Abstract
Background
The activity of palbociclib as a single agent in advanced breast cancer has not been extensively studied, with the only available clinical data limited to heavily pretreated patients. Preclinical data suggests palbociclib may partially reverse endocrine resistance, though this hypothesis has not been evaluated in previous clinical studies. This phase II, open-label, multicenter study examined the activity of palbociclib monotherapy, as well as palbociclib given in combination with the same endocrine therapy (ET) that was received prior to disease progression, in postmenopausal women with moderately pretreated, estrogen receptor-positive, HER2 negative advanced breast cancer.
Patients and methods
Eligible women with advanced disease which had progressed on one or two prior ETs were randomized 1 : 1 to receive either palbociclib alone, or palbociclib in combination with the ET as previously received. Primary end point was clinical benefit rate (CBR); secondary end points included progression-free survival (PFS).
Results
Between October 2012 and July 2016, a total of 115 patients were randomized. The CBR was 54% [95% confidence interval (CI): 41.5–63.7] for combination therapy, and 60% (95% CI: 47.8–72.9) for monotherapy. Median PFS was 10.8 months (95% CI: 5.6–12.7) for combination therapy, and 6.5 months (95% CI: 5.4–8.5) for monotherapy [hazard ratio (HR) 0.69; 95% CI: 0.4–1.1, exploratory P-value = 0.12]. Exploratory analyses revealed the PFS advantage for combination therapy was seen in the subgroup of patients who received prior ET for >6 months (HR 0.53; 95% CI: 0.3–0.9, exploratory P-value = 0.02), but not in those who received prior ET for ≤6 months.
Conclusion
Palbociclib has clinical activity as a single agent in women with moderately pretreated, oestrogen receptor-positive, HER2-negative advanced breast cancer. Palbociclib may have potential to reverse endocrine resistance in patients with a history of previous durable response to ET.
Clinical trial information
Keywords: CDK4/6 inhibitors, advanced breast cancer, endocrine resistance, palbociclib
Key Message
Palbociclib monotherapy has clinical activity in moderately pretreated advanced breast cancer. Palbociclib may potentially reverse endocrine resistance in cases wherein a previous durable response to endocrine therapy has been observed. Further investigation is warranted.
Introduction
Palbociclib is a selective, potent and orally available inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6) which has been shown to be preferentially active in luminal preclinical models of breast cancer [1, 2], and synergistic with endocrine therapy (ET) [3, 4]. The seminal PALOMA trials have shown that combining palbociclib with ET approximately doubles progression-free survival (PFS) rates when compared to ET plus placebo in estrogen receptor-positive, human epidermal grown factor receptor 2 (HER2)-negative advanced breast cancer (ABC) patients in both the setting of first-line treatment [5], and in patients who have previously progressed on ET [6]. PALOMA-3, which specifically enrolled women with ABC whose disease progressed whilst on ET, or within 12 months following completion of adjuvant therapy, produced a median PFS of 9.5 months in favor of combination palbociclib and fulvestrant, versus 4.6 months for the control group [hazard ratio (HR) 0.46; 95% confidence interval (CI): 0.36–0.59, P < 0.0001] [6]. This proven activity in the setting of endocrine resistance is of particular interest, given the existence of previous preclinical evidence that suggests adding palbociclib to ET may partially reverse resistance to the particular agent to which resistance has been acquired [4]. Resistance to ET is considered an eventual inevitability in the treatment of hormone receptor-positive ABC [7]; therefore, strategies that may ameliorate resistance (thereby postponing the need to progress to potentially more toxic lines of cytotoxic treatment) are important, and remains an unmet clinical need.
To date, the activity of palbociclib as a single agent has not been extensively studied, with the only available clinical data in this context limited to heavily pretreated patients [8]. Therefore, we planned a phase II clinical study to test the activity and safety of palbociclib as a single agent in a moderately pretreated population of women with estrogen receptor-positive ABC, as well as in combination with the same ET as was received at the time of disease progression.
Methods
TREnd is a randomized, open-label phase II trial (NCT02549430) at eight Italian centers. The study was approved by an independent ethics committee at each site, and carried out in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Patients provided written informed consent.
