Abstract
The amygdala receives cortical inputs from the medial prefrontal cortex (mPFC) and orbitofrontal cortex (OFC) that are believed to affect emotional control and cue-outcome contingencies, respectively. Although mPFC impact on the amygdala has been studied, how the OFC modulates mPFC–amygdala information flow, specifically the infralimbic (IL) division of mPFC, is largely unknown. In this study, combined in vivo extracellular single-unit recordings and pharmacological manipulations were used in anesthetized rats to examine how OFC modulates amygdala neurons responsive to mPFC activation. Compared with basal condition, pharmacological (N-Methyl-D-aspartate) or electrical activation of the OFC exerted an inhibitory modulation of the mPFC–amygdala pathway, which was reversed with intra-amygdala blockade of GABAergic receptors with combined GABAA and GABAB antagonists (bicuculline and saclofen). Moreover, potentiation of the OFC-related pathways resulted in a loss of OFC control over the mPFC–amygdala pathway. These results show that the OFC potently inhibits mPFC drive of the amygdala in a GABA-dependent manner; but with extended OFC pathway activation this modulation is lost. Our results provide a circuit-level basis for this interaction at the level of the amygdala, which would be critical in understanding the normal and pathophysiological control of emotion and contingency associations regulating behavior.
Keywords: amygdala, in vivo electrophysiology, medial prefrontal cortex, orbitofrontal cortex, rat
Introduction
The basolateral complex of the amygdala, including the lateral nucleus (LA) and the basolateral nucleus (BLA), has long been recognized as the key emotion center in the brain (LeDoux 2000; Phelps and LeDoux 2005; Roozendaal and McGaugh 2011). Traditionally, the basolateral complex is considered the sensory interface of the amygdala (LeDoux et al. 1990), where sensory information from thalamus, perirhinal cortex, and insular cortex, etc., converges (Maren 1999; Orsini and Maren 2012). It also receives inputs from the hippocampus (Canteras and Swanson 1992; Pitkanen et al. 2000; Quirk and Mueller 2008; Orsini et al. 2011) for spatial and contextual information. These emotional contingencies are then potently modulated by neocortical afferents, including the medial prefrontal cortex (mPFC), both the prelimbic (PL) and the infralimbic (IL) divisions (Sotres-Bayon and Quirk 2010). For example, the mPFC is related to the regulation of fear expression after extinction (Quirk and Mueller 2008; Sierra-Mercado et al. 2011; Milad and Quirk 2012).
Recently, interaction between the orbitofrontal cortex (OFC) and the amygdala has attracted attention because diverse high-level behaviors require functional integrity of this structure at the circuitry level (Orsini et al. 2015). OFC and the amygdala are heavily interconnected (Aggleton et al. 1980), and intact OFC–amygdala circuit is critical in development of cue-outcome contingencies (Schoenbaum and Roesch 2005; Lucantonio et al. 2015; Sharpe and Schoenbaum 2016). Shifting between different stimulus–reward associations (reversal learning) is facilitated by the OFC (Schoenbaum et al. 2007; Ghods-Sharifi et al. 2008), and damages to OFC disrupt the amygdala in integrating and updating information from multiple cue-outcome associations (Lucantonio et al. 2015).
The amygdala as the hub receives convergent inputs from the mPFC and the OFC (Vertes 2004; Rempel-Clower 2007). Furthermore, the mPFC and the OFC have been described as having regulating and facilitating effects over emotional learning, respectively (Phelps et al. 2004; Levens et al. 2011). However, the manner by which the mPFC and OFC activation interacts at the cellular level within the amygdala is largely unknown. In this study, we examined how activation of the OFC modulates the information flow from the mPFC to the amygdala, specifically targeting the IL division which modulates fear, using combined techniques of extracellular single-unit recordings, electrical stimulation, and local- and iontophoretic administration of drugs in anesthetized rats.
Materials and Methods
Subjects
Male Sprague-Dawley rats (300–400 g; Harlan Laboratories) were housed for at least 5 days upon arrival in pairs in a temperature (22 °C)- and humidity (47%)-controlled facility on a 12 h light/dark cycle (lights on at 7:00 AM) with food and water available ad libitum. Animals were handled in accordance with the guidelines outlined in the United States Public Health Service “Guide for the Care and Use of Laboratory Animals”, and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Surgery
All recordings were performed on anesthetized rats between 9:00 AM and 5:00 PM as previously described (Rosenkranz et al. 2003; Buffalari and Grace 2007; Chang and Grace 2015). Rats were anesthetized with 8% chloral hydrate (400 mg/kg, i.p.) and placed in a stereotaxic apparatus (David Kopf Instruments); core body temperature of 37 °C was maintained by a temperature-controlled heating pad (Fine Science Tools). Incisions were then made in the scalp to expose the skull. Supplemental doses of chloral hydrate were administered as needed throughout the entire recording session.
