Abstract
Dysregulated fear memory can lead to a broad spectrum of anxiety disorders. The brain systems underlying fear memory are manifold, with the hippocampus being prominently involved by housing fear-related spatial memories as engrams, which are created and stored through neural changes such as synaptic plasticity. Although metabotropic glutamate (mGlu) receptors contribute significantly to both fear behavior and hippocampal synaptic plasticity, the relationship between these two phenomena has not been fully elucidated. Here, we report that contextual fear extinction induces a novel form of metaplasticity mediated by mGlu5 at the hippocampal SC-CA1 synapse. Further, blockade of mGlu5 prevents both contextual fear extinction and expression of this metaplasticity. This form of metaplasticity was absent in a mouse model of MECP2-duplication syndrome, corresponding to a complete deficit in extinction learning. These findings suggest that mGlu5-dependent metaplasticity within the hippocampus may play a critical role in extinction of contextual fear.
Keywords: hippocampus, LTP, mGlu5, plasticity
Introduction
Fear memory is part of the normal adaptive response (Bolles 1969; Fanselow 1994); however, dysregulated fear memories can underlie anxiety states such as post-traumatic stress disorder (Johnson et al. 2012). While several brain structures contribute to fear memory formation and storage, the hippocampus is pivotal for the formation of contextual fear memory (Kim and Fanselow 1992; Liu et al. 2012) and has more recently been shown to be important for long-term storage of fear memories (Goshen et al. 2011). Formation and storage of the neural engram for fear memory is thought to be mediated within the brain by activity-dependent changes in the efficacy of synaptic transmission, or synaptic plasticity (Bliss and Collingridge 1993; Takeuchi et al. 2014; Eichenbaum 2016). Indeed, changes in synaptic plasticity have been demonstrated to occur after Pavlovian fear conditioning (Sacchetti et al. 2002), as well as simultaneously during fear learning in an inhibitory avoidance task in rodents (Whitlock et al. 2006). Experience-dependent changes in synaptic plasticity such as these are considered a form of “metaplasticity,” a term for plasticity regulation that occurs after prior synaptic activity at the specific synapse being investigated (Abraham and Bear 1996; Abraham 2008).
At the molecular level, the G-protein-coupled metabotropic glutamate (mGlu) receptors contribute significantly to both fear memory and long-term synaptic plasticity (Riedel and Reymann 1996; Swanson et al. 2005; Gravius et al. 2006; Niswender and Conn 2010). More specifically, mGlu receptor subtype 5 (mGlu5) plays a prominent role in many aspects of memory formation (Rodrigues et al. 2002; Simonyi et al. 2005; Ayala et al. 2009; Fontanez-Nuin et al. 2011; Rook et al. 2015). For example, fear conditioning leads to a dramatic temporal and subregion-specific upregulation of mGlu5 expression in the hippocampus (Riedel et al. 2000) and pharmacological inhibition of mGlu5 blocks fear conditioning (Schulz et al. 2001). Furthermore, genetic deletion of mGlu5 results in impaired acquisition and extinction of contextually conditioned fear (Xu et al. 2009) with corresponding deficits in hippocampal long-term potentiation (LTP) (Lu et al. 1997). The group I mGlu receptors (mGlu1 and mGlu5) have also been shown to be key mediators in another form of synaptic plasticity, termed long-term depression (LTD), at the Schaffer Collateral-CA1 (SC-CA1) synapse ex vivo (Fitzjohn et al. 1999; Huber et al. 2001) and in vivo (Popkirov and Manahan-Vaughan 2011). Despite its critical role, the effects of contextual fear conditioning on mGlu receptor-dependent synaptic plasticity in the hippocampus have not been specifically investigated. Therefore, we directly tested the hypothesis that contextual fear conditioning leads to an enhancement in mGlu receptor-dependent LTD at the SC-CA1 synapse. We found that fear learning does not enhance hippocampal LTD, but instead induces a form of metaplasticity by which mGlu receptor activation induces robust LTP at the SC-CA1 synapse 1 week after conditioning. Further studies demonstrate that this metaplastic switch from mGlu receptor-dependent LTD to LTP is not induced by the acquisition of conditioned fear, but rather by fear extinction training. The administration of a selective mGlu5 negative allosteric modulator (NAM) revealed that both contextual extinction behavior and extinction-induced metaplasticity are dependent on mGlu5 receptor activation. The role of hippocampal mGlu5-dependent metaplasticity in mediating fear extinction memory was strengthened by the finding that acute systemic or direct hippocampal injection of an mGlu5 NAM disrupted fear extinction recall. Finally, we found that an autism-related mouse model that displays impaired fear extinction also exhibits a lack of extinction-induced hippocampal metaplasticity. Taken together, these findings provide significant evidence that a novel form of mGlu5-dependent metaplasticity within the hippocampus is involved in contextual fear extinction.
Results
mGlu Receptor-Dependent LTD is Converted to LTP in the Hippocampus After Fear Conditioning
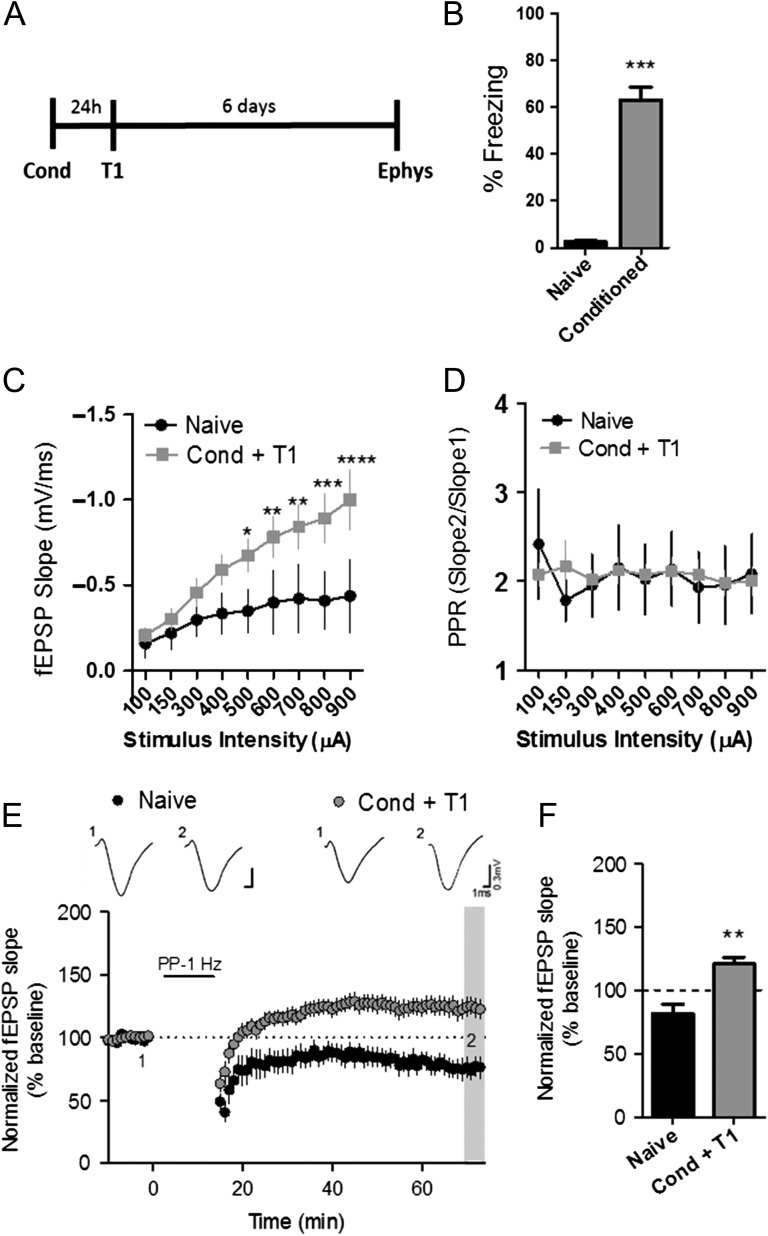
To test the effects of contextual fear conditioning on hippocampal mGlu receptor-dependent synaptic plasticity, mice underwent fear conditioning, followed by one day of context re-exposure to test for acquisition, and then sacrificed 1 week later to assess plasticity at the SC-CA1 synapse (Fig. 1A). Contextual fear conditioning is an associative learning paradigm that causes a long-lasting and robust fear memory, which manifests as freezing behavior when mice re-enter the context associated with an aversive stimuli, in this case, a mild foot shock (Kim and Fanselow 1992). In this study, fear conditioning resulted in significant freezing during the fear acquisition test (T1) 24 h later. Conversely, mice tested without previous conditioning (naïve), displayed little freezing behavior over the 3-min assessment time (% freezing: naïve: 2.5 ± 0.5; conditioned: 63 ± 5.5) (Fig. 1B). Fear behavior has been shown to be accompanied by an enhancement of basal excitatory synaptic responses at the SC-CA1 synapse that can be observed across a range of stimulus intensities (Sacchetti et al. 2001). In agreement with this, we found that the input–output function of evoked fEPSPs at the SC-CA1 synapse was significantly elevated in conditioned mice compare to naïve animals when acute slice electrophysiology experiments were performed 1 week after fear conditioning (Fig. 1C). No significant difference in paired-pulse ratio (PPR) was detected in slices among the groups, suggesting a strengthening of synaptic responses with no presynaptic change in synaptic transmission (Fig. 1D).