Patients
Eligible women were ≥18 years of age with estrogen receptor-positive, HER2-negative ABC, with a history of having received one or two lines of prior ET in the advanced setting. A maximum of one previous line of chemotherapy for ABC was also permitted. Only women who had received fulvestrant or an aromatase inhibitor as the most recent line of ET were eligible. Estrogen receptor status was locally determined by immunohistochemistry (IHC); positive classification was defined by ≥10% staining. HER2-negative status was locally determined by either fluorescence in situ hybridization (FISH) or IHC (IHC 0, 1+, 2+ and/or FISH HER2: CEP17 ratio <2.0). All patients were required to have measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) or measurable bone-only disease, with an Eastern Cooperative Oncology Group performance status of 0 to 2. Adequate organ function, determined by standard hematological and biochemistry tests, was mandatory. Subjects with unstable brain metastases or leptomeningeal disease were excluded. Previous treatment with any CDK inhibitor was not permitted.
Procedures
Patients were randomized 1 : 1. Those in the monotherapy arm received single-agent oral palbociclib 125 mg once daily, for 3 weeks, followed by 1 week off (28-day cycle). Those allocated to the combination arm received palbociclib at the same dose and regimen, plus continuation of the prior ET taken before progression (oral anastrozole 1 mg/day or letrozole 2.5 mg/day or exemestane 25 mg/day, or intramuscular fulvestrant 500 mg every 4 weeks). All ET was given continuously. Dose interruptions and reductions were allowed as required (see Appendix, available at Annals of Oncology online). The assigned study treatment was continued until disease progression, unacceptable toxicity or consent withdrawal. Randomization was stratified according to: number of previous ET lines (1 versus 2), duration of prior-line ET (≤6 months versus >6 months), metastatic disease site (visceral versus nonvisceral) and treating center. Crossover was not permitted at any time.
On-study evaluation
Response was assessed locally at baseline, after cycle 3, and every 12 weeks thereafter, utilizing RECIST version 1.1. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0.
Statistical methodology and end point analysis
The primary end point was clinical benefit rate (CBR) as defined by the percentage sum of complete responses (CR), partial responses (PR) and stable disease (SD) for at least 24 weeks according to RECIST 1.1 criteria. Secondary end points were AEs, objective response (OR) defined by the sum of confirmed CR plus PR, and investigator-assessed PFS, defined as the time from randomization to radiological disease progression or death on study. Other secondary end points included time to tumor progression and overall survival. A post hoc analysis of duration of clinical benefit was also performed.
We used a two-stage optimal design to assess treatment activity in each of the two randomized groups [9]. Assuming inactivity as a CBR of 20%, and activity as a CBR of 40%, with alpha set to 10% and power to 90%, the threshold for proceeding to the second stage was at least five responses among the 25 first patients in each group. In the second stage an additional 25 patients were treated in each group, resulting in a total sample size of at least 100 evaluable patients. The null hypothesis for each group could be rejected if at least 13 responses were observed among the first 50 evaluable patients.
An exploratory analysis of clinical benefit duration was conducted in the subgroup of patients with clinical benefit. This was defined as the time from the first response (PR, CR or SD) to progression or death from any cause (whichever came first). Further post hoc subgroup analyses were done according to duration of prior ET (arbitrarily set at > or ≤ 6 months) for the outcome of PFS.
CBR was compared between the two randomized groups by chi square tests, and by calculating the 95% CI of the difference of the proportions. Time-to-event outcomes were compared between the two groups using stratified log-rank tests. We plotted Kaplan–Meier product-limit estimators and calculated the hazard ratio using a stratified Cox regression model. The same stratification factors were used as for randomization, except for the treating center.
All reported P-values are two-sided, and exploratory in nature. All analyses were conducted by the International Drug Development Institute using SAS statistical software, version 9.3 (SAS Institute, Cary, NC).