Electrically Evoked Responses of mPFC–Amygdala Pathway
For electrical stimulation, a burr hole was drilled into the skull overlying the mPFC (from bregma: anteroposterior [AP], +3.5 mm; mediolateral [ML], +0.6 mm; dorsoventral [DV], −5.0 mm) for the placement of the electrode (Fig. 1A, both panels, left tracks). The stimulation electrode targeted the IL; however, current spread to adjacent PL subdivision of mPFC could not be ruled out; hence the stimulation site is identified as mPFC. A bipolar concentric electrode (NEX-100X; Rhodes Medical Instruments) was lowered into the target, and stimulation was delivered using a dual-output stimulator (S88; Grass Instruments) at an intensity of 1.0 mA and duration of 0.25 ms at 0.5 Hz in search of evoked responses in the amygdala, focused on the basolateral complex including the LA and BLA nuclei.
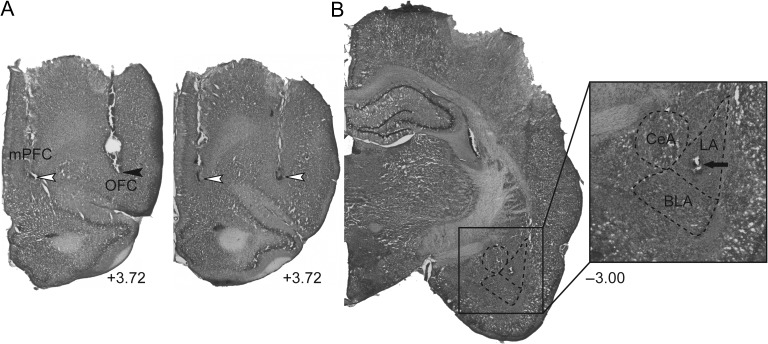
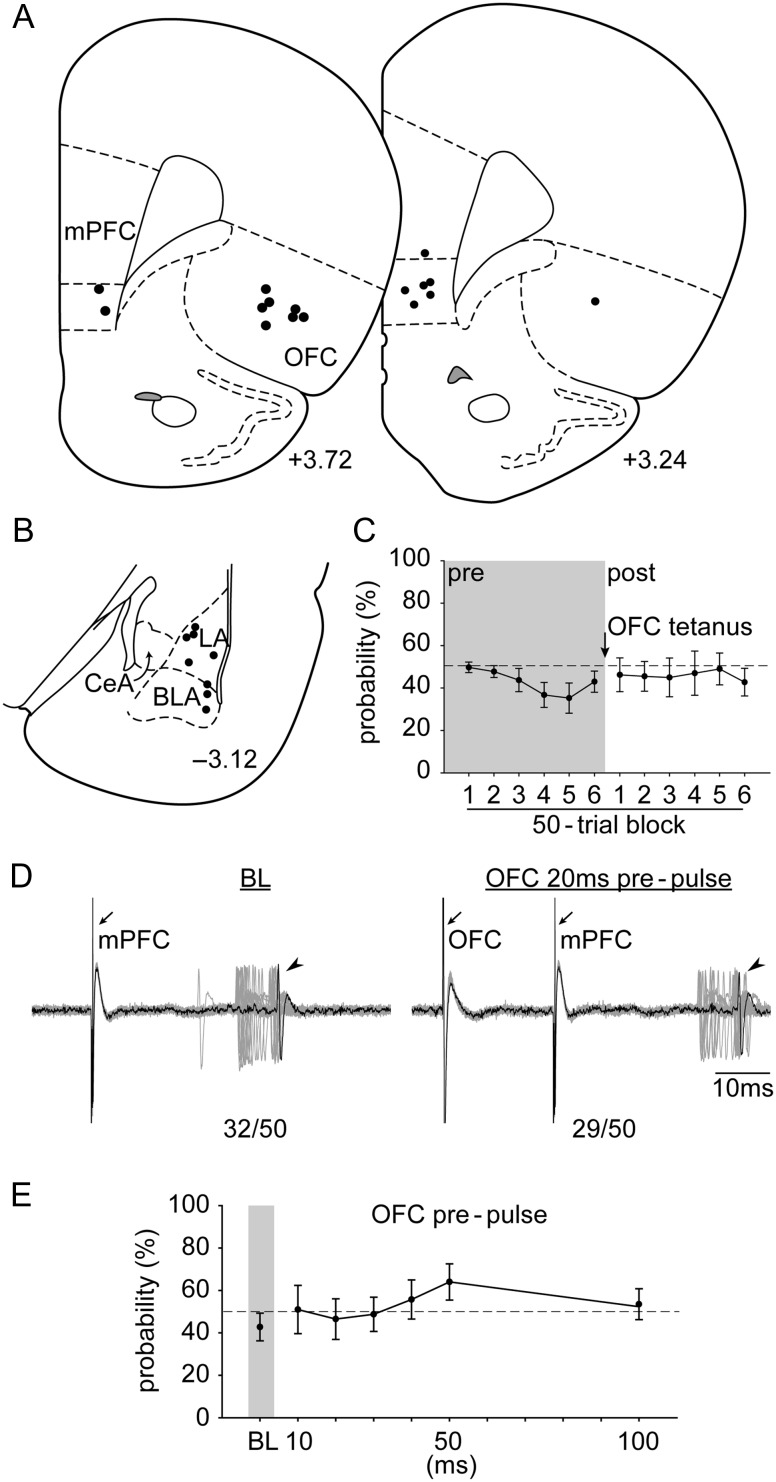
Figure 1.