Figure 1.
mGlu receptor-dependent LTD is converted to LTP in the hippocampus after fear conditioning. (A) Schematic of fear conditioning procedure. (B) Graph representing the percent time freezing to the context on T1 (***P < 0.001, compared with naïve, t(22) = 10.85, Student’s t test; naïve n = 12, Cond n = 12). (C) Input–output function measured at SC-CA1 synapse in hippocampal slices (black symbols, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with naïve; P < 0.0001, F(1,14) = 8.77, two-way ANOVA; naïve n = 9, Cond + T1 n = 10). (D) Paired-pulse ratio (PPR) at SC-CA1 synapse (P = 0.884, two-way ANOVA, naïve n = 9, Cond + T1 n = 10). (E) Time course showing the effects of PP-LFS on fEPSP slope in acute slices of naïve (black symbols, n = 9) and Cond + T1 (gray symbols, n = 10) mice. Representative traces (1) and (2) correspond to baseline (1) and 60 min post PP-LFS (2). (F) Averaged fEPSPs from last 5 min of a 60 min recording show significant difference in naïve compared with conditioned groups (**P < 0.01, compared with naïve, t(12) = 4.16, Student’s t test). Data are expressed as mean ± standard error of the mean (SEM).
Previous studies have revealed a delayed upregulation of mGlu5 expression in the CA1 region after fear conditioning (Riedel et al. 2000). Given the fact that mGlu5 activity contributes to mGlu receptor-dependent LTD at this synapse (Huber et al. 2001; Ayala et al. 2009), we tested the hypothesis that enhanced mGlu receptor-dependent LTD would also be observed in the hippocampus 1 week after conditioning. For these studies, we utilized an LTD induction paradigm consisting of 1 Hz paired-pulse stimulation for 900 pairs (paired-pulse low-frequency stimulation, PP-LFS), which has been shown to elicit mGlu receptor-dependent LTD at the SC-CA1 synapse (Huber et al. 2000). In contrast to our hypothesis, the naïve group displayed typical LTD, while PP-LFS induced a slow-onset LTP in slices from conditioned mice (Fig. 1E). This dramatic shift in the response to PP-LFS from induction of LTD to LTP was statistically significant when assessed by measuring the average fEPSP slope from the last 5 min of the 60 min recording (% of baseline, naïve: 81.4 ± 7.5; Cond + T1: 120.7 ± 5.5, black vs. grey hatched bars) (Fig. 1F). This effect was also observed when LTD was induced by application of the group I mGlu receptor agonist (S)-3,5-dihydroxyphenylglycine (DHPG; 25 μM); DHPG-induced LTD in slices from naïve mice and LTP in slices from conditioned mice (% of baseline, naïve: 80.5 ± 4.3, Cond + T1: 122 ± 3.4) (Supplemental Fig. S1A). Taken together, these data reveal that contextual fear conditioning can shift the plasticity response, or create a metaplasticity in the hippocampus, resulting in the induction of LTP in response to mGlu receptor activation using stimulation protocols that typically elicit mGlu-dependent LTD in slices from naïve mice.
Fear Conditioning-Induced Metaplasticity is Dependent on mGlu5, NMDA and CB1 Receptors
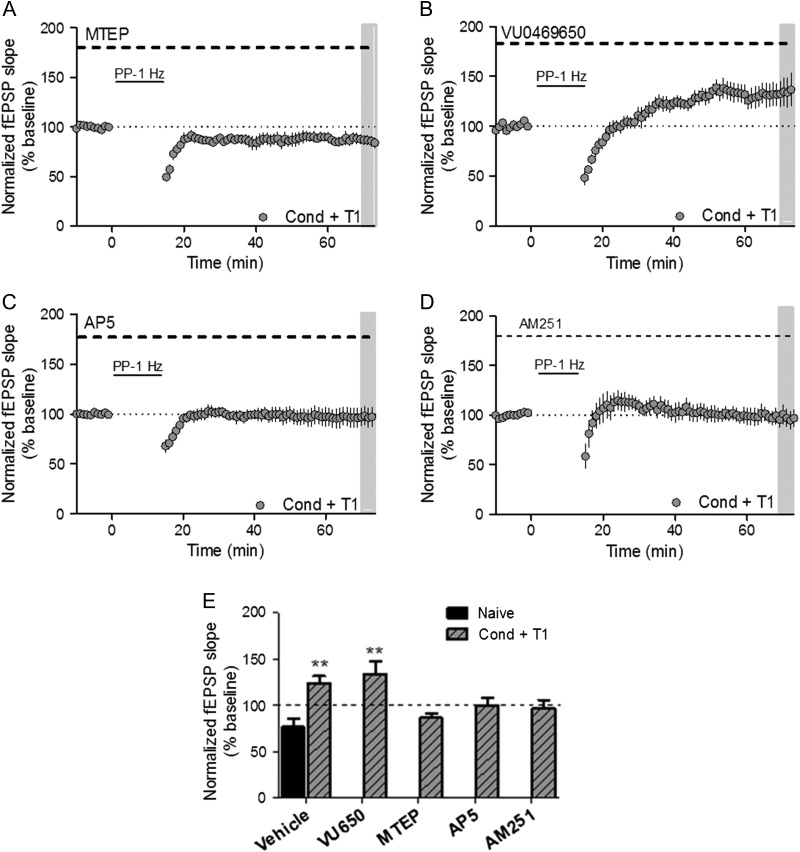
To identify the specific mGlu receptor subtype responsible for this mGlu receptor-dependent LTP, the contribution of each group I mGlu subtype was tested pharmacologically using the specific mGlu1 and mGlu5 NAMs VU0469650 and 3-((2-methyl-4-thiazolyl)ethynyl)pyridine (MTEP), respectively. Again, conditioned mice were tested for fear expression and sacrificed for electrophysiology 1 week later. Application of the mGlu5 NAM, MTEP, to slices from conditioned mice during PP-LFS experiments completely normalized long-term plasticity, resulting in LTD (% of baseline, 86.30 ± 4.9) (Fig. 2A). However, the mGlu1 NAM, VU0469650, did not significantly alter LTP in slices from the conditioned group (% of baseline, 133.9 ± 13.6) (Fig. 2B). Similar results were obtained in slices from conditioned mice when LTP was chemically induced with DHPG and blocked by MTEP, but not VU0469650 (% of baseline, MTEP: 74.8 ± 11.7; VU0469650: 133.5 ± 15.1) (Supplemental Fig. S1B, C).
Figure 2.
Fear conditioning-induced metaplasticity is dependent on mGlu5, NMDA and CB1 receptors. (A) Time course showing the effects of PP-LFS on fEPSP slope in acute slices from Cond + T1 mice with MTEP (1 μM) perfusion throughout experiment (n = 5). (B) Time course showing the effects of PP-LFS on fEPSP slope in acute slices with VU0649650 (10 μM) perfused throughout the entire experiment (n = 6). (C) Time course showing the effects of PP-LFS on fEPSP slope in acute slices with AP5 (50 μM) perfused throughout entire experiment (n = 8). (D) Time course showing the effects of PP-LFS on fEPSP slope in acute slices with AM251 (2 μM) perfused throughout entire experiment (n = 5). Summary of averaged fEPSPs from Figures 1F and 2A–D. (**P < 0.01 compared with naïve/vehicle; P = 0.0005, F(5,37) = 5.75, one-way ANOVA). Data are expressed as mean ± SEM.
Many classical induction paradigms used to evoke LTP at the SC-CA1 synapse, such as high-frequency stimulation, are dependent on NMDA receptor activation (Collingridge et al. 1983; Luscher and Malenka 2012). Therefore, NMDA receptor contribution to PP-LFS-induced LTP after fear conditioning was tested pharmacologically. Once again, mGlu receptor-dependent LTP was evoked in slices from conditioned mice using either the afferent stimulation PP-LFS protocol or chemically by DHPG application in the presence of the NMDA receptor antagonist D-(–)-2-amino-5-phosphonopentanoic acid (D-AP5). NMDA receptor antagonism prevented mGlu receptor-dependent LTP, with fEPSP slopes returning to baseline levels 60 min after induction by PP-LFS (% of baseline, AP5: 97.1 ± 9.5) (Fig. 2C) or DHPG (% of baseline, AP5: 101.5 ± 6.2) (Supplemental Fig. S1D).
Previous literature suggests that activation of mGlu5 receptors located on postsynaptic pyramidal neurons at this synapse can mediate a metaplasticity that enhances NMDA receptor-dependent LTP (Xu et al. 2014). This occurs through cannabinoid receptor type 1 (CB1) activation on GABAergic interneurons that disinhibits the glutamatergic afferents, thus lowering the threshold for LTP (Chevaleyre and Castillo 2003). Therefore, we applied the CB1 antagonist, AM251, to slices from conditioned mice and found that PP-LFS was unable to evoke LTP (Fig. 2D). Taken together, these results suggest that the metaplasticity induced by fear learning is dependent on mGlu5, NMDA, and CB1 receptors (Fig. 2E).
Contextual Fear Extinction Mediates the Shift to mGlu Receptor-Dependent LTP in the Hippocampus
To investigate whether this mGlu5-dependent metaplasticity underlies the original fear memory, we blocked mGlu5 receptors with a systemic injection of MTEP (5 mg/kg; intraperitoneal [i.p.]) prior to a recall trial 1 week after conditioning. MTEP treatment prior to the recall trial did not alter fear expression 1 week after conditioning when compared with vehicle controls (% freezing; vehicle: 53 ± 6.6, MTEP: 59.63 ± 7.23) (Supplemental Fig. S2). These data, together with the studies showing that ex vivo MTEP blocks expression of mGlu5-mediated LTP in acute slices at this time-point, suggest that mGlu5-dependent metaplasticity 1 week after fear conditioning is not related to the expression of the conditioned fear memory.
