Abstract
Metaplasticity refers to the ability of experience to alter synaptic plasticity, or modulate the strength of neuronal connections. Sleep deprivation has been shown to have a negative impact on synaptic plasticity, but it is unknown whether sleep deprivation also influences processes of metaplasticity. Therefore, we tested whether 5 h of total sleep deprivation (SD) in mice would impair hippocampal synaptic tagging and capture (STC), a form of heterosynaptic metaplasticity in which combining strong stimulation in one synaptic input with weak stimulation at another input allows the weak input to induce long-lasting synaptic strengthening. STC in stratum radiatum of area CA1 occurred normally in control mice, but was impaired following SD. After SD, potentiation at the weakly stimulated synapses decayed back to baseline within 2 h. Thus, sleep deprivation disrupts a prominent form of metaplasticity in which two independent inputs interact to generate long-lasting LTP.
Keywords: Metaplasticity, Synaptic tagging and capture, Hippocampus, Sleep deprivation, Mouse
1. Introduction
The ability to learn and remember, and thereby adapt behavior based on past experience, is critical for survival. These processes are thought to be mediated in large part by synaptic plasticity, in which the strength of particular synaptic links between neurons is modified and then maintained for varying amounts of time in this modified state (Bliss and Collingridge, 1993, Hebb, 1949, Martin, Grimwood, & Morris, 2000). Strengthening a synaptic connection is called potentiation, and when this alteration lasts more than a few minutes, it is referred to as long-term potentiation (LTP) (Bliss and Lomo, 1973, Lomo, 2003). LTP can occur in many locations throughout the nervous system, but in rodents it has been studied most thoroughly in the hippocampus (Huang, Nguyen, Abel, & Kandel, 1996, Nicoll, 2017). LTP is typically induced by applying tetanic, or high frequency, trains of stimulation, either once or repeatedly, to a bundle of axons that form synapses onto the neurons of interest. LTP in the CA1 region of the hippocampus has the property of being input-specific, in the sense that applying tetani to a population of neurons via one set of synaptic inputs does not cause non-specific potentiation in other non-tetanized inputs (Andersen, Sundberg, Sveen, & Wigström, 1977).
However, in the last 25 years, it has become increasingly clear that modulation of one set of synapses can in fact influence the ability of other synapses to undergo plasticity. This “plasticity of plasticity” has been termed metaplasticity (Abraham and Tate, 1997). When this phenomenon occurs due to prior activity at the same synapse under-going potentiation, this is called homosynaptic metaplasticity, whereas when prior activity at one synapse can influence the ability for plasticity at other synapses, this is called heterosynaptic metaplasticity (Hulme, Jones, Raymond, Sah, & Abraham, 2014, Young and Nguyen, 2005, Sharma and Sajikumar, 2015). A prominent example of heterosynaptic metaplasticity is synaptic tagging and capture (STC), in which induction of a long-lasting form of plasticity in one set of synapses causes conversion of short-lasting plasticity at another set of synapses into a long-lasting form. This is thought to occur because induction of either short-lasting or long-lasting forms of synaptic plasticity leaves a molecular “tag” at the affected synapses, which allows those synapses to “capture” the plasticity-related proteins created in response to the induction of long-lasting plasticity (Frey and Morris, 1997, Frey and Frey, 2008). This cellular phenomenon was originally described in hippocampal slices (Frey and Morris, 1997), but has also been observed in intact animals (Shires, Da Silva, Hawthorne, Morris, & Martin, 2012), and has potential behavioral correlates, such as the ability to remember otherwise innocuous details better when in the context of a traumatic event (Moncada and Viola, 2007, Reymann and Frey, 2007, Viola, Ballarini, Martínez, & Moncada, 2014). It is clearly important to better understand how an animal’s experiences and environmental and internal conditions influence these processes of metaplasticity.