Representative electrical stimulation sites and cannula placement in (A) the mPFC and OFC, as well as a representative recording site in (B) the LA (+3.72, and −3.00; AP distance [mm] to bregma). Open arrowheads, the lesion marks at the tips of the stimulation electrodes. Closed arrowhead, tip of the infusion cannula. Arrow, dye mark of the neuron recorded. LA, lateral nucleus of the amygdala; BLA, basolateral nucleus of the amygdala; CeA, central nucleus of the amygdala.
For recording, burr holes were drilled into the skull and the dura was removed in an area overlying the LA/BLA (from bregma: AP, −3.0 mm; ML, +5.3 mm; DV, −6.5 to −9.0 mm). Single- (Experiments 1, 2, and 4; 2 mm outer diameter Omegadot filament glass; World Precision Instruments) or 5-barrel microelectrodes (Experiment 3; ASI Instruments) were constructed using a vertical microelectrode puller (PE-2; Narishige), and the tip was broken back under microscopic control. The recording barrel of the microelectrode was filled with 2% Pontamine sky blue in 2 M NaCl with in situ impedance of 4–8 MΩ (measured at 1 kHz) for electrophysiological recordings. The microelectrode was slowly lowered into the LA/BLA using a hydraulic microdrive (Model 640; David Kopf Instruments) in search of neurons responsive to mPFC stimulation. Once a responsive single unit was identified, stimulation current was adjusted to determine a baseline (BL) evoked spike response probability of ~50% (40–60% evoked spikes per trial block).
Only single units with response onset latencies <30 ms (presumably monosynaptic) were included for further analyses. These LA/BLA neurons showed very little shift in latency when increasing the stimulus intensity, yet they showed some range (generally <5 ms) in latency distribution (“jitter”), ruling out antidromic activation. Moreover, all of the neurons reported in this study were putative projection neurons in that they exhibited very low spontaneous firing rates (<0.5 Hz) and long-duration action potential waveforms (>2.5 ms; the duration of the action was quantified as the time from the initial deflection from BL to the return to BL) as determined previously (Rosenkranz and Grace 1999).
OFC Local Administration of Drug
For local drug administration (Experiment 1), a 26-gauge stainless-steel guide cannula (Plastics One) was lowered into the OFC (relative to bregma: AP +3.5 mm; ML +3.1 mm; DV −4.0 mm), and a 33-gauge injector extended 1 mm beyond the guide was inserted for drug delivery (Fig. 1A, left panel, right track). All drugs were freshly mixed in Dulbecco's PBS buffer (VEH) (Sigma), with the doses chosen based on the previously published reports. Depending on the design of each experiment, 0.5 µL of N-methyl-D-aspartic acid (NMDA; 0.75 µg) (Sigma) (Chang and Grace 2014), NMDA antagonist “(2R)-amino-5-phosphonovaleric acid” (APV; 5 µg) (Sigma) (Zimmerman and Maren 2010), or VEH, was infused into the OFC. Drugs were delivered at 0.25 µL/min, and another 1 min was allowed for diffusion.
OFC Gating
For OFC gating (Experiments 2 and 4), another bipolar concentric electrode was lowered into the OFC (relative to bregma: AP +3.5 mm; ML +3.1 mm; DV −5.0 mm) (Fig. 1A, right panel, right track), and OFC electrical stimulation (1.0 mA and 0.25 ms pulse duration) was delivered prior to mPFC stimulation at various intervals (10, 20, 30, 40, 50, and 100 ms). OFC modulatory gating was tested in naive (Experiment 2) and OFC-tetanized animals (Experiment 4; 1.0 mA, 0.25 ms pulse duration, 200 trials at 20 Hz).
Intra-LA/BLA Iontophoretic Application of Drug
For iontophoretic application of drug (Experiment 3), 5-barrel microelectrodes were used. Other than the central recording barrel, one of the outer barrels was filled with 3 M NaCl for automatic current balancing, and the remaining barrels were filled with GABAA antagonist bicuculline methiodide (5 mM, pH 4.5) and GABAB antagonist saclofen (20 mM, pH 4.5) cocktail dissolved in 100 mM NaCl (Stutzmann and LeDoux 1999). The cocktail was held with (−) retaining current at 10 nA, and was ejected with (+) iontophoretic current at 40 nA during testing.
Data Acquisition
Signals from the recording electrode were amplified by a headstage before being fed into a window discriminator/amplifier (1000 gain, 200–16 kHz bandpass; Fintronics Inc.), then into an audio monitor (AM8; Grass Instruments), and displayed on an oscilloscope (Tektronix) for real-time monitoring. Data were collected using a data acquisition board interface, monitored online, and analyzed offline using computer software, Powerlab (AD Instruments).
Histology
A range of 1–4 neurons was recorded for a single track of search. At the conclusion of each experiment, the microelectrode was replaced to the depth of the neuron recorded, and the location verified via electrophoretic ejection (BAB-501; Kation Scientific) of Pontamine sky blue dye into the recording site for 30 min (−20 μA constant current) (Fig. 1B). If more than one neuron was recorded in a given electrode track, the first and the last neurons encountered were marked at their respective depths, and recording sites of all neurons were reconstructed according to their relative depth. To verify the placement of the stimulation electrode, a 10-s pulse at 100 μA was administered. Rats were then killed by an overdose of anesthetic (chloral hydrate, additional 400 mg/kg, i.p.). All rats were decapitated, their brains removed, fixed for at least 2 days (8% paraformaldehyde in 0.2 M PBS), and cryoprotected (25% sucrose in 0.1 M PBS) until saturated. Brains were sectioned (60 μm coronal sections), mounted onto gelatin-chrome alum-coated slides, and stained with a combination of neutral red and cresyl violet for histochemical verification of the stimulating and recording sites.
