Abstract
Background
It is unclear whether the established association between cutaneous melanoma (CM) and lymphoid neoplasms (LNs) differs across LN subtypes. This study quantifies risk for developing CM after specific LNs and, conversely, for developing specific LNs after CM, as well as assessing clinical impact.
Methods
We identified a cohort of Caucasian adults (age 20–83 years) initially diagnosed with CM or LN, as reported to 17 US population-based cancer registries, 2000–2014. Standardized incidence ratios (SIRs) quantified second cancer risk. We assessed impact of second cancer development on risk of all-cause mortality using Cox regression.
Results
Among 151 949 one-or-more-year survivors of first primary LN, second primary CM risk was statistically significantly elevated after chronic lymphocytic leukemia/small lymphocytic lymphoma (SIR = 1.96, 95% confidence interval [CI] = 1.74 to 2.21), follicular lymphoma (SIR = 1.32, 95% CI = 1.09 to 1.58), and plasma cell neoplasms (SIR = 1.33, 95% CI = 1.07 to 1.63). Risks for these same subtypes were statistically significantly elevated among 148 336 survivors of first primary CM (SIR = 1.44, 95% CI = 1.25 to 1.66; SIR = 1.47, 95% CI = 1.21 to 1.77; SIR = 1.25, 95% CI = 1.06 to 1.47; respectively). Risk for CM was statistically significantly elevated after diffuse large B-cell lymphoma (SIR = 1.22, 95% CI = 1.02 to 1.45) and Hodgkin lymphoma (SIR = 1.75, 95% CI = 1.33 to 2.26), but the reciprocal relationship was not observed. There were no statistically significant associations between marginal zone lymphoma and CM. Among survivors of most LN subtypes, CM statistically significantly increased risk of death (hazard ratio [HR] range = 1.52, 95% CI = 1.25 to 1.85, to 2.46, 95% CI = 1.45 to 4.16). Among survivors of CM, LN statistically significantly increased risk of death (HR range = 1.75, 95% CI = 1.15 to 2.65, to 6.28, 95% CI = 5.00 to 7.88), with the highest risks observed for the most aggressive LN subtypes.
Conclusions
Heterogeneous associations between CM and specific LN subtypes provide novel insights into the etiology of these malignancies, with the mutual association between CM and certain LN suggesting shared etiology. Development of second primary CM or LN substantially reduces overall survival.
An association between development of cutaneous melanoma (CM) and lymphoid neoplasms (LNs) was first recognized over half a century ago (1–4). Since that time, studies have consistently shown that LN survivors have increased risk of developing CM, and CM survivors have increased risk of developing LNs (5–8). Additionally, some studies have suggested that malignancies developing in this setting may adversely impact survival, particularly for CMs occurring after LN (9–12), despite these populations often undergoing careful surveillance following their first cancer.
Major advances in our understanding of LN have led to the recognition that specific disease subtypes are heterogeneous in terms of etiology and clinical course, including treatment approach, characteristic immune alterations, and prognosis. However, it is unclear whether the association between LNs and CM varies among the heterogeneous LN subtypes. A previous study using Surveillance, Epidemiology, and End Results (SEER) Program cancer registry data in the United States (US) during 1992–2006 reported elevated risk of CM after chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and follicular lymphoma (FL) but not diffuse large B-cell lymphoma (DLBCL) (13). However, risks for other LN subtypes were not evaluated in that study, and to our knowledge previous studies have not evaluated the risk of most LN subtypes after CM.
We conducted a comprehensive investigation of the association between development of CM and LN subtypes using SEER data from 2000 to 2014, coinciding with the introduction of the World Health Organization (WHO) classification of LN (14) and inclusion of additional SEER registries. The aims of our study were 1) to quantify risk of developing second primary CM after subtype-specific first primary LNs and assess clinical impact and, conversely, 2) to quantify risk of developing subtype-specific second primary LNs after first primary CM and assess clinical impact.
Methods
Study Population
Eligible patients included adults who were diagnosed with first primary LNs or CM during 2000–2013 at age 20 to 83 years and survived one or more years following diagnosis, as reported to 17 SEER registries (Table 1) (15). Collectively, these registries represent approximately one-quarter of the US population. We excluded non-Caucasian individuals and those younger than age 20 years at first primary diagnosis due to very low rates of CM in these populations. We also excluded individuals with first primary LNs who were known HIV-positive, which may affect CM risk (16).
