Abstract
STUDY QUESTION
Does anti-Müllerian hormone (AMH) inhibit activation (initiation of growth) of primordial follicles and attenuate the growth of primary follicles in cattle, an excellent animal model for human ovarian follicular development?
SUMMARY ANSWER
AMH inhibited activation of bovine primordial follicles and attenuated the growth of activated follicles in vitro.
WHAT IS KNOWN ALREADY
In mice null mutant for AMH, the pool of primordial follicles is depleted prematurely and AMH inhibits follicle activation in vitro. Results of studies with human ovarian tissue in vitro were inconsistent. Our previous work provided indirect evidence that AMH inhibits follicle activation in bovine ovaries.
STUDY DESIGN, SIZE, DURATION
Pieces of fetal bovine ovarian cortex (2 pieces/culture well), obtained during mid or late pregnancy, were cultured in control medium or with graded doses of AMH for 2, 10 or 12 days. Effects of treatment on follicle activation and growth were determined by histological morphometry; follicles in every 20th histological section were staged (primordial or primary), counted, and measured. In addition, AMH was immunolocalized in bovine ovaries obtained at various times during pregnancy (n = 20 ovaries).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Bovine fetal ovaries at mid or late gestation were obtained at a commercial abattoir. Pieces of ovarian cortex were cultured without or with AMH and fixed for histological morphometry on Day 0 and at the end of culture. Treatments were applied to duplicate cultures from each of two or three fetuses. In 12-day cultures, addition of AMH was delayed until the third day. Histological analysis provided information about the types, numbers and sizes of follicles in cortical pieces before and after treatments. Ovaries obtained during the second and third trimesters were assessed for the presence of AMH by immunohistochemistry.
MAIN RESULTS AND THE ROLE OF CHANCE
AMH (100–500 ng/ml) inhibited follicle activation in response to an activator (insulin) in ovarian cortical pieces from fetal ovaries in late gestation. Dose-dependent inhibitory effects on the diameters of primary follicles and their oocytes were also observed. These results were obtained only when AMH was added to cultures in advance of insulin (presumably because it penetrates tissue more slowly than insulin). Results of experiments with cortical pieces from fetal ovaries at mid-gestation, when follicles are forming, showed that AMH did not inhibit the formation of follicles. Immunohistochemical localization of AMH showed that it is not present in fetal ovaries until the third trimester, when it was localized to the granulosa cells of secondary and small antral follicles.
LIMITATIONS REASONS FOR CAUTION
The experiments were performed with fetal ovaries because follicles form and follicle activation begins during fetal life in cattle (as it does in humans), so fetal ovarian cortex of later gestation provides tissue rich in primordial follicles. We assume, but have no experimental evidence, that our findings also apply to post-natal ovaries.
WIDER IMPLICATIONS OF THE FINDINGS
Although circulating AMH is used as an indication of the follicular reserve in women, little is known about AMH in human ovaries. Cattle are an excellent non-primate model for human ovarian follicular development and, hence, the findings suggest similar roles for AMH in human follicular development.
LARGE SCALE DATA
Not applicable.
STUDY FUNDNG/COMPETING INTEREST(S)
This research was supported by National Research Initiative Competitive Grants no. 00-35203-9151, 2003-35203-13532, and 2008-35203-05989) from the U.S. Dept. of Agriculture's National Institute of Food and Agriculture to JEF and by an NIH National Research Service Award (F32 HD08264) to RAC. There are no conflicts of interest or competing interests.
Keywords: ovary, ovarian follicle, follicle activation, AMH, cattle
Introduction
Mammalian ovaries are endowed with a store of non-growing, primordial follicles. Individual follicles exit this resting pool (i.e. activate) continuously, providing the female with a steady stream of growing follicles for potential ovulation. What regulates the timing of activation of individual follicles is unknown. In mice and rats, primordial follicles form within a few days after birth and a subset of follicles leaves the resting pool to begin growth, both in vivo and in whole neonatal ovaries in vitro (Fortune and Eppig, 1979; Hirshfield, 1991; Parrott and Skinner, 1999). Thus, newborn rodent ovaries are a convenient model for studies of follicle formation and early development and studies with that model have revealed that various hormones and growth factors can increase the number of follicles that activate in cultured rodent ovaries. In contrast, there is evidence that anti-Müllerian hormone (AMH, also known as Müllerian inhibiting substance or MIS) negatively regulates follicle activation in rodents [reviewed in (Fortune, 2003; Skinner, 2005)].
AMH, a member of the transforming growth factor beta (TGF-β) super-family of growth factors, is responsible for regression of the Müllerian duct during sexual differentiation in males [for review see (Teixeira et al., 2001)]. In female mice null mutant for AMH, the pool of primordial follicles is depleted much more rapidly than in wild-type controls (Durlinger et al., 1999) and treating whole neonatal mouse ovaries with AMH in vitro decreased the number of activated follicles by approximately 40% after 2 or 4 days of culture (Durlinger et al., 2002a). AMH or its mRNA has been detected in the granulosa cells of activated follicles of sheep (Bezard et al., 1988) and rats (Baarends et al., 1995), respectively. However, activation was not completely inhibited by AMH in vitro and depletion of the primordial pool in AMH null mutant mice took more than 13 months (Durlinger et al., 1999, 2002a). This suggests that stimulatory factors and/or additional inhibitory factors are also important in the control of primordial follicle activation in vivo. Experiments with preantral follicles isolated from rodent ovaries have shown that AMH also affects the growth of activated follicles, but it was inhibitory to the growth of mouse secondary follicles (Durlinger et al., 2001) and stimulatory to rat secondary follicles (McGee et al., 2001).
Despite the strong evidence that AMH is a negative regulator of follicle activation in rodents, much less is known about whether it plays a similar role in other species, including species of practical interest, such as humans and domestic animals. To study follicle activation in cattle and baboons, species in which follicles form and follicle activation begins in the fetal ovary, our laboratory developed a serum-free organ culture system for small pieces of fetal ovarian cortex, obtained during the third trimester and rich in primordial follicles (Wandji et al., 1996, 1997). Primordial follicles in these ovarian cortical pieces, and similarly in cortical pieces from adult bovine ovaries, activated within 2 days in culture medium containing insulin and developed to the primary stage (Wandji et al., 1996, 1997; Braw-Tal and Yossefi, 1997). In contrast, activation was completely inhibited when fetal bovine cortical pieces (Cushman et al., 2002) or newborn mouse ovaries (Gigli et al., 2005) were grafted beneath the chorioallantoic membrane (CAM) of 6-day-old chick embryos. This indicated that one or more circulating factors, produced by the developing chick embryo, inhibited activation of primordial follicles in the CAM grafts. One potential inhibitory factor is AMH since it is produced by the gonads of chick embryos of both sexes during the developmental period when the cortical grafts were in place (di Clemente et al., 1992). Additional experiments with CAM-grafted whole mouse ovaries and bovine cortical pieces CAM-grafted to gonadectomized chick embryos provided indirect evidence that AMH is an inhibitor of follicle activation in cattle (Gigli et al., 2005), an excellent animal model for follicular development in women (Campbell et al., 2003). When cortical pieces from adult human ovaries were cultured with AMH, one group reported inhibition of follicle activation after a week in vitro (Carlsson et al., 2006), whereas another group reported that AMH increased follicle activation and growth of primary follicles after four weeks (Schmidt et al., 2005).
