Abstract
Although much progress has been made to uncover age-specific mortality patterns of the 1918 influenza pandemic in populations around the world, more studies in different populations are needed to make sense of the heterogeneous death impact of this pandemic. We assessed the absolute and relative magnitudes of 3 pandemic waves in the city of Madrid, Spain, between 1918 and 1920, on the basis of age-specific all-cause and respiratory excess death rates. Excess death rates were estimated using a Serfling model with a parametric bootstrapping approach to calibrate baseline death levels with quantified uncertainty. Excess all-cause and pneumonia and influenza mortality rates were estimated for different pandemic waves and age groups. The youngest and oldest persons experienced the highest excess mortality rates, and young adults faced the highest standardized mortality risk. Waves differed in strength; the peak standardized mortality risk occurred during the herald wave in spring 1918, but the highest excess rates occurred during the fall and winter of 1918/1919. Little evidence was found to support a “W”-shaped, age-specific excess mortality curve. Acquired immunity may have tempered a protracted fall wave, but recrudescent waves following the initial 2 outbreaks heightened the total pandemic mortality impact.
Keywords: 1918 pandemic, age-specific mortality patterns, excess mortality, herald wave, influenza, mortality baseline, Spain
The 1918–1920 influenza pandemic, or the so-called Spanish flu, was responsible for more than 50 million deaths worldwide (1, 2). In Europe, the excess mortality rate associated with the 1918–1919 influenza pandemic has been estimated at 1.1%, or approximately an 86% increase in all-cause mortality (3). This pandemic rapidly spread in a series of pandemic waves that gripped the world beginning in early 1918 (4). However, according to results of various phylogenetic and molecular-clock analyses, the initial circulation of the virus from avian or swine and other mammal species to humans may have occurred a few years earlier (5–7). Moreover, the symptoms and age-specific mortality patterns associated with this particular pandemic are unique. For example, the most severely affected patients were often young adults who had heliotrope cyanosis and acute respiratory distress. In fact, according to several detailed historical investigations, the highest excess mortality rates consistently were among young adults. This finding is in contrast to those indicating seasonal influenza epidemics primarily affect the very young and elderly (8, 9).
The name Spanish flu comes from the first news reports of influenza-like-illness in Madrid in the late spring of 1918. However, this pandemic gained its nickname because the first mentions of the virus were published in Spain, where the press faced no censorship during World War I, owing to the country’s neutrality (10). Many people fell ill with respiratory symptoms in May 1918, including King Alfonso XIII, which was well documented in the press (10). Because respiratory disease outbreaks occurred in neighboring France as early as April 1918, it is likely that the virus was introduced into Spain via Spanish and Portuguese labor migrants in southern France (11). Research has provided abundant information regarding the timing, severity, and excess mortality of the 1918 influenza pandemic in Spain (10–12), as well as some estimates of transmission potential of the virus within the city of Madrid (12–14). Nevertheless, these analyses provide a primarily descriptive picture of the pandemic in Spain through the lens of period press reports and midcentury publications, including a sense of the evolution of sanitation and health in Spain (10, 11, 15, 16), though newly digitized data sources provide increased opportunities to quantify the impact of the pandemic on the Spanish population (12). For instance, estimates of pandemic excess respiratory death rates have ranged from 6.1 per 10,000 for the Canary Islands to 169.7 per 10,000 for Burgos (12). Moreover, approximately 40% of between-province variation in cumulative excess death rates in Spain during 1918–1919 are explained by spatial factors, such as latitude, population density, and the proportion of children, have explained (12). However, in few of these analyses did researchers take into account a recrudescent wave in Spain, which peaked in Madrid in late December 1919 and in later months in the rest of Spain (3, 15, 16).
Although much progress has been made in uncovering the age-specific mortality patterns of this pandemic in several populations in Latin America (17–20), the United States, and Europe (21–24), more studies are needed to make sense of the heterogeneous death impact of this pandemic across different populations around the world. For instance, by characterizing and comparing the age-specific excess death rates across pandemic waves during 1918–1920 in different populations, researchers could suggest alternative hypotheses on the drivers of pandemic mortality risk at the time and place more emphasis on less-studied phenomena associated with the pandemic.
Despite previous efforts to characterize the impact of the 1918 influenza pandemic in Spain, prior studies have not systematically investigated differences in death impact between age groups and pandemic waves. In this study, we analyzed detailed series of deaths after retrieving more than 70,000 individual death certificates representing all-cause deaths during 1917–1920. We assessed the timing of pandemic waves and their magnitude in absolute and relative terms on the basis of all-cause and respiratory excess death rates across age groups and 3 pandemic waves in the city of Madrid during 1918–1920, including a recrudescent wave in winter 1919–1920.
METHODS
Spanish death data
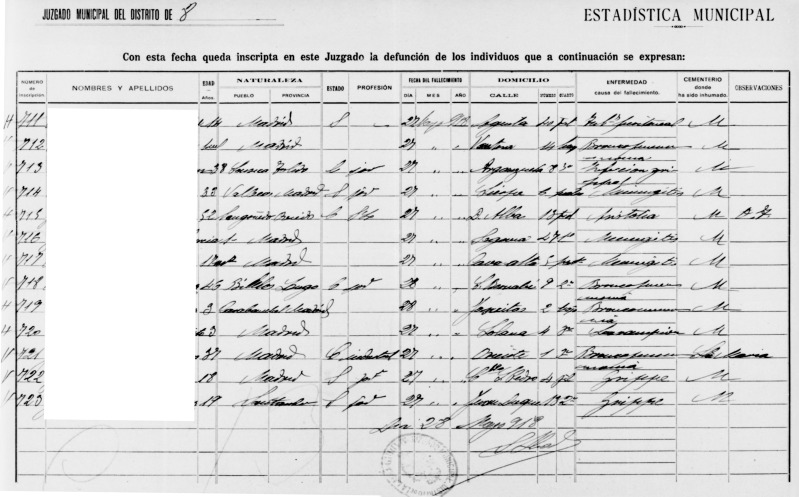
We retrieved all death certificates from the Madrid Civil Registry to construct time series of deaths during the 1918–1920 influenza pandemic (Figure 1). Each record provides specific details of the deceased, including the date of death, age, and causes of death. For years 1917–1920, the registry holds a total of 70,061 death records (an average, 17,650 deaths per year). Cause-of-death information for each death record allowed us to extract data on deaths attributed to influenza and respiratory causes.
