Abstract
Tyrosine phosphorylation is a key biochemical signal that controls growth and differentiation in multicellular organisms. Saccharomyces cerevisiae and nearly all other unicellular eukaryotes lack intact phosphotyrosine signaling pathways. However, many of these organisms have primitive phosphotyrosine-binding proteins and tyrosine phosphatases, leading to the assumption that the major barrier for emergence of phosphotyrosine signaling was the negative consequences of promiscuous tyrosine kinase activity. In this work, we reveal that the classic oncogene v-Src, which phosphorylates many dozens of proteins in yeast, is toxic because it disrupts a specific spore wall remodeling pathway. Using genetic selections, we find that expression of a specific cyclic peptide, or overexpression of SMK1, a MAP kinase that controls spore wall assembly, both lead to robust growth despite a continuous high level of phosphotyrosine in the yeast proteome. Thus, minimal genetic manipulations allow yeast to tolerate high levels of phosphotyrosine. These results indicate that the introduction of tyrosine kinases within single-celled organisms may not have been a major obstacle to the evolution of phosphotyrosine signaling.
Keywords: tyrosine kinase, cyclic peptide, cell wall remodeling, MAP kinase
Yeast can grow at normal rates despite having a large, unnatural level of phosphotyrosine modifications. This demonstrates that broad tyrosine phosphorylation is not intrinsically toxic to single-celled eukaryotes.
ABBREVIATIONS
- pTyr
phosphotyrosine
- CP
cyclic peptide
- SH2
Src-homology 2
- Hsp90
Heat-shock protein 90
INTRODUCTION
Tyrosine phosphorylation is a biochemical signal that evolved concurrently with the rise of multicellular organisms. Genome sequences of model organisms such as yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe, the choanoflagellate Monosiga brevicollis and the slime mold Dictyostelium discoideum have yielded some insight into how phosphotyrosine (pTyr) evolved as a biochemical signal (King 2004; Eichinger et al.2005; King et al.2008; Mayer 2008). These organisms possess some dual-specificity kinases, SH2-like domains and tyrosine phosphatases, but lack tyrosine kinases except in immediate precursors to metazoans such as M. brevicollis (Kennelly 2001; Pincus et al.2008). However, all organisms with tyrosine kinases have a large number of them, along with expanded numbers and variety of tyrosine phosphatases and SH2 domains (Manning et al.2008; Pincus et al.2008; Jin et al.2009; Srivastava et al.2010). This informs a model in which a handful of dual-specificity kinases, which phosphorylate serine/threonine as well as tyrosine, appeared first, and that these prompted the gradual appearance of early tyrosine phosphatases and pTyr-binding domains (Pincus et al.2008; Lim and Pawson 2010). The watershed event was the appearance of more potent tyrosine kinases, which spurred rapid expansion of tyrosine kinases, SH2 domains, and tyrosine phosphatases for growth and differentiation pathways and the development of metazoans (Lim and Pawson 2010). This model of how pTyr signaling evolved assumes that introduction of tyrosine kinases presented a formidable barrier due to the detrimental effects of introducing a new post-translational modification with, at least initially, little specificity (Tan et al.2009, 2011; Lim and Pawson 2010; Su et al.2011). However, there is little experimental evidence as to how early adopters of pTyr signaling might have balanced tyrosine kinase activity and selectivity. In fact, there is no evidence indicating whether widespread tyrosine phosphorylation necessarily results in a selective growth disadvantage for single-celled eukaryotes.
In this work, we explored the mechanism by which the classic oncogene v-Src is toxic to Saccharomyces cerevisiae. We applied transcriptional microarrays, overexpression selections and selections using genetically expressed cyclic peptide (CP) libraries in order to discover critical factors affecting v-Src toxicity (Kritzer et al.2009). In the process, we found that the basis of v-Src toxicity in yeast was very narrow, challenging some basic assumptions regarding the need for kinase selectivity.