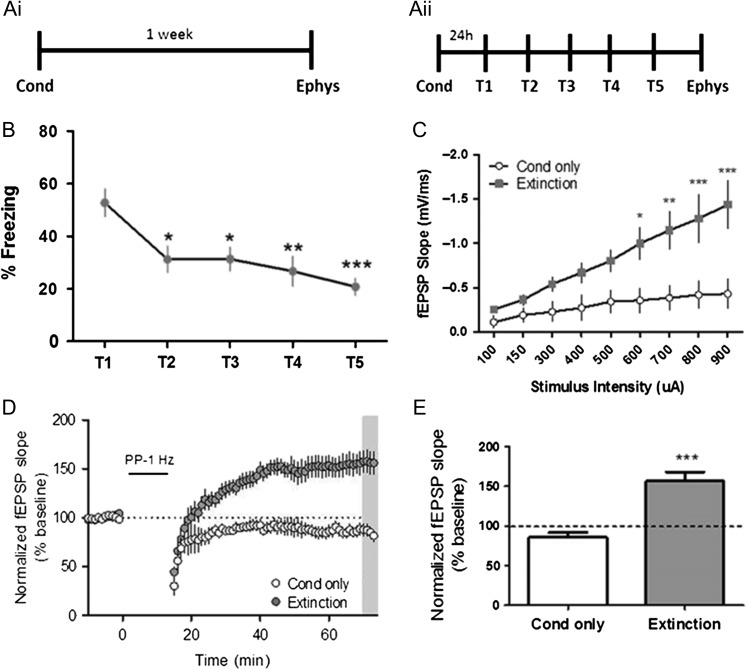
The lack of effect of MTEP on fear recall pointed to the possibility that the metaplastic switch from LTD to LTP could be important for a different aspect of fear memory. Fear memory is complex, and the mechanisms that drive acquisition, expression and extinction can be disparate (Abel and Lattal 2001). Our initial behavioral studies utilized a paradigm in which animals were fear conditioned and a test for fear memory (T1) was performed 24 h later. While the T1 fear testing was required to assess the acquisition of fear learning, this exposure to the conditioning chamber in the absence of shock also serves as an extinction session. Thus, we postulated that the hippocampal metaplasticity measured 1 week after conditioning could be related to extinction learning. To test this hypothesis, we performed experiments in which mice were exposed to conditioning without re-exposure to the contextual chamber (i.e., no expression testing) and sacrificed 1 week later to assess the effect of fear conditioning on synaptic physiology (Fig. 3Ai). A separate cohort of mice underwent contextual fear extinction for 5 trials with one trial per day before being sacrificed for electrophysiology experiments (Fig. 3Aii). Consistent with extinction occurring during the T1 testing trial, there was a significant decrease in freezing between T1 and T2. Freezing behavior gradually decreased when mice underwent daily extinction sessions over the total 5 days (Fig. 3B). Interestingly, fear extinction, but not fear conditioning, resulted in a large increase in the input–output function (Fig. 3C). In addition to displaying significantly enhanced synaptic responses to a given stimulus intensity, hippocampal slices from extinguished mice also exhibited PP-LFS-induced LTP (% of baseline, extinction: 156.6 ± 11.6) that was significantly more robust than that seen initially in mice that only underwent a single extinction trial (i.e., T1 only). Conversely, slices from the conditioned only group displayed PP-LFS-induced LTD (% of baseline, Cond only: 85.9 ± 5.9) (Fig. 3D, E). Overall, these experiments demonstrate that extinction of contextually conditioned fear leads to a robust form of metaplasticity in the hippocampus that induces a switch in the response to PP-LFS stimulation from induction of mGlu5-dependent LTD to a novel form of mGlu5-dependent LTP.
Figure 3.
Fear extinction mediates the shift to mGlu receptor-dependent LTP in the hippocampus. (Ai) Schematic of behavioral paradigm for mice that underwent conditioning without re-exposure to the context (Cond only). (Aii) Schematic of behavioral paradigm for mice that underwent conditioning (Cond) followed by contextual fear extinction (T1–T5). (B) Graph of percent time spent freezing over 5 contextual extinction sessions (*P < 0.05, **P < 0.01, ***P < 0.001 compared with T1; P = 0.0002, F(4,75) = 6.37, one-way repeated measures ANOVA, n = 16). (C) Input–output function of fEPSPs in acute slices (Extinction: gray symbols, *P < 0.05, **P < 0.01, ***P < 0.001 compared with Cond only: white symbols; P < 0.0001, F(1,9) = 10.08, two-way ANOVA with repeated measure, Cond only n = 6, extinction n = 7). (D) Time course showing the effects of PP-LFS on fEPSP slope in acute slices from Cond only (white symbols, n = 6) and extinction (gray symbols, n = 7) groups. (E) Averaged fEPSPs from last 5 min of recording (gray bar in (D)) show significant difference in slices from mice that underwent extinction compared with conditioned only (***P < 0.001, t(13) = 5.63, Student’s t test). Data are expressed as mean ± SEM.
Contextual Fear Extinction and Hippocampal Metaplasticity are Prevented by mGlu5 Antagonism
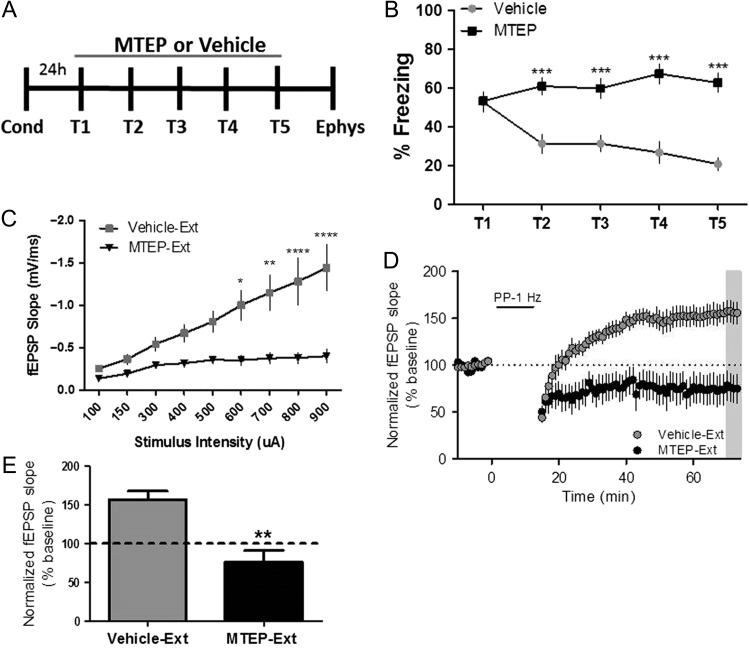
To further investigate the relationship between mGlu5-dependent metaplasticity and fear extinction, we tested the effects of mGlu5 receptor blockade in vivo during extinction learning. Using the contextual fear extinction paradigm (Fig. 4A), we found that mice that were administered MTEP (5 mg/kg; i.p.) prior to each extinction session did not extinguish freezing behavior over the 5 trials (T1–T5) (Fig. 4B). This is consistent with studies utilizing mGlu5 knockout mice, which display deficits in contextual fear extinction (Xu et al. 2009). Importantly, MTEP administration to behaviorally naïve mice did not affect basal freezing (Supplemental Fig. S3). Electrophysiology studies performed on acute slices from MTEP-treated mice revealed that the input–output function and LTD were both similar to that of behaviorally naïve mice (Fig. 4C–E). These data confirm previous findings that mGlu5 contributes to fear extinction regulation (Xu et al. 2009; Fontanez-Nuin et al. 2011; Sethna and Wang 2014) and reveal that (1) contextual fear extinction is accompanied by mGlu5-dependent metaplasticity at the hippocampal SC-CA1 synapse, (2) inhibition of extinction learning prevents the expression of this form of metaplasticity, and (3) blocking the metaplastic shift to mGlu5-dependent LTP prevents extinction learning.
Figure 4.
Contextual fear extinction and hippocampal metaplasticity are prevented by mGlu5 antagonism. (A) Schematic of behavioral paradigm. (B) Fear extinction was significantly impaired by MTEP treatment (black symbols, 5 mg/kg, i.p.; 30 min prior to each trial) compared with vehicle-treated mice (gray symbols) (***P < 0.001 compared with vehicle; P < 0.0001, F(1,29) = 29.68, two-way repeated measures ANOVA, vehicle n = 16, MTEP n = 15). (C) Input–output function in acute slices of vehicle-Ext compared with MTEP-Ext mice (gray vs. black symbols, respectively, *P < 0.05, **P < 0.01, ****P < 0.0001 compared with MTEP treated; P < 0.0001, F(1,9) = 9.93, two-way repeated measures ANOVA, vehicle-Ext n = 4, MTEP-Ext n = 7). (D) Time course showing the effects of PP-LFS on fEPSP slope in acute slices of MTEP-Ext mice (black symbols, n = 4) and vehicle-Ext slices (gray symbols, n = 7). (E) Averaged fEPSPs from last 5 min of a 60 min recording show significant difference in vehicle-Ext compared with MTEP-Ext (**P < 0.01 compared with vehicle-Ext; P < 0.01, t(11) = 4.23, Student’s t test). Data are expressed as mean ± SEM.
Contextual Fear Extinction and mGlu Receptor-Dependent LTP is Restored Following Termination of MTEP Treatment
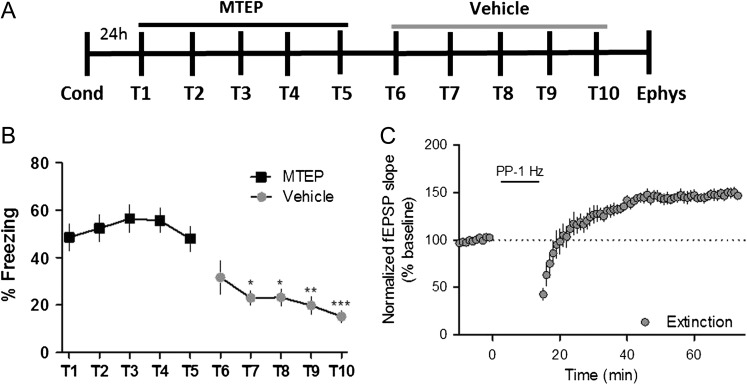
The finding that an mGlu5 NAM inhibits both extinction learning and mGlu5-dependent metaplasticity raises the exciting prospect that development of this novel form of mGlu5-mediated metaplasticity is required for acquisition of extinction learning. To test the hypothesis that acquisition of extinction learning is suppressed acutely by MTEP, we determined whether termination of mGlu5 antagonism would allow for extinction learning and expression of mGlu receptor-dependent LTP. Mice were contextually conditioned and underwent 10 days of extinction training, with MTEP treatment prior to T1–T5 and vehicle treatment prior to T6–T10 (Fig. 5A). As in previous experiments, mice treated with MTEP did not extinguish contextual fear behavior during extinction sessions T1–T5. However, the same cohort of mice demonstrated a statistically significant decrease in freezing when MTEP was replaced by vehicle for extinction sessions T6–T10 (Fig. 5B). In addition, termination of MTEP treatment restored PP-LFS-induced LTP in hippocampal slices from these mice (Fig. 5C). These results further support the role of mGlu5-mediated extinction learning in creating this hippocampal metaplasticity.