Results
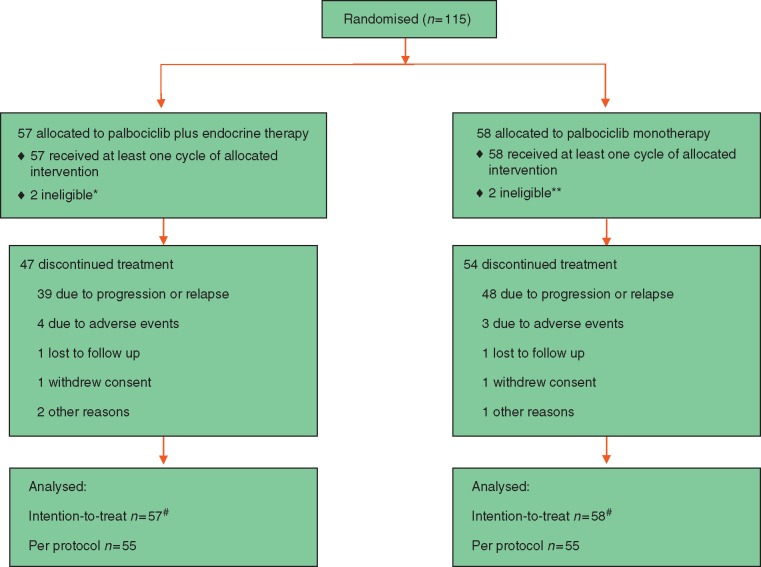
Between October 2012 and July 2016, a total 115 women were randomly assigned; 57 to receive palbociclib and ET, and 58 to receive palbociclib alone. Table 1 describes the baseline characteristics of the intention-to-treat population, which were generally well-balanced between the two arms. The type of treatment received most recently for ABC was ET [55 (96%) in the combination group; 56 (97%) in the single-agent group]. In both arms, approximately one half received an aromatase inhibitor as their most recent line of ET. The majority of patients had received one line of prior ET before study entry [39 (68%) and 40 (69%) in the combination and single-agent arms, respectively]; with just under a third in both arms having received two previous lines. Most of the patients [46 (80%) in the combination and 38 (66%) in the single-agent arm] received their prior line of ET for longer than six months. Approximately one third of patients in both groups had received prior chemotherapy. Overall, the majority of patients had visceral metastatic disease [42 (74%) in the combination arm, 45 (78%) in the monotherapy arm]. Conversely, bone-only disease comprised less than 10% overall in each group. Two patients in each arm were found to be ineligible subsequent to randomization, and are included in the intention-to-treat group (see Table 1). A separate analysis of the “per protocol” group (excluding ineligible patients) was conducted, leading to the same conclusions when compared to the intention-to-treat analyses. Here we report results from the intention-to-treat cohort (Figure 1).
Table 1.
Baseline characteristics (intention-to-treat population)
| Palbociclib plus endocrine therapy | Palbociclib monotherapy | |
|---|---|---|
| No. of patients | 57 (100) | 58 (100) |
| Median age in years (range) | 67 (37–82) | 63 (45–81) |
| ECOG performance status | ||
| 0 | 44 (77) | 44 (76) |
| 1 | 11 (19) | 14 (24) |
| 2 | 2 (4) | 0 |
| Disease classification at initial diagnosis | ||
| De novo metastatic | 13 (23) | 13 (22) |
| Early (adjuvant) | 41 (72) | 43 (74) |
| Not classified | 3 (5) | 2 (4) |
| Sites of metastases | ||
| Visceral | 42 (74) | 45 (78) |
| Bone only | 4 (7) | 5 (8) |
| Other sites (non-visceral) | 11 (19) | 8 (14) |
| Prior lines of endocrine therapy for advanced breast cancer | ||
| 0 | 1 (2)a | 0 |
| 1 | 39 (68) | 40 (69) |
| 2 | 16 (28) | 17 (29) |
| 3 | 1 (2)a | 1 (2)a |
| Duration of most recent prior line of endocrine therapy for advanced breast cancer | ||
| No prior endocrine therapy | 1 (2) | 0 |
| ≤6 months | 10 (18) | 20 (34) |
| >6 months | 46 (80) | 38 (66) |
| Most recent endocrine therapy for advanced breast cancer | ||
| None | 1 (2) | 0 |
| Aromatase inhibitor | 34 (60) | 29 (50) |
| Fulvestrant | 22 (38) | 29 (50) |
| Receipt of prior chemotherapy for advanced breast cancer | 19 (33) | 16 (28) |
Data are represented as number (%), unless otherwise indicated.
