Abstract
Both natural and synthetic brominated furanones are known to inhibit biofilm formation by bacteria, but their toxicity to mammalian cells is often not reported. Here, we designed and synthesized a new class of brominated furanones (BBFs) that contained a bicyclic structure having one bromide group with well-defined regiochemistry. This class of molecules exhibited reduction in the toxicity to mammalian cells (human neuroblastoma SK-N-SH) and did not inhibit bacteria (Pseudomonas aeruginosa and Escherichia coli) growth, but retained the inhibitory activity towards biofilm formation of bacteria. In addition, all the BBFs inhibited the production of virulence factor elastase B in P. aeruginosa. To explore the effect of BBFs on quorum sensing, we used a reporter gene assay and found that 6-BBF and 7-BBF exhibited antagonistic activities for LasR protein in the lasI quorum sensing circuit, while 5-BBF showed agonistic activity for the rhlI quorum sensing circuit. This study suggests that structural variation of brominated furanones can be designed for targeted functions to control biofilm formation.
Keywords: Furanones, Biofilm inhibition, Virulence factor, Cell signaling, Toxicity
1. Introduction
Biofilms on different surfaces cause enormous detrimental effects in medical and industrial settings1 and are the source of many diseases, including endocarditis, otitis media, chronic prostatitis, periodontal disease, chronic urinary tract infections, and osteomyelitis,2,3 and In particular, biofilms formed by Pseudomonas aeruginosa are often related to serious infections in immunocompromised patients,4 particularly lung infection in cystic fibrosis patients.3,5 The formation of biofilm is regulated by multiple genes, which results in highly complex film structures on the surface of microbes.6 Controlling the formation of biofilm has been challenging because inhibition of biofilm formation and dispersion of already formed biofilm are difficult.7 Also, the bacteria reside in the biofilm often appear to be more tolerant to antibiotic treatments than planktonic bacteria.8 One rational approach to control biofilm formation is to interfere with the chemical communication that results in a quorum sensing (QS) between bacteria, which is one of the key events leading to the biofilm formation.9 Several synthetic autoinducer analogs have been reported to induce or inhibit quorum sensing and biofilm formation of P. aeruginosa.10,11 Other small molecules that are not structurally similar to natural autoinducers have also been proven to modulate quorum sensing and inhibit and disperse proteobacterial biofilms.12,13 The agonistic/antagonistic activities of these molecules could be tuned by structural modifications. Chemical library screening has also been utilized to discover biofilm formation inhibitors.14
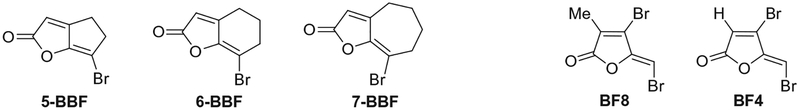
In this work, we aim to develop new structures of inhibitors of biofilm formation that are both nonmicrobicidal to bacteria and nontoxic to mammalian cells. Based on our previous study on what constitute the important structural elements of brominated furanones,15 we propose that a bicyclic version of brominated furanones, which retain the conjugated exocyclic vinyl bromide in the furanone moiety, that could potentially reduce their toxicity while retaining the biofilm inhibitory activities. Here, we designed a new class of bicyclic brominated furanones (BBFs), 5-BBF, 6-BBF, and 7-BBF, with [3,3,0], [4,3,0], and [5,3,0] fused ring structures, respectively (Fig. 1). Compared to the known brominated furanones, such as BF815 and BF4 (some literature use the name ‘C30’),16 the fused bicyclic systems bear only one bromo-substitution, and introduce bulkier but semi-rigid cyclic hydrocarbon skeletons into the molecules that can potentially increase the binding and selectivity to the receptor proteins.
Figure 1.
Structures of BBFs and known brominated furanones.
In this study, the toxicity of these molecules is evaluated for bacteria P. aeruginosa and Escherichia coli and human neuroblastoma SK-N-SH cells. We found that BBFs exhibited reduced toxicity to bacteria and mammalian cells compared to BF8 and BF4. It was also found that BBFs inhibited the production of virulence factor elastase B by P. aeruginosa. To explore a mechanistic understanding, we examined their interference (agonist or antagonist) with two quorum sensing pathways (las and rhl) in P. aeruginosa by using reporter gene assays.