Figure 5.
Contextual fear extinction and mGlu receptor-dependent LTP is restored following termination of MTEP treatment. (A) Schematic of behavioral paradigm. (B) Graphical representation of percentage of time freezing during extinction (T1–T5); MTEP (black symbols, T1–T5), vehicle (gray symbols, T6–T10) (*P < 0.05, **P < 0.01, ***P < 0.001 compared with T5; P < 0.0001, F(9110) = 10.53, one-way repeated measures ANOVA, n = 12). (C) Time course showing the effects of PP-LFS on fEPSP slope in acute slices from mice that underwent 10 total days of extinction (MTEP, T1–T5; Vehicle, T6–T10), which resulted in LTP (n = 6). Data are expressed as mean ± SEM.
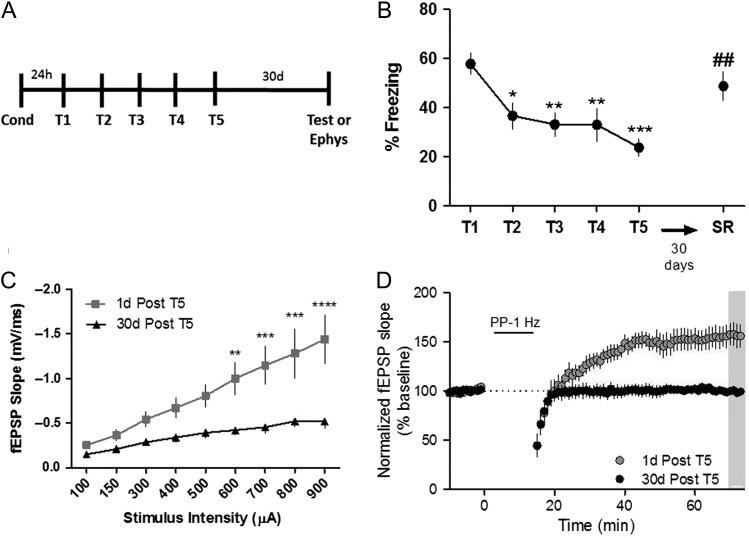
Spontaneous Recovery of Contextual Fear Corresponds to a Loss of mGlu Receptor-Dependent LTP
Taken together, the data presented above indicate that a metaplastic switch from mGlu5-dependent LTD to LTP may be integral to the acquisition and expression of fear extinction learning. However, if this mGlu5-dependent metaplasticity is directly tied to expression of extinction learning, we would predict that the expression of mGlu5-mediated LTP would be absent in animals after recovery of the conditioned fear. Indeed, after termination of fear extinction training, animals spontaneously recover the original fear memory over time (Rescorla 2004). To assess the effect of spontaneous recovery on mGlu receptor-dependent LTP, we extinguished fear behavior in a cohort of mice and performed a test for spontaneous recovery after a 30-day delay (Fig. 6A). Mice recovered the majority of the conditioned fear memory 30 days after the last extinction session (Fig. 6B). Basal hippocampal synaptic transmission, as measured by the input–output function, was significantly decreased 30 days after T5 compared with 1 day after T5 (Fig. 6C). Moreover, mGlu receptor-dependent LTP was lost 30 days after T5, corresponding to the loss of fear extinction recall (Fig. 6D; % of baseline: 99.38 ± 3.6). This is especially interesting in light of previous studies showing that activation of mGlu5 and protein synthesis contribute to inhibition of spontaneous recovery of fear memory in rats and mice (Mao et al. 2013). Taken together, these data suggest that the metaplasticity allowing for mGlu receptor-dependent LTP represents a neural correlate of the extinction memory within the hippocampus.
Figure 6.
Temporally mediated spontaneous recovery of contextual fear corresponds to a loss of mGlu receptor-dependent LTP. (A) Schematic of behavioral paradigm. (B) Graphical representation of percentage of time freezing during extinction (T1–T5) and spontaneous recovery test (SR, 30 days post T5). (*P < 0.05, **P < 0.01, ***P < 0.001 compared with T1, ##P < 0.01 compared with T5; P < 0.0001, F(5,11) = 8.01, one-way ANOVA repeated measures, n = 12). (C) Input–output function of fEPSPs in acute slices after a 30-day delay (black symbols, n = 7) compared with 1 day after T5 (gray symbols, n = 7) (**P < 0.01, ***P < 0.001, ****P < 0.0001 compared with 30-day delay; P < 0.0001, F(1,9) = 8.11, two-way ANOVA repeated measures). (D) Time course showing the effects of PP-LFS on fEPSP slope in acute slices from mice that underwent a 30-day delay (black symbols, n = 7) compared with one day after T5 (gray symbols, n = 7). Data are expressed as mean ± SEM.
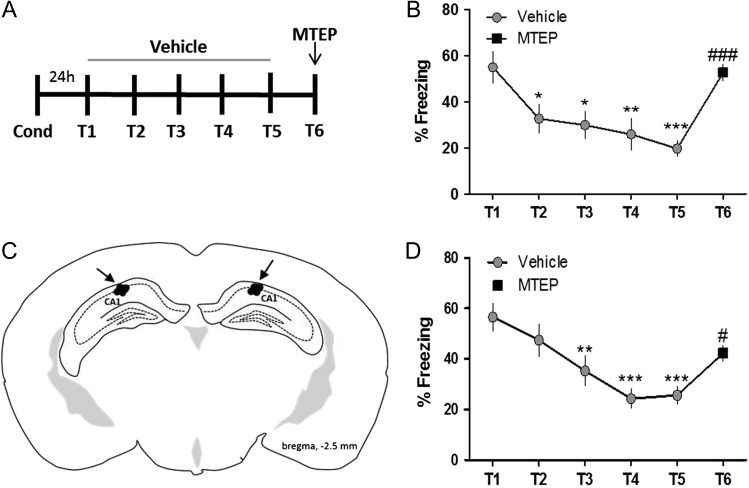
Contextual Fear Extinction Recall is Disrupted by mGlu5 Antagonism
To more directly test the hypothesis that mGlu5 receptor-dependent LTP is related to the extinction memory, we extinguished previously conditioned mice for 5 days (T1–T5) with vehicle, and on the sixth day of extinction (T6) mice were pretreated with the mGlu5 NAM MTEP (5 mg/kg; i.p.) (Fig. 7A). Systemic blockade of mGlu5 abolished recall of the extinction memory as indicated by increased freezing on T6 compared with T5 (Fig. 7B). To determine whether this is mediated by blockade of mGlu5 in the hippocampus, we directly targeted the dorsal hippocampal CA1 through bilateral infusion (Fig. 7C). Vehicle was infused prior to T1–T5, and MTEP (1 μg) prior to T6. Here, we found that mGlu5 blockade by direct infusion of the hippocampus was sufficient to significantly disrupt fear extinction, although not to the extent of systemic injection (Fig. 7D). These results provide evidence for the necessity of hippocampal mGlu5 activation in area CA1 of the hippocampus for the recall of contextual fear extinction memory.
Figure 7.
Contextual fear extinction recall is disrupted by mGlu5 antagonism. (A) Schematic of behavioral paradigm. (B) Graphical representation of percentage of time freezing during extinction after systemic administration of vehicle (gray symbols, T1–T5) or MTEP (5 mg/kg i.p., black symbols, T6) (*P < 0.05, **P < 0.01, ***P < 0.001 compared with T1, ###P < 0.001 compared with T5; P < 0.0001, F(5,10) = 8.38, one-way ANOVA repeated measures, n = 11). (C) Diagram of injector tip placement for all mice that received hippocampal CA1 infusions. (D) Graphical representation of percentage of time freezing during extinction after direct infusion of vehicle (gray symbols, T1–T5) or MTEP (1 μg/side, black symbols, T6) (**P < 0.01, ***P < 0.001 compared with T1, #P < 0.05 compared with T5; F(5,9) = 8.32, one-way ANOVA repeated measures, n = 10). Data are expressed as mean ± SEM.