Included in ITT population despite breach of eligibility criteria; one additional patient ineligible due to lack of measurable disease at baseline, is also included in ITT population.
ECOG, Eastern Cooperative Oncology Group.
Figure 1.
CONSORT diagram. *One patient had received no prior treatment for advanced disease; one patient had received more than two lines of endocrine therapy (ET) for advanced disease. **One patient had no measureable disease at baseline; one patient had received more than two lines of ET for advanced disease. #As all patient ineligibilities were discovered after the randomization process, those subjects are included in the intention-to-treat analysis. “Per protocol” population excludes ineligible subjects.
Efficacy
As of the data cut-off for the final analysis (8 February 2017), the mean follow-up was 12.2 months (95% CI: 9.7–14.6) for the combination arm, and 11.9 months (95% CI: 6.6–14.3) for the monotherapy group. Ten patients on combination therapy and four on monotherapy were still receiving their randomized treatment. The primary end point of the study was met, with both arms declared active based on study assumptions. CBR was 54% (95% CI: 41.5–63.7) in the palbociclib plus ET arm, and 60% (95% CI: 47.8–72.9) in the palbociclib monotherapy arm (exploratory P-value for the difference = 0.52) (see Table 2). Most of those patients achieving clinical benefit did so by way of stable disease exceeding six months. Table 3 illustrates best overall response reported for each arm.
Table 2.
Clinical benefit in intention-to-treat population overall, and subgroups according to duration of previous line of endocrine therapy
| Palbociclib plus endocrine therapy | Palbociclib monotherapy | Exploratory P valuea | |
|---|---|---|---|
| Overall | 31/57 (54)b | 35/58 (60) | 0.52 |
| 95% CI (%) | 41.5–67.3 | 47.8–72.9 | |
| Prior endocrine therapy >6 months | 27/46 (59) | 24/38 (63) | 0.68 |
| 95% CI (%) | 44.5–72.9 | 47.8–78.5 | |
| Prior endocrine therapy ≤6 months | 3/10 (30) | 11/20 (55) | 0.19 |
| 95% CI (%) | 1.6–58.4 | 33.2–76.8 |
Data are represented as number/total population (%), unless otherwise indicated. Clinical benefit defined by complete response + partial response + stable disease for duration ≥24 weeks.
Pearson chi-square test.
One patient did not receive any prior endocrine therapy, therefore excluded from subgroup analysis.
CI, confidence interval (%).
Table 3.
Best overall response in the intention-to-treat population
| Best overall response | Palbociclib plus endocrine therapy (n = 57) | Palbociclib monotherapy |
|---|---|---|
| Complete response | 0 (0) | 0 (0) |
| Partial response | 6 (10) | 4 (7) |
| Stable disease ≥24 weeks | 25 (44) | 31 (53) |
| Stable disease <24 weeks | 7 (12) | 2 (3) |
| Progressive disease | 14 (25) | 17 (29) |
| Not evaluablea | 5 (9) | 4 (7) |
Data are represented as number (%). Stable disease was defined by evidence of stability over a duration of at least 12 weeks.
Not evaluable due to absence of post baseline disease assessments.
An exploratory analysis of the duration of clinical benefit was performed, taking into account the time from achievement of clinical benefit to the point of disease progression or death. The median duration of clinical benefit in the combination arm was 11.5 months (95% CI: 8.5–17.8 months), and 6 months (95% CI: 3.9–10.8 months) in the single-agent arm (HR, 0.35; 95% CI: 0.18–0.70, exploratory P-value = 0.0021) (Figure 2). A similar trend was observed in an exploratory comparison of PFS in the two arms. The median PFS was 10.8 months (95% CI: 5.6–12.7 months) in the combination group, and 6.5 months (95% CI: 5.4–8.5 months) in the single agent group (HR 0.69; 95% CI: 0.4–1.1, exploratory P-value = 0.12) (Figure 3).
Figure 2.
