CI: 0.98, 2.15; P-trend = 0.03) and among women who had low PA levels and were also overweight or obese (HR: 1.41; 95% CI: 1.03, 1.93; P-trend = 0.009) (Figure 1, Supplemental Table 4). These differences seem to be driven by PA.
TABLE 3.
Multivariable-adjusted associations of the EDIH score and colorectal cancer risk among men and women in subgroups of body weight and physical activity1
| EDIH score quintile | P | ||||||
|---|---|---|---|---|---|---|---|
| Subgroups | 1 (reference) | 2 | 3 | 4 | 5 | Trend2 | Interaction3 |
| BMI (kg/m2) | |||||||
| Men | 0.62 | ||||||
| <25 (n cases = 604) | 1.00 | 0.84 (0.66, 1.08) | 0.93 (0.72, 1.19) | 1.03 (0.80, 1.33) | 1.31 (1.01, 1.70) | 0.03 | |
| ≥25 (n cases = 638) | 1.00 | 1.01 (0.77, 1.33) | 1.36 (1.05, 1.76) | 1.20 (0.92, 1.56) | 1.32 (1.00, 1.73) | 0.02 | |
| Women | 0.54 | ||||||
| <25 (n cases = 580) | 1.00 | 0.96 (0.76, 1.22) | 1.07 (0.83, 1.36) | 1.14 (0.88, 1.48) | 1.04 (0.78, 1.40) | 0.42 | |
| ≥25 (n cases = 859) | 1.00 | 1.00 (0.79, 1.26) | 1.00 (0.79, 1.25) | 1.01 (0.81, 1.28) | 1.27 (1.01, 1.59) | 0.03 | |
| Physical activity4 (MET-h/wk; below/above cohort-specific median) | |||||||
| Men | 0.13 | ||||||
| <28.4 (n cases = 638) | 1.00 | 1.09 (0.83, 1.42) | 1.31 (1.01, 1.69) | 1.24 (0.95, 1.61) | 1.62 (1.24, 2.12) | 0.0003 | |
| ≥28.4 (n cases = 604) | 1.00 | 0.84 (0.65, 1.08) | 1.04 (0.81, 1.33) | 1.07 (0.83, 1.38) | 1.14 (0.88, 1.48) | 0.15 | |
| Women | 0.14 | ||||||
| <13.4 (n cases = 820) | 1.00 | 1.04 (0.82, 1.31) | 1.03 (0.82, 1.31) | 1.21 (0.96, 1.52) | 1.30 (1.03, 1.65) | 0.01 | |
| ≥13.4 (n cases = 619) | 1.00 | 0.91 (0.72, 1.15) | 1.03 (0.81, 1.30) | 0.87 (0.67, 1.13) | 0.99 (0.75, 1.30) | 0.81 | |
1EDIH scores were adjusted for total energy intake with the use of the residual method. All of the analyses were conducted by using Cox models and except when stratifying by the potential effect modifier were adjusted for the following potential confounding variables: race, family history of cancer, history of endoscopy, multivitamin use, total alcohol intake, physical activity, pack-years of smoking, regular aspirin use, and regular NSAID use, and additionally for menopausal status and postmenopausal hormone use in women. EDIH, empirical dietary index for hyperinsulinemia; MET-h, metabolic equivalent task hours; NSAID, nonsteroidal anti-inflammatory drug.
2The P value for linear trend across EDIH quintiles was the P value of the ordinal variable constructed by assigning the EDIH score quintile medians to all of the participants in the quintile. Cox models for linear trend were adjusted for all covariates listed in footnote 1.
3The P value for interaction was the Wald P value of the interaction term.
4We used cohort-specific medians to categorize physical activity (MET-h/wk) because of the differences in physical activity levels between men and women, with men reporting much higher levels than women.
FIGURE 1.
RRs from Cox regression models for the association of the EDIH score and colorectal cancer development for men (A) and women (B) in the highest compared with the lowest EDIH quintile within strata or groups classified jointly by categories of BMI (kg/m2) and physical activity (MET-hours per week). Analyses were adjusted for age (months), calendar month of current questionnaire, family history of cancer, history of endoscopy, multivitamin use, total alcohol intake, physical activity, pack-years of smoking, regular aspirin use, and regular nonsteroidal anti-inflammatory drug use, and additionally for menopausal status and postmenopausal hormone use in women. P values for the 3-way interaction between the EDIH score, physical activity, and BMI were 0.20 in men and 0.09 in women. The P value for linear trend across EDIH quintiles was the P value of the ordinal variable constructed by assigning the EDIH score quintile medians to all participants in the quintile and adjusted for all listed potential confounding variables. EDIH, empirical dietary index for hyperinsulinemia; Hiact, high physical activity level; Lean, normal body weight (BMI <25); Lowact, low physical activity level; MET, metabolic equivalent task; Ovwt/Ob, overweight or obese (BMI ≥25).