MATERIALS AND METHODS
CP libraries and selections
Construction of CP libraries was described previously (Kritzer et al.2009). For the v-src screen, 3.4 million independent transformants were pooled, diluted to OD600 = 0.5 and plated on galactose media. After 3 days, colonies were scraped from the plate and pooled. Pooled plasmids were isolated using the ZymoPrep plasmid kit (Zymo Research), amplified in bacteria and subjected to a second round of selection.
Western blotting
For blotting, yeast were grown to mid-log phase in raffinose-containing medium before switching to galactose medium for 2 h. Cells were lysed and 10 mg total protein lysate was loaded in each lane. Anti-src mouse monoclonal antibody clone 327 (Abcam) and anti-pTyr mouse monoclonal antibody PY20 (Abcam) were used for blotting.
Microarray analysis
For microarray analysis, yeast were grown to mid-log phase in raffinose-containing medium before switching to galactose medium for 2 h. Cells were lysed and total RNA was isolated using phenol-chloroform extraction. Yeast-specific microarrays were prepared and analyzed as previously described (Su et al.2010). Three biological replicates were performed.
RESULTS
Transcriptional profiling of v-Src toxicity
To investigate the mechanism of v-Src toxicity, we used transcriptional profiling to catalog differences in gene expression upon v-Src expression. A suitable strain was constructed by cloning v-Src (pp60 from avian sarcoma virus, residues 1–587) into the Gal-pRS303 vector and integrating it into the W303 background strain. This allowed tight control over v-Src expression: little to no tyrosine phosphorylation was observed when cells were grown in glucose media, but a rapid increase in the number and intensity of pTyr-containing proteins were observed after switching to galactose media (Fig. 1A). Within hours of galactose induction, the cells underwent cell cycle arrest, as previously described for v-Src expression in yeast (Brugge et al.1987; Boschelli, Uptain and Lightbody 1993; Xu and Lindquist 1993). A matched strain expressing a kinase-inactive mutant of v-Src (K295M mutation) was used as a control (Xu and Lindquist 1993).
Figure 1.
SMK1 and V13 overexpression reverse v-src toxicity. (A) Spotting assays demonstrating the effects of SMK1 deletion and overexpression on v-Src toxicity and on the ability of V13 to reverse v-src toxicity. HPQ is a negative control cyclic peptide plasmid, and is representative of the level of toxicity observed for the parent v-src strain. (B) Western blots showing that SMK1 overexpression does not affect v-src levels, but shows a different overall pTyr pattern than v-Src alone or v-Src with V13.
Bulk mRNA from cultures expressing v-Src and v-SrcK295M was isolated and compared using a yeast-specific microarray (Table 1, first two columns) (Yeger-Lotem et al.2009). This comparison revealed that, despite phosphorylating many dozens of proteins, v-Src has an extremely limited effect at the transcriptional level. Of over 5800 ORFs analyzed, only one transcript was upregulated or downregulated by more than 5-fold, and only 21 were altered more than 2.5-fold. Of these 21 genes, 9 were cell wall remodeling enzymes such as endochitinases and glucanases. Also among these dysregulated genes were IME1, a master transcriptional regulator of meiosis (Chu et al.1998; McDonald et al.2009), and SET3, a key component of the SET3C histone deacetylase complex that represses meiosis and sporulation (Pijnappel et al.2001). Together, these data indicated that v-Src activates a subset of spore wall remodeling and meiosis-associated signaling pathways, without activation of other pathways critical for meiosis and sporulation.
Table 1.
Results from transcriptional profiling.

|
A pooled overexpression selection reveals SMK1 can suppress v-Src toxicity
To complement the mRNA microarrays, we also performed a pooled overexpression selection to identify yeast genes that reverse v-Src toxicity when overexpressed. Each member of the FLEXgene yeast overexpression library (over 5000 genes) was picked and pooled into a master bacterial plasmid stock (Brizuela, Braun and LaBaer 2001). DNA isolated from this stock was transformed into the v-Src expression strain and allowed to recover on glucose media. Then 100 000 independent transformants were plated onto galactose media. Thirty-six hit colonies were picked and the DNA within was isolated, amplified in bacteria and sequenced. Of these, 34 produced identifiable ORFs, and 32 contained the gene SMK1.