Table 1.
Select characteristics, by initial neoplasm, of ≥1-year Caucasian adult survivors of first primary lymphoid neoplasm subtypes or cutaneous melanoma, 17 SEER Program registries, 2000–2014*
| Characteristic | CLL/SLL | DLBCL | FL | MZL | PCN | HL | CM |
|---|---|---|---|---|---|---|---|
| (n = 36 784), % | (n = 33 443), % | (n = 26 212), % | (n = 11 406), % | (n = 26 548), % | (n = 17 556), % | (n = 148 336), % | |
| Age at first primary malignancy, y | |||||||
| 20–39 | 1.1 | 10.3 | 5.9 | 5.0 | 1.6 | 54.0 | 15.0 |
| 40–49 | 6.8 | 12.1 | 14.7 | 10.5 | 8.3 | 17.3 | 18.3 |
| 50–59 | 20.6 | 20.6 | 26.3 | 22.2 | 21.9 | 12.4 | 23.7 |
| 60–69 | 31.1 | 25.6 | 27.4 | 28.2 | 30.7 | 9.1 | 21.8 |
| 70–79 | 30.7 | 24.1 | 20.3 | 26.3 | 28.9 | 5.8 | 16.3 |
| 80–83 | 9.7 | 7.2 | 5.3 | 7.8 | 8.5 | 1.4 | 4.8 |
| Mean age at diagnosis, y | 66.2 | 60.8 | 60.6 | 63.2 | 65.2 | 41.6 | 56.5 |
| Sex | |||||||
| Male | 60.7 | 54.1 | 49.9 | 45.6 | 56.6 | 53.7 | 55.6 |
| Female | 39.3 | 45.9 | 50.1 | 54.4 | 43.4 | 46.3 | 44.4 |
| Year of first primary malignancy diagnosis | |||||||
| 2000–2004 | 34.5 | 33.7 | 34.9 | 30.8 | 31.5 | 36.1 | 33.0 |
| 2005–2009 | 37.0 | 36.0 | 37.7 | 38.0 | 35.6 | 37.0 | 37.4 |
| 2010–2013 | 28.5 | 30.2 | 27.4 | 31.3 | 32.9 | 26.9 | 29.5 |
| Mean follow-up time, y | 4.6 | 4.6 | 5.2 | 4.8 | 3.3 | 6.0 | 5.4 |
| Geographic region† | |||||||
| Northern | 38.3 | 34.1 | 34.9 | 37.0 | 34.9 | 35.7 | 32.2 |
| Central | 24.3 | 24.8 | 24.8 | 21.9 | 24.1 | 23.1 | 25.2 |
| Southern | 37.4 | 41.1 | 40.3 | 41.1 | 41.0 | 41.2 | 42.5 |
| Initial course of treatment | |||||||
| No/unknown treatment recorded | 72.0 | 6.5 | 16.5 | 26.2 | 23.8 | 5.7 | 2.9 |
| Any treatment | 28.0 | 93.5 | 83.5 | 73.8 | 76.2 | 94.3 | 97.1 |
| No/unknown CT or RT‡ | 6.1 | 5.2 | 17.2 | 20.7 | 4.4 | 5.0 | 94.4 |
| CT and RT | 0.6 | 24.8 | 7.6 | 4.6 | 16.1 | 34.0 | 0.4 |
| CT without RT | 20.6 | 61.5 | 49.4 | 28.9 | 51.5 | 51.5 | 1.3 |
| RT without CT | 0.7 | 2.0 | 9.3 | 19.6 | 4.2 | 3.8 | 1.1 |
| Stage at first primary diagnosis | |||||||
| Lymphoid neoplasm | |||||||
| I | – | 30.2 | 27.2 | 41.5 | – | 18.1 | – |
| II | – | 21.7 | 15.4 | 9.7 | – | 43.2 | – |
| III | – | 16.2 | 23.1 | 5.7 | – | 19.0 | – |
| IV | – | 26.7 | 27.3 | 28.4 | – | 14.7 | – |
| Unspecified | – | 5.2 | 6.9 | 14.6 | – | 5.0 | – |
| CLL/SLL§ | |||||||
| Early stage | 72.0 | – | – | – | – | – | – |
| Advanced stage | 28.0 | – | – | – | – | – | – |
| CM‖ | |||||||
| Localized and ≤1.0 mm thick | – | – | – | – | – | – | 62.5 |
| Localized and >1.0 mm thick | – | – | – | – | – | – | 18.3 |
| Regional/distant | – | – | – | – | – | – | 11.2 |
| Missing stage or missing thickness for localized disease | – | – | – | – | – | – | 8.0 |