Sleep deprivation (SD) is one particular condition that affects all too many of us. SD has been shown to impair hippocampus-dependent memory tasks such as contextual fear conditioning, object recognition, and water maze navigation (Graves, Heller, Pack, & Abel, 2003, Guan, Peng, & Fang, 2004, Palchykova, Winsky-Sommerer, Meerlo, Durr, & Tobler, 2006, Peigneux, Laureys, Delbeuck, & Maquet, 2001, Smith and Rose, 1996). In other tasks, such as the Y-maze or 8-box spatial task, performance is more or less maintained in the face of sleep deprivation, but the strategy the animal uses shifts away from a hippocampus-dependent mode (Bjorness, Riley, Tysor, & Poe, 2005, Hagewoud, Havekes, Tiba, et al., 2010b). SD also has a negative effect on synaptic plasticity in the hippocampus (Campbell, Guinan, & Horowitz, 2002, Davis, Harding, & Wright, 2003, Ishikawa et al., 2006, Kim, Mahmoud, Grover, 2005, Kopp, Longordo, Nicholson, & Luthi, 2006, Marks and Wayner, 2005, McDermott et al., 2003, Ravassard et al., 2009, Tartar et al., 2006, Vecsey et al., 2009). Relevant to the current study, we have shown in mice that a 5-h period of total SD specifically impairs long-lasting forms of LTP that depend on the second messenger cyclic AMP (cAMP) and its target, protein kinase A (PKA) (Vecsey et al., 2009).
Although there have been many studies of the effects of SD on LTP, we are unaware of any published tests of SD on metaplasticity. Therefore, in the current study, we examined the effects of 5 h of total SD on synaptic tagging in mouse hippocampal slices.
2. Materials and methods
2.1. Subjects
All experiments were carried out on young adult (2–3 months old) male C57BL/6J mice from Jackson Laboratories. Mice were individually housed in a temperature-controlled environment on a 12 h/12 h light/dark schedule, with ad libitum access to food and water. All experiments were conducted according to National Institutes of Health Guidelines for Animal Care and Use and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
2.2. Sleep Deprivation
Sleep deprivation was carried out as described previously (Vecsey et al., 2009, Vecsey, Park, Khatib, & Abel, 2015). Briefly, mice were handled for 2–3 min per day for 6 days prior to sleep deprivation. Sleep deprivation was carried out for 5 h beginning at zeitgeber time (ZT) 0–1 by the gentle handling method (Havekes, Vecsey, & Abel, 2012) to achieve nearly complete sleep loss.
2.3. Electrophysiology
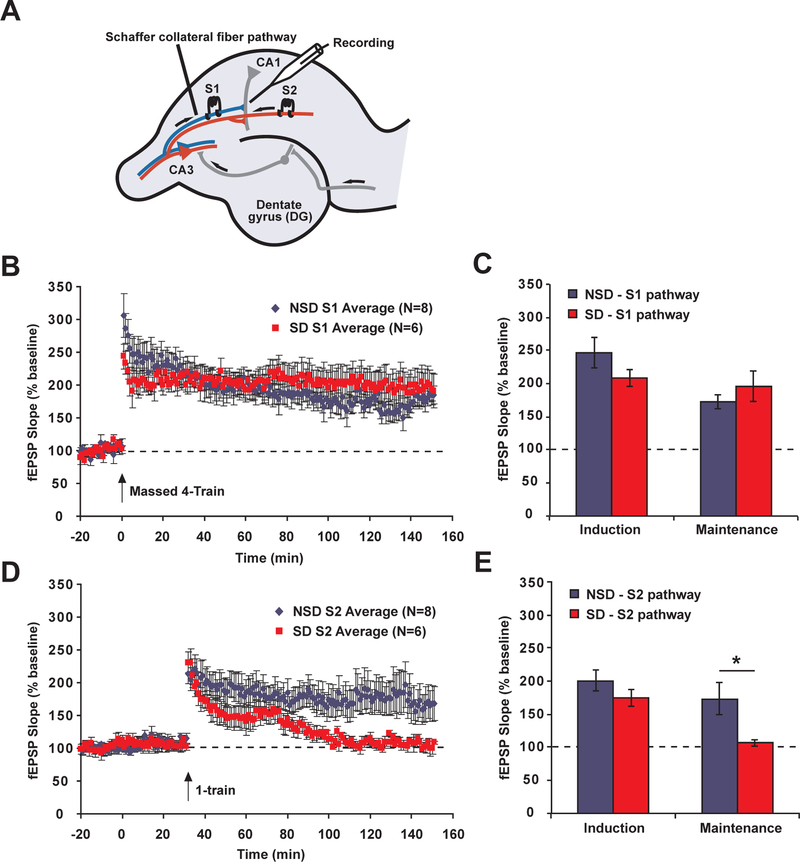
Studies of synaptic tagging were carried out by an experimenter blinded to the condition (sleep-deprived or non-sleep-deprived) of the animal. Protocols were based off of previously published work (Huang, McDonough, & Abel, 2006, Park et al., 2014). Mice were killed by cervical dislocation, and hippocampi were rapidly dissected in ice-cold, oxygenated artificial cerebrospinal fluid (aCSF – pH 7.4, containing 124 mM NaCl, 4.4 mM KCl, 1.3 mM MgSO4, 1 mM NaH2PO4, 26.2 mM NaHCO3, 2.5 mM CaCl2, and 10 mM D-glucose bubbled with 95% O2/5% CO2). Transverse slices (0.4 mm thick) were prepared using a tissue chopper and placed in an interface recording chamber maintained at 30 °C (Fine Science Tools, Foster City, CA). ACSF was constantly perfused over slices at a rate of approximately 1 ml/min. Following a recovery time of at least 1.5 h, field excitatory postsynaptic potentials (fEPSPs) were