Circulating AMH is a marker for the size of the ovarian follicular reserve in humans [reviewed in (Broer et al., 2014)] and cattle (Ireland et al., 2011), yet little is known about its effects in these species. To test more directly the hypothesis that AMH inhibits follicle activation in cattle, we cultured cortical pieces from fetal bovine ovaries obtained during late gestation with graded doses of AMH. Because Nilsson et al. (2011) reported that AMH inhibited follicle formation in newborn rat ovaries in vitro, we also cultured cortical pieces from bovine ovaries at mid-gestation to test whether AMH affects follicle formation, which occurs primarily during mid-gestation in cattle (Yang and Fortune, 2008). In addition, we determined where and when AMH is expressed in fetal ovaries by immunolocalization at various gestational ages.
Materials and Methods
Animals
Fetal bovine ovaries were collected at a local slaughterhouse from fetuses during mid-gestation (3.5–4 months) or late gestation (6–8 months; bovine gestational length = ~9.3 months). Gestational age was estimated by crown-rump length of the fetus (Evans and Sack, 1973). The ovaries were transported to the laboratory at ambient temperature (20–22°C) in Leibovitz's L-15 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 1% fetal bovine serum, 50 U penicillin/ml and 50 μg streptomycin/ml (Invitrogen) (Wandji et al., 1996).
Isolation and culture of ovarian cortical pieces
The ovarian cortex was dissected from the medullary tissue and cut into 0.5 mm3 pieces. The pieces were either fixed immediately as Day 0 controls (4 pieces/ovary) or placed in culture for 2, 10 or 12 days, depending on the experiment. Cortical pieces (2/well) were cultured in 24-well Costar plates (Corning Inc., Corning, NY, USA) on uncoated culture plate inserts (Millicell-CM, 0.4 μm pore size; EMD Millipore Corporation, Bedford, MA, USA) with 300 μl of culture medium at 38.5°C with 5% CO2 in air. The control culture medium was Waymouth's medium MB 752/1 (Invitrogen) supplemented with antibiotics (50 μg/ml streptomycin sulfate and 75 μg/ml penicillin; Sigma, St. Louis, MO, USA), ITS+ (6.25 μg/ml insulin, 6.25 μg/ml transferrin, 6.25 ng/ml selenious acid, 1.25 mg/ml bovine serum albumin (BSA), 5.35 μg/ml linoleic acid; Corning), and 25 mg/l pyruvic acid sodium salt (Sigma). In all experiments, treatments were applied to duplicate cultures from each fetus. When the duration of culture was longer than 2 days, cultures were refreshed every second day by drawing off 200 µl of medium and adding 200 µl of fresh medium.
In the first experiment, cortical pieces were isolated from ovaries obtained during late gestation (6–8 months) and cultured as described above for 2 days, in defined medium without or with graded doses (0, 100, 200, 500 or 1000 ng/ml; 0–7.1 nM) of recombinant human AMH (rhAMH; kindly provided by Dr R.L. Cate, Biogen Inc., Cambridge, MA, USA). During late gestation, bovine fetal ovaries have primarily primordial and primary follicles, with a few secondary follicles and occasional small antral follicles (small antral follicles were not cultured). The second experiment was similar in design, but the duration of culture was extended to 10 days.
The results of the first two experiments suggested that AMH (potential inhibitor of activation) diffuses into cortical pieces more slowly than the insulin (activation stimulator) in ITS+. Because culturing bovine cortical pieces in medium with TS+ (identical to ITS+, but without insulin) maintains follicular health without activation (Fortune et al., 2011), in the third experiment we used a two-step design to re-examine the effects of AMH on follicle activation and growth in cortical pieces from late gestation (6–8 months). Cortical pieces were first cultured with graded doses of rhAMH in TS+ medium for 2 days, to ensure time for AMH to diffuse into the cortical pieces, and then cultured for 10 more days with graded doses of rhAMH but in ITS+ (activation stimulator present).
The aim of the fourth experiment was to test the effects of AMH on formation and activation of primordial follicles in vitro in fetal ovaries obtained during mid-gestation (~Days 105–120). Follicles begin to form around Day 90 of gestation, but primary follicles are not observed until around Day 140 in vivo (Yang and Fortune, 2008). Activation of primordial follicles in fetal ovaries <140 days old can be induced in vitro, but only if cortical pieces are cultured longer than 2 days, implying that follicles gain the capacity to activate during culture (Yang and Fortune, 2008).
Assessment of survival and growth of follicles and oocytes by histological morphometry
On Day 0 or after termination of cultures on Day 2, 10 or 12, cortical pieces were fixed for 1 h in Tousimis fixative (2.5% glutaraldehyde, 2.5% formaldehyde in 0.075 M cacodylate buffer, pH 7.3), post-fixed for 45 min in 2% osmium tetroxide, and embedded in LR White plastic (EMS, Fort Washington, PA, USA). Blocks were sectioned at 2 μm with a glass knife. Every other set of 10 consecutive sections was mounted on gelatin-coated slides at 30–40 °C and stained with toluidine blue. One cross-section in each set of 10 consecutive sections was examined and only follicles with the germinal vesicle present in that section were counted and measured to avoid counting or measuring the same follicle twice.
Follicles were classified as primordial, primary, or secondary and health was assessed, as described previously (Wandji et al., 1996). Follicles with an oocyte surrounded by a single layer of flattened somatic cells were classified as primordial follicles (van Wezel and Rodgers, 1996). In primary follicles, the oocyte is surrounded by a single layer of large cuboidal granulosa cells and in secondary follicles, there are two or more complete layers of granulosa cells. Follicles in transition from primordial to primary were considered primordial if <50% of the granulosa cells were cuboidal and primary if >50% were cuboidal. Examples of bovine primordial and primary follicles are shown in Supplementary Fig. S1, in Fig. 5 and in our previous publications (Wandji et al., 1996; Cushman et al., 2002). After staging, follicles were classified as healthy (intact basal lamina, oocyte with no more than three cytoplasmic vacuoles, intact germinal vesicle and nucleolus) or atretic. Atresia was virtually absent in fetal ovaries at mid-gestation and the percentage of atretic follicles was low (~10%) in ovaries at late gestation and was not affected by treatment in vitro.
Figure 5.