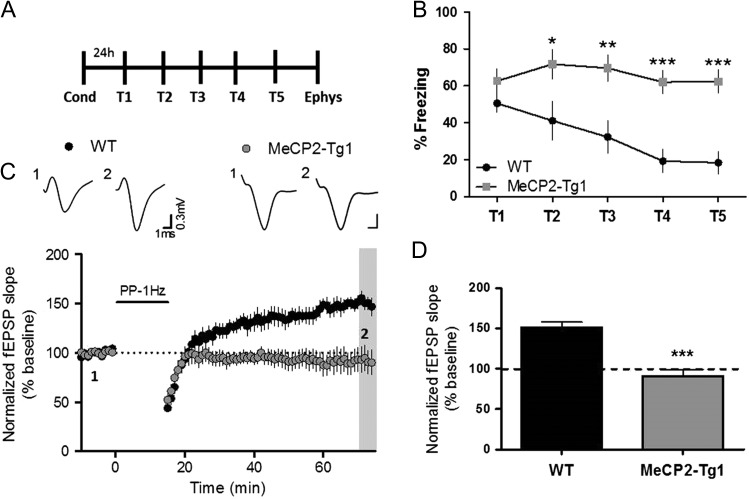
MECP2 Duplication Syndrome Model Mice Display Impairments in Contextual Fear Extinction and mGlu5-Dependent Metaplasticity
Genetic overexpression of the transcriptional regulator MECP2 in mice has been shown to result in hippocampal synaptic plasticity alterations as well as impairments in contextual fear conditioning and extinction learning (Collins et al. 2004; Na et al. 2012). The current findings put forth the intriguing possibility that deficits in extinction learning in MECP2-overexpressing mice could reflect an underlying deficit in the mGlu5-dependent synaptic plasticity changes that are induced in response to extinction training. Thus, we performed a series of studies using MECP2 duplication syndrome model mice (MeCP2-Tg1), which express approximately 2-fold more MeCP2 protein compared with wild-type (WT) littermates (Collins et al. 2004), to confirm the loss of extinction learning and determine whether these mice have a corresponding deficit in mGlu5-dependent metaplasticity. Consistent with a previous report (Collins et al. 2004), we found MeCP2-Tg1 mice exhibited increased contextual fear expression on T1 compared with WT littermates (% freezing, WT: 22.83 ± 8.78, MeCP2-Tg1: 61 ± 7.75), a difference likely attributable to changes in acquisition learning as opposed to pain sensitivity, given that we measured no differences in nociception between genotypes. Additionally, MeCP2-Tg1 mice did not display any overt physical impairment as animal weight and time spent freezing prior to foot shock was comparable to that of WT littermate controls (Supplemental Fig. S4). The low level of freezing on T1 in the WT littermates rendered subsequent fear extinction difficult to achieve. Therefore, we increased the number of foot shocks in our conditioning paradigm for the WT littermates (see Experimental Procedures), which increased T1 freezing in WT to levels more comparable to that of MeCP2-Tg1 mice. As demonstrated in similar models (Na et al. 2012), MeCP2-Tg1 mice displayed a lack of extinction learning in contrast to WT littermates, which displayed significant reduction of freezing from T1–T5 (Fig. 8A, B). Therefore, MeCP2-Tg1 mice provide a useful model to examine how genetic alterations that lead to impaired extinction learning may induce corresponding deficits in hippocampal metaplasticity.
Figure 8.
MECP2 Duplication Syndrome model mice display impairments in contextual fear extinction and mGlu5-dependent metaplasticity. (A) Schematic of behavioral paradigm. (B) Graphical representation of percentage of time freezing during extinction in WT littermate mice (black symbols) and MeCP2-Tg1 mice (gray symbols) (*P < 0.05, **P < 0.01, ***P < 0.001 compared with WT; P < 0.001, F(1,15) = 16.98, two-way ANOVA with repeated measures, WT n = 9, MeCP2-Tg1 n = 8). (C) Time course showing the effects of PP-LFS on fEPSP slope in acute slices from WT mice (black symbols, n = 6) and MeCP2-Tg1 mice (gray symbols, n = 9). Representative traces (1) and (2) correspond to baseline (1) and 60 min post PP-LFS (2). (D) Averaged fEPSPs from the last 5 min of a 60 min recording (gray bar in (C)) (***P < 0.001 compared with WT; P < 0.001, t(13) = 4.97 Student’s t-test). Data are expressed as mean ± SEM.
In our initial electrophysiological characterization of hippocampal slices, we discovered that behaviorally naïve MeCP2-Tg1 mice have impairments in synaptic transmission as indicated by a decreased input–output function of fEPSPs at the SC-CA1 synapse (Supplemental Fig. S5A). Furthermore, slices from MeCP2-Tg1 mice displayed enhanced PPR, as described previously (Collins et al. 2004), suggesting a decrease in presynaptic release probability (Supplemental Fig. S5B). To our knowledge, hippocampal LTD has not been reported for MeCP2 overexpressing mice; therefore, we first investigated PP-LFS-induced LTD in hippocampal slices from naïve MeCP2-Tg1 and WT littermate mice. While WT littermate control mice did show modest LTD (% of baseline, WT: 84.5 ± 9.7), the MeCP2-Tg1 mice did not display any change from baseline 60 min after induction (% of baseline, MeCP2-Tg1: 98.8 ± 9.8) (Supplemental Fig. S5C, D). To improve our ability to detect differences in LTD, we next used 50 μM DHPG to induce LTD in MeCP2-Tg1 and WT littermate slices. We found a statistically significant attenuation in DHPG-induced LTD when we compared MeCP2-Tg1 with WT slices (% of baseline, WT: 48.9 ± 6.1, MeCP2-Tg1: 70.8 ± 7.0) (Supplemental Fig. S5E, F). This plasticity impairment is likely attributable to functional deficits in mGlu5 as opposed to changes in expression, as the analysis of hippocampal mGlu5 immunoreactivity using Western blots did not reveal differences between behaviorally naïve MeCP2-Tg1 and WT littermate mice (Supplemental Fig. S6A). These results confirm this mouse model to have significant basal synaptic transmission and plasticity impairments and reveal the novel finding that LTD is also disrupted in mice that overexpress MeCP2. More importantly, in keeping with our current findings, the extinction impairment in MeCP2-Tg1 mice corresponded to the absence of hippocampal metaplasticity after extinction training, as MeCP2-Tg1 mice did not display mGlu-dependent LTP (% of baseline, extinction: 90.85 ± 8.6) in contrast to WT littermates (% of baseline, extinction: 156.6 ± 5.7) (Fig. 8C, D). We further attempted to correlate these plasticity deficits with changes in mGlu5 protein expression using whole hippocampal preparations. However, there were no significant effects of extinction either in WT or MeCP2-Tg1 mice on hippocampal mGlu5 expression (Supplemental Fig. S6B–D). Nonetheless, these data provide further validation that contextual fear extinction and hippocampal mGlu-dependent metaplasticity are linked.
Discussion
Although there is significant evidence for mGlu receptor regulation of both hippocampal plasticity and conditioned fear behavior, the relationship between these two highly overlapping phenomena has not been fully elucidated. The current set of studies demonstrates that contextual fear extinction induces a novel form of metaplasticity in the hippocampus. Together, these findings indicate that mGlu5 activation during extinction is crucial for this hippocampal metaplasticity, and in turn, this mGlu5-dependent metaplasticity may mediate subsequent extinction behavior.
Synaptic plasticity provides an activity-dependent mechanism through which the brain creates neural engrams to store and retrieve memories (Martin et al. 2000; Takeuchi et al. 2014), and is well documented through structural morphology and electrophysiological measures to occur after associative learning (Sacchetti et al. 2001; Fukazawa et al. 2003; Fischer et al. 2004; Lamprecht and LeDoux 2004; Song et al. 2012). In the current studies, extinction learning caused a fundamental change in the hippocampal plasticity response, or metaplasticity, resulting in an mGlu5-dependent switch from LTD to LTP after PP-LFS (Fig. 1). The dependence of this metaplasticity on mGlu5 is in line with the role of group I mGlu receptors in hippocampal plasticity, as they have been shown to be involved in both LTP and LTD at the SC-CA1 synapse (Lu et al. 1997; Volk et al. 2006). At the molecular level, the lack of mGlu1 dependence is not surprising given the fact that synaptic expression of mGlu5, but not mGlu1, is upregulated after contextual fear conditioning in the CA1 region (Riedel et al. 2000). The absence of PPR changes after conditioning also suggests these effects on synaptic transmission are likely due to postsynaptic mechanisms, consistent with data from mGlu5 knockout mice demonstrating a lack of change in PPR compared with WT controls (Lu et al. 1997).
While mGlu receptor-dependent LTD induced by either afferent PP-LFS or group I mGlu agonism is typically NMDA receptor-independent (Huber et al. 2000), enhancing high-frequency stimulation-induced LTP through “priming” or activating mGlu receptors (Bortolotto et al. 1994; Cohen et al. 1998; Raymond et al. 2000; Ayala et al. 2009) may involve interaction with postsynaptic NMDA receptors (Moutin et al. 2012). This is highlighted by research utilizing mGlu5 knockout mice, which display a reduction in the NMDA receptor component of LTP (Jia et al. 1998). In the current studies, the PP-LFS-induced LTP seen after contextual fear extinction was NMDA receptor-dependent (Fig. 2), suggesting that the metaplasticity caused by fear extinction may alter the synaptic environment so that mGlu activation leads to the recruitment of NMDA receptors, resulting in LTP. Our data point to mGlu5 activation inducing release of cannabinoids, leading to CB1 receptor activation and subsequent disinhibition of glutamatergic afferents at the SC-CA1 synapse that facilitates NMDA receptor-dependent LTP (Fig. 2), as described previously (Chevaleyre and Castillo 2003). While this mechanism enhances NMDA receptor-dependent LTP, it does not necessarily require direct modulation of NMDA receptors by mGlu5. Instead, the disinhibition allows greater depolarization during afferent stimulation and thus facilitates LTP. However, we cannot rule out mGlu5 interaction with NMDA receptors through structural connections with scaffolding proteins such as Homer and Shank (Tu et al. 1999). Interestingly, mRNA encoding certain scaffold proteins associated with mGlu5, such as Homer1a, have been reported to be increased after fear conditioning (Mahan et al. 2012), perhaps leading to enhanced mGlu5/NMDA receptor interaction in the current studies.
It is important to note that the PP-LFS-induced LTP after fear extinction described here is in fact a novel form of mGlu-dependent LTP, which does not recapitulate all of the aspects of similar forms of LTP. For example, previous studies have shown that broad mGlu activation pharmacologically results in a slow-onset LTP that is NMDA receptor-independent (Bortolotto and Collingridge 1995). In addition, low-frequency stimulation for a short duration results in a slow-onset LTP in vivo that is mGlu5, but not NMDA, receptor-dependent (Lante et al. 2006). The PP-LFS-induced LTP described in the current studies also develops with a slow-onset; however, the later component of LTP is NMDA receptor-dependent, similar to the time-course in which NMDA-dependent LTP is produced by high-frequency stimulation protocols (Collingridge et al. 1983). Taken together, future studies will be required to fully address possible mechanisms by which NMDA receptors contribute to this form of experience-induced metaplasticity.