elicited from Schaffer collateral (area CA3 to area CA1) synapses using bipolar nichrome wire (0.5 mm; AM Systems, Carlsborg, WA) extracellular stimulating electrodes placed in stratum radiatum of CA1. For synaptic tagging experiments, two stimulating electrodes were positioned in such a way as to activate two separate sets of inputs (S1 and S2) onto the same postsynaptic population of neurons (Fig. 1A). Pathway independence was assessed by the absence of paired-pulse facilitation (50 ms interval) between the two pathways. Extracellular fEPSPs in the apical dendrites were recorded using a glass micropipette (1.5 mm OD; AM Systems, Carlsborg, WA) electrode filled with aCSF with a resistance of 2–4 MΩ. Data were acquired using ClampEx 9.2 and a Digidata1322 A/D converter (Axon Instruments, Union City, CA) at 20 kHz and low pass filtered at 2 kHz with a 4-pole Bessel filter. To examine basal synaptic transmission, input-output curves were generated by measuring the initial slope of the fEPSP in response to systematic increases in the strength of the stimulus. Slices that had maximum amplitude responses of less than 4 mV were rejected. Stimulus strength was set to elicit approximately 40% of the maximum initial fEPSP amplitude. Paired pulse facilitation was then examined at interpulse intervals between 25 and 300 ms. For synaptic tagging longterm potentiation (LTP) experiments, test pulses were delivered to Schaffer collaterals once every minute for 20 min. Slices that did not have stable baseline responses for 20 min were rejected. After 20 min, LTP was induced electrically by using one of two protocols. A massed 4-train protocol (four 1 s, 100 Hz tetanic stimulus trains delivered 5 s apart) was used to induce long-lasting L-LTP in input pathway S1. 30 min later, a 1-train protocol (one 1 s, 100 Hz) in input pathway S2 was used to induce short-lasting S-LTP (see Vecsey et al. (2007): Figs. 1A and 4A/B/C/D, Isiegas et al. (2008): Fig. 3A, Vecsey et al. (2009): Fig. 1D, Bridi et al. (2013): Fig. 4A/B, and Bridi, Hawk, Chatterjee, Safe, and Abel (2017): Figs. 3A and A) for examples de-monstrating the decremental nature of this form of LTP in isolation). After induction of synaptic potentiation in S2, test pulses were delivered once per minute for two hours to assess the efficacy of the synaptic tagging and capture process.
Fig. 1.
Sleep deprivation impairs synaptic tagging. C57BL/6J mice were deprived of sleep for 5 h by gentle handling (SD) or were left undisturbed in their home cages (NSD). In vitro field recordings were made in hippocampal slices taken immediately following the deprivation period. (A) Two stimulating electrodes were positioned along the Schaffer collateral fiber pathways in such a way as to activate two separate sets of stimulus inputs (S1 and S2) onto the same postsynaptic population of neurons in CA1. (B and C) In S1, at 0 min, strong stimulation of the massed 4-train type (four 1 s, 100 Hz trains of electrical stimuli with a 5-s inter-train interval) produced normal long-lasting potentiation in NSD and SD mice. (D and E) In S2, at 30 min, weak stimulation (one 1 s, 100 Hz train) in NSD mice resulted in long-lasting potentiation, and this synaptic tagging and capture process was impaired in SD mice. Arrows indicate timing of tetanic stimulation. Error bars indicate SEM. * indicates a post hoc test with p > 0.05.