Smk1p is a MAP kinase responsible for orchestrating spore wall assembly (Huang, Doherty and Herskowitz 2005; McDonald et al.2009). It is required for specific spore wall remodeling events in mid-to-late sporulation, and interacts directly with remodeling factors such as glucan synthase Gsc2p (Huang, Doherty and Herskowitz 2005). Its kinase activity is constitutively repressed until it is dually phosphorylated on its activation loop, which only occurs when Smk1 is in a complex with the activator protein Ssp2 (McDonald et al.2009; Tio et al.2015, 2017; Omerza et al.2018). The signaling pathways directly upstream and downstream of SMK1 remain an area of active investigation (McDonald, Cooper and Winter 2005; Chen and Thorner 2007; McDonald et al.2009; Tio et al.2015, 2017; Omerza et al.2018). We verified that overexpression of SMK1 reversed v-Src toxicity (Fig. 1A), and western blots showed that SMK1 overexpression did not alter v-Src levels or overall pTyr levels (Fig. 1B). We also constructed SMK1 deletion strains, and observed that SMK1 deletion did not alter v-Src toxicity (Fig. 1A). These data are consistent with v-Src activating spore wall remodeling at a point downstream from SMK1, and that overexpression of SMK1 can override this activation without altering overall v-Src activity in the cell. Alternatively, since Smk1 is catalytically inactive outside of the Ssp2 complex, these results may hint at a protein-protein interaction involving Smk1 that is responsible for reversal of v-Src toxicity.
CP inhibitors of v-Src toxicity in yeast
Since the mechanism by which SMK1 controls spore wall remodeling is not well understood, we sought another means of reversing v-Src toxicity. To this end, we transformed the v-Src-expressing strain with a genetically encoded library of CPs (Horswill and Benkovic 2005). This library consists of a single intein-based gene that encodes randomized eight-amino-acid sequences that are post-translationally spliced into head-to-tail CPs. We previously described the construction of this library and its application to a yeast model of Parkinson's disease (Kritzer et al.2009). Approximately 3.4 million independent transformants were plated on galactose media at a high plating density (Fig. 2A). After 3 days, some background growth and many colonies were visible among the plates. These colonies were scraped from the plate and pooled, and plasmids from this pool were isolated and amplified in bacteria. These plasmids were subjected to a second round of selection at a smaller scale and a lower plating density (Fig. 2A). This second round of selection produced only 14 colonies after 3 days of incubation on galactose. These were picked, and the plasmids within were isolated, amplified and re-transformed individually into the v-Src strain. Thirteen of these plasmids were shown to independently suppress v-Src toxicity (Fig. 2B).
Figure 2.
Selections for cyclic peptides that reverse v-Src toxicity in yeast. (A) Representative selection plates showing (from left to right) growth of transformed yeast on glucose medium, growth on galactose medium during the first round of selection and growth on galactose medium during the second round of selection. The high background from the first round of selection is attributed to the high plating density (cells were normalized to an optical density of 0.5 before plating 100 μl on a standard-size plate). This was required for statistical coverage of the 3.4 million independent transformants. Because the second round had much lower theoretical diversity, it could be plated at a much lower density (optical density of 0.1), resulting in very low background. (B) Spotting assays showing the relative potencies of the 13 hits. Hit plasmids were isolated, amplified in bacteria, re-transformed into the original selection strain and serial dilutions of the transformed yeast were spotted onto glucose and galactose plates. HPQ refers to a negative control CP plasmid (Naumann, Savinov and Benkovic 2005; Kritzer et al.2009). V1–V13 refer to CP plasmids selected in the v-Src strain, and CP1 and CP2 are unrelated cyclic peptide plasmids from a prior selection in a yeast model of Parkinson's disease (Kritzer et al.2009). Protein sequences of the CP regions of the 13 hit plasmids are shown, with highly-conserved positions 4 and 7 shown in red. (D) Consensus histogram showing the distributions of amino acids within the CP regions of the hit plasmids. Color coding has been used to emphasize amino acids of common physicochemical character.