DISCUSSION
We applied a food-based dietary index in a large prospective study in men and women to characterize the ability of whole diets to contribute to hyperinsulinemia and to elucidate the role of the dietary insulinemic potential in colorectal cancer development. Our findings showed that the intake of diets with hyperinsulinemic potential was associated with a higher risk of developing colorectal cancer in men and women. The positive associations appeared to be limited to the distal colon and rectum in men and to the distal colon and proximal colon in women; however, combined risk estimates were significant for all anatomic locations except for the rectum. Furthermore, in both men and women, the influence of hyperinsulinemic diets appeared to be stronger among those who reported low levels of PA, although interactions were not significant.
Our group previously examined associations between circulating C-peptide concentrations and colorectal cancer risk in several case-control studies nested within the NHS and HPFS (28–30). Wei et al. (28) reported an OR of 1.76 (95% CI: 0.85, 3.63) for colorectal cancer risk comparing NHS women in extreme C-peptide quartiles, whereas Wu et al. (29) found a 37% higher risk of colorectal cancer (HR: 1.37; 95% CI: 1.05, 1.78) comparing women and men above the median C-peptide concentrations with those below the median. Ma et al. (30) also found a 150% higher risk of colorectal cancer comparing men in extreme C-peptide quintiles in the Physicians’ Health Study (HR: 2.5; 95% CI: 1.5, 5.6), even after adjusting for BMI, insulin-like growth factor (IGF) I, and IGF binding protein. Results in the current analysis closely align with results from our group's previous study in which the original unweighted C-peptide dietary pattern (19) was applied to examine colorectal cancer risk in women in the NHS. That study found a 29% higher risk of colorectal cancer comparing the highest with the lowest dietary index quintiles (HR: 1.29; 95% CI: 1.05, 1.58; P-trend = 0.048) in up to ∼20 y of follow-up and 985 colorectal cancer cases. The same comparison among a combined category of overweight or obese and sedentary women showed a 58% (P-trend = 0.002) higher risk (19). Similarly, in validation studies of the EDIH score, we found that, in men, there were differences in C-peptide concentrations across EDIH quintiles stratified by combined categories of BMI and PA, with overweight or obese and sedentary men having the highest C-peptide concentrations across all EDIH quintiles compared with overweight or obese and active men or with lean and sedentary men or with lean and active men (18).
Although interactions were not significant, results from the subgroup analyses suggested that adiposity and PA may influence the association of a hyperinsulinemic dietary pattern and colorectal cancer risk. Indeed, energy balance factors (body weight and PA) and metabolic factors (e.g., insulin, adiponectin, inflammatory cytokines) are correlated (27); therefore, insulin can be a marker of metabolic dysfunction, and a useful marker of metabolic risk, that can help identify dietary patterns that increase cancer risk (27). Adiposity and PA levels have been associated with circulating insulin concentrations (31, 32) and are established factors in colorectal cancer development, with lower PA levels and higher adiposity associated with higher risk (20). Therefore, the insulinemic potential of diet may influence colorectal cancer risk through energy balance mechanisms of insulin regulation. For example, higher levels of PA are associated with reduced circulating concentrations of insulin and bioavailable IGF-I (31). These are major mitogenic hormones implicated in carcinogenesis (9). Insulin/IGF signaling, in the context of chronic systemic low-grade inflammation, as in an environment of low levels of PA and a higher intake of diets with hyperinsulinemic and proinflammatory potential (33), contributes to the cancer-promoting effects of obesity. Indeed, there is a close and reciprocal relation between higher insulin/IGF signaling and inflammatory signaling (9).
Major strengths of our study include the use of a food-based EDIH score that is correlated with concentrations of C-peptide, which are associated with colorectal cancer risk. The large number of colorectal cancer cases allowed us to stratify analyses by levels of other colorectal cancer risk factors. We also had comprehensive and prospectively collected data on diet and important covariates, which reduces the potential for residual confounding and for recall bias. In addition, dietary and covariate data were assessed at multiple times throughout follow-up, which enabled us to use long-term cumulative average exposures, thus reducing within-person variation. In addition, we used a 2-y lagged approach as our main analytic approach to reduce potential reverse causation by subclinical colorectal cancer symptoms, which may influence dietary intake.