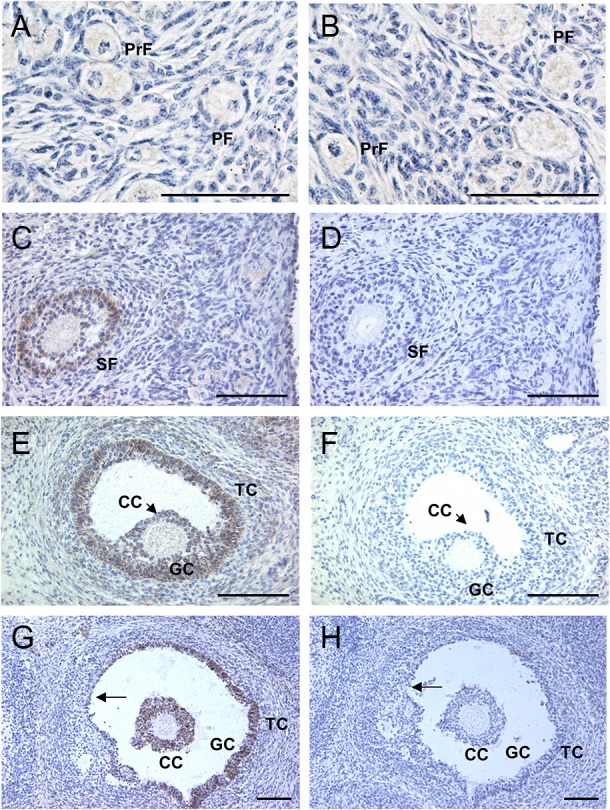
Photomicrographs of AMH immunostaining in sections from bovine fetal ovaries after 210 days of gestation (panels A, C, E and G). (A) No staining was detected in granulosa cells of primordial follicles (PrF). Granulosa cells of primary follicles (PF) were generally negative for AMH immunostaining. (C) Granulosa cells of secondary follicles (SF) displayed staining. (E) Strong AMH staining was observed in granulosa cells (GC) and cumulus cells (CC) of small antral follicles. Some theca cells (TC) showed weak immunoreactivity. (G) In an antral follicle undergoing atresia, AMH staining was absent in degenerating granulosa cells (arrow), whereas cumulus cells and remaining healthy granulosa cells still displayed staining. (B, D, F and H) Negative controls; sections incubated with AMH antibody and an excess of blocking peptide. Bars = 100 µm. Results in A-H are representative of ovaries from five fetuses.
An inverted microscope equipped with Hoffman modulation contrast optics was used to examine sections. Images were projected onto a video monitor and the diameters of individual healthy follicles and enclosed oocytes were measured by a computer-driven image analysis program (NIH Image, NIH, Bethesda, MD, USA). The diameter of each follicle and its oocyte was calculated by averaging measurements taken in 2D.
Immunohistochemistry for AMH
Fetal bovine ovaries were collected from fetuses (n = 20) between 88 and 244 days of gestation. Female fetuses were divided into four groups (n = 3–6 ovaries/group) based on the expected transitions in follicular development: (i) Days 80–90, oogonia and oocytes present, but no follicles; (ii) Days 91–140, primordial follicles forming; (iii) Days 141–210, primary follicles appear; and (iv) >210 days, a few secondary follicles observed (Yang and Fortune, 2008). Whole fetal ovaries were fixed in Bouin's solution, dehydrated through a graded series of ethanol and embedded in paraffin. Sections (5-μm) were cut, mounted on slides coated with poly-l-lysine (Poly-prep slides; Sigma), and dried overnight at 37°C and for 15 min at 50°C. After deparaffinization in xylene and rehydration, the sections were pretreated by heating for 15 min in 10 mM citric acid buffer (pH 6) in a microwave oven at 700 W to enhance antigen retrieval, with evaporated water replaced every 5 min. Endogenous peroxidases were blocked by incubating sections with 3% H2O2 for 15 min. To prevent nonspecific reactions, sections were incubated with 1% BSA in phosphate buffered saline for 1 h and then with normal rabbit serum (Zymed Laboratories, San Francisco, CA, USA) for 30 min at room temperature.
The primary antibody used for AMH immunohistochemistry was a goat polyclonal antibody against an epitope of human AMH (C-20, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). This antibody was chosen because it detects AMH in both human and mouse ovaries (Durlinger et al., 2002a; Weenen et al., 2004). Each section was incubated with AMH antibody (diluted 1:1000) overnight at 4°C. The biotinylated secondary antibody (Zymed Laboratories) was then applied for 15 min at room temperature. All incubations were carried out in a humid chamber. The location of the antigen/antibody/enzyme complex was detected and visualized using a Histostain-SP kit (Zymed Laboratories). Diaminobenzidine chromogen substrate was administered for 1 min and produced a brown positive stain. Negative controls were included in each run and consisted of the adjacent sections incubated with AMH antibody and a 5-fold excess of a blocking peptide (C-20 P; Santa Cruz Biotechnology, Inc.). Sections were counterstained with hematoxylin.
Statistical analysis
Treatments were applied to duplicate cultures from each of two or three fetuses (depending on the experiment), obtained on separate days. Mean numbers of primordial and primary follicles per section and mean diameters of follicles and their oocytes were calculated for each replicate of each treatment. The numbers of follicles were smaller and more variable in Exp. 4 (ovaries at mid-gestation) than in Exps. 1 and 2 because follicle formation is still occurring at that stage of ovarian development. Therefore, in Exp. 4 numbers of primordial and primary follicles were expressed as percentages of the total number of follicles for each replicate of each treatment and mean percentages were calculated. If Hartley's test indicated heterogeneity of variance, data were transformed to logarithms before they were subjected to analysis of variance (ANOVA). Differences among the means were evaluated by two-way ANOVA with fetus and treatment as the two factors. After ANOVA, differences among individual means were determined using Duncan's multiple range test.
Results
Effects of AMH on activation and growth of follicles in cortical pieces from fetal ovaries in late gestation after 2 days in vitro
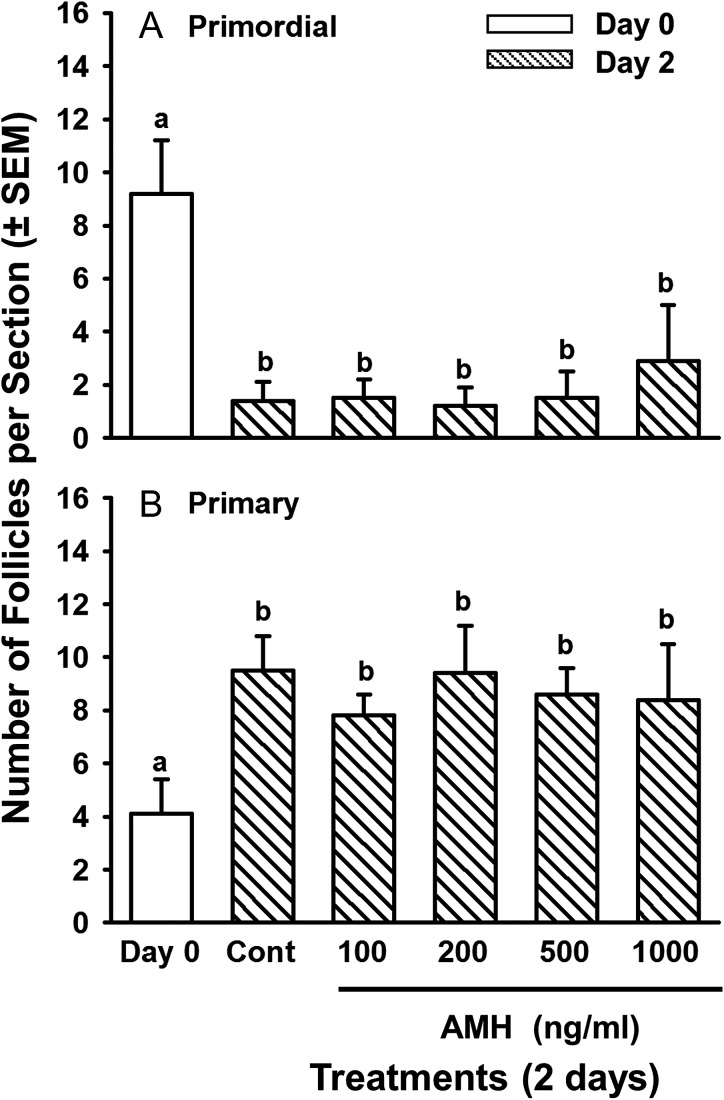
Ovarian cortical pieces from third trimester fetuses (6–8 months) were cultured for 2 days with graded doses of AMH. After 2 days in control medium, the number of primordial follicles had decreased (P < 0.01) and the number of primary follicles had increased concomitantly (P < 0.01) compared to Day 0, indicating that primordial follicles had activated in vitro, as expected (Fig. 1). However, contrary to the hypothesis that AMH inhibits activation, there was no effect of rhAMH, at any dose, on activation of primordial follicles (Fig. 1).