Statistics
All data are represented as the mean ± SEM and were submitted to analysis of variance (ANOVA). Post hoc comparisons using Fisher's LSD test were performed for ANOVAs that achieved a significance of P < 0.05. All statistics were calculated using SPSS (IBM) or SigmaStat (Systat Software Inc.).
Results
Experiment 1: OFC Pharmacological Activation Decreased the Ability of the mPFC to Drive LA/BLA Neurons
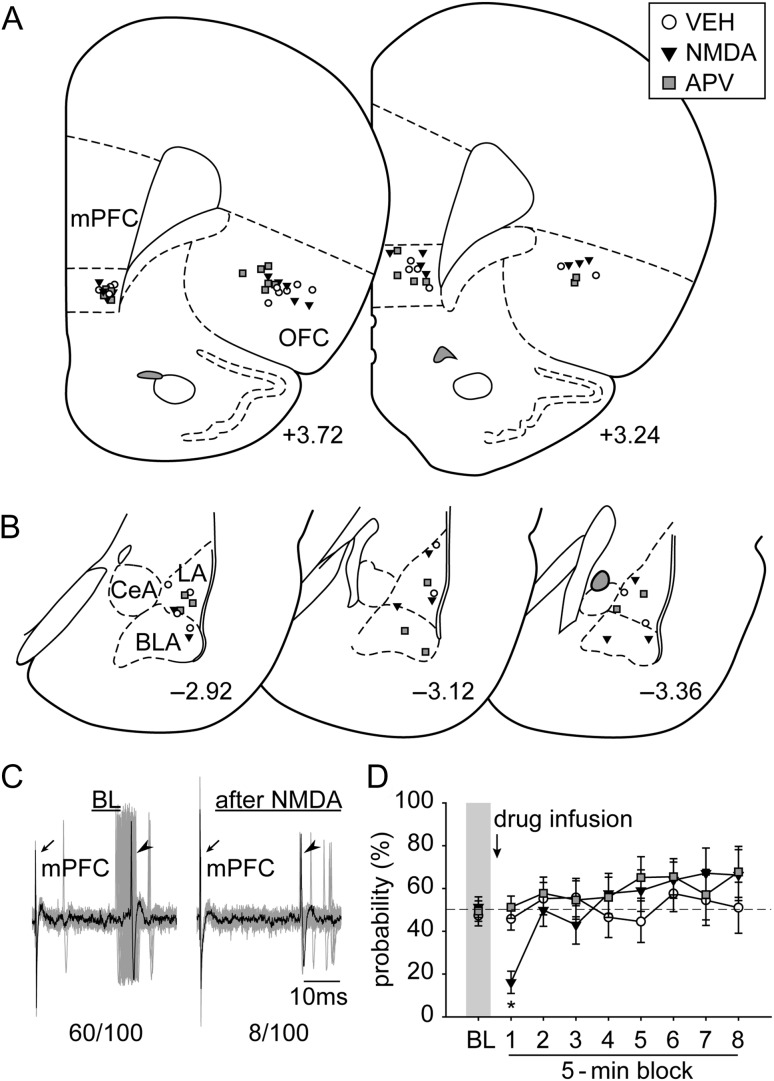
In this experiment, we pharmacologically modulated the activity of the OFC and examined its effects on the mPFC in driving LA/BLA neuronal activities. A total of 24 neurons (n = 8 each; VEH, NMDA, and APV) in the LA/BLA that were responsive to mPFC stimulation were recorded in this experiment (Fig. 2A,B). Pharmacological activation of the OFC with NMDA decreased the mPFC drive on LA/BLA evoked probability, but blockade of the glutamatergic excitatory drive of the OFC with APV did not change the evoked firing probability (Fig. 2C,D; significant main effect of “Time Block” [5 min] [F8,168 = 5.482, P < 0.001] and a significant interaction between “treatment” and “Time Block” [F16,168 = 2.342, P = 0.004]). Planned comparison among treatments suggested that at the first 5-min time block after drug treatment, OFC activation with NMDA significantly decreased mPFC drive on LA/BLA evoked probability (P < 0.05) compared with VEH or APV groups, while there was no statistically significant difference between the later 2. There were no statistical differences among treatments at the remaining time blocks.
Figure 2.
The placements of (A) all the stimulation electrodes in the mPFC and infusion cannulae in the OFC and (B) the distribution of all the neurons recorded (+3.72, +3.24, −2.92, −3.12, and −3.36; AP distance [mm] to bregma). (C) Electrophysiological recording of an amygdala neuron responsive to mPFC stimulation (left) that decreased its evoked responses after OFC pharmacological activation with NMDA (right; n/100 = evoked spikes out of 100 trials). Arrows, electrical stimulation artifacts from mPFC stimulation. Arrowheads, evoked spikes in amygdala. (D) Pharmacological activation of the OFC with NMDA exerted an inhibitory modulation on the mPFC–amygdala pathway (*P < 0.05 relative to VEH and APV). VEH, vehicle. Other abbreviations refer to Figure 1.