2. Results and discussion
2.1. Synthesis of BBFs
Bicyclic brominated furanones, 5-BBF, 6-BBF and 7-BBF, were synthesized via a similar route starting with keto acids (Scheme 1). The synthesis of 7-BBF is described as an example here. Under basic condition, coupling of cycloheptanone and dimethyl carbonate provided methyl 2-oxo-1-cycloheptanecarboxylate 2, which underwent acetoacetic ester synthesis to give 2-oxocycloheptaneacetic acid 3. This intermediate was then subjected to bromination and dehydration to build the fused ring framework, followed by elimination to yield the conjugated final product 7-BBF without isolating the intermediates (Scheme 1). Known brominated furanones BF815 and BF417 were also synthesized to compare their toxicities and biofilm inhibition activities with BBFs.
Scheme 1.
Synthesis of BBFs. Reagents and conditions: (a) Br2, CH2Cl2, 0 °C–rt; (b) P2O5, DCM, 0 °C to reflux; (c) Et3N, DCM, 0 °C to reflux; (d) LiOH, THF/H2O (9:4), 23 h, 1 M HCl (aq), rt; (e) NaH, benzene, rt to 85 °C; (f) ethyl bromoacetate, K2CO3, acetone, rt to reflux, 16 h; (g) 6 M HCl (aq), AcOH, rt to reflux, 2 d.
2.2. BBFs inhibit the biofilm formation by P. aeruginosa and E. coli
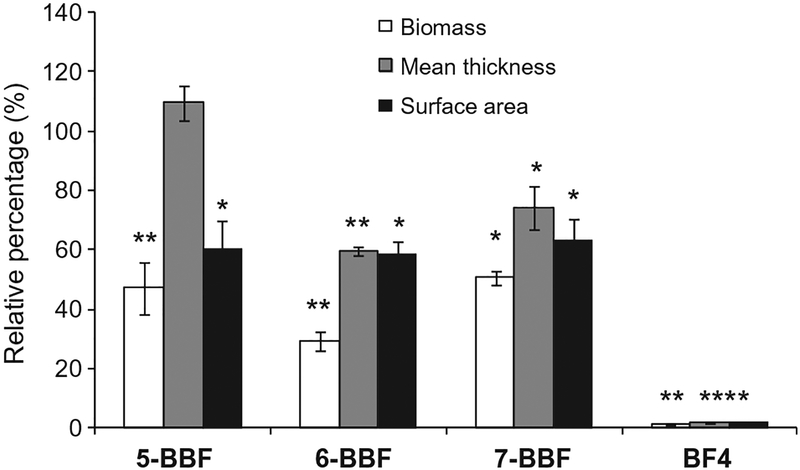
We used a wild type strain PA01-GFP to study biofilm formation on steel coupons with and without BBFs. This strain constitutively expresses green fluorescent proteins (GFP)18 and enables easy and direct visualization of biofilm by confocal laser scanning microscopy. In the initial screening, all three BBFs resulted in much less green fluorescence signals in the PA01 biofilms than the BF-free control at 400 μM, with 6-BBF provided more inhibition than that by 5-BBF and 7-BBF (Fig. 2). Biofilm grown in the presence of 400 μM BF4 showed the least fluorescence signals (see Fig. S1). To characterize the biofilm inhibition quantitatively, we accessed the biomass, mean thickness and surface area of biofilms formed with and without agents using COMSTAT software (Fig. 3).19 The results were normalized by the controls without agents. Compound 6-BBF exhibited the strongest inhibition as it reduced the biofilm formation by 71% (P < 0.01), followed by 5-BBF with 53% inhibition (P < 0.01) and 7-BBF with 50% inhibition (P < 0.05). The mean thickness of biofilm was reduced by 6-BBF to 60% (P < 0.01) and by 7-BBF to 74% (P < 0.05). We note that the mean thickness of biofilm treated by 5-BBF did not seem to reduce. One possibility is that the biofilm becomes more ‘fluffy’ in the presence of 400 μM 5-BBF. The surface areas of biofilm in the presence of all three BBFs were about 60% of that formed in the absence of BFs (P < 0.05). Similar results were obtained when E. coli RP437 (pRSH103) biofilms were grown in the absence and presence of 200 μM BBFs and BF8 (see Fig. S2).
Figure 2.