Figure 1.
Numbers of (A) primordial and (B) primary follicles (mean per histological section ± SEM) in ovarian cortical pieces from bovine fetuses in late-gestation (6–8 months) after 0 (white bars) or 2 (striped bars) days in culture without (Cont = control cultures) or with graded doses of rhAMH (100–1000 ng/ml). Within each panel, means (bars) with no common superscripts are different, as determined by two-way ANOVA and Duncan's multiple range test (P < 0.01; n = 6 cultures, 2 from each of three fetuses; 21–29 sections examined per treatment per fetus).
Primary follicles in Day 2 controls were larger than the primary follicles in freshly isolated pieces (38.3 ± 0.4 vs. 32 ± 0.5 μm, respectively; P < 0.05). However, primary follicles in cortical pieces cultured with the highest dose of AMH (1000 ng/ml) were intermediate in size between Day 0 and the Day 2 controls and tended be smaller than primary follicles in Day 2 control cultures (35.3 ± 0.7 vs. 38.3 ± 0.4 μm, respectively; P < 0.1), suggesting that AMH may attenuate follicular growth in cattle. The diameters of the oocytes of primary follicles did not change during 2 days of culture for any of the treatment groups, compared with Day 0 (data not shown).
Effects of AMH on activation and growth of follicles in cortical pieces from fetal ovaries in late gestation after 10 days in vitro
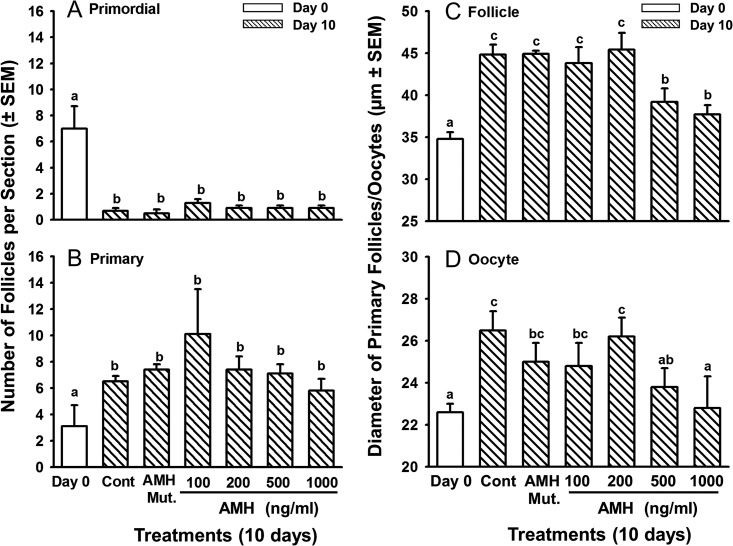
Results of the 2-day cultures described above did not support the hypothesis that AMH inhibits follicle activation in cattle, but suggested that AMH inhibits the growth of primary follicles. Although follicle activation occurs within 2 days of culture, follicle growth is a slower process (Fortune et al., 2000). Hence the culture period was extended to 10 days to test the hypothesis that AMH inhibits the growth of bovine primary follicles in vitro. After 10 days in culture, AMH had no effects on the activation of primordial follicles (Fig. 2A and B), as expected based on the results of the 2-day cultures. In the 10 day cultures we had an additional negative control, a biologically inactive cleavage mutant of human AMH (also supplied by Dr. Cate). In the cleavage mutant, Arg 427 was replaced with threonine, which prevents the protein from being cleaved by plasmin to its biologically active form (Cate et al., 1990).
Figure 2.
Numbers of (A) primordial and (B) primary follicles (mean per histological section ± SEM) and diameters (mean ± SEM) of healthy primary follicles (C) and their oocytes (D) in ovarian cortical pieces from bovine fetuses in late-gestation (6–8 months) after 0 (white bars) or 10 (striped bars) days in culture without (Cont = control cultures) or with graded doses of rhAMH (100–1000 ng/ml) or with an inactive, mutant form of AMH (AMH Mut.; 1000 ng/ml). Within each panel, means (bars) with no common superscripts are different, as determined by two-way ANOVA and Duncan's multiple range test (P < 0.01; n = 6 cultures, 2 from each of three fetuses; 22–40 sections examined per treatment per fetus; 11–122 primary follicles/oocytes measured per treatment per fetus).
In the control groups, primary follicles were larger after 10 days in culture than on Day 0 (44.8 ± 1.2 vs. 34.8 ± 0.8 μm, respectively; Fig. 2C). The negative effect of AMH on primary follicle growth was much more dramatic in the cortical pieces treated with AMH for 10 days than for 2 days. Primary follicles were smaller in cultures treated with 500 or 1000 ng/ml AMH for 10 days, compared to untreated controls or to cultures treated with the biologically inactive mutant AMH, with diameters about 87.5 and 83.1%, respectively of those in Day 10 controls (P < 0.001; Fig. 2C). Although the difference in diameters may seem small, it corresponds to about a 40% smaller volume. Likewise, the oocytes of primary follicles treated with 500 or 1000 ng/ml AMH were smaller than those of Day 10 controls (P < 0.05) and did not differ from Day 0 controls (Fig. 2D). These results support the hypothesis that AMH attenuates the growth of primary follicles in cattle.