Figure 1.
Sample of death records from May 27, 1918, from the Civil Register of Madrid (49).
It is now well recognized that a significant fraction of the pandemic deaths resulted from secondary respiratory ailments (e.g., most commonly bacterial pneumonia) following influenza infection, rather from influenza infection alone (25, 26). Additional influenza-related deaths have been attributed to other types of bacterial infections and severe acute respiratory distress, often evidenced by the appearance of bluish-gray skin shortly before death (25). As such, estimates of death attributed to respiratory causes also provide key information regarding the impact of influenza-specific deaths. As was done in prior studies (e.g., Chowell et al. (17)), we have estimated in this study excess death rates for all-cause deaths and for pneumonia- and influenza-related deaths, a category that comprises all death records indicating influenza, pneumonia, bronchopneumonia, or bronchitis as a cause of death after removing death certificates reporting tuberculosis as a cause of death.
Furthermore, to estimate death rates, information regarding the population composition of Madrid was obtained from the city’s yearly population books (27). With this information, we were able to standardize our results according to the age structure of the population of Madrid at the time. We describe methods to estimate baseline and excess mortality rates as well as excess mortality ratios across age groups and pandemic waves.
Spain experienced one of the highest excess mortality rates during the 1918 influenza pandemic in Europe (3), although this country did not take part in World War I. Perhaps this pandemic outcome is associated with the fact that Spain was going through a demographic transition and experiencing elevated death rates that were only comparable to those of eastern Europe. Of note, the life expectancy in Spain was 41 years in 1910 and 40 years in 1920 (28).
Estimating mortality baselines with quantified uncertainty
Using mortality data for 1917, we characterized baseline death levels using weekly death rates and a simple, cyclical, Serfling linear regression model (29). However, this initial attempt to characterize the baseline did not capture a small but noticeable summer mortality peak. To account for this variation, we modified the initial Serfling model with additional parameters, as was done in another study of the 1957 influenza pandemic in Maricopa County, Arizona (30). The added coefficients in the model account for time (α) and seasonal (β and γ) variations in normal influenza activity, such that the oscillations (at time t) may be written as:
To account for uncertainty in our 1917 baseline death level, we used a parametric bootstrap approach (31). With this method, we first simulated data before fitting the regression model displayed in the previous paragraph, accounting for fluctuations in the annual timing of winter and summer death peaks. For each of the weekly sets of death counts, we simulated a Poisson-distributed number of expected deaths, because the number of deaths each week is a “count” variable that must be 0 or greater. Our Poisson estimations assumed the mean and variance of a week were equal to the observed total number of deaths in a given week of 1917.
From each of 500 simulated data sets, α and β parameters were estimated according to the aforementioned modified seasonal regression model. We calculated the 5-year baseline from the mean values of the coefficients from 500 models and computed the upper baseline from the upper quartile value of the 95% confidence interval of coefficients. As in other reports in which Serfling regression was used to estimate baseline death rates, we defined weeks with death counts above the upper baseline as “pandemic weeks” (18, 30, 32). We defined 3 distinct wave periods: May to July 1918, August 1918 to April 1919, and November 1919 to February 1920. Although there is evidence to suggest the city of Madrid experienced a 1918 fall wave and a 1918/1919 winter wave, these become unclear when disaggregating the data into smaller categories such as age groups. For this reason and to facilitate comparisons with prior studies (12, 33, 34), we analyzed the successive fall and winter increases in excess mortality as 1 pandemic wave.
We characterized excess mortality for each wave by summing the total death rate above the baseline rate during the epidemic periods. To aid in the comparison of our results with other research, we also provide relative estimates for each wave and age group to allow relative comparisons across age groups (12, 35). For each wave, we defined relative risk as the ratio of total excess mortality observed to expected baseline number of deaths during pandemic weeks, when total mortality exceeded the 95% confidence interval of the baseline. This aids in the direct comparison of the total influenza pandemic between study groups, because baseline death counts varied substantially by age group (18).
RESULTS
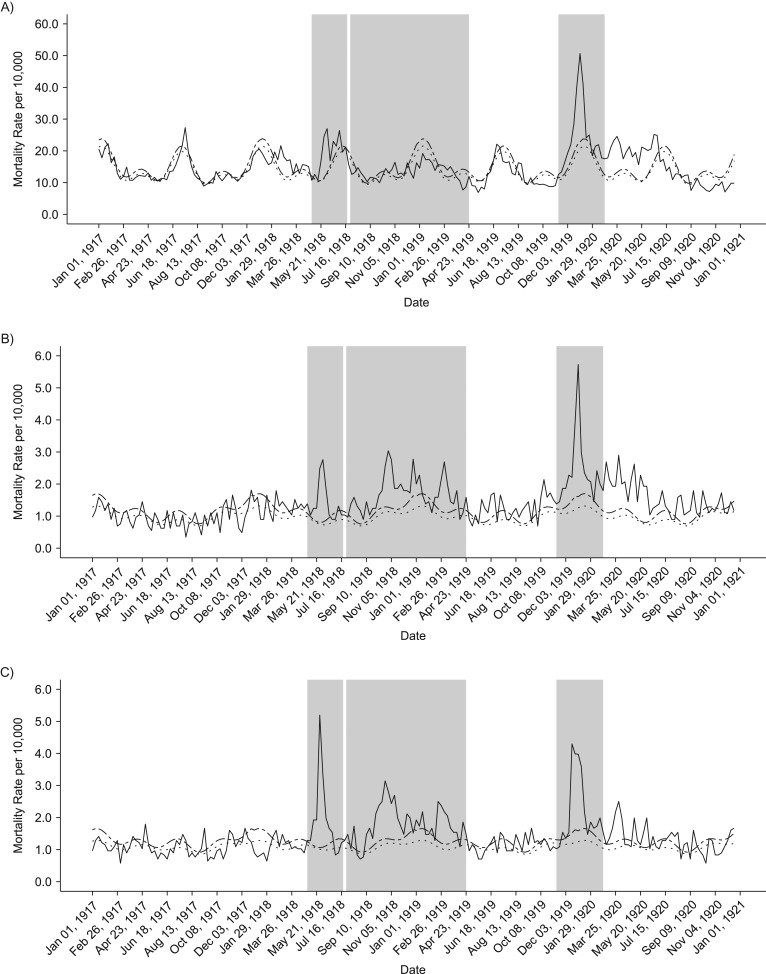
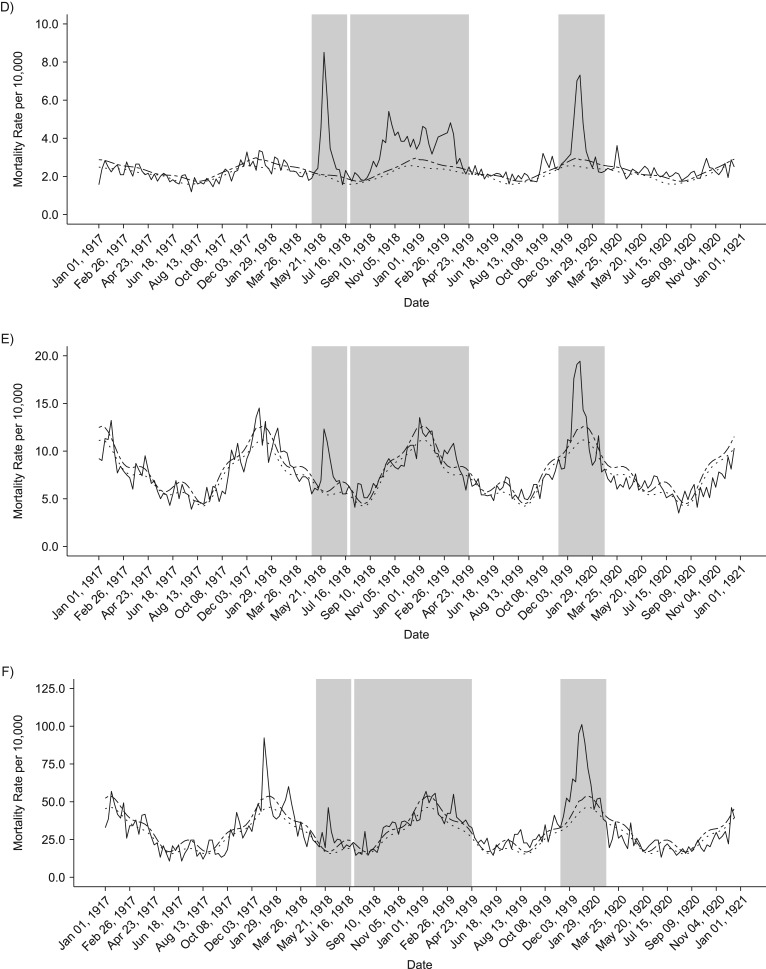
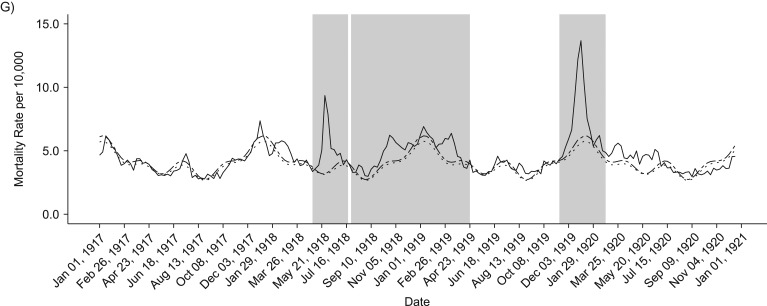
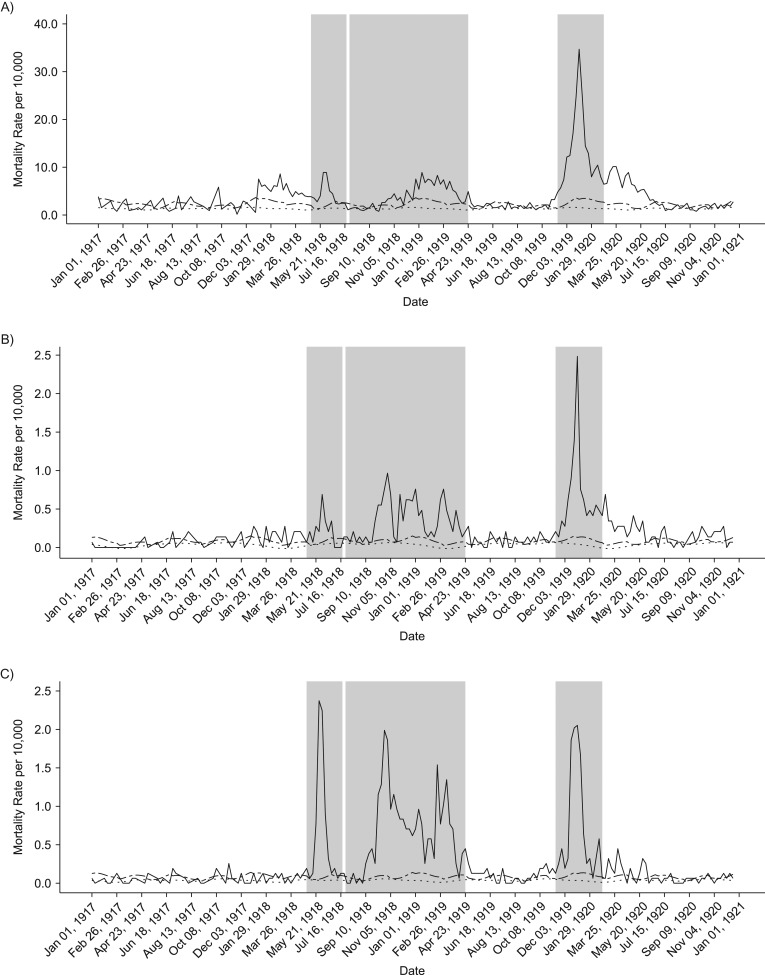
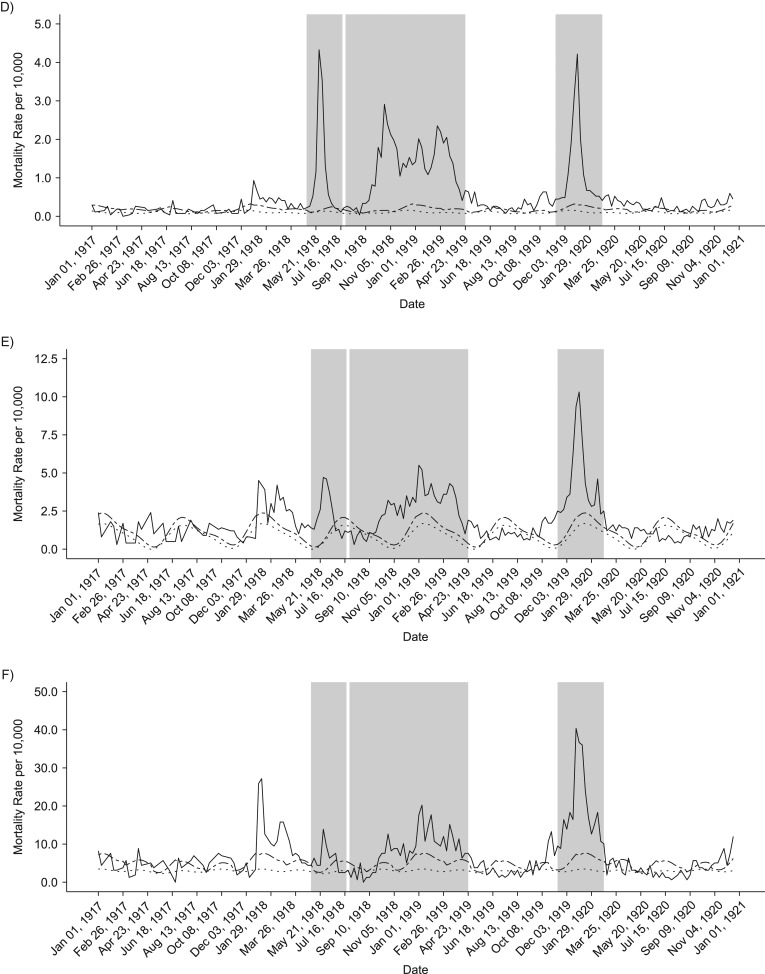