Sequencing revealed that the 13 hit plasmids each represented independent, non-redundant hit sequences (Fig. 2B). There was a striking consensus among them, summarized in Fig. 2C. The library has an invariant cysteine as the first amino acid, because a nucleophile is required for peptide splicing (Kritzer et al.2009). However, all 13 hit plasmids contained a tryptophan in the fourth CP position. Also, nearly all had valine or leucine in the seventh position. There were also clear preferences for glycine or valine in the third position, and a hydrophilic amino acid in the eighth position. The observed consensus among the 13 individually selected hits suggested that they operate via the same mode of action.
The most potent plasmid, V13, reversed the v-Src toxicity phenotype such that growth on galactose plates was nearly indistinguishable from growth on glucose plates (Figs 1A and 2B). To determine whether V13 requires intein splicing to reverse v-Src toxicity, we introduced a point mutation into the intein region of V13 such that the mutant plasmid, V13*, encodes the same CP sequence but cannot splice it out of the larger polypeptide (Ghosh, Sun and Xu 2001; Kritzer et al.2009). V13* could no longer reverse v-Src toxicity (Fig. 3). This demonstrated that plasmid V13 required not only the specific encoded CP sequence, but also the ability to splice it out of the larger polypeptide. This effectively ruled out the involvement of a nucleic acid or protein aptamer, and provided strong evidence that the CP encoded by plasmid V13 was reversing v-Src toxicity.
Figure 3.
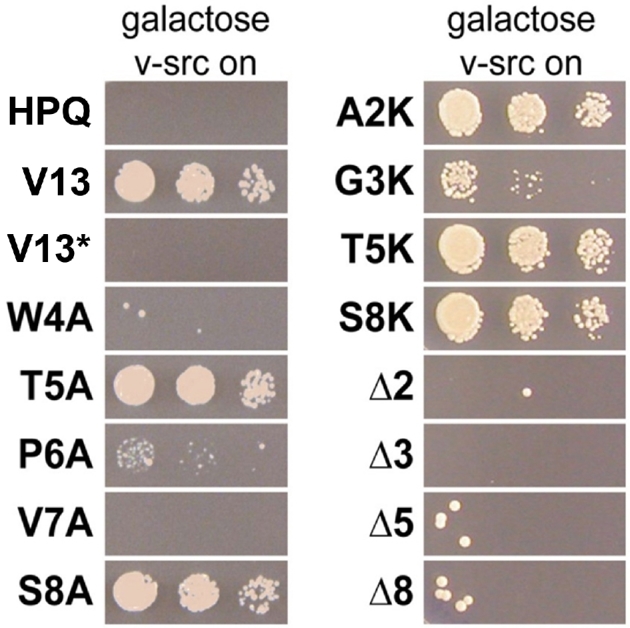
Structure-activity relationships for V13, the most potent CP. (A) Spotting assays of serial dilutions of yeast on galactose media show differential ability to reverse v-Src toxicity among V13 variants. V13* denotes a plasmid that encodes for the same CP sequence as V13, but possesses an intein-inactivating mutation rendering it unable to splice the CP. Indicated amino acids were mutated to alanine, mutated to lysine or deleted to produce a plasmid that encoded a seven-residue CP.
We next assessed the roles of each individual amino acid within V13 by constructing alanine-scanning mutants and expressing each mutant in the v-Src strain. Growth assays confirmed that the tryptophan in the fourth position and the valine in the seventh position, which were highly conserved among selected plasmids, were absolutely required for V13 to reverse v-Src toxicity (Fig. 3). Mutating the proline in the sixth position to alanine resulted in a dramatic loss of potency. This proline was present in 3 of 13 hits, so its role may be to directly contact the target or to structure the overall macrocycle. The threonine and serine in the fifth and eighth positions, respectively, could be replaced by alanine with no effect. Previous selections with these same libraries generated plasmid-encoded CPs that could be truncated into smaller CPs with little loss of function (Kritzer et al.2009). However, point deletions of V13 at positions 2, 3, 5 and 8 (all positions at which the side chain was not required for function) each yielded plasmids that could not reverse v-Src toxicity (Fig. 3). Thus, it appears that the CP encoded by V13 requires, at minimum, the tryptophan and valine as well as a specific overall structure of the eight-amino-acid macrocycle.