Experiment 2: OFC Stimulation Exerted an Inhibitory Gating on mPFC-LA/BLA Evoked Responses
Pharmacological activation was used to provide a long-duration effect. In this experiment, we electrically engaged the activity of the OFC and examined its modulatory gating on the mPFC in driving LA/BLA neuronal activities. A total of 23 LA/BLA neurons responsive to mPFC stimulation were recorded from 11 rats (Fig. 3A,B). The mPFC evoked response was attenuated in 20 out of 23 neurons by OFC (50 trials each delay; changes in evoked probability >15% relative to BL), and 5 out of 20 received convergent inputs from both the mPFC and the OFC.
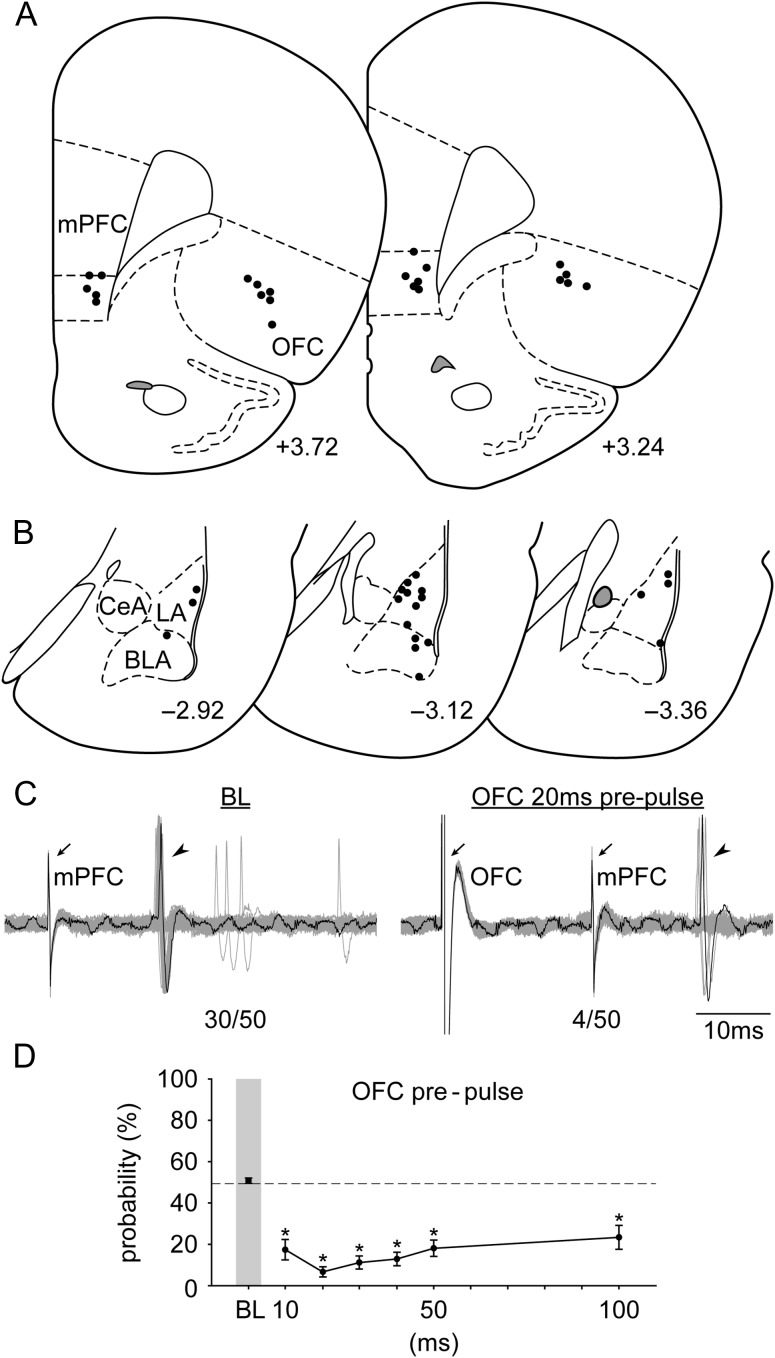
Figure 3.
The placements of (A) all the stimulation electrodes in the mPFC and the OFC and (B) the distribution of all the neurons recorded (+3.72, +3.24, −2.92, −3.12, and −3.36; AP distance [mm] to bregma). (C) Electrophysiological recording of an amygdala neuron responsive to mPFC stimulation (left) that showed a decrease in evoked responses with OFC 20 ms prepulse (n/50 = evoked spikes out of 50 trials). Arrows, electrical stimulation artifacts from OFC and mPFC stimulation, respectively. Arrowheads, evoked spikes in amygdala. (D) OFC activation exerted an inhibitory gating on the mPFC–amygdala pathway at all delays tested (relative to BL; *P < 0.05). Abbreviations refer to Figure 1.