Seventeen Surveillance, Epidemiology, and End Results Program registry areas (Atlanta, Georgia; Connecticut; Detroit, Michigan; Hawaii; Iowa; New Mexico; San Francisco-Oakland, Los Angeles, and San Jose-Monterey, California; Seattle-Puget Sound, Washington; Utah; Kentucky; Louisiana; New Jersey; and areas of Rural Georgia, Greater Georgia, and Greater California). Morphology codes: CLL/SLL: 9670, 9823; DLBCL: 9678–9680, 9684 [B-cell immunophenotype only], 9688, 9712, 9737–9738; FL: 9690–9691, 9695, 9698; MZL: 9689, 9699, 9760, 9764; PCN: 9732–9733; HL: 9650–9655, 9659, 9661–9667; melanoma: 8720–8790; and topography codes for skin (melanoma): C440–449. – = not applicable; CLL/SLL = chronic lymphocytic leukemia/small lymphocytic leukemia; CM = cutaneous melanoma; CT = chemotherapy; DLBCL = diffuse large B-cell lymphoma; FL = follicular lymphoma; HL = Hodgkin lymphoma; MZL = marginal zone lymphoma; NOS = not otherwise specified; PCN = plasma cell neoplasm; RT = radiation; SEER = Surveillance, Epidemiology, and End Results.
SEER region was categorized according to the Melanoma Risk Assessment Tool, available at https://www.cancer.gov/melanomarisktool/. Northern region includes the SEER registry areas of Connecticut; Detroit, Michigan; Iowa; Seattle-Puget Sound, Washington; and New Jersey. Central region includes SEER registry areas of San Francisco-Oakland, California; Utah; and Kentucky; as well as the following California counties: Alpine, Amador, Butte, Calaveras, Colusa, Del Norte, El Dorado, Glenn, Humboldt, Lake, Lassen, Madera, Mariposa, Mendocino, Merced, Modoc, Mono, Napa, Nevada, Placer, Plumas, Sacramento, San Joaquin, Santa Clara, Santa Cruz, Shasta, Sierra, Siskiyou, Solano, Sonoma, Stanislaus, Sutter, Tehama, Trinity, Tuolumne, Yolo, Yuba. Southern region includes the SEER registry areas of Hawaii; New Mexico; Georgia; Los Angeles, California; and Louisiana; and the following California counties: Fresno, Imperial, Inyo, Kern, Kings, Monterey, Orange, Riverside, San Benito, San Bernardino, San Diego, San Luis Obispo, Santa Barbara, Tulare, Ventura.
Includes “surgery alone.”
No initial treatment for CLL/SLL was used as a proxy for early stage, and any initial treatment for CLL/SLL was used as a proxy for advanced disease.
Per SEER summary stage, localized disease includes papillary dermis invaded (Clark's level II), papillary-reticular dermal interface invaded (Clark’s level III), reticular dermis invaded (Clark’s level IV), skin/dermis, NOS, and localized, NOS. Regional/distant includes those with unknown stage who have “no mass found” for thickness. Thickness was missing for 6990 CM patients, and stage was missing for 4819 CM patients.
Lymphoid Neoplasm and Cutaneous Melanoma Data
SEER captures patient demographics, vital status (including cause of death), and all incident malignancy diagnoses that occur among residents in the registry areas (≥95% case ascertainment). For each diagnosis, SEER collects morphology and topography (defined by the International Classification of Disease for Oncology, 3rd edition [ICD-O-3]) (17), stage of disease, sequence (eg, first primary, second primary), initial course of treatment, and other disease-specific information (eg, CM thickness).