Mode of action of CP effectors
There were three possible modes of action for V13: it could alter the production of active v-Src (for instance, through inhibiting Gal4p or Hsp90), it could directly inhibit v-Src or it could prevent v-Src toxicity by modulating cellular processes related to the spore wall remodeling pathway. V13-expressing cells grew robustly in galactose, but cells expressing HPQ, a negative control CP, could not grow in galactose. However, both these cultures had identical v-Src and pTyr levels after 2 h of induction (Fig. 1B). By contrast, cells treated with 10 μM radicicol, an Hsp90 inhibitor, also grew under induction conditions, but had greatly reduced v-Src and pTyr levels since Hsp90 is required for v-Src folding (Fig. 1B). In light of these findings, it appears unlikely that V13 prevents v-Src toxicity by inhibiting Hsp90 or v-Src directly.
We next tested V13’s effects in the SMK1 deletion strain (Fig. 1A). V13 was still able to reverse v-Src toxicity when SMK1 was deleted, indicating that it operates downstream from SMK1 or in an independent pathway. Transcriptional profiling was used to provide more data regarding how V13 reverses v-Src toxicity. W303 cells expressing v-SrcK295M were used as a background strain, and cultures expressing V13 and a negative control CP were compared to reveal whether V13 had any basal effects. These two cultures were nearly identical, with zero transcripts having 2.5-fold differences (P ≤ 0.01). However, when V13 expression was compared to expression of negative control CP in the presence of active v-Src, the same subset of genes that were modified in response to v-Src expression were modified in the opposite direction when V13 was co-expressed (Table 1, Fig. 4). Expression of V13 clearly prevents v-Src toxicity in a potent and very specific manner, such that cells co-expressing v-Src and V13 are nearly indistinguishable from normal cells with respect to transcriptional activity. The most concise explanation for these data is that V13 modulates cellular processes related the spore wall remodeling, downstream from SMK1.
Figure 4.

V13 reverses the v-Src-associated phenotype. This scatter plot correlates the log2 fold change in transcript levels attributed to v-Src (y-axis) to those attributed to V13 in the presence of v-Src (x-axis). All 6580 independent transcripts monitored by the yeast-specific DNA microarray are shown. Transcripts with changes between 2-fold and 2.5-fold (log2 fold changes between 1 and 1.3, listed in Table 1) are shown in red. Transcripts with changes greater than 2.5-fold (log2 fold changes greater than 1.3, listed in Table 1) are shown in orange. A linear fit to all the data (diagonal line) is provided to highlight the inverse correlation.
DISCUSSION
Historically, the observation in 1987 by Brugge et al. that v-Src causes rapid cell death in Saccharomyces cerevisiae led to investigations into the roles of v-Src's catalytic and interaction domains (Brugge et al.1987; Boschelli, Uptain and Lightbody 1993). Later, Xu and Lindquist (1993) used this phenotype to describe the dependence of v-Src activity on heat-shock protein 90 (Hsp90). Most of the subsequent work using v-Src in yeast used v-Src as a reporter for Hsp90 activity (Nathan and Lindquist 1995; Dey, Caplan and Boschelli 1996; Nathan, Vos and Lindquist 1999; Goeckeler et al.2002; Lee et al.2002; Panaretou et al.2002; Montalibet and Kennedy 2004). In this work, we reveal that v-Src is toxic to yeast because it activates a spore wall remodeling pathway. Despite the fact that v-Src phosphorylates many dozens of proteins in yeast cells (Brugge et al.1987; Boschelli, Uptain and Lightbody 1993; Florio et al.1994), our results imply that dysregulation of the spore wall remodeling pathway is likely the sole source of toxicity. One consequence of this conclusion is to warn against using v-Src toxicity as a functional readout of Hsp90 function in yeast, due to possible interference from signaling pathways controlled by IME1/IME2, SMK1, and possibly other factors.