2.4. Statistics
We performed a mixed-model ANOVA using Sleep Condition (SD vs. NSD) as a between-subject factor, and Time Point (average of first 20 min vs. average of last 20 min) and Input Pathway (S1 vs. S2) as within-subject factors. When significant overall effects and interactions were found, Student’s post hoc tests were used to look for specific differences. JMP11 was used for all statistical analysis.
3. Results
In our synaptic tagging protocol, we chose to use massed 4-train 100 Hz LTP as our strong stimulus because this stimulation protocol induces a long-lasting form of plasticity that engages synaptic tagging mechanisms (Park et al., 2014) and that is itself resistant to the effects of sleep deprivation (SD) (Vecsey et al., 2009). We chose 1-train 100 Hz LTP as our weak stimulus because synapses experiencing that form of stimulation can be enhanced through synaptic tagging and capture (Huang et al., 2006, Park et al., 2014), and because 1-train LTP is unaffected by SD (Vecsey et al., 2009). By choosing these two SD-independent protocols, we hoped to observe whether the synaptic tagging process, in which a weak stimulus is enhanced by pairing it with a strong stimulus, was susceptible to disruption by SD.
In non-sleep-deprived (NSD) animals, tagging occurred effectively between massed 4-train through pathway S1 and 1-train through pathway S2 – both pathways experienced sustained potentiation (Fig. 1B and C). On the other hand, in SD animals, tagging was blocked – although plasticity induced by massed 4-train was long lasting, the paired 1-train stimulation returned to baseline (Fig. 1D and E). Statistical analysis found that there was an overall interaction between sleep deprivation, input pathway, and time point (F(1, 24) = 4.01, p = 0.05). Post-hoc analysis found that, during the first 20 min following tetanization, SD did not have a significant effect on either pathway S1 or S2. However, during the last 20 min of the recording, SD significantly reduced synaptic strength in the S2 pathway, but did not affect the S1 pathway.
4. Discussion
We originally hypothesized that a short period of total sleep deprivation (SD) would affect the synaptic tagging and capture (STC) form of heterosynaptic metaplasticity in the mouse hippocampus. The results supported this hypothesis – homosynaptic massed 4-train LTP and 1-train LTP were normal in SD mice, as had previously been demonstrated (Vecsey et al., 2009), but the STC process when these two stimulus protocols were applied 30 min apart was strongly impaired. These results demonstrate that SD can negatively affect the capacity for metaplasticity between inputs. Thus, in SD animals, plasticity induced in one set of synapses in the hippocampus may have an altered ability to influence the flexibility of other synapses in the same neurons, which could have widespread behavioral significance.
A major question resulting from this finding is which molecular and cellular mechanisms underlie this effect, and future experiments will be required to explore this issue. Synaptic tagging has previously been shown to rely on cAMP and PKA (Young, Isiegas, Abel, & Nguyen, 2006), and SD clearly negatively regulates cAMP signaling (Vecsey et al., 2009, Havekes et al., 2014). Thus, this molecular pathway may be the relevant target of SD that blocks the STC processes. SD also causes widespread misregulation of protein synthesis (Vecsey et al., 2012, Tudor et al., 2016), and may cause insufficient and/or incorrect products to be produced that would be needed for either tag or capture mechanisms. Assuming that strongly stimulated synapses are more likely to capture PRPs than are weakly stimulated synapses, if SD reduces the overall pool of PRPs, strongly stimulated synapses may still be able to capture enough PRPs to undergo stable potentiation, whereas weakly stimulated will not. This could explain why synapses receiving strong stimulation had stable long-lasting LTP even in SD conditions, whereas capture of PRPs at weakly stimulated synapses was impaired.