We identified LNs using ICD-O-3 morphology codes, grouped according to the WHO classification (14,18), and included the six most common LN subtypes: DLBCL, FL, CLL/SLL, marginal zone lymphoma (MZL), plasma cell neoplasm (PCN), and Hodgkin lymphoma (HL) (Table 1). For all subtypes, initial course of treatment was defined as any treatment (yes vs no/unknown), chemotherapy (any vs no/unknown), and radiotherapy (any vs no/unknown). LN stage was categorized according to Ann Arbor staging for DLBCL, FL, MZL, and HL (19). Because CLL stage is not captured in SEER, CLL patients with no initial treatment were approximated as early stage, and those who received any initial treatment as advanced stage. SLL was categorized similarly because the WHO classification has grouped it with CLL since 2010. PCN stage was not analyzed because it is not captured in SEER and cannot be approximated by initial treatment.
We identified CM using ICD-O-3 morphology and topography codes. Extent of disease was categorized based on a combination of stage and tumor thickness: localized and 1.0 mm thick or less, localized and greater than 1.0 mm thick, regional/distant, and missing (unknown stage or unknown thickness with localized disease). More than 91% of first primary CM patients received surgery alone as initial treatment (no known chemotherapy or radiation); therefore, CM treatment was not analyzed. Because sun exposure is a major risk factor for CM but this information is not collected in SEER, we approximated sun exposure based on geographic region (Table 1) and evaluated anatomic location of CM as a proxy for sun exposure (head and neck, trunk, limb, and other).
Statistical Analysis
We conducted a series of analyses to address the aims of this study. First, we evaluated risk for developing incident second primary CM after first primary subtype-specific LN. Risk was quantified using standardized incidence ratios (SIRs) and exact, Poisson-based 95% confidence intervals (CIs), comparing the number of observed CM cases after LN with that expected in the general population (SEER*Stat version 8.3.2). The expected number of cases was calculated by multiplying general population CM incidence rates for Caucasians (stratified by age [five-year groups], sex, and calendar year [2000–2004, 2005–2009, 2010–2014]) by the person-time at risk of the LN patient cohort. Patients were followed from one year after their first primary LN diagnosis to the earliest of: second primary malignancy diagnosis, last known follow-up, death, attained age 85 years, or end of study (December 31, 2014). The first year following first primary LN diagnosis was excluded to avoid biases from increased medical surveillance, whereas person-time at age 85 years or older was excluded to avoid potential underascertainment due to decreased medical surveillance in this oldest age group. For each LN subtype investigated, SIRs were calculated overall and stratified by key patient characteristics (Table 1). We then quantified risk of developing subtype-specific LNs after CM compared with the general population by estimating SIRs overall and by key patient characteristics.
Using the observed and expected numbers of cases computed in SEER*Stat, we then constructed multivariable Poisson regression models to evaluate whether SIRs varied across strata of patient characteristics for each LN subtype separately (Epicure version 2.0) (20). In these Poisson models, the observed number of cases was the outcome and the log of the corresponding expected number of cases was included as an offset to indirectly adjust for attained age and calendar year (21). Models were further adjusted for sex, age at first primary diagnosis, and time since first primary cancer diagnosis through stratification. Two-sided P values for heterogeneity and trend tests were derived from likelihood ratio tests, comparing models’ fit with and without the factor of interest. Ordinal variables were treated as continuous variables in models testing for trends. In separate analyses including all patients, we tested for overall SIR heterogeneity across LN subtypes.
Finally, we assessed the clinical impact of developing second primary CM or LN by comparing risk of death from any cause after diagnosis of a second primary cancer of interest (modeled as a time-dependent covariate) with risk of death in the absence of that second primary cancer. Separate models were fit for each LN subtype. Hazard ratios (HRs) and 95% CIs were calculated from Cox regression models using age as the time scale and adjusting for sex and year of first primary diagnosis (SAS 9.4, Cary, NC). Patients were followed from one year after their first primary diagnosis until the earliest of: death, age 85 years, loss to follow-up, or study end date (December 31, 2014).