We have described two independent ways to reverse v-Src toxicity in yeast. The first is to overexpress the MAP kinase SMK1. This finding, along with the transcriptional array data, is evidence that the rapid cell-cycle arrest that characterizes v-Src expression in yeast is caused by inappropriate remodeling of the cell wall, which causes a catastrophic failure during mitosis (Brugge et al.1987). These data also provide several new footholds for studying late events in spore formation in yeast and for studying SMK1, which is by far the most poorly understood of the five yeast MAP kinases (Chen and Thorner 2007). Another means of reversing v-Src toxicity in yeast is to express one of a family of CPs exemplified by V13. V13 has no discernible effect on yeast cells by itself, yet potently reverses v-Src toxicity in an SMK1-independent manner. These results suggest that the molecular target of V13 could be a signaling protein or glycan-remodeling enzyme downstream from SMK1.
Finally, we have shown that yeast can grow and divide robustly despite a high pTyr load, provided that spore wall remodeling pathways are protected from inappropriate activation. If this finding extends to other systems, it would help to explain the rapid expansion of pTyr-mediated signaling pathways during the transition from unicellular to multicellular organisms (Mayer 2008). A model for this transition was presented by Lim and Pawson in 2010 (Lim and Pawson 2010). It describes the appearance of the first tyrosine kinases as a transformative event that helped catalyze metazoan development. One question that arises in this model is how organisms dealt with widespread tyrosine phosphorylation caused by these first, presumably promiscuous, tyrosine kinases. Some findings have suggested that there was negative selective pressure against tyrosine within the proteome of multicellular organisms as pTyr signaling evolved (Tan et al.2009). This was attributed to the need to limit the negative impacts of tyrosine kinases on the proteome (Tan et al.2009; Lim and Pawson 2010; Su, Huang and Gu 2011). However, our findings demonstrate that these negative impacts are likely more narrow than originally assumed. If kinase selectivity is only important for protecting a small subset of critical signaling proteins, as we observed for spore wall remodeling, then the reason for decreased tyrosine utilization in metazoans might need to be re-examined (Tan et al.2009, 2011; Su, Huang and Gu 2011). Indeed, recent tests of the correlation between tyrosine usage and tyrosine kinase activity support that the negative effects of promiscuous tyrosine phosphorylation may not be the best explanation for overall tyrosine usage patterns in metazoans (Pandya et al.2015).
Our results argue that widespread tyrosine phosphorylation may have been only a speedbump to the rapid expansion of pTyr as a biochemical signal. We also note that cancer-associated mutant kinases tend to have lower specificity (Blume-Jensen and Hunter 2001; Miller et al.2008). If kinase specificity is indeed less critical than previously assumed, the oncogenic effects of lower kinase specificity may reside in the ability to phosphorylate a very small subset of targets. Perhaps these targets, rather than the kinases themselves, could be the subject of future target-oriented drug discovery.
Acknowledgements
This article is dedicated to the enduring warmth, generosity and spirit of discovery of Dr. Susan Lindquist.
We thank Eric Spooner for assistance in mass spectrometric identification of proteins from SDS-PAGE gels, Catherine McLellan and Luke Whitesell for expertise and assistance in Hsp90 and v-Src assays, and Daniel Tardiff for the pooled overexpression library. We also thank Esti Leger-Yotem, Jennifer Love, George Bell and the Whitehead Institute Center for Genome Research for assistance with transcriptional profiling and data processing.
FUNDING
This work was supported by a Ruth L. Kirschstein National Research Service Award (NRSA) Individual Postdoctoral Fellowship from the National Institutes of Health (NIH) to JAK (5F32NS055492).
Conflict of interest. None declared.