A variant of this idea is that the products are made normally, but are not localized correctly to the appropriate synapses. This could be true of the “tag” molecules, which should normally be maintained for at least 60 min at the weakly and strongly stimulated synapses, or the plasticity-related products (PRPs), which should only be produced robustly in response to strong stimulation (Frey and Morris, 1997, Frey and Morris, 1998). There appears to be control over the subcellular localization of the PRPs. For example, in hippocampal area CA1, cross-tagging between apical and basal dendritic compartments is absent or at least has a higher threshold for synaptic tagging and capture (Alarcon, Barco, & Kandel, 2006, Sajikumar, Navakkode, & Frey, 2007). Placing and maintaining a synaptic tag are also dependent on precise localization of cAMP/PKA signaling via A Kinase Anchoring Proteins (AKAPs) (Huang et al., 2006, Young et al., 2006), which are known to interact with PKA itself as well as other components of the cAMP pathway, such as adenylate cyclases and phosphodiesterases (Colledge and Scott, 1999). Interestingly, AKAP150 protein is downregulated in the mouse hippocampus following 6–12 h of total SD (Hagewoud, Havekes, Novati, et al., 2010a). If SD were capable of disrupting these processes of localization, PRPs might be distributed throughout the post-synaptic neuron and thus diluted to a point where they would not be adequately captured, and/or PRPs might simply not be captured by the poorly localized tags.
However, it is important to note that we found that synapses stimulated with strong massed 4-train are still capable of capturing PRPs and maintaining stable potentiation normally. Thus, PRPs do in fact appear to be made correctly and distributed normally, at least to the strongly stimulated synapses. This argues against some of the explanations we have laid out in the previous paragraphs. We therefore believe that the most likely explanation for SD’s ability to impair STC is that SD disrupts the process of placing a long-lived tag at weakly potentiated synapses. It is currently unknown why tags set by 1 tetanus are disrupted by SD but tags set by 4 tetani spaced 5 s apart are unaffected by SD. As touched on above, a possible explanation is that the 5-h period of SD used in this study has been shown to impair cAMP/PKA signaling via an increase in phosphodiesterase PDE4A5 activity (Vecsey et al., 2009). Tag setting by 1 tetanus in a very similar protocol to our own was shown to be dependent on PKA, whereas tag setting during massed 4-train LTP was shown to be PKA-independent (Young et al., 2006), confirming other findings (Kim, Huang, Abel, & Blackwell, 2010, Woo, Duffy, Abel, & Nguyen, 2003). Thus, brief SD and disruption of cAMP/PKA have very similar patterns of effects on these forms of synaptic plasticity and metaplasticity, suggesting that cAMP/PKA blockade may mediate the effects of SD on synaptic tagging.
In the current study, we applied the strong massed 4-train stimulation first, followed 30 min later by weak 1-train stimulation to the second input. In future studies, this order could be reversed, which would allow us to alter the delay between weak and strong stimuli in order to determine if the tag at weak synapses decays faster in SD animals than in controls (Frey and Morris, 1998). It will also be of interest to test if SD alters additional forms of metaplasticity. For example, in this study we examined STC between two forms of synaptic potentiation. However, synapses given prolonged low-frequency stimulation undergo long-term depression (LTD), and strong LTD and weak LTD protocols can engage in STC as well, and “cross-tagging” can even occur between LTD and LTP (Sajikumar and Frey, 2004). For example, pairing a strong LTP protocol through one input pathway with a weak LTD protocol through a second input pathway results in enhanced longevity of the synaptic weakening at the synapses receiving the LTD stimulus. The mechanisms for these forms of cross-tagging appear to be different, depending on whether LTP is capturing LTD or vice versa (Sajikumar et al., 2007). For example, calmodulin-dependent kinase II (CaMKII) is required to set the tag at synapses undergoing weak LTP, such that those synapses cannot capture PRPs produced by either strong LTP or strong LTD through independent inputs. However, extracellular signal-related kinase (ERK) is not required for those actions, but IS required to set the tag at synapses undergoing weak LTD (Sajikumar et al., 2007). Thus, future study could examine whether SD selectively impairs STC processes associated with LTP or whether metaplasticity involving LTD is also affected, and the results could provide insight as to the relevant molecular mechanisms targeted by SD.