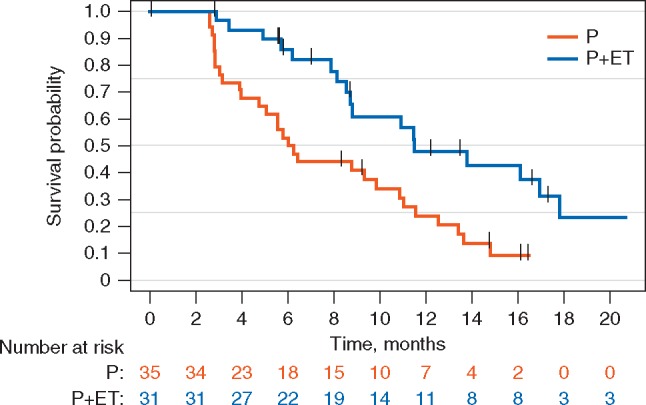
Kaplan–Meier plot of the duration of clinical benefit (CB) in the intention-to-treat (ITT) population. P, palbociclib; P + ET, palbociclib plus endocrine therapy.
Figure 3.
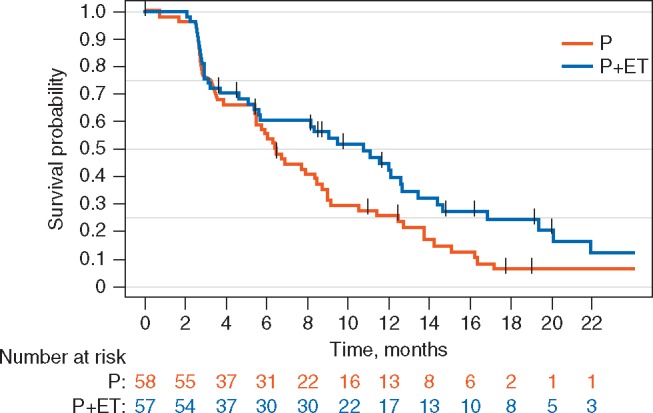
Kaplan–Meier plot of progression-free survival (PFS) in the ITT population.
Further exploratory analyses were performed to determine if there was a patient subgroup that received a larger benefit from either study treatment. The PFS advantage favoring the combination arm was seen in the subgroup of patients who received prior ET for more than six months (HR 0.53; 95% CI: 0.3–0.9, exploratory P-value = 0.02) (Figure 4A). This was not observed in the subgroup who previously received ET for a short duration only (HR 1.59; 95% CI: 0.6–4.0, exploratory P-value = 0.33) (Figure 4B).
Figure 4.
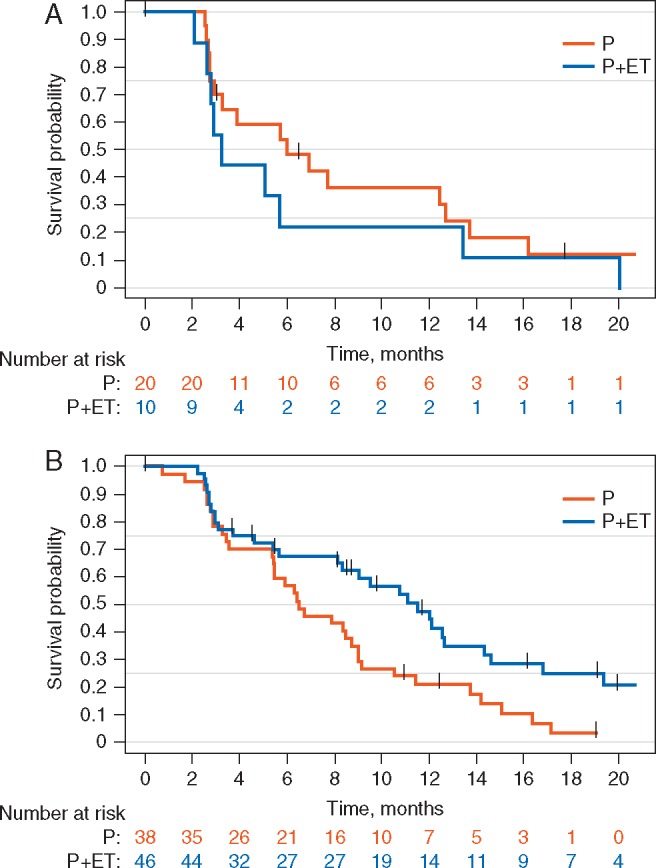
Kaplan–Meier plot of PFS in the ITT population according to the duration of prior ET. (A) Patients who received prior ET for ≤6 months and (B) patients who received prior ET for >6 months.