Our analyses of weekly death rates from January 1917 to December 1921 revealed 3 distinct periods of pandemic-related mortality: a brief but well-defined spring wave (May to July 1918), an intense fall-winter wave during August 1918 to April 1919, and a recrudescent winter wave during November 1919 to February 1920 (Figures 2 and 3). Overall, peaks in respiratory and all-cause death rates were well synchronized. All-cause and respiratory-related excess deaths for all age groups generally followed the same pattern of excess mortality by wave: The fall-/winter wave had the highest excess rates, followed by the third recrudescent wave, then the herald wave in spring 1918 (Tables 1 and 2). In addition, the pattern of the age-specific standardized mortality risk (SMR) remains the same, but the total elevated risk in all waves is much more pronounced when considering only respiratory mortality. Our cumulative estimates of excess mortality for these 3 pandemic waves were 86.8 per 10,000 from all-cause death and 44.6 per 10,000 from respiratory-related deaths, or approximately 6,500 total excess deaths, of which 3,300 were respiratory related.
Figure 2.
Weekly time series of all-cause death rates, 1917–1920. Solid lines show the real weekly mortality rates from 1917 to 1922, and dotted and dashed lines show the mean and upper 95% bound baseline rates from simulated 1917 death data. Shaded gray blocks indicate the 3 epidemic wave periods. A–F) The figure panels correspond to the following age groups: <5, 5–14, 15–24, 25–49, 50–69, and ≥70 years. G) Graph shows data for all ages combined.
Figure 3.
Weekly time series of respiratory-related death rates, 1917–1920. Solid lines show the real weekly mortality rates from 1917 to 1922, and dotted and dashed lines show mean and upper 95% bound baseline rates from simulated 1917 death data. Shaded gray blocks indicate the 3 epidemic wave periods. A–F) Figure panels correspond to the following age groups: <5, 5–14, 15–24, 25–49, 50–69, and ≥70 years. G) Graph shows data for all ages combined.
Table 1.
Age-Specific Excess All-Cause Deaths by Wave in Madrid During 3 Epidemic Periods From 1918 to 1920
| Age Group, years | Total No. of Excess Deaths | Total Excess Mortality Rate per 10,000 | Standardized Mortality Risk |
|---|---|---|---|
| Spring Wave, 1918 | |||
| Overall | 1,456 | 19.42 | 1.57 |
| <5 | 375 | 57.57 | 1.40 |
| 5–14 | 95 | 6.58 | 2.03 |
| 15–24 | 165 | 10.59 | 2.28 |
| 25–49 | 486 | 18.13 | 1.95 |
| 50–69 | 213 | 21.32 | 1.55 |
| ≥70 | 127 | 80.39 | 1.68 |
| Fall and Winter Wave, 1918/1919 | |||
| Overall | 2,511 | 33.50 | 1.27 |
| <5 | 293 | 44.90 | 1.22 |
| 5–14 | 364 | 25.11 | 1.82 |
| 15–24 | 401 | 25.73 | 1.83 |
| 25–49 | 1,250 | 46.63 | 1.58 |
| 50–69 | 262 | 26.22 | 1.24 |
| ≥70 | 275 | 173.58 | 1.24 |
| Winter Wave 1919/1920 | |||
| Overall | 2,538 | 33.86 | 1.52 |
| <5 | 823 | 126.34 | 1.59 |
| 5–14 | 261 | 17.98 | 2.18 |
| 15–24 | 235 | 15.08 | 2.14 |
| 25–49 | 467 | 17.41 | 1.63 |
| 50–69 | 344 | 34.45 | 1.41 |
| ≥70 | 485 | 306.51 | 1.63 |
Table 2.