Effects of AMH on activation and growth of follicles in cortical pieces from fetal ovaries in late gestation after 12 days in vitro (with delayed addition of insulin)
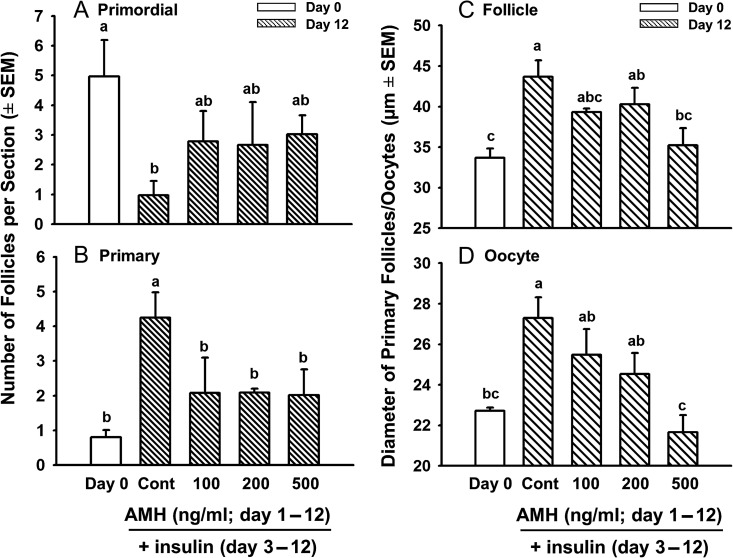
The results of the studies described above indicated that AMH slowed the growth of activated bovine follicles, but had no effect on follicle activation, in contrast to its inhibitory effect on follicle activation in mice (Durlinger et al., 1999, 2002b). We hypothesized that because AMH is a large molecule (~140 kDa), it did not penetrate the relatively dense bovine ovarian stroma in time to inhibit activation in vitro. In our culture system with ITS+, activation is first evident between 12 and 24 h of culture (Fortune et al., 2000) and therefore, the molecular and biochemical changes responsible for activation must occur even earlier during culture. We found that the insulin in ITS+ stimulates activation and that culturing bovine cortical pieces with TS+ (identical to ITS+ but without insulin) maintains follicular health in vitro without activating follicles (Fortune et al., 2011). The experimental design in this study was based on this finding. Cortical pieces from late gestation were first cultured with graded doses of AMH in TS+ medium (i.e. no insulin) for 2 days, to allow AMH penetration into cortical tissue, and then with the same graded doses of AMH in ITS+ medium for an additional 10 days, to test the effects of AMH on insulin-stimulated follicle activation and growth.
After 12 days in culture (10 days with insulin), there was the expected dramatic decrease in the number of primordial follicles and concomitant increase in primary follicles in control cultures (2 days in TS+ medium, then 10 days in ITS+ medium) compared to Day 0 (P < 0.05; Fig. 3A and B), indicating that primordial follicles had activated. Interestingly, AMH at all tested doses decreased the number of primary follicles by >50%, compared to Day 12 controls (P < 0.05; Fig. 3B), showing that AMH can inhibit primordial follicle activation in cattle.
Figure 3.
Effects of AMH on numbers of (A) primordial and (B) primary follicles (mean ± SEM) and on the mean diameters (µm ± SEM) of healthy primary follicles (C) and their oocytes (D) in cortical pieces from bovine fetal ovaries obtained during late-gestation (6–8 months) and cultured for 12 days. White bars = freshly isolated cortical pieces (Day 0); striped bars = cortical pieces cultured without (Cont = Day 12 control culture; first 2 days in TS+ medium, then 10 more days in ITS+ medium) or with graded doses of rhAMH (first 2 days with 100, 200, or 500 ng/ml AMH in TS+, then 10 more days with 100, 200, or 500 ng/ml AMH in ITS+). Within each panel, means (bars) with no common superscripts are different, as determined by two-way ANOVA and Duncan's multiple range test (P < 0.05, n = 4 cultures, 2 from each of two fetuses; 13–21 sections examined per treatment per fetus; 9–87 primary follicles/oocytes measured per treatment per fetus).
Similar to the results in Fig. 2C, the diameters of primary follicles in controls treated with insulin for 10 days were larger than the diameters of the primary follicles in freshly isolated tissues (Day 0; Fig. 3C; P < 0.05). Likewise, the diameters of oocytes in primary follicles were also larger in cultured controls than those in freshly isolated tissues (Fig. 3D; P < 0.05). AMH at 500 ng/ml significantly reduced the diameters of primary follicles and their oocytes, compared to Day 12 controls, to levels not different from Day 0 controls, suggesting that AMH retards follicle and oocyte growth in vivo (Fig. 3C and D; P < 0.05).
Effects of AMH on formation of primordial follicles and their capacity to activate in cortical pieces from fetal ovaries at mid-gestation
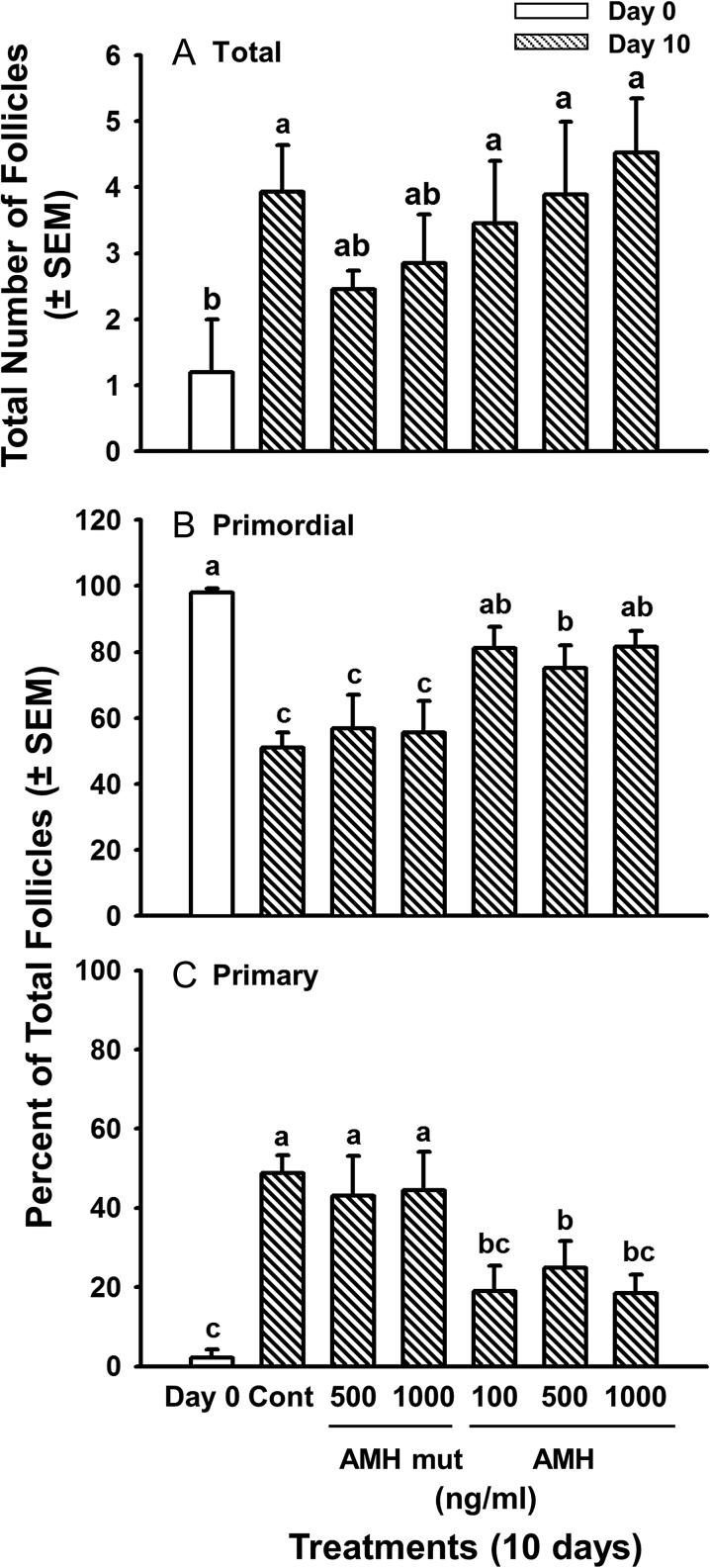
Because Nilsson et al. (2011) reported that AMH inhibited follicle formation in newborn rat ovaries, we tested the effects of AMH on the formation of primordial follicles in fetal bovine ovaries at mid-gestation (Days 105–120) and on their development of the capacity to activate. Follicles begin to form around Day 90 of gestation and new follicles can form in vitro in medium with ITS+. At this stage of ovarian development (before Day 140) primordial follicles do not activate in vivo. They also do not activate after 2 days in vitro, but activation can occur during 10 days in culture (i.e. they acquire the capacity to activate in vitro) (Yang and Fortune, 2008). We assumed that the gradual formation of new follicles and the slower activation of primordial follicles in cortical pieces during mid-gestation would allow time for AMH to diffuse into the tissue before follicle formation/activation started and that delayed addition of AMH would not be necessary. Ovarian cortical pieces were cultured for 10 days with ITS+ in the presence or absence of graded doses of AMH.