However, our study is not without limitations. Some methodologic issues to be considered in interpreting our findings include potential measurement error in the self-reported dietary and lifestyle data; however, previous studies in the NHS and HPFS that evaluated the relative validity of FFQ data have shown reasonably good correlations between FFQ and diet records, which suggests that dietary intake is generally well measured (21, 34, 35). In addition, the multiple FFQ administrations during follow-up approximate habitual long-term diet and reduce measurement error. Although the diet effect is quantified directly through C-peptide concentrations in the EDIH score, a hyperinsulinemic dietary pattern may also be associated with other factors not included in the current study. Another potential limitation is that although we adjusted for several potential confounding variables, we cannot completely rule out confounding by unmeasured variables. In addition, participants were not a random sample of the US population; however, although the prevalence of risk factors often differs across population subgroups, risk factors have been documented to operate across ethnic and different populations, as expected from a common underlying biology (36). Exposure-cancer associations in our cohorts are highly similar across different populations (20).
In conclusion, a higher EDIH score, which reflects a higher insulinemic potential of diet, was directly associated with the risk of colorectal cancer development in 2 large prospective cohort studies in men and women. Our results suggest that the insulinemic potential of diet may partly underlie the influence of dietary patterns on colorectal cancer development, especially among men and women with low PA. Dietary interventions to reduce the adverse role of a hyperinsulinemic dietary pattern may therefore be a means of preventing colorectal cancer.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—FKT and ELG: designed the research; FKT: conducted the research, performed statistical analysis, and wrote the manuscript; ELG: provided study oversight; and all authors: analyzed and interpreted the data and provided critical input, assume full responsibility for analyses and interpretation of these data, and read and approved the final manuscript. None of the authors declared a conflict of interest.
Notes
FKT was supported by National Cancer Institute grant K99 CA207736. CSF was supported by NIH grants P50 CA127003, R01 CA118553, and R01 CA169141 and a Stand Up To Cancer (SU2C) Colorectal Cancer Dream Team Translational Research Grant. The Health Professionals Follow-Up Study (HPFS) and Nurses’ Health Study (NHS) cohorts are supported by NIH grants UM1CA167552 (HPFS), P01 CA55075 (HPFS), UM1CA186107 (NHS), and P01 CA87969 (NHS). The funding agencies played no role in the project planning, data analysis, results interpretation, and manuscript preparation
Supplemental Figure 1 and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: EDIH, empirical dietary index for hyperinsulinemia; FFQ, food-frequency questionnaire; GI, glycemic index; HPFS, Health Professionals Follow-Up Study; IGF, insulin-like growth factor; NHS, Nurses’ Health Study; PA, physical activity.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 3. Giovannucci E. Insulin and colon cancer. Cancer Causes Control 1995;6:164–79. [DOI] [PubMed] [Google Scholar]
- 4. Fung TT, Hu FB, Fuchs C, Giovannucci E, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC. Major dietary patterns and the risk of colorectal cancer in women. 2003;163:309–14. [DOI] [PubMed] [Google Scholar]
- 5. Mehta RS, Song M, Nishihara R, Drew DA, Wu K, Qian ZR, Fung TT, Hamada T, Masugi Y, da Silva A et al. Dietary patterns and risk of colorectal cancer: analysis by tumor location and molecular subtypes. Gastroenterology 152:1944–53, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC, Hu FB. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr 2001;73:61–7. [DOI] [PubMed] [Google Scholar]
- 7. Cespedes EM, Hu FB, Tinker L, Rosner B, Redline S, Garcia L, Hingle M, Van Horn L, Howard BV, Levitan EB et al. Multiple healthful dietary patterns and type 2 diabetes in the Women's Health Initiative. Am J Epidemiol 2016;183:622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heron M. Deaths: leading causes for 2011. Natl Vital Stat Rep 2015;64:1–96. [PubMed] [Google Scholar]