Age-Specific Excess Respiratory-Related Deaths by Wave in Madrid During 3 Epidemic Periods From 1918 to 1920
| Age Group, years | Total No. of Excess Deaths | Total Excess Mortality Rate per 10,000 | Standardized Mortality Risk |
|---|---|---|---|
| Spring Wave, 1918 | |||
| Overall | 613 | 8.17 | 2.59 |
| <5 | 253 | 38.81 | 2.62 |
| 5–14 | 19 | 1.31 | 3.11 |
| 15–24 | 49 | 3.12 | 4.43 |
| 25–49 | 114 | 4.25 | 2.89 |
| 50–69 | 100 | 9.98 | 2.27 |
| ≥70 | 77 | 48.72 | 3.15 |
| Fall and Winter Wave, 1918/1919 | |||
| Overall | 1,670 | 22.28 | 1.82 |
| <5 | 308 | 47.25 | 1.69 |
| 5–14 | 82 | 5.64 | 2.27 |
| 15–24 | 185 | 11.87 | 4.20 |
| 25–49 | 524 | 19.56 | 2.77 |
| 50–69 | 346 | 34.66 | 1.65 |
| ≥70 | 250 | 157.79 | 1.88 |
| Winter Wave 1919/1920 | |||
| Overall | 1,061 | 14.15 | 1.86 |
| <5 | 397 | 61.01 | 2.04 |
| 5–14 | 58 | 3.97 | 2.35 |
| 15–24 | 83 | 5.33 | 3.56 |
| 25–49 | 180 | 6.71 | 2.18 |
| 50–69 | 168 | 16.85 | 1.67 |
| ≥70 | 193 | 121.84 | 1.89 |
Total excess mortality for epidemic weeks during the observed period was highest during the second fall-winter wave in 1918/1919. We found a total excess rate of approximately 33.5 deaths per 10,000 persons, based on all-cause deaths and 22.3 per 10,000 based on respiratory-related deaths. In contrast, the spring-summer wave was associated with an excess death rate at 8.2 per 10,000 persons, based on respiratory-related deaths and 19 per 10,000 for all-cause deaths. It is interesting that the third wave in winter of 1919–1920 generated a substantial death rate at 34 deaths per 10,000 persons, based on all-cause deaths, which is comparable to that of the intense fall-winter 1918/1919 wave. However, it is worth noting that the first and third waves were relatively brief and had a pointed shape, whereas some age groups had 2 well-defined death peaks during the protracted second wave in fall-winter 1918/1919 (Figure 3).
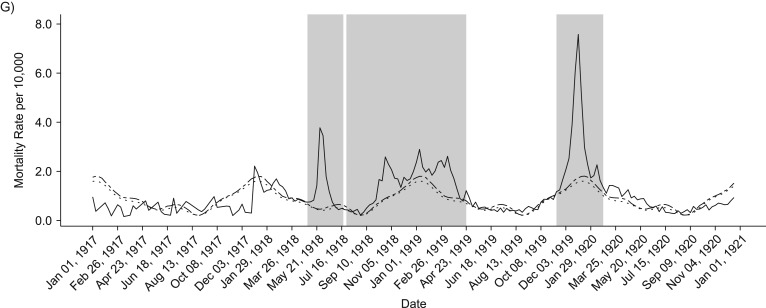
In general, age-specific excess mortality rates were lowest during the spring wave and highest during the protracted second wave, as shown in Figure 4. Compared with the first 2 pandemic waves, the youngest and oldest groups were particularly affected during the recrudescent wave in the winter of 1919–1920. In fact, during the third wave, those older than 70 years faced excess all-cause and respiratory-related death rates that were more than 3 times higher than in the first wave. Furthermore, during the last wave, infants and children aged up to 15 years experienced more than double the all-cause and respiratory-related excess mortality rates estimated for the first 2 waves. The age groups 5–14 years and 15–24 years maintained similar patterns in each of the waves, facing the lowest excess rates in the spring herald wave and highest in the combined fall and winter waves of 1918/1919. The highest excess mortality rate in the age group 25–49 years occurred in the second wave in fall-winter 1918/1919.
Figure 4.
Total excess mortality rates per 10,000 persons for all-cause (A) and respiratory-related (B) deaths according to age groups for each wave. The solid line represents the first spring wave, the fall and winter waves are represented by the dashed line, and the final winter wave is represented by the dotted line.
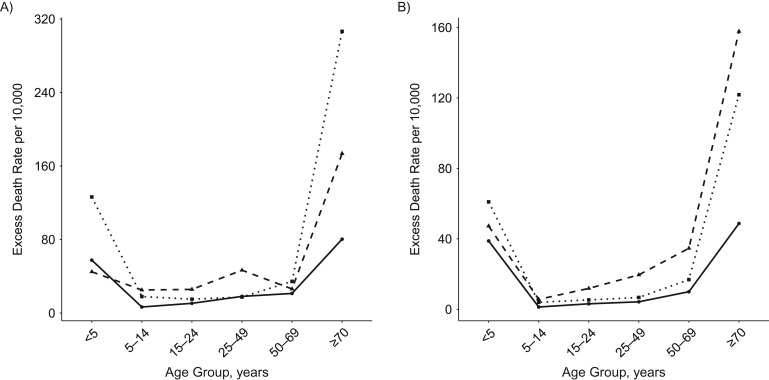
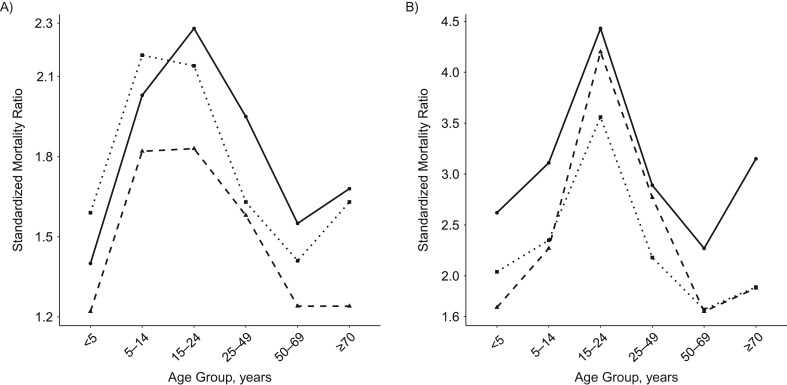
Although the herald spring wave accounted for slightly more than 20% of all total excess deaths, we note that the SMR during this period was higher than in the succeeding waves, due to lower baseline numbers of deaths during spring and summer (see Figure 5). Remarkably, although individuals 15–24 years of age had low excess mortality rates relative to other age groups, this age group had the highest SMR across all pandemic waves. Generally, the age-specific pattern of the SMR is that of an inverted “v,” with the exception of the oldest age group. During the first and third waves, those older than 70 years experienced a higher SMR than individuals aged 50–69 years. Most generally, the highest SMRs occur in the first and last waves, though the highest calculated SMRs for respiratory-related deaths (4.4 and 4.2, respectively,) occurred in those 15–24 years of age in the first and second waves.