All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant.
Results
Study Population
Analyses of first primary LN included 151 949 adults diagnosed with one of the six most common first primary LN subtypes: CLL/SLL (n = 36 784), DLBCL (n = 33 443), FL (n = 26 212), MZL (n = 11 406), PCN (n = 26 548), and HL (n = 17 556) (Table 1). Mean age at diagnosis ranged from 60 to 66 years for LN subtypes other than HL (41.6 years), and mean follow-up time ranged from 3.3 to 6.0 years. A male predominance was observed for most subtypes other than FL (49.9%) and MZL (45.6%). Reported first course of treatment varied substantially by LN subtype; the majority of CLL/SLL patients had no known treatment, whereas patients with other subtypes received a range of chemotherapy (33.5% to 86.5%) and/or radiotherapy (1.3% to 37.8%).
For first primary CM survivors, analyses included 148 336 adults with a mean follow-up of 5.4 years (Table 1). Mean age at diagnosis was 56.5 years with a male predominance (55.6%). CM stage was most commonly localized and 1.0 mm thick or less (62.5%).
SIR Analyses
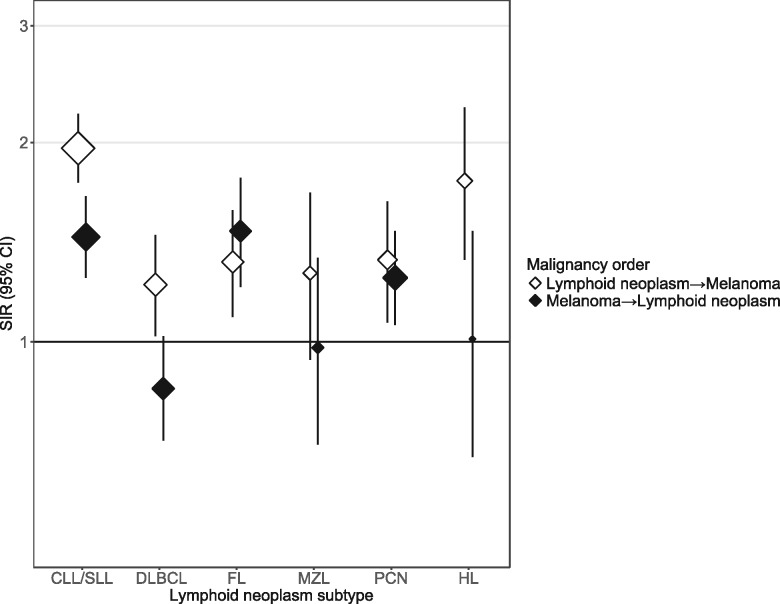
Compared with the general population, the risk of developing CM was statistically significantly elevated among survivors of CLL/SLL (SIR = 1.96, 95% CI = 1.74 to 2.21), DLBCL (SIR = 1.22, 95% CI = 1.02 to 1.45), FL (SIR = 1.32, 95% CI = 1.09 to 1.58), PCN (SIR = 1.33, 95% CI = 1.07 to 1.63), and HL (SIR = 1.75, 95% CI = 1.33 to 2.26) and elevated, although not statistically significantly, after MZL (SIR = 1.27, 95% CI = 0.94 to 1.68) (Figure 1, Table 2). The magnitude of risk differed statistically significantly across subtypes of LN (Pheterogeneity < .001). For the reciprocal relationship (Figure 1, Table 3), risks of CLL/SLL (SIR = 1.44, 95% CI = 1.25 to 1.66), FL (SIR = 1.47, 95% CI = 1.21 to 1.77), and PCN (SIR = 1.25, 95% CI = 1.06 to 1.47) were statistically significantly higher among CM survivors compared with the general population, whereas no increase was observed for DLBCL (SIR = 0.85, 95% CI = 0.71 to 1.02), MZL (SIR = 0.98, 95% CI = 0.70 to 1.34), or HL (SIR = 1.01, 95% CI = 0.67 to 1.47, across LN subtypes Pheterogeneity < .001).
Figure 1.