Behaviorally, the contribution of NMDA receptors to fear extinction is well known and is in agreement with our data. For example, pharmacological antagonism of NMDA receptors prevents fear extinction learning in rodents (Baker and Azorlosa 1996; Santini et al. 2001; Liu et al. 2009) while NMDA receptor partial agonists such as d-cycloserine enhance the consolidation of extinction memory (Ledgerwood et al. 2003). Fittingly, mGlu5 activity can regulate NMDA receptor currents in hippocampal pyramidal neurons (Mannaioni et al. 2001), providing support for the novel finding that mGlu5 activity-dependent fear extinction induces a metaplasticity involving NMDA receptors.
We have also identified fear extinction, as opposed to fear expression, to be critical for inducing this form of hippocampal metaplasticity (Fig. 3). Moreover, in our current studies, mGlu receptor-dependent LTP correlated positively with fear extinction and not fear expression, as inhibition of fear extinction with MTEP or through spontaneous recovery resulted in loss of mGlu receptor-dependent LTP (Figs 4 and 6). These results point to mGlu5-dependent metaplasticity as being crucial for extinction acquisition and recall, although the specific time course by which these metaplastic effects are engaged after fear learning needs to be further investigated. These results are reinforced in light of the fact that mGlu5 antagonism has been shown to be anxiolytic in certain rodent assays (Tatarczynska et al. 2001), suggesting that enhanced fear behavior during extinction in MTEP-treated mice is not a result of anxiogenic effects of the drug.
The relationship between extinction behavior and metaplasticity was substantiated by the lack of extinction-induced metaplasticity in a mouse model that overexpresses MeCP2, which acquired but did not extinguish contextual fear behavior (Fig. 8). Importantly, these mice have been shown to have enhanced homeostatic forms of LTP (Collins et al. 2004), illustrating that the plasticity deficits in MeCP2-Tg1 mice are specific to the extinction-induced metaplastic shift from LTD to LTP and not LTP in general. From a preclinical standpoint, these data reveal important potential neural mechanisms underlying the autism-related disorder, MECP2-duplication syndrome. Indeed, proper MeCP2 dosage in the brain is critical for normal synaptic plasticity function (Chao and Zoghbi 2012). Given that MeCP2 is a transcriptional regulator, MeCP2 overexpression likely acts upstream of mGlu5 activity and leads to dysregulation metaplasticity and fear extinction behavior. While further studies are needed to fully elucidate these mechanisms, previous work has highlighted GABAA receptor involvement in hippocampal plasticity impairments found in similar mouse models of MeCP2 overexpression (Na et al. 2014). Our data showing a reduction in DHPG-induced LTD suggests a dysfunction of mGlu5 receptors, which has been noted recently in other MeCP2-related disorder models in our lab and by others (Bedogni Gigli et al. 2015; Gogliotti et al. 2016; Tao et al. 2016). This is in line with previous studies demonstrating that MeCP2 modulates homeostatic plasticity through mGlu5-dependent mechanisms (Zhong et al. 2012). The lack of mGlu5 expression changes in MeCP2-Tg1 mice (Supplemental Fig. 6) further illustrates that the alterations in mGlu5-plasticity seen in MeCP2-Tg1 mice are likely related to functional deficits. However, the absence of expression change in WT mice after extinction also suggests that perhaps changes occur specifically to synaptic fractions of mGlu5 within the CA1 area, as described previously (Riedel et al. 2000), but are undetectable in our whole-hippocampus preparation.
Previous literature indicates that activation of mGlu5 is important for contextual fear extinction, as mGlu5 knockout mice display deficits in extinction (Xu et al. 2009). Furthermore, pharmacological inhibition of mGlu5 activity with MPEP prevents appetitive extinction learning in the absence of context change (Andre et al. 2015). Enhancement of mGlu5 activity through positive allosteric modulation (PAM) can increase the rate of extinction in certain paradigms of fear conditioning or drug reinforcement (Cleva et al. 2011; Kufahl et al. 2012; Sethna and Wang 2014). However, these results are not unanimous across all forms of extinction learning or laboratories (Xu et al. 2013). Discrepancies between previous studies in regard to the efficacy of mGlu5 PAMs in enhancing extinction may be due to behavioral paradigm differences or to the individual pharmacological properties of specific mGlu5 PAMs used. Certainly, mGlu5 PAMs can display unique signal bias (Rook et al. 2015), and may have differential effects on hippocampal mGlu5 activity during extinction; this is a topic that should be investigated in future studies. Nevertheless, our current findings define a neural mechanism by which mGlu5 activity during extinction learning results in a hippocampal metaplasticity that correlates with extinction expression.
In addition to the novel effects described here in the hippocampus, there is also previous research demonstrating the importance of mGlu5 receptor-mediated plasticity within the mPFC in contributing to fear extinction (Fontanez-Nuin et al. 2011; Sepulveda-Orengo et al. 2013). Together, our data supplement this previous research that suggests a seemingly parallel contribution of mGlu5 to fear extinction in the mPFC. It may be that hippocampal mGlu5 activity is more important for extinction of contextual fear, whereas mPFC mGlu5 activity may contribute more to extinction of auditory-induced fear conditioning. Alternatively, it may be that these limbic structures act in concert during both types of extinction learning through long-projecting neuronal connections (Preston and Eichenbaum 2013). Indeed, the hippocampus and mPFC have been shown to be co-activated during recall of fear extinction in humans (Milad et al. 2007). By acting in concert, the extinction engram may be stored continuously between structures. This is evidenced by the fact that direct infusion of MTEP into the hippocampus only partially impaired fear extinction recall, while systemic MTEP administration caused fear extinction recall to be fully blocked (Fig. 7), suggesting other mGlu5-containing brain structures, such as the mPFC or amygdala, might also be involved. The hippocampus is thought to gate contextual information through its projections to both the mPFC and the amygdala (Maren 2011). There are direct, albeit sparse, projections from the dorsal hippocampus to mPFC, which are functionally relevant for fear behavior (Ye et al. 2016). In addition to structural connectivity, mGlu5 is expressed in the hippocampus, mPFC and amygdala, and has been demonstrated to modulate different aspects of fear memory and plasticity within each respective area (Lu et al. 1997; Gravius et al. 2006; Kim et al. 2007; Rudy and Matus-Amat 2009; Fontanez-Nuin et al. 2011). In a simplistic view, mGlu5 activation could possibly be thought of as a molecular lynchpin, unifying these limbic structures during fear extinction processes.
The discovery of this extinction-induced metaplasticity, and its mGlu5 dependence, allows for insight to be gleaned about the neural mechanisms mediating fear extinction. While drawing causation between neural plasticity and memory is a difficult task, applying a theoretical framework to empirical data can strengthen associative correlations. The shift in frequency-response after fear extinction is highly reminiscent of seminal studies on experience-dependent plasticity changes in the visual cortex (Kirkwood et al. 1996; Rittenhouse et al. 1999). Like those studies, our current data fit with the prominent BCM (Bienenstock, Cooper and Munro) theory (Bienenstock et al. 1982), which posits that a “sliding” modification threshold keeps the synapse in a useful dynamic range given the history of postsynaptic firing. Therefore, after episodes of low postsynaptic activity, the modification threshold shifts to the left, enabling LTP to be more likely to occur (Abraham 2008; Cooper and Bear 2012). In keeping with our current data, mGlu5 activation has been shown to shift the modification threshold ex vivo, as bath applying a low concentration of the group I mGlu agonist DHPG results in a leftward-shift of the entire frequency-response of long-term plasticity at the SC-CA1 synapse (van Dam et al. 2004). Overall, these studies combine to indicate that mGlu5 activity during contextual extinction may work to keep the synapse in a functionally primed state and allow the persistence of the extinction memory engram within the hippocampus.
Experimental Procedures
Drugs
3-((2-Methyl-1,3-thiazol-4-yl)ethynyl)pyridine hydrochloride (MTEP) and D-(–)-2-amino-5-phosphonopentanoic acid (D-AP5), (S)-3,5-dihydroxyphenylglycine (DHPG) and AM251 were purchased from Tocris (Minneapolis, MN). VU0649650 was synthesized at the Vanderbilt Center for Neuroscience Drug Discovery according to previously published methods (Lovell et al. 2013). All drugs used for electrophysiology experiments were diluted in artificial cerebrospinal fluid (aCSF). MTEP was diluted in 10% Tween 80 or water for behavioral experiments.
Animals
All of the animals used in the present study were group housed with food and water given ad libitum and maintained on a 12-h light/dark cycle. Animals were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All studies were approved by the Institutional Animal Care and Use Committee for Vanderbilt University School of Medicine and took place during the light phase. The 8–10 weeks old C57BL6/J (Jackson Labs strain #000664) male mice or 6–10 weeks old FVB-Tg(MECP2)1Hzo/J (Jackson Labs strain #008679) male mice were used for all studies.
Surgery
Mice were anesthetized by isoflurane gas (2% isoflurane in 100% oxygen) and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA). The skin was retracted and holes were drilled in the skull. Mice were implanted with bilateral stainless-steel guide cannulas (Plastics One) in the dorsal hippocampus as described previously (Kenney et al. 2012). Stereotaxic coordinates in relation to bregma were −2.5 mm posterior, ±1.8 mm mediolateral, and −1.5 mm ventral. Mice were allowed 7 days to recover from surgery, after which, behavioral training was initiated. Injection site placement was verified immediately following the last behavioral trial.