Safety
AEs were in line with previous data [10], with no new signals detected (see Table 4). All 57 patients in the combination arm, and 56 (97%) of those in the monotherapy arm reported at least one AE occurring in one or more cycles. Hematological toxicities, mainly neutropenia, were the most common AEs in both arms. Neutropenia occurred in 53 (93%) of those in the combination arm [35 (61%) and 6 (11%) at grades 3 and 4, respectively], and in 55 (95%) of those in the monotherapy group [28 (48%) and 11 (19%) at grades 3 and 4, respectively]. Infections and febrile neutropenia was uncommon in both groups. Three (5%) patients in each arm reported at least one serious treatment-related AE. The combination therapy arm had two reported incidences of overdose, and one diagnosis of diabetes mellitus. One report each of febrile neutropenia, nonfebrile neutropenia and ischemic colitis/intestinal hemorrhage occurred in the monotherapy group.
Table 4.
Treatment-attributed adverse events that occurred in 5% or more of subjects at grade 2 or above (safety population)
| Palbociclib plus endocrine therapy (n = 57) |
Palbociclib monotherapy (n = 58) |
|||||||
|---|---|---|---|---|---|---|---|---|
| All grades | Grade 2 | Grade 3 | Grade 4 | All grades | Grade 2 | Grade 3 | Grade 4 | |
| Neutropenia | 53 (93) | 9 (16) | 35 (61) | 6 (11) | 55 (95) | 16 (28) | 28 (48) | 11 (19) |
| Leukopenia | 54 (95) | 21 (37) | 20 (35) | 2 (4) | 51 (88) | 28 (48) | 17 (29) | 1 (2) |
| Anemia | 42 (74) | 13 (23) | 2 (4) | 0 | 41 (71) | 10 (17) | 0 | 0 |
| Thrombocytopenia | 27 (47) | 5 (9) | 1 (2) | 0 | 28 (48) | 4 (7) | 1 (2) | 0 |
| Fatigue | 33 (58) | 14 (25) | 0 | 0 | 26 (45) | 7 (12) | 1 (2) | 0 |
| Mucositis | 13 (23) | 2 (4) | 3 (5) | 0 | 10 (17) | 2 (3) | 0 | 0 |
| Arthralgia/myalgia | 13 (23) | 1 (2) | 1 (2) | 0 | 8 (14) | 4 (7) | 0 | 0 |
| Infection | 10 (18) | 4 (7) | 2 (4) | 0 | 10 (17) | 3 (5) | 2 (3) | 0 |
| Nausea | 13 (23) | 3 (5) | 0 | 0 | 6 (10) | 1 (2) | 0 | 0 |
| Hepatic toxicity | 7 (12) | 2 (4) | 2 (4) | 0 | 6 (10) | 0 | 0 | 0 |
Data are represented by number (%).
All patients received at least one cycle of study treatment, and all were treated within their assigned groups with no subsequent crossover. Five hundred and twenty-five cycles in total were delivered in the combination arm, and 464 in the palbociclib-alone group, with a median relative dose intensity of 100% in both study arms. Dose reductions occurred in 120 (23%) and 58 (13%) in the combination and monotherapy groups, respectively. The predominant reason for dose reduction was hematological toxicity [80 (67%) in the combination group versus 37 (64%) in the monotherapy arm]. Similarly, dose delays occurred in 183 (35%) cycles in the combination group [142 (78%) secondary to hematological toxicity], and 130 (28%) in the monotherapy group [109 (84%) attributed to hematological toxicity].
The most common reason for study discontinuation in both arms was disease progression [39 (83%) in the combination group versus 48 (89%) in the monotherapy arm]. Treatment discontinuations due to AEs occurred in 4 (9%) and 3 (6%) patients in the combination and single-agent arms, respectively. There were no treatment-related deaths.
Discussion
This open label, phase II study presents the first data obtained regarding the activity and safety of palbociclib given either as single agent or in combination with ET received beyond disease progression in women with moderately pretreated estrogen receptor-positive, HER2-negative ABC. The CBR for the single-agent group was 60%, and 54% in the combination group.