The effect of brominated furanones on biofilm formation by P. aeruginosa. Representative confocal laser scan microscopy (CLSM) images of biofilm formed by PA01-GFP (expresses green fluorescence on plasmid pSMC2) (A) in the absence of agents, and in the presence of (B) 5-BBF, (C) 6-BBF, (D) 7-BBF. The control is supplemented with the same amount (0.8%) of DMSO as present in the BF-treated conditions. Scale bar = 50 μm.
Figure 3.
Quantification of biofilm formation by PA01-GFP in the absence and presence of 400 μM brominated furanones. Biomass, mean thickness, and surface area were quantified from fluorescence image using COMSTAT software. Z-Stack images from four different locations were used. Values are normalized by that of the BF-free control and represent the means ± standard deviation from 4 replicates. Significant differences in biofilm formation with BF-free control are indicated by asterisks: *P < 0.05; **P < 0.01.
These results suggest that 6-BBF is a stronger biofilm inhibitor than 5-BBF and 7-BBF for both P. aeruginosa and E. coli. We followed up with a dose-dependence study on inhibitory activity of BBFs on P. aeruginosa biofilm using a colorimetric assay in 96-well plates employing a dye molecule, crystal violet (CV) (see Fig. S3).20 The half maximal inhibitory concentration (IC50) values obtained from the dose–response curves were more than 400 μM for 5-BBF, and 145.8 and 139.7 μM for 6-BBF and 7-BBF, respectively. These results suggest that 6-BBF and 7-BBF are stronger biofilm inhibitors than 5-BBF for P. aeruginosa. We note that the percentage of relative biofilm formation in the presence of 400 μM BBFs obtained from confocal laser scan microscopy did not match exactly that obtained from the CV-dye staining assay. However, the general trend that 6-BBF and 7-BBF is more active than 5-BBF against Pseudomonas biofilm formation is consistent for both assays.
2.3. BBFs do not influence the growth of P. aeruginosa or E. coli
The toxicity of brominated furanones to the growth of bacteria is unpredictable based on structures. For example, BF8 does not inhibit the growth of E. coli, whereas similar structures do.15 Here, we compared the toxicity of BBFs and BF4 to the growth of P. aeruginosa and E. coli to study if the biofilm inhibition was due to bactericidal effect. At 400 μM, none of the three BBFs inhibited the growth of P. aeruginosa PA01 (Fig. 4), and bacteria grown in the presence of BBFs reached the same optical density (OD600) as those in the absence of BBFs after 24 h. Under the same conditions, BF4 completely inhibited bacterial growth (P < 0.01), which suggested that the significant biofilm inhibition observed for BF4 at this concentration was due to bactericidal effect. The effect of the BBFs on the growth of E. coli strain RP437 was also studied, with BF8 (a known biofilm inhibitor to this strain at 200 μM) as a positive control. At 200 μM, none of the BBFs exhibited obvious impact on the growth of E. coli RP437 (see Fig. S4). The growth curve of bacteria in the presence of BF8 deviated from that of the control for up to 8 h and then the OD600 values were essentially the same to the control after then.
Figure 4.
Growth curves of P. aeruginosa PA01 in the absence (control) and presence of 400 μM brominated furanones. Values represent the means ± standard deviation from six replicates. Significant differences in the optical density with BF-free control are indicated by asterisks: **P < 0.01.
2.4. BBFs interfere with the quorum sensing in P. aeruginosa
To investigate whether BBFs inhibit biofilm formation via interference with QS, we studied the effects of BBFs on the QS systems in P. aeruginosa. There are two identified N-acyl homoserine lactone (AHL)-mediated quorum sensing circuits in P. aeruginosa. One is the las circuit that includes an AHL synthase gene lasI responsible for the synthesis of N-(3-oxo-dodecanoyl)-L-homoserine lactone (3-oxo-C12-HSL or PAI1);21 the PAI1 binds to LasR to activate a range of quorum sensing genes. The other is the rhl circuit that includes rhlI gene responsible for synthesis of N-butanoyl-L-homoserine lactone (C4-HSL, or PAI2);22 the PAI2 binds to RhlR to further activate other genes.