Figure 5.
Standardized mortality ratio for all-cause (A) and respiratory-related (B) deaths according to age groups for each wave. The solid line represents the first spring wave, the fall and winter waves are represented by the dashed line, and the final winter wave is represented by the dotted line.
DISCUSSION
Although estimates of excess mortality reveal variability in age-specific patterns throughout the world, our results are unique in that the highest absolute excess rates occurred among older populations (≥70 years) compared with findings in previous reports from Europe and the United States (9, 21, 22). Specifically, the Madrid age-specific excess dominant pattern resembles that of seasonal influenza epidemics in which the highest excess rates occurred in the youngest and oldest groups (17, 18, 36). However, much of the elderly population of Madrid would have been exposed to other viruses; for example, in the decades preceding the Spanish flu, the “Russian” influenza pandemic that struck Madrid in the winter of 1889–1990 produced overall all-cause excess mortality rates of 58.3 per 10,000 persons and produced an age-specific excess mortality pattern similar to each of the 3 pandemic waves in Madrid (34).
Our results also confirm those of earlier analyses of a particularly lethal spring wave in Madrid relative to smaller peaks in numbers of deaths, but high incidence rates in some locations, such as Norway and Denmark (4, 21, 22). In Madrid, weekly excess death rates during the spring wave nearly rivaled that of the protracted fall-winter 1918/1919 wave.
We can contextualize the timing of this first wave in Madrid relative to herald pandemic waves in North America and outbreaks among civilian populations in Europe. Many of the first purported spring outbreaks occurred in US military camps; these outbreaks spread to larger cities in April and May, before the herald wave in Madrid (4). However, the mid-late May outbreak was the first reported in civilian populations in Europe. In the following months, reported influenza outbreaks in Europe occurred eastward and northward to other parts of Spain and Italy, then England, Sweden, and Norway, and Switzerland and Poland (4, 12, 22–25, 37, 38). However, it remains difficult to distinguish to what extent the virus spread through military rather than civilian population movement (4).
According to analyses of hospitalization, deaths, and other surveillance sources in military and civilian settings, there is evidence of cross-protection between spring and fall influenza outbreaks during waves of the 1918–1919 epidemic (39–42). The high pandemic death rate we found, together with evidence of high incidence rates during spring-summer waves (10, 15, 43), could have provided some immunity and cross-protection to the strain of virus in the succeeding fall wave. Conversely, in New York City, a noticeable age shift in influenza death patterns occurred in early 1918, perhaps suggesting the presence of the new virus strain. Yet, there was little total excess mortality until the strong fall wave, which killed more than 9 times as many people (21). This pattern may partially explain the slower growth and protracted wave in Madrid that began in September 1918 and continued through the winter and early spring of 1919.
We also found evidence of a powerful recrudescent wave after the enduring second wave; the recrudescent wave peaked at the very end of 1919 and appeared throughout the world in the spring of 1920 (1, 3, 9, 17, 19–21, 24, 37, 44). In Madrid, all-cause excess rates were on par with those of the elongated second wave, and all-cause and respiratory-related excess mortality rates were higher than in the spring 1918 wave. In other countries and cities where this wave has been documented, a slight shift in the age-specific mortality often occurred, with a return to high excess mortality among people older than 65 years (9, 17, 19, 21, 24). As in our study, the death rate of young adults reported in these locations often dropped slightly but remained persistently high and well above the prepandemic level. In line with previous studies in Spain, during this fourth wave, death rates of infants and young children were particularly high (10, 15, 16). Because high rates of excess mortality existed in all age groups, lack of acquired immunity from earlier waves may only explain the excess mortality among infants and young children. Antigenic shift or mutation in the virus also could have contributed to the elevated mortality remaining elevated across all ages, but it remains difficult to ascertain the exact mechanisms that shaped the strong wave.
Another all-cause and respiratory-related peak in deaths occurred in late December 1921; although we did not specifically analyze this peak, it was present in all age groups and predominately in those age 50 years and those younger than 5 years (45). Recrudescent waves can still occur years after the initial and main pandemic waves, echoing the initial impact of an outbreak, such as in the 2011 A/H1N1 influenza epidemic recurrence in Mexico following the 2009 A/H1N1 influenza pandemic (46). The presence and impact of recrudescent waves of the pandemic should continue to be studied and quantified because they may substantially change the overall death impact of the influenza pandemic.
Our estimates of the influenza pandemic death impact in Madrid can be compared with those derived from a previous study in which excess monthly all-cause and respiratory-related deaths were analyzed in all provinces of Spain during the herald spring wave and second fall-winter wave (12). We found higher overall excess rates in the spring wave (19.4 vs. 11.7 per 10,000 persons) but lower excess rates in the second wave (33.5 vs. 55.0 per 10,000 persons). We also found lower excess respiratory-related mortality rates in both the herald and protracted second waves. These differences may stem from various factors, including differences in death data sources and the fact that the earlier study (12) analyzed pandemic impact in the entire province of Madrid, whereas our study focused on the capital city alone. Moreover, the spring wave may have largely affected the city itself (which we analyzed) and the surrounding province (the subject of the prior study (12)) to a lesser degree, resulting in different excess mortality estimates. The total impact of the spring wave could also extend to the second wave; perhaps those living in the city gained some immunity from exposure to the first wave, whereas those without this exposure did not benefit from cross-protection. Disentangling additional factors that drove these differences could be the focus of future study.