OFC prepulse exerted an inhibitory gating on the mPFC-LA/BLA evoked response (Fig. 3C,D; significant main effect of “delay” [F6,114 = 22.921, P < 0.001]). Evoked probability was significantly decreased at all delay latencies tested (10, 20, 30, 40, 50, and 100 ms) compared with BL (all Ps < 0.05). The long-delay inhibitory modulation was likely not due to the accumulation of GABA because with repeated OFC stimulation there was no residual effect when tested at later time points. In a separate group of neurons recorded (n = 5), we re-examined their basal response after OFC gating. There was no statistical difference before and after serial OFC gating (50.0 ± 2.45% and 50.8 ± 2.72%, respectively; paired-t(4) = 0.21, P = 0.85).
Experiment 3: OFC Inhibitory Gating on mPFC-LA/BLA Pathway Is Reversed by Blockade of Local GABAergic Receptors in the Amygdala
Experiment 1 and Experiment 2 suggested that activation of the OFC produced an inhibitory modulation of the mPFC to LA/BLA pathway. In this experiment, we examined whether intra-amygdala inhibitory circuitry may play a role in this effect. A total of 15 LA/BLA neurons responsive to mPFC stimulation were recorded from 7 rats (Fig. 4A,B). Consistent with the findings in Experiment 2 above, OFC prepulse (20 ms) imposed an inhibitory gating in 12 out of 15 neurons. Moreover, 11 out of 12 neurons responded to GABAA/GABAB antagonist co-administration.
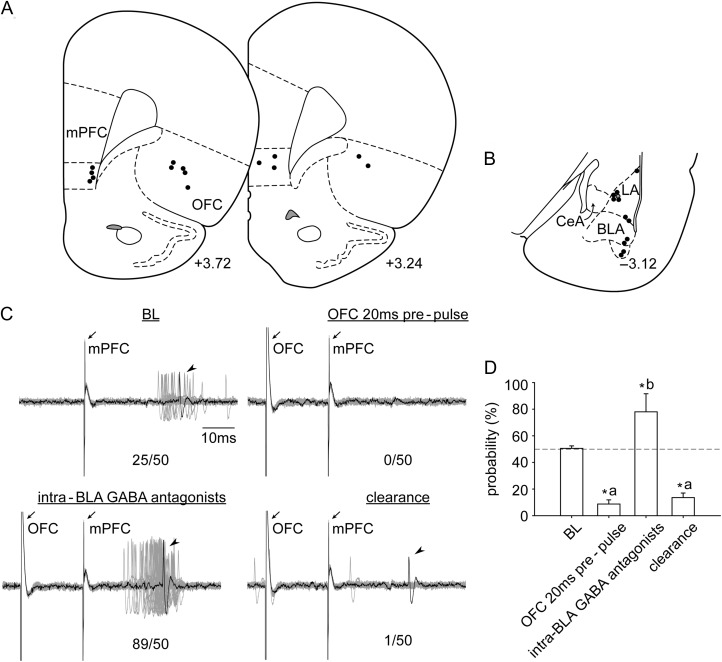
Figure 4.
The placements of (A) all the stimulation electrodes in the mPFC and the OFC and (B) the distribution of all the neurons recorded (+3.72, +3.24, and −3.12; AP distance [mm] to bregma). (C) Electrophysiological recording of an amygdala neuron that is responsive to mPFC stimulation that exhibited a decrease in evoked responses following OFC 20 ms prepulse. This was reversed to a facilitation upon local administration of GABA antagonists, followed by a rapid return to inhibitory gating (n/50 = evoked spikes out of 50 trials). (D) Compared with BL, evoked probability was significantly decreased with OFC prepulse (20 ms; “a”, P < 0.05), and significantly increased under the influence of GABA antagonists (“b”, P < 0.05). Abbreviations refer to Figures 1 and 3.
Iontophoretic blockade of the intra-amygdala GABAergic receptors reversed the OFC inhibitory gating to excitatory gating (Fig. 4C,D; significant main effect of “treatment” [F3,28 = 18.388, P < 0.001]). Compared with BL, evoked probability was significantly decreased with OFC prepulse (20 ms; “a”, P < 0.05). This inhibitory gating was reversed to facilitation under the influence of GABA antagonists (“b”, P < 0.05). Importantly, the antagonist effect was transient and returned rapidly to inhibitory gating when the ejection current was turned off and the drug was cleared from the system (clearance; “a”, P < 0.05).
Experiment 4: OFC Tetanus Abolished the Inhibitory Gating on mPFC-LA/BLA Pathway
The previous 3 experiments examined how activation of the OFC affected mPFC drive of LA/BLA neurons and the potential mechanism underlying this action. In this experiment, we further examined whether altered OFC functional connectivity, rather than the structures per se, played a role. A total of 8 LA/BLA neurons responsive to mPFC stimulation were recorded in this experiment (Fig. 5A,B). OFC tetanus did not change LA/BLA baseline evoked response to mPFC stimulation (Fig. 5C; no significant difference in the main effect of “Trial Block” [50 trials] [F11,77 < 1]). However, OFC tetanus abolished the OFC inhibitory gating of the mPFC-LA/BLA pathway (Fig. 5D). There was no significant difference in the main effect of “delay” [F6,42 = 1.914, P = 0.101] (Fig. 5E).
Figure 5.