After 10 days of culture, the number of follicles had increased about 3.5-fold relative to Day 0, as expected (Yang and Fortune, 2008), but AMH had no effect on follicle formation (Fig. 4A). Because the number of follicles is low and varies among fetuses during the period when follicles are beginning to form, follicle activation was assessed in this experiment by expressing primordial and primary follicles as percentages of the total number of follicles. On Day 0, almost all follicles (98%) were primordial, but after 10 days in control medium, about 50% of the follicles were primary, confirming that primordial follicles can acquire the capacity to activate in vitro (they do not begin to activate in vivo until after Day 140) (Fig. 4B, C). All three concentrations of AMH inhibited the decrease in the percentage of primordial follicles that was observed on Day 10 in control cortical pieces and in pieces treated with the AMH mutant (the negative control) (P < 0.05; Fig. 4B) and concomitantly inhibited the dramatic increase in the percentage of primary follicles observed in Day 10 and AMH mutant controls (P < 0.05; Fig. 4C). Compared to Day 10 controls and cultures treated with the AMH mutant, all three doses of AMH prevented the decrease in primordial follicles and the increase in follicles at the primary stage (P < 0.05; Fig. 4), strongly suggesting that AMH inhibited primordial follicle activation. There was no difference among AMH doses (P > 0.05; Fig. 4). In contrast to the results with cortical pieces from late gestation, there were no effects of AMH on follicle or oocyte diameters when cortical pieces were from fetal ovaries in mid-gestation (data not shown).
Figure 4.
Effects of AMH on total number of follicles (A) and on percentages of primordial (B) and primary (C) follicles in cortical pieces from bovine fetal ovaries obtained during mid-gestation (3.5–4 months) and cultured for 10 days (mean ± SEM). White bars = freshly isolated cortical pieces (Day 0); striped bars = cortical pieces cultured without (Cont, Day 10 control culture) or with graded doses of rhAMH (100, 500 or 1000 ng/ml) or an inactive, mutant form of AMH (AMH mut; 500 or 1000 ng/ml). Means (bars) with no common superscripts are different, as determined by two-way ANOVA and Duncan's multiple range test (P < 0.05, n = 6 cultures, 2 from each of three fetuses; 23–38 sections examined per treatment per fetus; the AMH mutant was included in 2 of the three experiments).
Localization of AMH by immunohistochemistry
To determine when and where AMH is produced during the development of fetal bovine ovaries, immunohistochemistry was used to localize AMH in bovine ovaries obtained from 88- to 244-day-old-fetuses (n = 20), which were divided into four groups based on the expected transitions in follicular development. AMH immunoreactivity was completely absent in ovarian sections from the first three groups: (i) Days 80–90, oogonia and oocytes present, but no follicles (n = 4); (ii) Days 91–140, primordial follicles are forming (n = 6); and (iii) Days 141–210, primary follicles appear (n = 5). In contrast, AMH-positive staining was observed in sections from 211- to 244-day-old fetal ovaries (n = 5), which had secondary and antral follicles, as well as earlier follicular stages. AMH immunoreactivity was limited to the cytoplasm of granulosa cells of follicles at certain developmental stages. No granulosa cells of primordial follicles expressed AMH and granulosa cells of primary follicles were rarely AMH-positive (Fig. 5A). Stronger AMH expression was observed in the granulosa cells of secondary follicles and small antral follicles (Fig. 5C and E). Cumulus cells of antral follicles were also AMH positive (Fig. 5E and G). Some theca cells showed faint AMH immunoreactivity, but AMH staining was never detected in stromal cells (Fig. 5A, C, E and G). During follicular atresia, granulosa and cumulus cells continued to show AMH staining as long as they remained viable, but degenerating granulosa cells did not stain and staining was absent from the theca interna (Fig. 5G). Staining was absent in adjacent sections exposed to the blocking peptide (Fig. 5B, D, F and H).
Discussion
The ovarian follicular reserve is a determinant of female reproductive potential. In humans and more recently in cattle, the level of circulating AMH has become an important indicator of the size of the reserve (Ireland et al., 2011; Broer et al., 2014). However, the function of AMH in females has been studied primarily in rodents, where there is strong evidence that it acts as a ‘brake’ to inhibit follicular activation and growth. In this study we have shown that in cattle, an excellent model for follicular development in women (Campbell et al., 2003), AMH inhibited follicle activation and attenuated the growth of primary follicles in fetal ovarian cortical pieces in vitro. The effects of AMH on activation were only apparent when AMH was added to cortical cultures in advance of insulin, which stimulates activation in cattle. The addition of AMH to cortical pieces obtained from ovaries in mid-gestation, when follicles are still actively forming and activation has not yet begun in vivo, showed that AMH did not inhibit follicle formation. In addition, the concentrations of AMH that were effective in these experiments were low (0.7–7.1 nM) and within the range of concentrations measured in follicular fluid from small antral follicles of cattle, sheep and goats (Monniaux et al., 2013). AMH was strongly expressed in the granulosa cells of the secondary and early antral follicles present during late gestation. Together the results of these experiments provide support for AMH, derived presumably from granulosa cells of growing follicles, as a factor that regulates the rate of loss of follicles from the primordial pool and the rate of follicular growth in a non-rodent model.
Mice null mutant for AMH are fertile and initially no reproductive phenotype was noted. The important study of Durlinger et al. (1999) established that in AMH null mice more primordial follicles are activated early in life, leading to a premature depletion of ovarian follicles, relative to wild-type controls. Nilsson et al. (2007) reported that AMH inhibited activation in cultured ovaries of newborn rats, consistent with the findings for mice, and also that it attenuated the stimulatory effects of basic fibroblast growth factor (bFGF), kit ligand (KITLG) and kerotinocyte growth factor (KGF) on activation. Bovine follicles in ovarian cortical pieces activate in <2 days in vitro in response to insulin (Wandji et al., 1996; Fortune et al., 2000) and addition of AMH to the culture medium in the first two studies had no effect on follicle activation. This was puzzling because transplantation of cortical pieces beneath the CAM of chick embryos, which have high circulating levels of AMH (Hutson et al., 1981; Teng, 1987), completely inhibited activation of bovine follicles and the inhibition was reversed by removing the embryonic gonads (Cushman et al., 2002; Gigli et al., 2005), which produce AMH in both male and female embryos (Hutson et al., 1981; Teng, 1987). However, when addition of the stimulator (insulin) to bovine cortical pieces cultured with AMH was delayed until the third day of culture, all doses of AMH completely inhibited activation. This finding supports our hypothesis that failure of AMH to inhibit follicle activation in the first two studies was due to slower penetration of the cortical pieces by AMH (mol wt = 140 000 Da) vs. insulin (mol wt = 5808 Da). The inhibition of bovine follicle activation by AMH in vitro is consistent with the results for mice and rats. In contrast, AMH inhibited the initiation of follicle growth in cultured pieces of human ovaries in one study (Carlsson et al., 2006), but was reported to stimulate follicular development and increase the diameter of human primary follicles in vitro in another study (Schmidt et al., 2005).