Considering the pandemic events collectively known as the Spanish influenza, the case of Madrid provides additional insights into how, in a large urban environment, individual waves and their progression contributed to the overall death impact on the city. Although other analyses looked at herald waves and questioned the impacts of acquired immunity from spring to fall (21, 22), the force of the spring wave in Madrid, relative to the successive fall and winter outbreaks, does appear to indicate some type of protective influence of the initial outbreak on succeeding waves, possibly due to a small amount of antigenic shift in the virus between the 2 periods. Only strains from the spring and fall waves of 1918 have been studied, to our knowledge, meaning that the extent to which earlier and later strains differed cannot be confirmed (5, 47, 48). Yet, continued analyses of successive waves using new data sources and innovative approaches should be undertaken to better understand acquired immunity and the protection it may provide against successive outbreaks. Using contemporary and historic demographic death and surveillance data of recent and historic epidemics, additional insights into the ways early outbreaks affected immunity and disease transmission can influence the way public health officials respond to contain outbreaks.
ACKNOWLEDGMENTS
Author affiliations: Institute of Economy, Geography and Demography, Center for Humanities and Social Sciences Spanish National Research Council, Madrid, Spain (Laura Cilek, Diego Ramiro Fariñas); School of Public Health, Division of Epidemiology & Biostatistics, Georgia State University, Atlanta, Georgia (Gerardo Chowell); and Division of International Epidemiology and Population Studies, Fogarty International Center, National Institutes of Health, Bethesda, Maryland (Gerardo Chowell).
This work was supported by the LONGPOP (Methodologies and Data Mining Techniques for the Analysis of Big Data Based on Longitudinal Population and Epidemiological Registers) project, which has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant 676060 and the project LONGHEALTH (Early Life Conditions and Health and Mortality in Adult Life CSO2015-69834-R) funded by the Spanish Ministry of Economy and Competitiveness. G.C. received support from the Multinational Influenza Seasonal Mortality Study, an ongoing international collaborative effort to understand influenza epidemiologic and evolutionary patterns that is led by the Fogarty International Center, National Institutes of Health (http://www.origem.info/misms/index.php).
Conflict of interest: none declared.
Abbreviation
- SMR
standardized mortality ratio.
REFERENCES
- 1. Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76(1):105–115. [DOI] [PubMed] [Google Scholar]
- 2. Murray CJ, Lopez AD, Chin B, et al. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. Lancet. 2006;368(9554):2211–2218. [DOI] [PubMed] [Google Scholar]
- 3. Ansart S, Pelat C, Boelle PY, et al. Mortality burden of the 1918–1919 influenza pandemic in Europe. Influenza Other Respir Viruses. 2009;3(3):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simonsen L, Chowell G, Andreasen V, et al. A review of the 1918 herald pandemic wave: importance for contemporary pandemic response strategies. Ann Epidemiol. 2018;28(5):281–288. [DOI] [PubMed] [Google Scholar]
- 5. Smith GJ, Bahl J, Vijaykrishna D, et al. Dating the emergence of pandemic influenza viruses. Proc Natl Acad Sci U S A. 2009;106(28):11709–11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oxford JS, Lambkin R, Sefton A, et al. A hypothesis: the conjunction of soldiers, gas, pigs, ducks, geese and horses in northern France during the Great War provided the conditions for the emergence of the “Spanish” influenza pandemic of 1918–1919. Vaccine. 2005;23(7):940–945. [DOI] [PubMed] [Google Scholar]
- 7. Worobey M, Han GZ, Rambaut A. Genesis and pathogenesis of the 1918 pandemic H1N1 influenza A virus. Proc Natl Acad Sci U S A. 2014;111(22):8107–8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller MA, Viboud C, Balinska M, et al. The signature features of influenza pandemics—implications for policy. N Engl J Med. 2009;360(25):2595–2598. [DOI] [PubMed] [Google Scholar]
- 9. Simonsen L, Clarke MJ, Schonberger LB, et al. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis. 1998;178(1):53–60. [DOI] [PubMed] [Google Scholar]
- 10. Echeverri B. Spanish influenza seen from Spain In: Phillips H, Killingray D, eds. The Spanish Influenza Pandemic of 1918–1919: New Perspectives. Abingdon, UK: Routledge; 2003:173–190. [Google Scholar]
- 11. Trilla A, Trilla G, Daer C. The 1918 “Spanish flu” in Spain. Clin Infect Dis. 2008;47(5):668–673. [DOI] [PubMed] [Google Scholar]
- 12. Chowell G, Erkoreka A, Viboud C, et al. Spatial-temporal excess mortality patterns of the 1918–1919 influenza pandemic in Spain. BMC Infect Dis. 2014;14:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erkoreka A. The Spanish influenza pandemic in occidental Europe (1918–1920) and victim age. Influenza Other Respir Viruses. 2010;4(2):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oeppen J, Ramio Fariñas D, Garcia Ferrero S, et al. Estimating reproduction numbers for the 1889–90 and 1918–20 influenza pandemics in the city of Madrid. Presented at the IX Congreso de la Asociación de Demografía Histórica, Ponta Delgada, Portugal, Universidades dos Azores, June 16–19, 2010.