Contextual Fear Conditioning and Extinction
Mice were fear conditioned to the context by methods previously described (Rook et al. 2015). Briefly, mice were placed in a sound-attenuating conditioning chamber with a shock grid (Med Associates, St. Albans, VT) in the presence of 1 mL, 10% vanilla odor cue. Following a 3-min habituation period, mice were exposed to two 1 s, 0.7 mA foot shocks spaced 30 s apart. The only deviation from this paradigm was for experiments that involved WT littermate controls for the MeCP2-Tg1 mouse line. In these experiments, WT littermate mice received 4 foot shocks in order to enhance percent time freezing (Fig. 7). Mice were removed from the context immediately after the last foot shock. Test 1 (T1) occurred 24h later, mice were placed back into the same shock chamber with a 10% vanilla odor cue without foot shock and the percent of time spent freezing during a 3-min testing period was assessed by video motion detection software (Video freeze; Med Associates, St. Albans, VT). Conditioned mice underwent both days of training (Cond + T1) whereas naïve mice experienced T1 alone (Fig. 1). To assess the effects of conditioning alone (Cond only), mice were conditioned without T1 (Fig. 2). For all groups, mice were returned to housing room for 1 week before electrophysiology experiments were conducted.
Extinction trials consisted of the same protocol and conditions used for the test day (T1); 5 trials with 24 h Intertrial-interval (T1–T5). For behavioral pharmacology experiments, each extinction session was preceded by i.p. injections 30 min prior to the session with vehicle (1 mL/kg of 10% Tween 80) or MTEP (5 mg/kg). For the infusions of the selective mGlu5 NAM, MTEP, cannula dummies were removed from guide cannulas and replaced with 33 gauge injectors, which were connected by polyethylene tubing to 10-mL syringes mounted in an infusion pump (Harvard Apparatus). Water (vehicle) or MTEP (1.0 μg) was infused 30 min prior to extinction training at a rate of 0.5 μL/min for 1 min.
Acute Slice Electrophysiology
Mice were anesthetized with isoflurane and brains were rapidly removed and submerged in ice-cold sucrose cutting buffer containing: 230 mM sucrose, 2.5 mM KCl, 8 mM MgSO4, 0.5 mM CaCl2, 1.25 mM NaH2PO4, 10 mM glucose, and 26 mM NaHCO3 saturated with 95%/5% O2/CO2. Coronal slices 400 μm thick were cut using a CompresstomeTM VF-200 (Precisionary Instruments). Slices were transferred to a holding chamber containing N-methyl-d-glucamine (NMDG)-HEPES recovery solution (in mM, 93 NMDG, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 D-glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 10 MgSO4, 0.5 CaCl2, pH 7.3, 305 mOsm) for 15 min at 31 °C. Slices were then transferred to a room temperature modified artificial cerebral spinal fluid (ACSF) containing (in mM) 126 NaCl, 1.25 NaH2PO4, 2.5 KCl, 10 d-glucose, 26 NaHCO3, 2 CaCl2 and 1 MgSO4, and 600 μM sodium ascorbate for at least 1 h. Subsequently, slices were transferred to a submersion recording chamber and continuously perfused (2 mL/min) with ACSF continuously bubbled with 95%/5% O2/CO2 at 31 °C. Glass electrodes were pulled using a Flaming/Brown micropipette puller (Sutter Instruments, CA) (resistance of 3–5 MΩ with ACSF). A concentric bi-polar stimulating electrode was positioned near the CA3–CA1 border and paired-pulse field excitatory postsynaptic potentials (fEPSPs) were evoked (200 μs duration, every 20 s spaced 20–500 ms apart) by placing a glass recording electrode in the stratum radiatum of CA1. Input–output curves were generated for each slice and the stimulation intensity was adjusted to 50% of the maximum response for subsequent experiments. PPR were calculated as the ratio between the slope of the second fEPSP divided by the slope of the first fEPSP. Drugs were dissolved in either DMSO (<0.05% final) or water and then diluted to the appropriate concentration in ACSF. Chemically induced LTD was induced by the application of DHPG (25 or 50 μM) for 10 min. Synaptically evoked mGlu receptor-dependent LTD was induced by paired-pulse low-frequency stimulation (PP-LFS) consisting of 900 pairs of stimuli (50-ms interstimulus interval) delivered at 1 Hz. For all electrophysiological experiments, the slopes of 3 consecutive sweeps were averaged and normalized to the average slope during the baseline period. Data were digitized using a Multiclamp 700B, Digidata 1322A, and pClamp 10 software (Molecular Devices). Multiple slices (n) were used per mouse with at least 3 mice contributing to each group.
Western Blotting
Western blots were performed using standard quantitative fluorescent western blotting techniques (Odyssey). Hippocampal tissues were dissected from WT or MeCP2-Tg1 mice after behavioral assays. Twenty-five micrograms of total protein was loaded per well for mGlu5 blots. Antibodies were diluted in Odyssey block (LiCor #927-40000) and used at the following concentrations: mGlu5 (Millipore #AB5675, 1:1000); Tubulin (E7, DHB, 1:4000); Goat Anti-Mouse 680 (LiCor # 926-68020, 1:5000); Goat Anti-Rabbit 800 (LiCor #926-32211, 1:5000). mGlu5 immunoreactivity was normalized to the loading control, Tubulin.
Statistical Analyses
Adequate sample size was determined based on previous experiments of conditioned fear behavior (Gogliotti et al. 2016) and acute slice electrophysiology (Ayala et al. 2009) in mice, as borne out the by results. Statistical analyses were performed using Graphpad Prism Version 5.01. The Bartlett’s multiple sample test was used to determine equal variance. All data shown represent mean ± SEM. Statistical significance between groups was determined using unpaired or paired Student’s t tests, one- or two-way ANOVA with Bonferroni’s post hoc test, and one- or two-way repeated measures ANOVA with Bonferroni’s post hoc test, as specified in the figure legends.
Supplementary Material
Notes
The authors thank Jerri Rook and Weimin Peng for their technical guidance. Conflict of Interest: None declared.
Authors’ Contributions
Conceptualization: B.J.S., P.J.C., and C.M.N.; Methodology, B.J.S., R.G.G., and N.M.F; Investigation: B.J.S.; Writing Original Draft: B.J.S.; Writing, Review & Editing: B.J.S., R.G.G., N.M.F, P.J.C, and C.M.N.; Visualization: B.J.S.; Funding Acquisition: B.J.S., P.J.C., and C.M.N.; Resources: C.W.L., P.J.C., and C.M.N.; Supervision: P.J.C. and C.M.N.
Funding
NIH grants (R21MH102548) and Autism Speaks (to C.M.N.), and NIH grants (R01MH062646 and R37NS031373) to P.J.C. NIMH (F32MH111124-01 to B.J.S.). P.J.C. C.W.L. and C.M.N. are inventors on patents that protect multiple classes of mGlu5 PAMs and NAMs.
References
- Abel T, Lattal KM. 2001. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 11(2):180–187. [DOI] [PubMed] [Google Scholar]
- Abraham WC. 2008. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 9(5):387. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. 1996. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 19(4):126–130. [DOI] [PubMed] [Google Scholar]
- Andre MA, Gunturkun O, Manahan-Vaughan D. 2015. The metabotropic glutamate receptor, mGlu5, is required for extinction learning that occurs in the absence of a context change. Hippocampus. 25(2):149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, et al. 2009. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 34(9):2057–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JD, Azorlosa JL. 1996. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci. 110(3):618–620. [DOI] [PubMed] [Google Scholar]
- Bedogni F, Cobolli Gigli C, Pozzi D, Rossi RL, Scaramuzza L, Rossetti G, Pagani M, Kilstrup-Nielsen C, Matteoli M, Landsberger N. 2015. Defects during Mecp2 null embryonic cortex development precede the onset of overt neurological symptoms. Cereb Cortex. 26(6):2517–2529. [DOI] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. 1982. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 2(1):32–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. 1993. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 361(6407):31–39. [DOI] [PubMed] [Google Scholar]
- Bolles RC. 1969. Avoidance and escape learning: simultaneous acquisition of different responses. J Comp Physiol Psychol. 68(3):355–358. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Bashir ZI, Davies CH, Collingridge GL. 1994. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature. 368(6473):740–743. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Collingridge GL. 1995. On the mechanism of long-term potentiation induced by (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD) in rat hippocampal slices. Neuropharmacology. 34(8):1003–1014. [DOI] [PubMed] [Google Scholar]
- Chao HT, Zoghbi HY. 2012. MeCP2: only 100% will do. Nat Neurosci. 15(2):176–177. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. 2003. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 38(3):461–472. [DOI] [PubMed] [Google Scholar]
- Cleva RM, Hicks MP, Gass JT, Wischerath KC, Plasters ET, Widholm JJ, Olive MF. 2011. mGluR5 positive allosteric modulation enhances extinction learning following cocaine self-administration. Behav Neurosci. 125(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Raymond CR, Abraham WC. 1998. Priming of long-term potentiation induced by activation of metabotropic glutamate receptors coupled to phospholipase C. Hippocampus. 8(2):160–170. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. 1983. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 334:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, David Sweatt J, Zoghbi HY. 2004. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet. 13(21):2679–2689. [DOI] [PubMed] [Google Scholar]
- Cooper LN, Bear MF. 2012. The BCM theory of synapse modification at 30: interaction of theory with experiment. Nat Rev Neurosci. 13(11):798–810. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. 2016. Still searching for the engram. Learn Behav. 44(3):209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. 1994. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev. 1(4):429–438. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. 2004. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J Neurosci. 24(8):1962–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzjohn SM, Kingston AE, Lodge D, Collingridge GL. 1999. DHPG-induced LTD in area CA1 of juvenile rat hippocampus; characterisation and sensitivity to novel mGlu receptor antagonists. Neuropharmacology. 38(10):1577–1583. [DOI] [PubMed] [Google Scholar]
- Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT. 2011. Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb Cortex. 21(3):727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. 2003. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 38(3):447–460. [DOI] [PubMed] [Google Scholar]
- Gogliotti RG, Senter RK, Rook JM, Ghoshal A, Zamorano R, Malosh C, Stauffer SR, Bridges TM, Bartolome JM, Daniels JS, et al. 2016. mGlu5 positive allosteric modulation normalizes synaptic plasticity defects and motor phenotypes in a mouse model of Rett syndrome. Hum Mol Genet. 25(10):1990–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K. 2011. Dynamics of retrieval strategies for remote memories. Cell. 147(5):1197–1197. [DOI] [PubMed] [Google Scholar]