The placements of (A) all the stimulation electrodes in the mPFC and the OFC and (B) the distribution of all the neurons recorded (+3.72, +3.24, and −3.12; AP distance [mm] to bregma). (C) OFC tetanus did not change evoked probability of the amygdala neuron response to mPFC stimulation. (D) Electrophysiological recording of an amygdala neuron that responded to mPFC stimulation after OFC tetanus. (E) Stimulation of the OFC failed to produce an inhibitory gating over the mPFC–amygdala pathway following OFC tetanus. Abbreviations refer to Figures 1 and 3.
Discussion
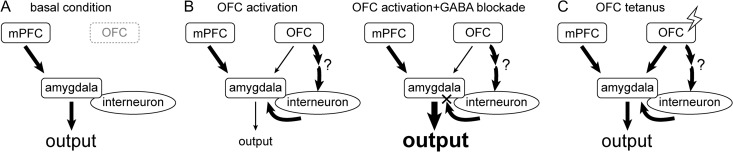
In this study, combined techniques of in vivo electrophysiology, electrical stimulation, local- and iontophoretic administration of drugs were used in anesthetized rats. Our results suggest that compared with basal condition (Fig. 6A), pharmacological or electrical activation of the OFC exerted an inhibitory modulation of the mPFC–amygdala pathway (Fig. 6B, left), which was reversed with intra-amygdala blockade of GABAergic receptors (Fig. 6B, right). Moreover, potentiation of the OFC-related pathways, which strengthened the functional connectivity between structures, attenuated the OFC control over the mPFC–amygdala pathway (Fig. 6C).
Figure 6.
One potential model that may account for the OFC modulation of the mPFC–amygdala pathway. Compared with (A) basal condition, (B) pharmacological or electrical activation of the OFC exerted an inhibitory modulation of the mPFC–amygdala pathway (left), which was reversed by intra-amygdala blockade of GABAergic receptors (right). (C) Tetanization of the OFC-related pathways results in a loss of OFC control over the mPFC–amygdala pathway, presumably because of a selective enhancement of the OFC–amygdala principle neuron input. Abbreviations refer to Figure 1.
IL and PL of the mPFC have opposite effects in regulating fear expression (Sierra-Mercado et al. 2011). In this study, we targeted the IL subdivision and reported responsive neurons in both the LA and the BLA nuclei, which constitute the sensory interface of the amygdala (LeDoux et al. 1990), and both receive inputs from the IL (Vertes 2004), although we cannot rule out potential current spread to adjacent PL in some cases. Nonetheless, stimulation at central versus dorsal aspects of IL produced the same effect.
Pharmacological activation of the OFC decreased the ability of the mPFC to drive LA/BLA neurons, likely due to the engagement of the feed-forward inhibition as demonstrated in the inhibitory gating experiments. Interestingly, when the excitatory drive of the OFC was blocked with the NMDA antagonist, there was no modulatory effect compared with the control group. This result suggests that there is no active drive of the OFC on the mPFC–amygdala pathway during the basal condition or the ongoing NMDA tone in the OFC is low, and the OFC only interacts with the mPFC at the amygdala when it is brought online. This result renders an interesting question of whether these 2 independent pathways would start interacting with each other, and the circumstances under which this would occur.
We found that the inhibitory gating of the OFC on the mPFC–amygdala pathway was effective at a wide range of interstimulus intervals (ISIs), up to 100 ms in our study. This result was surprising in that if it were just a simple disynaptic feed-forward inhibition at the synaptic level within the amygdala (Fig. 6B), OFC to mPFC prepulse intervals longer than 40 ms should have started losing the modulatory effect, as demonstrated in an earlier study looking into the similar interaction within the nucleus accumbens (Asher and Lodge 2012). Although the long-delay (up to 100 ms) inhibitory modulation could potentially result from antidromic recruitment of intra-amygdala inhibitory networks, this is unlikely given that the latencies observed are not consistent with local circuit effects. It is also unlikely that antidromic recruitment of the OFC itself plays a significant role because for some neurons we sampled during our search (n = 14), the antidromic latency from amygdala to the OFC ranged from 7.6 to 31.3 ms (mean latency 16.9 ± 2.0 ms). Although this type of long-delay inhibitory modulation suggests a more complicated multisynaptic process that involves at least one feed-forward inhibitory connection, it is also possible that this was driven by a GABAB action, which is known to last of long duration (Palmer et al. 2012). The pathways through which such an extended action could take place will require further analysis, including whether the amygdala is the common node of feed-forward inhibition.
Intra-amygdala blockade of the GABA was found to reverse the inhibitory modulation at the short ISI of 20 ms OFC prepulse. This was not due to an offsetting activation due to blockade of tonic inhibition, since there was no increase in spontaneous firing of the neurons recorded when GABA receptors were blocked. This result also suggests that the inhibitory modulation at the short ISI is mediated at least partially via the feed-forward inhibition within the amygdala (Fig. 6B).