- 15. Echeverri Dávila B. La Gripe Española. La Pandemia de 1918–19. Colección Monografías (No. 132). Madrid, Spain: Centro de Investigaciones Sociológicas;1993. [Google Scholar]
- 16. Gomez Redondo R. La Mortalidad Infantil Española En el Siglo XX. Colección Monografías. (Vol. 123). Madrid, Spain: Centro de Investigaciones Sociológicas;1992. [Google Scholar]
- 17. Chowell G, Viboud C, Simonsen L, et al. Mortality patterns associated with the 1918 influenza pandemic in Mexico: evidence for a spring herald wave and lack of preexisting immunity in older populations. J Infect Dis. 2010;202(4):567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chowell G, Viboud C, Simonsen L, et al. The 1918–19 influenza pandemic in Boyaca, Colombia. Emerg Infect Dis. 2012;18(1):48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chowell G, Viboud C, Simonsen L, et al. The 1918–1920 influenza pandemic in Peru. Vaccine. 2011;29(suppl 2):B21–B26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chowell G, Simonsen L, Flores J, et al. Death patterns during the 1918 influenza pandemic in Chile. Emerg Infect Dis. 2014;20(11):1803–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olson DR, Simonsen L, Edelson PJ, et al. Epidemiological evidence of an early wave of the 1918 influenza pandemic in New York City. Proc Natl Acad Sci U S A. 2005;102(31):11059–11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andreasen V, Viboud C, Simonsen L. Epidemiologic characterization of the 1918 influenza pandemic summer wave in Copenhagen: implications for pandemic control strategies. J Infect Dis. 2008;197(2):270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Viboud C, Eisenstein J, Reid AH, et al. Age-and sex-specific mortality associated with the 1918–1919 influenza pandemic in Kentucky. J Infect Dis. 2013;207(5):721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dahal S, Jenner M, Dinh L, et al. Excess mortality patterns during 1918–1921 influenza pandemic in the state of Arizona, USA. Ann Epidemiol. 2018;28(5):273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195(7):1018–1028. [DOI] [PubMed] [Google Scholar]
- 26. Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198(7):962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Municipal Archive of the City of Madrid (Archivo Villa) Demographic Statistical Yearly Books of the City of Madrid, 1900–1920 Madrid, Spain: Archivo Villa; 1918.
- 28. Dopico F, Reher DS. El Declive de la Mortalidad en España, 1860–1930; vol. 1. Barcelona, Spain: Asociación de Demografía Histórica;1998 [Google Scholar]
- 29. Serfling RE. Methods for current statistical analysis of excess pneumonia-influenza deaths. Public Health Rep. 1963;78(6):494–506. [PMC free article] [PubMed] [Google Scholar]
- 30. Cobos AJ, Nelson CG, Jehn M, et al. Mortality and transmissibility patterns of the 1957 influenza pandemic in Maricopa County, Arizona. BMC Infect Dis. 2016;16(1):405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Monographs on Statistics and Applied Probability 57. London, UK: Chapman and Hall; 1993 [Google Scholar]
- 32. Mamelund SE. A socially neutral disease? Individual social class, household wealth and mortality from Spanish influenza in two socially contrasting parishes in Kristiania 1918–19. Soc Sci Med. 2006;62(4):923–940. [DOI] [PubMed] [Google Scholar]
- 33. García Ferrero Sara. La Gripe de 1889–1890 en Madrid PhD dissertation. Madrid, Spain: Universidad Complutense de Madrid; 2017.
- 34. Ramiro D, Garcia S, Casado Y, et al. Age-specific excess mortality patterns and transmissibility during the 1889–1890 influenza pandemic in Madrid, Spain. Ann Epidemiol. 2018;28(5):267–272. [DOI] [PubMed] [Google Scholar]
- 35. Serfling RE, Sherman IL, Houseworth WJ, et al. Excess pneumonia-influenza mortality by age and sex in three major influenza A2 epidemics, United States, 1957–58, 1960 and 1963. Am J Epidemiol. 1967;86(2):433–441. [DOI] [PubMed] [Google Scholar]
- 36. Mamelund SE. Geography may explain adult mortality from the 1918–20 influenza pandemic. Epidemics. 2011;3(1):46–60. [DOI] [PubMed] [Google Scholar]
- 37. Hoffman BL. Influenza activity in Saint Joseph, Missouri 1910–1923: evidence for an early wave of the 1918 pandemic. PLoS Curr. 2011;2:RRN1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ammon C. Spanish flu epidemic in 1918 in Geneva, Switzerland. Euro Surveill. 2002;7(12):190–192. [DOI] [PubMed] [Google Scholar]
- 39. Barry JM, Viboud C, Simonsen L. Cross-protection between successive waves of the 1918–1919 influenza pandemic: epidemiological evidence from US Army camps and from Britain. J Infect Dis. 2008;198(10):1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shanks GD, Mackenzie A, McLaughlin R, et al. Mortality risk factors during the 1918–1919 influenza pandemic in the Australian army. J Infect Dis. 2010;201(12):1880–1889. [DOI] [PubMed] [Google Scholar]
- 41. Rios-Doria D, Chowell G. Qualitative analysis of the level of cross-protection between epidemic waves of the 1918–1919 influenza pandemic. J Theor Biol. 2009;261(4):584–592. [DOI] [PubMed] [Google Scholar]
- 42. Mathews JD, McBryde ES, McVernon J, et al. Prior immunity helps to explain wave-like behaviour of pandemic influenza in 1918–19. BMC Infect Dis. 2010;10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Davis R. The Spanish Flu: Narrative and Cultural Identity in Spain, 1918. New York, NY: Palgrave Macmillan; 2013. [Google Scholar]
- 44. Revuelta-Eugercios B. The Uses of the Foundling Hospital of Madrid, Mortality and Retrieval at the Beginning of the 20th Century (1890–1935) PhD thesis. Madrid, Spain: Universidad Complutense de Madrid; 2011.
- 45. Chowell G, Casado Y, Garcia Ferrero S, et al. The transmissibility of influenza pandemics of 1889–1890 and 1918–1920 in a large urban environment: Age-specific excess mortality. Presented at the Social Science History Conference, Baltimore, MD, November 12–15, 2015.
- 46. Chowell G, Echevarría-Zuno S, Viboud C, et al. Recrudescent wave of pandemic A/H1N1 influenza in Mexico, winter 2011–2012: age shift and severity. PLoS Curr. 2012;4:RRN1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Holmes EC, Ghedin E, Miller N, et al. Whole-genome analysis of human influenza A virus reveals multiple persistent lineages and reassortment among recent H3N2 viruses. PLoS Biol. 2005;3(9):e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sheng ZM, Chertow DS, Ambroggio X, et al. Autopsy series of 68 cases dying before and during the 1918 influenza pandemic peak. Proc Natl Acad Sci U S A. 2011;108(39):16416–16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Municipal Archive of the City of Madrid (Archivo Villa) Madrid Civil Register Series, Death Records Madrid, Spain: Archivo Villa; 1918.