- Gravius A, Barberi C, Schafer D, Schmidt WJ, Danysz W. 2006. The role of group I metabotropic glutamate receptors in acquisition and expression of contextual and auditory fear conditioning in rats—a comparison. Neuropharmacology. 51(7–8):1146–1155. [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. 2000. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 288(5469):1254–1257. [DOI] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF. 2001. Chemical induction of mGluR5- and protein synthesis—dependent long-term depression in hippocampal area CA1. J Neurophysiol. 86(1):321–325. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J. 1998. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem. 5(4–5):331–343. [PMC free article] [PubMed] [Google Scholar]
- Johnson LR, McGuire J, Lazarus R, Palmer AA. 2012. Pavlovian fear memory circuits and phenotype models of PTSD. Neuropharmacology. 62(2):638–646. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Raybuck JD, Gould TJ. 2012. Nicotinic receptors in the dorsal and ventral hippocampus differentially modulate contextual fear conditioning. Hippocampus. 22(8):1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee S, Park K, Hong I, Song B, Son G, Park H, Kim WR, Park E, Choe HK, et al. 2007. Amygdala depotentiation and fear extinction. Proc Natl Acad Sci USA. 104(52):20955–20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. 1992. Modality-specific retrograde amnesia of fear. Science. 256(5057):675–677. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF. 1996. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 381(6582):526–528. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Hood LE, Nemirovsky NE, Barabas P, Halstengard C, Villa A, Moore E, Watterson LR, Olive MF. 2012. Positive allosteric modulation of mGluR5 accelerates extinction learning but not relearning following methamphetamine self-administration. Front Pharmacol. 3:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. 2004. Structural plasticity and memory. Nat Rev Neurosci. 5(1):45–54. [DOI] [PubMed] [Google Scholar]
- Lanté F, de Jésus Ferreira MC, Guiramand J, Récasens M, Vignes M. 2006. Low-frequency stimulation induces a new form of LTP, metabotropic glutamate (mGlu5) receptor- and PKA-dependent, in the CA1 area of the rat hippocampus. Hippocampus. 16(4):345–360. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. 2003. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 117(2):341–349. [DOI] [PubMed] [Google Scholar]
- Liu JL, Li M, Dang XR, Wang ZH, Rao ZR, Wu SX, Li YQ, Wang W. 2009. A NMDA receptor antagonist, MK-801 impairs consolidating extinction of auditory conditioned fear responses in a Pavlovian model. PLoS One. 4(10):e7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. 2012. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 484(7394):381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell KM, Felts AS, Rodriguez AL, Venable DF, Cho HP, Morrison RD, Byers FW, Daniels JS, Niswender CM, Conn PJ, et al. 2013. N-Acyl-N’-arylpiperazines as negative allosteric modulators of mGlu1: identification of VU0469650, a potent and selective tool compound with CNS exposure in rats. Bioorg Med Chem Lett. 23(13):3713–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. 1997. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 17(13):5196–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. 2012. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol. 4(6):a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Mou L, Shah N, Hu JH, Worley PF, Ressler KJ. 2012. Epigenetic modulation of Homer1a transcription regulation in amygdala and hippocampus with pavlovian fear conditioning. J Neurosci. 32(13):4651–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. 2001. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 21(16):5925–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao SC, Chang CH, Wu CC, Orejanera MJ, Manzoni OJ, Gean PW. 2013. Inhibition of spontaneous recovery of fear by mGluR5 after prolonged extinction training. PLoS One. 8(3):e59580. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Maren S. 2011. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 70(5):830–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. 2000. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 23:649–711. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. 2007. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 62(5):446–454. [DOI] [PubMed] [Google Scholar]
- Moutin E, Raynaud F, Roger J, Pellegrino E, Homburger V, Bertaso F, Ollendorff V, Bockaert J, Fagni L, Perroy J. 2012. Dynamic remodeling of scaffold interactions in dendritic spines controls synaptic excitability. J Cell Biol. 198(2):251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na ES, Morris MJ, Nelson ED, Monteggia LM. 2014. GABAA receptor antagonism ameliorates behavioral and synaptic impairments associated with MeCP2 overexpression. Neuropsychopharmacology. 39(8):1946–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na ES, Nelson ED, Adachi M, Autry AE, Mahgoub MA, Kavalali ET, Monteggia LM. 2012. A mouse model for MeCP2 duplication syndrome: MeCP2 overexpression impairs learning and memory and synaptic transmission. J Neurosci. 32(9):3109–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. 2010. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 50:295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkirov SG, Manahan-Vaughan D. 2011. Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb Cortex. 21(3):501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. 2013. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 23(17):R764–R773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CR, Thompson VL, Tate WP, Abraham WC. 2000. Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation. J Neurosci. 20(3):969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. 2004. Spontaneous recovery. Learn Mem. 11(5):501–509. [DOI] [PubMed] [Google Scholar]
- Riedel G, Casabona G, Platt B, Macphail EM, Nicoletti F. 2000. Fear conditioning-induced time- and subregion-specific increase in expression of mGlu5 receptor protein in rat hippocampus. Neuropharmacology. 39(11):1943–1951. [DOI] [PubMed] [Google Scholar]
- Riedel G, Reymann KG. 1996. Metabotropic glutamate receptors in hippocampal long-term potentiation and learning and memory. Acta Physiol Scand. 157(1):1–19. [DOI] [PubMed] [Google Scholar]
- Rittenhouse CD, Shouval HZ, Paradiso MA, Bear MF. 1999. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature. 397(6717):347–350. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE. 2002. The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci. 22(12):5219–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook JM, Xiang Z, Lv X, Ghoshal A, Dickerson JW, Bridges TM, Johnson KA, Foster DJ, Gregory KJ, Vinson PN, et al. 2015. Biased mGlu5-positive allosteric modulators provide in vivo efficacy without potentiating mGlu5 modulation of NMDAR currents. Neuron. 86(4):1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Matus-Amat P. 2009. DHPG activation of group 1 mGluRs in BLA enhances fear conditioning. Learn Mem. 16(7):421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Lorenzini CA, Baldi E, Bucherelli C, Roberto M, Tassoni G, Brunelli M. 2001. Long-lasting hippocampal potentiation and contextual memory consolidation. Eur J Neurosci. 13(12):2291–2298. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Lorenzini CA, Baldi E, Bucherelli C, Roberto M, Tassoni G, Brunelli M. 2002. Time-dependent inhibition of hippocampal LTP in vitro following contextual fear conditioning in the rat. Eur J Neurosci. 15(1):143–150. [DOI] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. 2001. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 21(22):9009–9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz B, Fendt M, Gasparini F, Lingenhohl K, Kuhn R, Koch M. 2001. The metabotropic glutamate receptor antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) blocks fear conditioning in rats. Neuropharmacology. 41(1):1–7. [DOI] [PubMed] [Google Scholar]
- Sepulveda-Orengo MT, Lopez AV, Soler-Cedeno O, Porter JT. 2013. Fear extinction induces mGluR5-mediated synaptic and intrinsic plasticity in infralimbic neurons. J Neurosci. 33(17):7184–7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethna F, Wang H. 2014. Pharmacological enhancement of mGluR5 facilitates contextual fear memory extinction. Learn Mem. 21(12):647–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyi A, Schachtman TR, Christoffersen GR. 2005. The role of metabotropic glutamate receptor 5 in learning and memory processes. Drug News Perspect. 18(6):353–361. [DOI] [PubMed] [Google Scholar]
- Song CH, Detert JA, Sehgal M, Moyer JR. 2012. Trace fear conditioning enhances synaptic and intrinsic plasticity in rat hippocampus. J Neurophysiol. 107(12):3397–3408. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. 2005. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 4(2):131–144. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ, Morris RG. 2014. The synaptic plasticity and memory hypothesis: encoding, storage and persistence. Philos Trans R Soc Lond B Biol Sci. 369(1633):20130288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Wu H, Coronado AA, de Laittre E, Osterweil EK, Zhang Y, Bear MF. 2016. Negative allosteric modulation of mGluR5 partially corrects pathophysiology in a mouse model of Rett syndrome. J Neurosci. 36(47):11946–11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarczynska E, Klodzinska A, Chojnacka-Wojcik E, Palucha A, Gasparini F, Kuhn R, Pilc A. 2001. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br J Pharmacol. 132(7):1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, et al. 1999. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 23(3):583–592. [DOI] [PubMed] [Google Scholar]
- van Dam EJ, Kamal A, Artola A, de Graan PN, Gispen WH, Ramakers GM. 2004. Group I metabotropic glutamate receptors regulate the frequency-response function of hippocampal CA1 synapses for the induction of LTP and LTD. Eur J Neurosci. 19(1):112–118. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Daly CA, Huber KM. 2006. Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression. J Neurophysiol. 95(4):2427–2438. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. 2006. Learning induces long-term potentiation in the hippocampus. Science. 313(5790):1093–1097. [DOI] [PubMed] [Google Scholar]
- Xu J, Antion MD, Nomura T, Kraniotis S, Zhu Y, Contractor A. 2014. Hippocampal metaplasticity is required for the formation of temporal associative memories. J Neurosci. 34(50):16762–16773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. 2009. mGluR5 has a critical role in inhibitory learning. J Neurosci. 29(12):3676–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Kraniotis S, He Q, Marshall JJ, Nomura T, Stauffer SR, Lindsley CW, Conn PJ, Contractor A. 2013. Potentiating mGluR5 function with a positive allosteric modulator enhances adaptive learning. Learn Mem. 20(8):438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kapeller-Libermann D, Travaglia A, Inda MC, Alberini CM. 2016. Direct dorsal hippocampal-prelimbic cortex connections strengthen fear memories. Nat Neurosci. 20(1):52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Li H, Chang Q. 2012. MeCP2 phosphorylation is required for modulating synaptic scaling through mGluR5. J Neurosci. 32(37):12841–12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.