REFERENCES
- Blume-Jensen P., Hunter T.. Oncogenic kinase signalling. Nature 2001;411:355–65. [DOI] [PubMed] [Google Scholar]
- Boschelli F, Uptain SM, Lightbody JJ.. The lethality of P60(V-Src) in Saccharomyces cerevisiae and the activation of P34(Cdc28) kinase are dependent on the integrity of the Sh2 domain. J Cell Sci 1993;105:519–28. [DOI] [PubMed] [Google Scholar]
- Brizuela L, Braun P, LaBaer J.. FLEXGene repository: from sequenced genomes to gene repositories for high-throughput functional biology and proteomics. Mol Biochem Parasitol 2001;118:155–65. [DOI] [PubMed] [Google Scholar]
- Brugge JS, Jarosik G, Andersen J et al. Expression of Rous sarcoma virus transforming protein pp60v-src in Saccharomyces cerevisiae cells. Mol Cell Biol 1987;7:2180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RE, Thorner J.. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. BBA-Mol Cell Res 2007;1773:1311–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JM, Hong EL, Amundsen C et al. Saccharomyces Genome Database: the genomics resource of budding yeast. Nucleic Acids Res 2012;40:D700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, DeRisi J, Eisen M et al. The transcriptional program of sporulation in budding yeast. Science 1998;282:699–705. [DOI] [PubMed] [Google Scholar]
- Dey B, Caplan AJ, Boschelli F.. The Ydj1 molecular chaperone facilitates formation of active p60v-src in yeast. Mol Biol Cell 1996;7:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger L, Pachebat JA, Glockner G et al. The genome of the social amoeba Dictyostelium discoideum. Nature 2005;435:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio M, Wilson LK, Trager JB et al. Aberrant protein phosphorylation at tyrosine is responsible for the growth-inhibitory action of pp60v-src expressed in the yeast Saccharomyces cerevisiae. Mol Biol Cell 1994;5:283–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh I, Sun L, Xu MQ.. Zinc inhibition of protein trans- splicing and identification of regions essential for splicing and association of a split intein. J Biol Chem 2001;276:24051–8. [DOI] [PubMed] [Google Scholar]
- Goeckeler JL, Stephens A, Lee P et al. Overexpression of yeast Hsp110 homolog Sse1p suppresses ydj1-151 thermosensitivity and restores Hsp90-dependent activity. Mol Biol Cell 2002;13:2760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horswill AR, Benkovic SJ.. Cyclic peptides, a chemical genetics tool for biologists. Cell Cycle 2005;4:552–5. [DOI] [PubMed] [Google Scholar]
- Huang LS, Doherty HK, Herskowitz I.. The Smk1p MAP kinase negatively regulates Gsc2p, a 1,3- -glucan synthase, during spore wall morphogenesis in Saccharomyces cerevisiae. P Natl Acad Sci USA 2005;102:12431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Xie XY, Chen C et al. Eukaryotic protein domains as functional units of cellular evolution. Sci Signal 2009;2:ra76. [DOI] [PubMed] [Google Scholar]
- Kennelly PJ. Protein phosphatases—a phylogenetic perspective. Chem Rev 2001;101:2291–312. [DOI] [PubMed] [Google Scholar]
- King N. The unicellular ancestry of animal development. Dev Cell 2004;7:313–25. [DOI] [PubMed] [Google Scholar]
- King N, Westbrook MJ, Young SL et al. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 2008;451:783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer JA, Hamamichi S, McCaffery JM et al. Rapid selection of cyclic peptides that reduce alpha-synuclein toxicity in yeast and animal models. Nat Chem Biol 2009;5:655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Rao J, Fliss A et al. The Cdc37 protein kinase–binding domain is sufficient for protein kinase activity and cell viability. J Cell Biol 2002;159:1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim WA, Pawson T. Phosphotyrosine signaling: evolving a new cellular communication system. Cell 2010;142:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Young SL, Miller WT et al. The protist, Monosiga brevicollis, has a tyrosine kinase signaling network more elaborate and diverse than found in any known metazoan. P Natl Acad Sci USA 2008;105:9674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer BJ. Clues to the evolution of complex signaling machinery. P Natl Acad