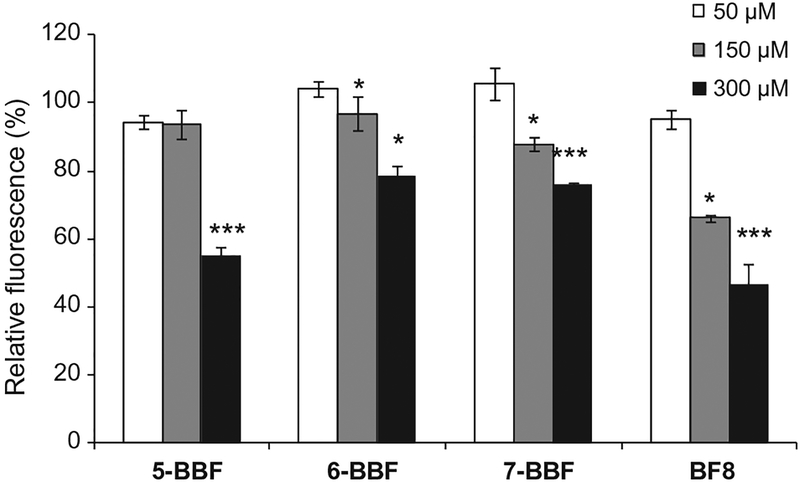
Reporter strain PA01 (plasI-LVAgfp)12 was used to evaluate the las quorum sensing system. The GFP production is quantified by correcting the measured fluorescence signal for cell density. Both 6-BBF and 7-BBF decreased fluorescent signals as the concentration was increased from 50 to 150 to 300 μM (Fig. 5), indicating an inhibition of GFP production. At 300 μM, the presence of 6-BBF or 7-BBF caused a reduction in GFP production to 78 ± 1.1% (P < 0.05) and 69 ± 4.0% (P < 0.001), respectively, as compared to that of the BF-free controls. These results suggest that 6-BBF and 7-BBF are weak antagonists of LasR protein. The known biofilm inhibitor BF815 resulted in less GFP production, 46 ± 4.8%, compared to that of the BF-free control (P < 0.001). Compound 5-BBF inhibited the GFP production slightly at 50 and 150 μM, and reduced the GFP expression by approximately half at 300 μM. These results suggest that the BBFs are weak to moderate antagonists of the las QS circuit, possibly by binding to LasR receptor protein. The BBFs were also examined for antagonism of QS using a double-knockout reporter strain P. aeruginosa PAO-JP223 (ΔlasIΔrhlI) harboring the plasmid plasI-LVAgfp.24 When bacteria are growing in the presence of exogenously introduced autoinducer 3-oxo-C12-HSL or analogues, LasR receptor binds to the AI and activates transcription of the lasI promoter which controls green fluorescence protein (GFP) expression. Similar inhibition of GFP production was obtained when PAO-JP2 (plasI-LVAgfp) was used (see Fig. S5). We note that the inhibition of GFP production within the concentration range tested is not due to bactericidal effect.
Figure 5.
GFP expression by PA01 (plasI-LVAgfp) in the absence or presence of 5-BBF, 6-BBF, 7-BBF, or BF8 at 50, 150, and 300 μM. Fluorescence signals were corrected for cell density by dividing by OD600 of cell culture and the results were normalized to that of the BF-free control. Values represent the means ± standard deviation of the mean. Data shown is a representative of at least three separate experiments. Significant differences in the GFP expression with the control are indicated by asterisks: *P < 0.05;***, P < 0.001.
We also tested the effects of BBFs on the rhl quorum sensing system using PA01 (prhlI-LVAgfp). Interestingly, 5-BBF promoted, instead of suppressed, the GFP expression significantly as its concentration was increased (See Fig. S5). At 150 and 300 μM, 5-BBF increased the GFP expression by 82.6 ± 4.4% and 165 ± 17.6%, respectively (P < 0.01). On the contrary, 6-BBF, 7-BBF and BF8 had no effect on the GFP expression at all concentrations tested. These results suggest that 5-BBF is a strong agonist of RhlR, while 6-BBF and 7-BBF are not. Similar results were also obtained when reporter strain PAO-JP2 (prhlI-LVAgfp) was used (see Fig. S6).