- 9. Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, Rexrode KM, Rimm EB, Hu FB. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J Am Coll Cardiol 2017;70:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varraso R, Chiuve SE, Fung TT, Barr RG, Hu FB, Willett WC, Camargo CA. Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: prospective study. BMJ 2015;350:h286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med 2016;13:e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Besser REJ, Ludvigsson J, Jones AG, McDonald TJ, Shields BM, Knight BA, Hattersley AT. Urine C-peptide creatinine ratio is a noninvasive alternative to the mixed-meal tolerance test in children and adults with type 1 diabetes. Diabetes Care 2011;34:607–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jenab M, Riboli E, Cleveland RJ, Norat T, Rinaldi S, Nieters A, Biessy C, Tjønneland A, Olsen A, Overvad K et al. Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal cancers in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2007;121:368–76. [DOI] [PubMed] [Google Scholar]
- 14. Giovannucci E. Diet, body weight, and colorectal cancer: a summary of the epidemiologic evidence. J Womens Health 2003;12:173. [DOI] [PubMed] [Google Scholar]
- 15. Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS Jr, Brehm BJ, Bucher HC. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med 2006;166:285–93. [DOI] [PubMed] [Google Scholar]
- 16. Jenkins D, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981;34:362–6. [DOI] [PubMed] [Google Scholar]
- 17. Nimptsch K, Brand-Miller JC, Franz M, Sampson L, Willett WC, Giovannucci E. Dietary insulin index and insulin load in relation to biomarkers of glycemic control, plasma lipids, and inflammation markers. Am J Clin Nutr 2011;94:182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tabung FK, Wang W, Fung TT, Hu FB, Smith-Warner SA, Chavarro JE, Fuchs CS, Willett WC, Giovannucci EL. Development and validation of empirical indices to assess the insulinaemic potential of diet and lifestyle. Br J Nutr 2016:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fung TT, Hu FB, Schulze M, Pollak M, Wu T, Fuchs CS, Giovannucci E. A dietary pattern that is associated with C-peptide and risk of colorectal cancer in women. Cancer Causes Control 2012;23:959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Cancer Research Fund/American Institute for Cancer Research Continuous Update Project Report Summary: diet, nutrition, physical activity, and colorectal cancer. London, UK: World Cancer Research Fund; 2017. [Google Scholar]
- 21. Willett W, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 22. Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food frequency questionnaire. Am J Clin Nutr 1999;69:243–9. [DOI] [PubMed] [Google Scholar]
- 23. Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology 1996;7:81–6. [DOI] [PubMed] [Google Scholar]
- 24. Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–9. [DOI] [PubMed] [Google Scholar]
- 25. Willett W, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–8S. [DOI] [PubMed] [Google Scholar]
- 26. Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics 1995;51:524–32. [PubMed] [Google Scholar]
- 27. Giovannucci E. A framework to understand diet, physical activity, body weight, and cancer risk. Cancer Causes Control 2018;29:1–6 . [DOI] [PubMed] [Google Scholar]
- 28. Wei EK, Ma J, Pollak MN, Rifai N, Fuchs CS, Hankinson SE, Giovannucci E. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 2005;14:850. [DOI] [PubMed] [Google Scholar]
- 29. Wu K, Feskanich D, Fuchs CS, Chan AT, Willett WC, Hollis BW, Pollak MN, Giovannucci E. Interactions between plasma levels of 25-hydroxyvitamin D, insulin-like growth factor (IGF)-1 and C-peptide with risk of colorectal cancer. PLoS One 2011;6:e28520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma J, Giovannucci E, Pollak M, Leavitt A, Tao Y, Gaziano JM, Stampfer MJ. A prospective study of plasma C-peptide and colorectal cancer risk in men. J Natl Cancer Inst 2004;96:546–53. [DOI] [PubMed] [Google Scholar]
- 31. Tabung FK, Steck SE, Burch JB, Chen C-F, Zhang H, Hurley TG, Cavicchia P, Alexander M, Shivappa N, Creek KE et al. A healthy lifestyle index is associated with reduced risk of colorectal adenomatous polyps among non-users of non-steroidal anti-inflammatory drugs. J Prim Prev 2015;36:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 2007;86:S836–42. [DOI] [PubMed] [Google Scholar]
- 33. Tabung FK, Liu Li, Wang W, Fung TT, Wu K, Smith-Warner SA, Cao Y, Hu FB, Ogino S, Fuchs CS, Giovannucci EL. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncol 2018;4:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 35. Rimm E, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26. [DOI] [PubMed] [Google Scholar]
- 36. Tabung FK, Giovannucci EL, Giulianini F, Liang L, Chandler PD, Balasubramanian R, Manson JE, Cespedes Feliciano EM, Hayden KM, Van Horn L et al. An empirical dietary inflammatory pattern score is associated with circulating inflammatory biomarkers in a multi-ethnic population of postmenopausal women in the United States. J Nutr 2018;148:771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.