Abnormal activity of the amygdala is the core to several psychiatric disorders (MacNamara et al. 2016). For example, obsessive-compulsive disorder (OCD) is associated with the overactive cortico–striato–thalamocortical circuitry including hyperactive OFC (Monteiro and Feng 2016), and aberrant amygdala hyperactivity may mediate the anxiety component in OCD (Milad and Rauch 2012; Simon et al. 2014). The dysfunction of the network that would result from aberrant functional connectivity, rather than the structures per se, may be central for understanding the pathophysiology of these disorders. In searching for amygdala neurons responsive to mPFC electrical stimulation, only 5 out of 23 neurons were found to receive convergent inputs from mPFC and OFC. In contrast, most of the OFC responsive amygdala neurons also respond to mPFC stimulation (data not shown). This result suggests that projection from OFC onto the amygdala is relatively weak compared with mPFC inputs (Fig. 6B). Although only suggestive, it is interesting that 1 neuron (out of 8) began responding to previously subthreshold OFC stimulation after tetanus, which supports the model in which the OFC–amygdala projection is strengthened after tetanus (Fig. 6C). Whether this strengthening of the basal connectivity leads to a loss of inhibitory modulatory gating of the OFC over the mPFC–amygdala pathway remains to be determined. Moreover, given that mPFC drive of the amygdala is believed to contribute to several anxiety disorders, and that OCD involves overactivity in the OFC, the expected loss of inhibitory modulation of the OFC over the mPFC that may occur in OCD could serve as a physiological basis for increasing susceptibility for comorbidity in these individuals.
Author's contribution
C.-h.C. and A.A.G. designed the research and wrote the paper; C.-h.C. performed the research and analyzed data.
Funding
This work was supported by United States Public Health Service Grant MH57440 (A.A.G.). Dr Chang and Dr Grace reported no biomedical financial interests. Dr Grace received funds from Johnson & Johnson, Lundbeck, Pfizer, GSK, Puretech Ventures, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, Alkermes, and Asubio.
Notes
We thank Nicole MacMurdo for her assistance in histology. Conflict fo Interest: None declared.
References
- Aggleton JP, Burton MJ, Passingham RE. 1980. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta). Brain Res. 190:347–368. [DOI] [PubMed] [Google Scholar]
- Asher A, Lodge DJ. 2012. Distinct prefrontal cortical regions negatively regulate evoked activity in nucleus accumbens subregions. Int J Neuropsychopharmacol. 15:1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. 2007. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. J Neurosci. 27:12358–12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. 1992. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 324:180–194. [DOI] [PubMed] [Google Scholar]
- Chang CH, Grace AA. 2014. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry. 76:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Grace AA. 2015. Dopaminergic modulation of lateral amygdala neuronal activity: differential D1 and D2 receptor effects on thalamic and cortical afferent inputs. Int J Neuropsychopharmacol. 18:pii: pyv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB. 2008. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem. 89:567–573. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. 2000. Emotion circuits in the brain. Annu Rev Neurosci. 23:155–184. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. 1990. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 10:1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens SM, Devinsky O, Phelps EA. 2011. Role of the left amygdala and right orbital frontal cortex in emotional interference resolution facilitation in working memory. Neuropsychologia. 49:3201–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantonio F, Gardner MP, Mirenzi A, Newman LE, Takahashi YK, Schoenbaum G. 2015. Neural estimates of imagined outcomes in basolateral amygdala depend on orbitofrontal cortex. J Neurosci. 35:16521–16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, DiGangi J, Phan KL. 2016. Aberrant spontaneous and task-dependent functional connections in the anxious brain. Biol Psychiatry. 1:278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. 1999. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci. 22:561–567. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. 2012. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 63:129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. 2012. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 16:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro P, Feng G. 2016. Learning from animal models of obsessive-compulsive disorder. Biol Psychiatry. 79:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. 2011. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 31:17269–17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Maren S. 2012. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 36:1773–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Moorman DE, Young JW, Setlow B, Floresco SB. 2015. Neural mechanisms regulating different forms of risk-related decision-making: Insights from animal models. Neurosci Biobehav Rev. 58:147–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LM, Schulz JM, Murphy SC, Ledergerber D, Murayama M, Larkum ME. 2012. The cellular basis of GABA(B)-mediated interhemispheric inhibition. Science. 335:989–993. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. 2004. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 43:897–905. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. 2005. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 48:175–187. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. 2000. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 911:369–391. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. 2008. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 33:56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel-Clower NL. 2007. Role of orbitofrontal cortex connections in emotion. Ann N Y Acad Sci. 1121:72–86. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. 2011. Memory modulation. Behav Neurosci. 125:797–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. 1999. Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci. 19:11027–11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. 2003. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 23:11054–11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch M. 2005. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 47:633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Stalnaker TA. 2007. Reconciling the roles of orbitofrontal cortex in reversal learning and the encoding of outcome expectancies. Ann N Y Acad Sci. 1121:320–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe MJ, Schoenbaum G. 2016. Back to basics: Making predictions in the orbitofrontal-amygdala circuit. Neurobiol Learn Mem. 131:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. 2011. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 36:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D, Adler N, Kaufmann C, Kathmann N. 2014. Amygdala hyperactivation during symptom provocation in obsessive-compulsive disorder and its modulation by distraction. NeuroImage Clin. 4:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. 2010. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 20:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE, LeDoux JE. 1999. GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: a mechanism for modulation of sensory inputs related to fear conditioning. J Neurosci. 19:Rc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 51:32–58. [DOI] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S. 2010. NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats. Eur J Neurosci. 31:1664–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]