The expression of the virulence factor metalloprotease elastase B (LasB) in P. aeruginosa is also controlled by the QS.25 This highly toxic virulence factor facilitates the invasion and destruction of host tissues,26 induces inflammatory responses from the host27 and also signals the biofilm formation.28 Thus, molecules that inhibit virulence factor production without bactericidal effect are of potential use without invoking the bacterial resistance. Because BBFs appeared to be potent antagonists of LasR, we also tested their ability to inhibit elastase B production. At 300 μM, all BBFs significantly reduced the production of elastase B by ~80% (see Fig. S7). In a dose-dependent study, the expression of elastase B decreased as the concentration of 6-BBF increased. These results are consistent with BBFs being antagonists of LasR in P. aeruginosa.
These results suggest that the ring size of BBFs have an impact on their ability to modulate QS in P. aeruginosa. For the las system, BBFs with larger rings (6-BBF and 7-BBF) seemed to have slightly stronger antagonistic activity than that with smaller rings (5-BBF). For the rhl system, the agonistic activity seemed to be more sensitive to the ring size, as 5-BBF was able to enhance the activity of RhlR protein while 6-BBF or 7-BBF had no effect. We believe that the interference of only one quorum sensing signaling pathway will likely elicit an effect on biofilm formation, and response in an in vivo environment, but does not ensure a therapeutic development.29
2.5. BBFs are less cytotoxic than other BFs to human cells
To avoid invoking bacterial resistance,30–33 nonmicrobicidal agents that do not kill bacteria or influence bacterial growth are being sought over. However, for any potential use of the agents, the toxicity of the molecules towards mammalian or human cells should be minimized. Although many natural and non-natural brominated furanones inhibit biofilm formation by a wide range of bacteria, few studies have addressed their toxicity to mammalian or human cells.11,34 Here, we compare the toxicity of BBFs to a non-microbicidal brominated furanone, BF8, against human neuroblastoma SK-N-SH. This human cell line were allowed to grow in 96-well tissue culture-treated microtiter plates for 24 h, after which they were treated with at 400 μM (the highest concentration tested for biofilm inhibition) of each agent (5-BBF, 6-BBF, 7-BBF and BF8) for 1 h. The agent-containing medium was replaced with fresh medium and the cells were then allowed to recover for 0, 24, and 48 h (recovery time), at which time the number of live cells was determined by the CCK 8 assay.35 Survival (%) was calculated based on BF-free control.
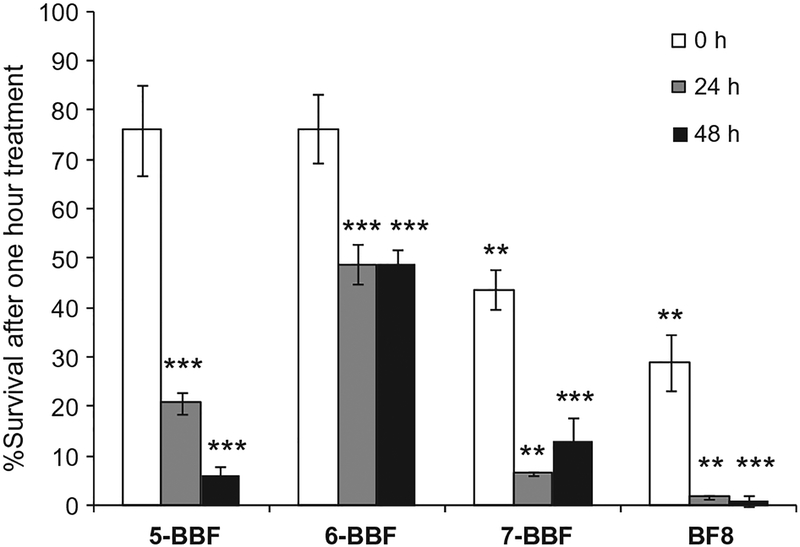
After 1 h treatment of agents and 0 h recovery time, BF8 exhibited the strongest cytotoxic effect toward human neuroblastoma SK-N-SH cells; 29.0 ± 14.1% of cells were alive compare to the BF-free control (P < 0.001). Bicyclic brominated furanones 5-BBF and 6-BBF showed mild toxicity (~76% cell survival for both), and 7-BBF resulted in ~44% of cell survival (Fig. 6). After 24 and 48 h of recovery time, survival (%) for cells treated with 5-BBF dropped to ~21% and ~6%, respectively. For cells treated with BF8, almost no live cell remained (<2%) after 24 and 48 h. In the presence of 7-BBF, cell survival dropped to ~7% after 24 h of recovery time, but appeared to increase to 13% after 48 h. In contrast, 6-BBF exhibited the least cytotoxic effect to this human cell line. After 24 and 48 h of recovery time, about 50% survival was observed for cells treated with 6-BBF. We note this cytotoxicity assay was also performed at lower concentration (100 μM). At this lower concentration, all three BBFs showed little to no toxicity, with 6-BBF being the least toxic one. Brominated furanone, BF8, was still toxic at this low concentration (see Fig. S8). The cytotoxicity exhibited by the BBFs to the human neuroblastoma limited their use in further internal clinical study.