Similar to the results for pieces from fetal ovaries at late-gestation, AMH inhibited the primordial to primary transition in ovarian cortical pieces from fetal ovaries at mid-gestation (105–120 days). Follicle formation begins around Day 90 of gestation, but primary follicles are not observed before Day 140 in vivo and primordial follicles do not activate in vitro after 2 days in culture (Yang and Fortune, 2008). However, primordial follicles in mid-gestation ovaries (90–140 days) develop the capacity to activate during longer (10-day) cultures (Yang and Fortune, 2008). AMH was apparently able to diffuse into the ovarian cortical pieces in time to inhibit activation, i.e. before follicles had acquired the capacity to activate, and thus inhibited the stimulation of activation by insulin. These results strongly suggest that AMH inhibits the primordial to primary transition in cattle. In contrast, Campbell et al. (2012) found no difference in the number of primordial follicles after sheep were actively immunized against AMH, but they concluded that their data were not sufficient to rule out an effect of AMH on initiation of follicular growth.
In addition to its inhibitory effect on follicle activation, AMH also slowed the growth rate of activated bovine follicles. After only 2 days of culture in the first study, there was a tendency for primary follicles to be smaller in the presence of the highest dose of AMH, than in control medium. When the culture period was extended to 10 days, there were dose-dependent and dramatic inhibitory effects on the diameters of both primary follicles and their oocytes. These effects are consistent with those of Durlinger et al. (2001) who reported that AMH attenuated the growth of isolated mouse preantral (secondary) follicles in the presence or absence of FSH in vitro. In contrast, McGee et al. (2001) observed an increase in the rate of growth of isolated rat secondary follicles cultured with AMH, in the absence or presence of FSH and Schmidt et al. (2005) reported that AMH increased the diameter of human primary follicles in vitro.
Nilsson et al. (2011) found that AMH decreased the number of follicles formed in newborn rat ovaries cultured for 2 days, similar to the effect of exogenous progesterone, which inhibited follicle formation (assembly) in rats and mice (Kezele and Skinner, 2003; Chen et al., 2007). In cattle, follicles form more gradually than in rodents, beginning about Day 90 of gestation and follicles form in vitro in cortical pieces from 90 to140 day-old fetuses (Yang and Fortune, 2008). Culturing cortical pieces from bovine ovaries (105–120 days of gestation) for 10 days increased the total number of follicles about 3.5-fold, but addition of AMH to the culture medium had no effect on the number of follicles. This suggests that AMH does not play a role in regulating follicle formation in cattle, in contrast to the results for rats. However, Nilsson et al. (2011) found that when they extended the culture of newborn rat ovaries to 10 days, neither AMH nor progesterone was inhibitory; the effects of both inhibitors were temporary. This finding may not be applicable to cattle since the period of follicle formation is much longer than in rodents and steroids are inhibitory to follicle formation and activation over 10 days of culture (Yang and Fortune, 2008; Fortune et al., 2013). Another question is whether AMH is present in the ovary at the time of follicle formation. Nilsson et al. (2011) immunolocalized AMH to stromal tissue around oocyte nests and primordial follicles of 3-day-old rat ovaries and this is consistent with the effect of AMH they observed. On the other hand, we detected AMH only in follicular cells of activated follicles and this observation in vivo is consistent with the lack of AMH effect on follicle formation.
Since we observed effects of AMH on follicular activation and growth in fetal bovine ovarian tissue, we immunolocalized AMH to begin to determine when and where AMH acts in bovine ovaries in vivo. Immunoreactivity for AMH was absent until around the beginning of the eighth month of pregnancy (gestation length = 279 days), suggesting that this is when AMH begins to exert its function as a “brake” to conserve the follicular reserve. Both secondary follicles and the small antral follicles that develop near the end of gestation stained strongly for AMH, with occasional weak staining in large primary follicles and no staining in primordial follicles. Similarly, immunoreactivity for AMH was first detected in fetal sheep ovaries during the third trimester (Bezard et al., 1987; 1988) and in fetal human ovaries during the ninth month of gestation (Rajpert-De Meyts et al., 1999). In the studies on ovine and human fetal ovaries, AMH was localized to the granulosa cells of secondary and small antral follicles, but not to primordial or primary follicles, in agreement with our findings for cattle. However, in adult human ovaries AMH was observed by immunohistochemistry beginning at the primordial stage (Stubbs et al., 2005). In mice and rats, follicles form and begin to activate a few days after birth and staining for AMH was observed in both primary and secondary follicles (Ueno et al., 1989; Durlinger et al., 2002a). Nilsson et al. (2011) detected AMH in the ovarian stroma around oocyte nests and primordial follicles in ovaries from 3-day-old rats. Detection of AMH in growing preantral follicles is consistent with its postulated role as a negative regulator of follicle activation and growth, but the mechanisms by which AMH inhibits activation and growth are poorly understood.
In summary, the results show that AMH inhibited the activation of primordial follicles in bovine ovarian tissue in vitro. AMH also retarded the growth of primary follicles in a dose-dependent fashion, suggesting for the first time that it may regulate ovarian follicular growth in cattle. Localization of AMH to granulosa cells, beginning at the secondary follicle stage, suggests that AMH from growing follicles restrains follicle activation and attenuates the rate of follicular growth in cattle in vivo. Thus, the results have provided strong evidence for a role for AMH in regulating the size of the follicular reserve in cattle, an excellent model for human follicular development.
Supplementary Material
Acknowledgments
The authors thank Cargill Regional Beef (Wyalusing, PA, USA) for the fetal bovine ovaries. We are most grateful to Dr Richard Cate for providing the rhAMH used in these experiments and to Drs Ned Place and Jeremy Allen for reading the article.
Supplementary data
Supplementary data are available at Molecular Human Reproduction online.
Authors’ roles
Study design and data analysis: all three authors; M.Y.Y. and R.A.C. performed experiments; all three authors contributed to writing.
Funding
National Research Initiative Competitive Grants no. 00-35203-9151, 2003-35203-13532, and 2008-35203-05989 from the U.S. Dept. of Agriculture's National Institute of Food and Agriculture to J.E.F. and by an NIH National Research Service Award (F32 HD08264) to R.A.C.
Conflict of interest
None declared.