Collective data remains limited on the activity of single-agent CDK4/6 inhibitors in estrogen receptor-positive, HER2-negative ABC. Previous data with single agent palbociclib showed positive, albeit modest, activity exists in patients with heavily pretreated hormone receptor-positive disease [8], with a CBR of 21%—the majority of whom had received two or more lines of ET, and/or two or more chemotherapy regimens for ABC. Similarly, the MONARCH-1 trial [11] explored the activity and safety of single-agent abemaciclib in a heavily pretreated population of patients with estrogen receptor-positive, HER2-negative ABC who had received a median of three prior systemic therapies, yielding a CBR of 42% [11]. Our study demonstrated similar findings in a less pretreated population, wherein the majority of subjects had received only one prior line of ET, with a minority having received previous chemotherapy in the metastatic setting. At the time of the original conception and early stages of this trial, there was limited data on the activity of CDK4/6 inhibition in estrogen receptor positive, HER2 negative ABC. Multiple large phase III studies have subsequently confirmed the activity of combined ET with CDK4/6 agents in both upfront and later-line treatment settings, consistently and uniformly demonstrating an approximate doubling of PFS with combination therapy compared to ET alone [5, 6, 12–14]. Consequently, the utility of CDK4/6 monotherapy is of arguably less relevance today, as combining CDK4/6 inhibitors with ET is already established in clinical practice. Nevertheless, the CBR obtained with single agent palbociclib achieved in this study was still clinically meaningful for this patient population. Furthermore, the design and subsequent findings of this study are likely to remain unique; contemporary approaches to evaluating CDK4/6 agents are most likely to involve doublet (or triplet) therapy, rather than exploring the utility of single-agent CDK4/6 inhibition.
ET remains a cornerstone of the management of ABC, and resistance to ET is inevitable in almost all hormone receptor-positive breast cancers [7], therefore strategies to overcome resistance are of ongoing interest. Upon disease progression on ET monotherapy, the strategy of switching to an alternative ET agent plus a CDK4/6 inhibitor has been explored in large phase III clinical trials, showing superiority of the combination over ET alone [6, 13]. Based on preclinical evidence suggesting that palbociclib may be able to overcome conditioned resistance to a given ET [4], we explored whether reversal of resistance by adding CDK4/6 inhibition to the same ET continued beyond progression could be clinically established and evaluated.
Our exploratory analysis of the duration of clinical benefit showed a significant difference in favor of the combination arm over palbociclib alone (11.5 versus 6 months, respectively; HR 0.35, exploratory P-value = 0.0021). This might suggest that in spite of the similar CBR between the two treatment arms, the duration of observed benefit may be longer when palbociclib is combined with the same ET received beyond progression. This is also supported by an exploratory comparison of PFS between both arms, with the combination arm trending towards (but not achieving) superiority compared to the monotherapy arm (median PFS 10.8 versus 6.5 months respectively; HR, 0.69; 95% CI: 0.4–1.1, exploratory P-value = 0.12). Further subgroup analysis according to the duration of the previous line of ET suggests that the advantage in terms of PFS for the combination arm is seen predominantly in the subgroup of patients who had a history of ET for 6 months or greater (HR 0.53; 95% CI: 0.3–0.9, P = 0.02). No analogous benefit for combination therapy was observed in the subgroup who previously received ET for less than 6 months. Of note, the activity of single agent palbociclib was very similar in these two groups. These analyses are limited by their exploratory nature, but do generate the compelling hypothesis that palbociclib may have potential to reverse endocrine resistance in patients with a previous history of durable response to ET. It is possible to speculate that, in patients with acquired endocrine resistance after prolonged benefit on single agent ET, the known synergism between ET and palbociclib may still be maintained and exploited by extending ET beyond disease progression. Whether this strategy would prove superior to switching to an alternative ET and adding palbociclib upon disease progression, as in PALOMA-3, is unknown. Approximately 75% of the patients enrolled in PALOMA-3 had received more than one prior line of systemic therapy for ABC, with about one third having received prior chemotherapy in the metastatic setting. These characteristics are similar to those seen in TREnd. In PALOMA-3, the median PFS for patients in the fulvestrant plus palbociclib arm was 9.5 months (95% CI: 9.2–11.0) [6], whilst in TREnd, the median PFS in patients in the combination arm was 10.8 months (95% CI: 5.6–12.7), reaching 11.5 months (95% CI: 8.2–14.3) in the smaller population of patients who received prior ET for more than 6 months. Within the limitations of cross-trial comparison, the small sample size of TREnd, and the exploratory nature of its secondary end point analyses, our data collectively suggest that a strategy of adding a CDK4/6 inhibitor to the same ET beyond disease progression may merit further studies in a selected population of patients who obtained prolonged benefit during their prior line of ET.