The synaptic homeostasis hypothesis (Tononi and Cirelli, 2014) posits that during wakefulness there is a net increase in synaptic strength due to daily learning. Sleep then allows for restitution of the overall set point for synaptic weight by non-specifically downscaling the strength of all synapses. We highlight two recent reports that are supportive of this concept. The first found that structural down-regulation of synapses occurs in the motor cortex during sleep as compared with wake (de Vivo et al., 2017). The second broadly examined mouse forebrain tissue and found widespread sleep-associated reductions in the synaptic levels of GluA1 and GluA2 AMPA-type receptors, which are strong determinants of glutamatergic synaptic strength (Diering et al., 2017). However, in our studies focused on the hippocampus, SD does not appear to cause widespread synaptic strengthening, as baseline synaptic strength is unchanged between animals receiving 5–6 h of total SD and undisturbed, sleeping controls (Vecsey et al., 2009, Florian, Vecsey, Halassa, Haydon, & Abel, 2011). In fact, 5 h of SD may have the opposite effect, predisposing hippocampal synapses to weaken. Havekes et al. (2016) recently reported that SD decreases CA1 dendritic length and spine density, via a cell signaling pathway mediated by increased activity of cofilin, a F-actin severing protein. We found here that SD prevented time-limited metaplasticity between synaptic inputs onto the same neurons. These findings highlight the fact that SD can have much more targeted effects on synaptic plasticity than proposed by the synaptic homeostasis hypothesis.
Acknowledgments
We would like to thank Robbert Havekes and Mahesh Shivarama Shetty for their helpful input during the preparation of this manuscript. This research was supported by NIH training grant HL07953 (to C.G.V., A. I. Pack PI), the National Institutes of Health (AG017628; to T.A., A. I. Pack PI), SCOR grant HL060287 (to T.A., A. I. Pack PI), and HFSP grant RGSP/2005 (to T.A.).
Footnotes
Conflict of interest
The authors declare no competing financial interests.
References
- Abraham WC, & Tate WP (1997). Metaplasticity: A new vista across the field of synaptic plasticity. Progress in Neurobiology, 52(4), 303–323. [DOI] [PubMed] [Google Scholar]
- Alarcon JM, Barco A, & Kandel ER (2006). Capture of the late phase of long-term potentiation within and across the apical and basilar dendritic compartments of CA1 pyramidal neurons: Synaptic tagging is compartment restricted. Journal of Neuroscience, 26(1), 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Sundberg SH, Sveen O, & Wigström H (1977). Specific long-lasting potentiation of synaptic transmission in hippocampal slices. Nature, 266(5604), 736–737. [DOI] [PubMed] [Google Scholar]
- Bjorness TE, Riley BT, Tysor MK, & Poe GR (2005). REM restriction persistently alters strategy used to solve a spatial task. Learning and Memory, 12(3), 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, & Collingridge GL (1993). A synaptic model of memory: Long-term potentiation in the hippocampus. Nature, 361(6407), 31–39. [DOI] [PubMed] [Google Scholar]
- Bliss TV, & Lomo T (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. Journal of Physiology, 232(2), 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridi MS, & Abel T (2013). The NR4A orphan nuclear receptors mediate transcription-dependent hippocampal synaptic plasticity. Neurobiology of Learning and Memory, 105, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridi MS, Hawk JD, Chatterjee S, Safe S, & Abel T (2017). Pharmacological activators of the NR4A nuclear receptors enhance LTP in a CREB/CBP-dependent manner. Neuropsychopharmacology, 42(6), 1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Guinan MJ, & Horowitz JM (2002). Sleep deprivation impairs long-term potentiation in rat hippocampal slices. Journal of Neurophysiology, 88(2), 1073–1076. [DOI] [PubMed] [Google Scholar]
- Colledge M, & Scott JD (1999). AKAPs: From structure to function. Trends in Cell Biology, 9(6), 216–221. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Harding JW, & Wright JW (2003). REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Research, 973(2), 293–297. [DOI] [PubMed] [Google Scholar]
- de Vivo L, Bellesi M, Marshall W, Bushong EA, Ellisman MH, Tononi G, & Cirelli C (2017). Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science, 355(6324), 507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering GH, Nirujogi RS, Roth RH, Worley PF, Pandey A, & Huganir RL (2017). Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science, 355(6324), 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian C, Vecsey CG, Halassa MM, Haydon PG, & Abel T (2011). Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. Journal of Neuroscience, 31(19), 6956–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, & Frey JU (2008). Synaptic tagging’ and ‘cross-tagging’ and related associative reinforcement processes of functional plasticity as the cellular basis for memory formation. Progress in Brain Research, 169, 117–143. [DOI] [PubMed] [Google Scholar]