Figure 6.
Survival (%) of human neuroblastoma SK-N-SH cells at 0, 24, and 48 h after 1 h treatment of 400 μM 5-BBF, 6-BBF, 7-BBF and BF8, respectively. Values represent the means ± standard deviation from six replicates. Significant differences between BF-treated conditions in the survival (%) and BF-free control at each time point are indicated by asterisks: *P < 0.05; **P < 0.01; ***P < 0.001.
3. Conclusion
The bicyclic brominated furanones are a new series of synthetic brominated furanones that interfere with quorum sensing and QS-controlled activities in Gram-negative bacteria. They inhibit biofilm formation by E. coli and P. aeruginosa and inhibit elastase B production by P. aeruginosa at nonmicrobicidal concentrations. The BBFs are also less cytotoxic to human neuroblastoma cells compared to other known brominated furanones. This work indicates some structure–bioactivity relationship for brominated furanones. The relative high IC50 of biofilm inhibition and moderate cytotoxicity to human cells of these BBFs need further improvement for pharmaceutical area. However, they may be useful in industrial and other ex vivo settings. The exploration of structures that give high potency for biofilm inhibition while maintaining a low toxicity is an ongoing subject of our research.
Supplementary Material
Acknowledgments
This work was partially supported by NSF-CAREER (#0845686, PI Y.Y.L), NSF-EFRI (#1137186, coPI, Y.Y.L), and NIH HL096007 (G.W.). We thank Bonnie B. Toms (Upstate Medical University, SUNY) for the generous donation of human neuroblastoma cell line SK-N-SH. We thank Dr. Helen E. Blackwell (Univ. of Wisconsin-Madison) for the generous donation of strains PAO-JP2 (plasI-LVAgfp) and PAO-JP2 (prhlI-LVAgfp), Dr. Dacheng Ren (SU) for the generous donation of strains E. coli RP437, E. coli RP437(pRSH103), and Dr. Hiroaki Suga (The University of Tokyo) for the generous donation of plasmids plasI-LVAgfp and prhlI-LVAgfp. We also thank Dr. James L. Hougland (SU) for the use of fluorescence plate reader and the review of the manuscript.
Footnotes
Supplementary data
Supplementary data (the synthesis procedures, NMR characterization, protocols for biological assays, and supplemental graph of data for biological activities) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmc.2014.01.004.
References and notes
- 1.Costerton JW; Lewandowski Z; Caldwell DE; Korber DR; Lappin-Scott HM Annu. Rev. Microbiol 1995, 49, 711. [DOI] [PubMed] [Google Scholar]
- 2.Costerton JW; Stewart PS; Greenberg EP Science 1999, 284, 1318. [DOI] [PubMed] [Google Scholar]
- 3.Parsek MR; Singh PK Annu. Rev. Microbiol 2003, 57, 677. [DOI] [PubMed] [Google Scholar]
- 4.Van Delden C; Iglewski BH Emerg. Infect. Dis 1998, 4, 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campana S; Taccetti G; Ravenni N; Masi I; Audino S; Sisi B; Repetto T; Doring G; de Martino MJ Cyst. Fibrosis 2004, 3, 159. [DOI] [PubMed] [Google Scholar]
- 6.Stoodley P; Sauer K; Davies DG; Costerton JW Annu. Rev. Microbiol 2002, 56, 187. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan JB J. Dent. Res 2010, 89, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies D Nat. Rev. Drug Disc 2003, 2, 114. [DOI] [PubMed] [Google Scholar]