Sci USA 2008;105:9453–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CM, Cooper KE, Winter E.. The Ama1-directed anaphase-promoting complex regulates the Smk1 mitogen-activated protein kinase during meiosis in yeast. Genetics 2005;171:901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CM, Wagner M, Dunham MJ et al. The Ras/cAMP pathway and the CDK-like kinase Ime2 regulate the MAPK Smk1 and spore morphogenesis in Saccharomyces cerevisiae. Genetics 2009;181:511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Jensen LJ, Diella F et al. Linear motif atlas for phosphorylation-dependent signaling. Sci Signal 2008;1:ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalibet J, Kennedy BP. Using yeast to screen for inhibitors of protein tyrosine phosphatase 1B. Biochem Pharmacol 2004;68:1807–14. [DOI] [PubMed] [Google Scholar]
- Nathan DF, Lindquist S.. Mutational analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol Cell Biol 1995;15:3917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DF, Vos MH, Lindquist S. Identification of SSF1, CNS1, and HCH1 as multicopy suppressors of a Saccharomyces cerevisiae Hsp90 loss-of-function mutation. P Natl Acad Sci USA 1999;96:1409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann TA, Savinov SN, Benkovic SJ.. Engineering an affinity tag for genetically encoded cyclic peptides. Biotechnol Bioeng 2005;92:820–30. [DOI] [PubMed] [Google Scholar]
- Omerza G, Tio CW, Phillips T et al. The meiosis-specific Cdc20 family-member Ama1 promotes binding of the Ssp2 activator to the Smk1 MAP kinase. Mol Biol Cell 2018;29:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaretou B, Siligardi G, Meyer P et al. Activation of the ATPase activity of Hsp90 by the stress-regulated cochaperone Aha1. Mol Cell 2002;10:1307–18. [DOI] [PubMed] [Google Scholar]
- Pandya S, Struck TJ, Mannakee BK et al. Testing whether metazoan tyrosine loss was driven by selection against promiscuous phosphorylation. Mol Biol Evol 2015;32:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnappel W, Schaft D, Roguev A et al. The S. cerevisiae SET3 complex includes two histone deacetylases, Hos2 and Hst1, and is a meiotic-specific repressor of the sporulation gene program. Genes Dev 2001;15:2991–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus D, Letunic I, Bork P et al. Evolution of the phospho-tyrosine signaling machinery in premetazoan lineages. P Natl Acad Sci USA 2008;105:9680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava M, Simakov O, Chapman J et al. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature 2010;466:720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LJ, Auluck PK, Outeiro TF et al. Compounds from an unbiased chemical screen reverse both ER-to-Golgi trafficking defects and mitochondrial dysfunction in Parkinson's disease models. Dis Model Mech 2010;3:194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZX, Huang W, Gu X. Comment on "positive selection of tyrosine loss in metazoan evolution”. Science 2011;332:917. [DOI] [PubMed] [Google Scholar]
- Tan CSH, Pasculescu A, Lim WA et al. Positive selection of tyrosine loss in metazoan evolution. Science 2009;325:1686–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CSH, Schoof EM, Creixell P et al. Response to comment on "positive selection of tyrosine loss in metazoan evolution”. Science 2011;332:917. [DOI] [PubMed] [Google Scholar]
- Tio CW, Omerza G, Sunder S et al. Autophosphorylation of the Smk1 MAPK is spatially and temporally regulated by Ssp2 during meiotic development in yeast. Mol Biol Cell 2015;26:3546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tio CW, Omerza G, Phillips T et al. Ssp2 Binding Activates the Smk1 Mitogen-Activated Protein Kinase. Mol Biol Cell 2017;37:e00607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Lindquist S. Heat-shock protein hsp90 governs the activity of pp60v-src kinase. P Natl Acad Sci USA 1993;90:7074–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeger-Lotem E, Riva L, Su LJ et al. Bridging high-throughput genetic and transcriptional data reveals cellular responses to alpha-synuclein toxicity. Nat Genet 2009;41:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]