- Frey U, & Morris RG (1997). Synaptic tagging and long-term potentiation. Nature, 385(6616), 533–536. [DOI] [PubMed] [Google Scholar]
- Frey U, & Morris RG (1998). Weak before strong: Dissociating synaptic tagging and plasticity-factor accounts of late-LTP. Neuropharmacology, 37(4–5), 545–552. [DOI] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, & Abel T (2003). Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learning and Memory, 10(3), 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Peng X, & Fang J (2004). Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus’. Brain Research, 1018(1), 38–47. [DOI] [PubMed] [Google Scholar]
- Hagewoud R, Havekes R, Novati A, Keijser JN, Van der Zee EA, & Meerlo P (2010a). Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. Journal of Sleep Research, 19(2), 280–288. [DOI] [PubMed] [Google Scholar]
- Hagewoud R, Havekes R, Tiba PA, Novati A, Hogenelst K, Weinreder P, … Meerlo P (2010b). Coping with sleep deprivation: Shifts in regional brain activity and learning strategy. Sleep, 33(11), 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes R, Bruinenberg VM, Tudor JC, Ferri SL, Baumann A, Meerlo P, & Abel T (2014). Transiently increasing cAMP levels selectively in hippocampal excitatory neurons during sleep deprivation prevents memory deficits caused by sleep loss. Journal of Neuroscience, 34(47), 15715–15721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes R, Park AJ, Tudor JC, Luczak VG, Hansen RT, Ferri SL, … Abel T (2016). Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. Elife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes R, Vecsey CG, & Abel T (2012). The impact of sleep deprivation on neuronal and glial signaling pathways important for memory and synaptic plasticity. Cellular Signalling, 24(6), 1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO (1949). The organization of behavior: A neuropsychological theory New York: Wiley. [Google Scholar]
- Huang T, McDonough CB, & Abel T (2006). Compartmentalized PKA signaling events are required for synaptic tagging and capture during hippocampal late-phase long-term potentiation. European Journal of Cell Biology, 85(7), 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Nguyen PV, Abel T, & Kandel ER (1996). Long-lasting forms of synaptic potentiation in the mammalian hippocampus. Learning and Memory, 3(2–3), 74–85. [DOI] [PubMed] [Google Scholar]
- Hulme SR, Jones OD, Raymond CR, Sah P, & Abraham WC (2014). Mechanisms of heterosynaptic metaplasticity. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 369(1633), 20130148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Kanayama Y, Matsumura H, Tsuchimochi H, Ishida Y, & Nakamura S (2006). Selective rapid eye movement sleep deprivation impairs the maintenance of long-term potentiation in the rat hippocampus. European Journal of Neuroscience, 24(1), 243–248. [DOI] [PubMed] [Google Scholar]
- Isiegas C, McDonough C, Huang T, Havekes R, Fabian S, Wu LJ, … Abel T (2008). A novel conditional genetic system reveals that increasing neuronal cAMP enhances memory and retrieval. Journal of Neuroscience, 28(24), 6220–6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Mahmoud GS, & Grover LM (2005). REM sleep deprivation inhibits LTP in vivo in area CA1 of rat hippocampus. Neuroscience Letters, 388(3), 163–167. [DOI] [PubMed] [Google Scholar]
- Kim M, Huang T, Abel T, & Blackwell KT (2010). Temporal sensitivity of protein kinase a activation in late-phase long term potentiation. PLoS Computational Biology, 6(2), e1000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C, Longordo F, Nicholson JR, & Luthi A (2006). Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. Journal of Neuroscience, 26(48), 12456–12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T (2003). The discovery of long-term potentiation. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358(1432), 617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks CA, & Wayner MJ (2005). Effects of sleep disruption on rat dentate granule cell LTP in vivo. Brain Research Bulletin, 66(2), 114–119. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, & Morris RG (2000). Synaptic plasticity and memory: An evaluation of the hypothesis. Annual Review of Neuroscience, 23, 649–711. [DOI] [PubMed] [Google Scholar]
- McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, & Magee JC (2003). Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. Journal of Neuroscience, 23(29), 9687–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada D, & Viola H (2007). Induction of long-term memory by exposure to novelty requires protein synthesis: Evidence for a behavioral tagging. Journal of Neuroscience, 27(28), 7476–7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA (2017). A brief history of long-term potentiation. Neuron, 93(2), 281–290. [DOI] [PubMed] [Google Scholar]
- Palchykova S, Winsky-Sommerer R, Meerlo P, Durr R, & Tobler I (2006). Sleep deprivation impairs object recognition in mice. Neurobiology of Learning and Memory, 85(3), 263–271. [DOI] [PubMed] [Google Scholar]
- Park AJ, Havekes R, Choi JH, Luczak V, Nie T, Huang T, & Abel T (2014). A presynaptic role for PKA in synaptic tagging and memory. Neurobiology of Learning and Memory, 114, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Delbeuck X, & Maquet P (2001). Sleeping brain, learning brain. The role of sleep for memory systems. Neuroreport, 12(18), A111–A124. [DOI] [PubMed] [Google Scholar]
- Ravassard P, Pachoud B, Comte JC, Mejia-Perez C, Scote-Blachon C, Gay N, … Salin PA (2009). Paradoxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hippocampus. Sleep, 32(2), 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymann KG, & Frey JU (2007). The late maintenance of hippocampal LTP: Requirements, phases, ‘synaptic tagging’, ‘late-associativity’ and implications. Neuropharmacology, 52(1), 24–40. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, & Frey JU (2004). Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiology of Learning and Memory, 82(1), 12–25. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Navakkode S, & Frey JU (2007). Identification of compartment- and process-specific molecules required for “synaptic tagging” during long-term potentiation and long-term depression in hippocampal CA1. Journal of Neuroscience, 27(19), 5068–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, & Sajikumar S (2015). Metaplasticity of synaptic tagging and capture: Memory beyond the circle. In Sajikumar S (Ed.). Synaptic tagging and capture (pp. 197–213). New York, NY: Springer. [Google Scholar]
- Shires KL, Da Silva BM, Hawthorne JP, Morris RG, & Martin SJ (2012). Synaptic tagging and capture in the living rat. Nature Communications, 3, 1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, & Rose GM (1996). Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiology & Behavior, 59(1), 93–97. [DOI] [PubMed] [Google Scholar]
- Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, … Strecker RE (2006). Hippocampal synaptic plasticity and spatial learning are im-paired in a rat model of sleep fragmentation. European Journal of Neuroscience, 23(10), 2739–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, & Cirelli C (2014). Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron, 81(1), 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor JC, Davis EJ, Peixoto L, Wimmer ME, van Tilborg E, Park AJ, Poplawski SG, Chung CW, Havekes R, Huang J, Gatti E, Pierre P, & Abel T (2016). Sleep deprivation impairs memory by attenuating mTORC1-dependent pro-tein synthesis. Science Signaling, 9(425) ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, … Abel T (2009). Sleep deprivation impairs cAMP signalling in the hippocampus. Nature, 461(7267), 1122–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, … Wood MA (2007). Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. Journal of Neuroscience, 27(23), 6128–6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Park AJ, Khatib N, & Abel T (2015). Effects of sleep deprivation and aging on long-term and remote memory in mice. Learning and Memory, 22(4), 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Peixoto L, Choi JH, Wimmer M, Jaganath D, Hernandez PJ, … Abel T (2012). Genomic analysis of sleep deprivation reveals translational regula-tion in the hippocampus. Physiological Genomics, 44(20), 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola H, Ballarini F, Martínez MC, & Moncada D (2014). The tagging and capture hypothesis from synapse to memory. Progress in Molecular Biology and Translational Science, 122, 391–423. [DOI] [PubMed] [Google Scholar]
- Woo NH, Duffy SN, Abel T, & Nguyen PV (2003). Temporal spacing of synaptic stimulation critically modulates the dependence of LTP on cyclic AMP-dependent protein kinase. Hippocampus, 13(2), 293–300. [DOI] [PubMed] [Google Scholar]
- Young JZ, Isiegas C, Abel T, & Nguyen PV (2006). Metaplasticity of the late-phase of long-term potentiation: A critical role for protein kinase A in synaptic tagging. European Journal of Neuroscience, 23(7), 1784–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JZ, & Nguyen PV (2005). Homosynaptic and heterosynaptic inhibition of synaptic tagging and capture of long-term potentiation by previous synaptic activity. Journal of Neuroscience, 25(31), 7221–7231. [DOI] [PMC free article] [PubMed] [Google Scholar]