- 9.Camilli A; Bassler BL Science 2006, 311, 1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geske GD; O’Neill JC; Blackwell HE Chem. Soc. Rev 2008, 37, 1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowery CA; Abe T; Park J; Eubanks LM; Sawada D; Kaufmann GF; Janda KD J. Am. Chem. Soc 2009, 131, 15584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frei R; Breitbach AS; Blackwell HE Angew. Chem., Int. Ed. 2012, 51, 5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards JJ; Melander C Anti-Infect. Agents Med. Chem 2009, 8, 295. [Google Scholar]
- 14.Junker LM; Clardy J Antimicrob. Agents Chemother 2007, 51, 3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Y; Hou S; Simon KA; Ren D; Luk Y-Y Bioorg. Med. Chem. Lett 2008, 18, 1006. [DOI] [PubMed] [Google Scholar]
- 16.Hentzer M; Wu H; Andersen JB; Riedel K; Rasmussen TB; Bagge N; Kumar N; Schembri MA; Song Z; Kristoffersen P; Manefield M; Costerton JW; Molin S; Eberl L; Steinberg P; Kjelleberg S; Hoiby N; Givskov M EMBO J. 2003, 22, 3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorg A; Siegel K; Brueckner R Synlett 2004, 321. [Google Scholar]
- 18.Bloemberg GV; O’Toole GA; Lugtenberg BJJ; Kolter R Appl. Environ. Microbiol 1997, 63, 4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heydorn A; Nielsen AT; Hentzer M; Sternberg C; Givskov M; Ersboll BK; Molin S Microbiology 2000, 146, 2395. [DOI] [PubMed] [Google Scholar]
- 20.Huigens RW III; Richards JJ; Parise G; Ballard TE; Zeng W; Deora R; Melander CJ Am. Chem. Soc 2007, 129, 6966. [DOI] [PubMed] [Google Scholar]
- 21.Pearson JP; Gray KM; Passador L; Tucker KD; Eberhard A; Iglewski BH; Greenberg EP Proc. Natl. Acad. Sci. U.S.A 1994, 91, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson JP; Passador L; Iglewski BH; Greenberg EP Proc. Natl. Acad. Sci. U.S.A 1995, 92, 1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pesci E; Pearson JP; Seed PC; Iglewski BH J. Bacteriol 1997, 179, 3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Kievit TR; Gillis R; Marx S; Brown C; Iglewski BH Appl. Environ. Microbiol 2001, 67, 1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gambello MJ; Iglewski BH J. Bacteriol 1991, 173, 3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamura Y; Kijima M; Suzuki S; Takahashi T; Nakamura MJ Vet. Med. Sci 1992, 54, 1049. [DOI] [PubMed] [Google Scholar]
- 27.Kon Y; Tsukada H; Hasegawa T; Igarashi K; Wada K; Suzuki E; Arakawa M; Gejyo F FEMS Immunol. Med. Microbiol 1999, 25, 313. [DOI] [PubMed] [Google Scholar]
- 28.Kamath S; Kapatral V; Chakrabarty AM Mol. Microbiol 1998, 30, 933. [DOI] [PubMed] [Google Scholar]
- 29.Jensen Peter O; Bjarnsholt T; Phipps R; Rasmussen Thomas B; Calum H; Christoffersen L; Moser C; Williams P; Pressler T; Givskov M; Hoiby N Microbiology (Reading, England) 2007, 153, 1329. [DOI] [PubMed] [Google Scholar]
- 30.Smith AW Hugo and Russell’s Pharmaceutical Microbiology, 8th ed.; Wiley-Blackwell, 2011, 217. [Google Scholar]
- 31.Neu HC Science 1992, 257, 1064. [DOI] [PubMed] [Google Scholar]
- 32.Wu H; Song Z; Hentzer M; Andersen JB; Molin S; Givskov M; Hoiby NJ Antimicrob. Chemother 2004, 53, 1054. [DOI] [PubMed] [Google Scholar]
- 33.Rogers SA; Huigens RW III; Cavanagh J; Melander C Antimicrob. Agents Chemother 2010, 54, 2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuehl R; Al-Bataineh S; Gordon O; Luginbuehl R; Otto M; Textor M; Landmann R Antimicrob. Agents Chemother 2009, 53, 4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishiyama M; Tominaga H; Shiga M; Sasamoto K; Ohkura Y; Ueno K Biol. Pharm. Bull 1996, 19, 1518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.