References
- Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, Grootegoed JA. Anti-mullerian hormone and anti-Mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology 1995;136:4951–4962. [DOI] [PubMed] [Google Scholar]
- Bezard J, Vigier B, Tran D, Mauleon P, Josso N. Immunocytochemical study of anti-Mullerian hormone in sheep ovarian follicles during fetal and post-natal development. J Reprod Fertil 1987;80:509–516. [DOI] [PubMed] [Google Scholar]
- Bezard J, Vigier B, Tran D, Mauleon P, Josso N. Anti-mullerian hormone in sheep follicles. Reprod Nutr Dev 1988;28:1105–1112. [DOI] [PubMed] [Google Scholar]
- Braw-Tal R, Yossefi S. Studies in vivo and in vitro on the initiation of follicle growth in the bovine ovary. J Reprod Fertil 1997;109:165–171. [DOI] [PubMed] [Google Scholar]
- Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Mullerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update 2014;20:688–701. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Clinton M, Webb R. The role of anti-Mullerian hormone (AMH) during follicle development in a monovulatory species (sheep). Endocrinology 2012;153:4533–4543. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Souza C, Gong J, Webb R, Kendall N, Marsters P, Robinson G, Mitchell A, Telfer EE, Baird DT. Domestic ruminants as models for the elucidation of the mechanisms controlling ovarian follicle development in humans. Reproduction 2003;61(Suppl):429–443. [PubMed] [Google Scholar]
- Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod 2006;21:2223–2227. [DOI] [PubMed] [Google Scholar]
- Cate RL, Donahoe PK, MacLaughlin DT. Mullerian-inhibiting substance In: Sporn MB, Roberts AB (eds).. Peptide Growth Factors and Their Receptors, II. Berlin: Springer-Verlag, 1990,179–210. [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology 2007;148:3580–3590. [DOI] [PubMed] [Google Scholar]
- Cushman RA, Wahl CM, Fortune JE. Bovine ovarian cortical pieces grafted to chick embryonic membranes: a model for studies on the activation of primordial follicles. Hum Reprod 2002;17:48–54. [DOI] [PubMed] [Google Scholar]
- di Clemente N, Ghaffari S, Pepinsky RB, Pieau C, Josso N, Cate RL, Vigier B. A quantitative and interspecific test for biological activity of anti-Mullerian hormone: the fetal ovary aromatase assay. Development 1992;114:721–727. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 2002. a;143:1076–1084. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JT, Grootegoed JA et al. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 2001;142:4891–4899. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 1999;140:5789–5796. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction 2002. b;124:601–609. [DOI] [PubMed] [Google Scholar]
- Evans HE, Sack WO. Prenatal development of domestic and laboratory mammals: growth curves, external features and selected references. Anat Histol Embryol 1973;2:11–45. [DOI] [PubMed] [Google Scholar]
- Fortune JE. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Anim Reprod Sci 2003;78:135–163. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Cushman RA, Wahl CM, Kito S. The primordial to primary follicle transition. Mol Cell Endocrinol 2000;163:53–60. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Eppig JJ. Effects of gonadotropins on steroid secretion by infantile and juvenile mouse ovaries in vitro. Endocrinology 1979;105:760–768. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Yang MY, Allen JJ, Herrick SL. Triennial Reproduction Symposium: the ovarian follicular reserve in cattle: what regulates its formation and size. J Anim Sci 2013;91:3041–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune JE, Yang MY, Muruvi W. In vitro and in vivo regulation of follicular formation and activation in cattle. Reprod Fertil Dev 2011;23:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I, Cushman RA, Wahl CM, Fortune JE. Evidence for a role for anti-Mullerian hormone in the suppression of follicle activation in mouse ovaries and bovine ovarian cortex grafted beneath the chick chorioallantoic membrane. Mol Reprod Dev 2005;71:480–488. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol 1991;124:43–101. [DOI] [PubMed] [Google Scholar]
- Hutson J, Ikawa H, Donahoe PK. The ontogeny of Mullerian inhibiting substance in the gonads of the chicken. J Pediatr Surg 1981;16:822–827. [DOI] [PubMed] [Google Scholar]
- Ireland JJ, Smith GW, Scheetz D, Jimenez-Krassel F, Folger JK, Ireland JL, Mossa F, Lonergan P, Evans AC. Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti-Mullerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reprod Fertil Dev 2011;23:1–14. [DOI] [PubMed] [Google Scholar]
- Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology 2003;144:3329–3337. [DOI] [PubMed] [Google Scholar]
- McGee EA, Smith R, Spears N, Nachtigal MW, Ingraham H, Hsueh AJ. Mullerian inhibitory substance induces growth of rat preantral ovarian follicles. Biol Reprod 2001;64:293–298. [DOI] [PubMed] [Google Scholar]
- Monniaux D, Drouilhet L, Rico C, Estienne A, Jarrier P, Touze J-L, Regulapa J, Phocas F, Dupont J, Dalbies-Tran R et al. Regulation of anti-Müllerian hormone production in domestic animals. Reprod Fertil Dev 2013;25:1–16. [DOI] [PubMed] [Google Scholar]
- Nilsson E, Rogers N, Skinner MK. Actions of anti-Mullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction 2007;134:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson EE, Schindler R, Savenkova MI, Skinner MK. Inhibitory actions of Anti-Mullerian Hormone (AMH) on ovarian primordial follicle assembly. PLoS One 2011;6:e20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology 1999;140:4262–4271. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Jorgensen N, Graem N, Muller J, Cate RL, Skakkebaek NE. Expression of anti-Mullerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab 1999;84:3836–3844. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Kryger-Baggesen N, Byskov AG, Andersen CY. Anti-Mullerian hormone initiates growth of human primordial follicles in vitro. Mol Cell Endocrinol 2005;234:87–93. [DOI] [PubMed] [Google Scholar]
- Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update 2005;11:461–471. [DOI] [PubMed] [Google Scholar]
- Stubbs SA, Hardy K, Da Silva-Buttkus P, Stark J, Webber LJ, Flanagan AM, Themmen AP, Visser JA, Groome NP, Franks S. Anti-mullerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab 2005;90:5536–5543. [DOI] [PubMed] [Google Scholar]
- Teixeira J, Maheswaran S, Donahoe PK. Mullerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr Rev 2001;22:657–674. [DOI] [PubMed] [Google Scholar]
- Teng CS. Quantification of Mullerian inhibiting substance in developing chick gonads by a competitive enzyme-linked immunosorbent assay. Dev Biol 1987;123:255–263. [DOI] [PubMed] [Google Scholar]
- Ueno S, Kuroda T, Maclaughlin DT, Ragin RC, Manganaro TF, Donahoe PK. Mullerian inhibiting substance in the adult rat ovary during various stages of the estrous cycle. Endocrinology 1989;125:1060–1066. [DOI] [PubMed] [Google Scholar]
- van Wezel IL, Rodgers RJ. Morphological characterization of bovine primordial follicles and their environment in vivo. Biol Reprod 1996;55:1003–1011. [DOI] [PubMed] [Google Scholar]
- Wandji SA, Srsen V, Nathanielsz PW, Eppig JJ, Fortune JE. Initiation of growth of baboon primordial follicles in vitro. Hum Reprod 1997;12:1993–2001. [DOI] [PubMed] [Google Scholar]
- Wandji SA, Srsen V, Voss AK, Eppig JJ, Fortune JE. Initiation in vitro of growth of bovine primordial follicles. Biol Reprod 1996;55:942–948. [DOI] [PubMed] [Google Scholar]
- Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 2004;10:77–83. [DOI] [PubMed] [Google Scholar]
- Yang MY, Fortune JE. The capacity of primordial follicles in fetal bovine ovaries to initiate growth in vitro develops during mid-gestation and is associated with meiotic arrest of oocytes. Biol Reprod 2008;78:1153–1161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.