Acknowledgements
We thank the patients and their families who participated in our clinical trial, and the associated staff in all participating centers who contributed their time and expertise in completing TREnd, in particular Daniela Baldari for her assistance in coordinating data management, and Sophie Dekeyser for her contributions to statistical programming and quality control. We would like to acknowledge the ongoing support of the Fondazione Sandro Pitigliani. The study was funded by an investigator-initiated research grant from Pfizer; we would particularly like to thank Maria Koehler and Cynthia Huang.
Funding
The trial was an academic study sponsored by the not-for-profit foundation “Sandro Pitigliani per la lotta contro i Tumori ONLUS” and was funded by Pfizer through an investigator-initiated research grant (no grant number is applicable). Pfizer had no influence on the design or conduct of the trial, and were not involved in the reporting of the data and its interpretation.
Disclosure
LM has received research funding from Pfizer and has had a consulting/advisory role with AstraZeneca. GA has received research funding from Novartis, and has had a consulting/advisory role with Pfizer, Novartis and Lilly. FP has had a consulting/advisory role with Pfizer, Amgen, Celgene, Eisai, Eli Lilly, Ipsen, Novartis, Roche and Takeda. MB has declared employment with and stock/other ownership in IDDI. ADL has received research funding from AstraZeneca, Pfizer and Pierre-Fabre, and has had a consulting/advisory role with AstraZeneca, Bayer, Celgene, Daichi-Sankyo, Eisai, Genomic Health, Ipsen, Lilly, Novartis, Pierre-Fabre, Pfizer and Roche. All remaining authors have declared no conflicts of interest.
References
- 1. Miller TW, Balko JM, Fox EM. et al. ERalpha-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov 2011; 1(4): 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thangavel C, Dean JL, Ertel A. et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer 2011; 18(3): 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wardell SE, Ellis MJ, Alley HM. et al. Efficacy of SERD/SERM Hybrid-CDK4/6 Inhibitor Combinations in Models of Endocrine Therapy-Resistant Breast Cancer. Clin Cancer Res 2015; 21(22): 5121–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Finn RS, Dering J, Conklin D. et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009; 11(5): R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finn RS, Martin M, Rugo HS. et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375(20): 1925–1936. [DOI] [PubMed] [Google Scholar]
- 6. Cristofanilli M, Turner NC, Bondarenko I. et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016; 17(4): 425–439. [DOI] [PubMed] [Google Scholar]
- 7. Osborne CK, Schiff R.. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011; 62: 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeMichele A, Clark AS, Tan KS. et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res 2015; 21(5): 995–1001. [DOI] [PubMed] [Google Scholar]
- 9. Simon R, Thall PF, Ellenberg SS.. New designs for the selection of treatments to be tested in randomized clinical trials. Stat Med 1994; 13(5-7): 417–429. [DOI] [PubMed] [Google Scholar]
- 10. Verma S, Bartlett CH, Schnell P. et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist 2016; 21: 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dickler MN, Tolaney SM, Rugo HS. et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(-) metastatic breast cancer. Clin Cancer Res 2017; 23(17): 5218–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hortobagyi GN, Stemmer SM, Burris HA. et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375(18): 1738–1748. [DOI] [PubMed] [Google Scholar]
- 13. Sledge GW Jr, Toi M, Neven P. et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017; 35(25): 2875–2884. [DOI] [PubMed] [Google Scholar]
- 14. Goetz MP, Toi M, Campone M. et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; JCO2017756155; 35: 3638–3646. [DOI] [PubMed] [Google Scholar]