Standardized incidence ratios for second primary cutaneous melanoma after first primary subtype-specific lymphoid neoplasm, and second primary subtype-specific lymphoid neoplasm after first primary cutaneous melanoma among one-or-more-year Caucasian adult survivors in 17 Surveillance, Epidemiology, and End Results Program registries, 2000–2014. Standardized incidence ratios and exact, Poisson-based 95% confidence intervals (represented by error bars) compared the number of observed cases with that expected in the general population. See Tables 2 and 3 for the population sizes and observed number of cases. CLL/SLL = chronic lymphocytic leukemia/small lymphocytic leukemia; DLBCL = diffuse large B-cell lymphoma; FL = follicular lymphoma; MZL = marginal zone lymphoma; PCN = plasma cell neoplasm; HL = Hodgkin lymphoma; SIR = standardized incidence ratio.
Table 2.
Standardized incidence ratios for second primary cutaneous melanoma by age, sex, latency, thickness, and stage among ≥1-year Caucasian adult survivors of first primary lymphoid neoplasms, 17 SEER Program registries, 2000–2014*
| Characteristic | Lymphoid neoplasms |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLL/SLL |
DLBCL |
FL |
MZL |
PCN |
HL |
|||||||
| (n = 36 784) |
(n = 33 443) |
(n = 26 212) |
(n = 11 406) |
(n = 26 548) |
(n = 17 556) |
|||||||
| O | SIR (95% CI) | O | SIR (95% CI) | O | SIR (95% CI) | O | SIR (95% CI) | O | SIR (95% CI) | O | SIR (95% CI) | |
| Overall | 287 | 1.96 (1.74 to 2.21) | 128 | 1.22 (1.02 to 1.45) | 119 | 1.32 (1.09 to 1.58) | 49 | 1.27 (0.94 to 1.68) | 91 | 1.33 (1.07 to 1.63) | 59 | 1.75 (1.33 to 2.26) |
| Age at first primary lymphoid neoplasm, y | ||||||||||||
| 20–39 | 15† | 2.47 (1.38 to 4.07) | 9 | 2.66 (1.22 to 5.05) | 13† | 1.25 (0.67 to 2.15) | 6† | 1.87 (0.69 to 4.08) | 9† | 2.48 (1.14 to 4.72) | 19 | 2.15 (1.29 to 3.35) |
| 40–49 | 9 | 1.09 (0.50 to 2.06) | 8 | 1.26 (0.54 to 2.48) | ||||||||
| 50–59 | 46 | 1.83 (1.34 to 2.44) | 24 | 1.18 (0.75 to 1.75) | 40 | 1.82 (1.30 to 2.47) | 9 | 1.16 (0.53 to 2.20) | 23 | 1.77 (1.12 to 2.65) | 18 | 2.65 (1.57 to 4.19) |
| 60–69 | 120 | 2.27 (1.88 to 2.72) | 48 | 1.41 (1.04 to 1.87) | 31 | 0.99 (0.68 to 1.41) | 19 | 1.47 (0.89 to 2.30) | 34 | 1.41 (0.98 to 1.98) | 14§ | 1.20 (0.65 to 2.01) |
| 70–79 | 94 | 1.71 (1.38 to 2.10) | 31 | 0.90 (0.61 to 1.28) | 35‡ | 1.30 (0.91 to 1.81) | 15‡ | 1.02 (0.57 to 1.68) | 25‡ | 0.90 (0.58 to 1.33) | ||
| 80–83 | 12 | 1.68 (0.87 to 2.94) | 7 | 1.64 (0.66 to 3.38) | ||||||||
| Ptrend | .07 | .15 | .29 | .17 | .02 | .33 | ||||||
| Sex | ||||||||||||
| Male | 225 | 2.01 (1.76 to 2.29) | 86 | 1.17 (0.94 to 1.45) | 80 | 1.34 (1.07 to 1.67) | 29 | 1.19 (0.80 to 1.71) | 54 | 1.08 (0.81 to 1.41) | 33 | 1.57 (1.08 to 2.21) |
| Female | 62 | 1.81 (1.39 to 2.32) | 42 | 1.33 (0.96 to 1.80) | 39 | 1.27 (0.90 to 1.73) | 20 | 1.41 (0.86 to 2.18) | 37 | 2.03 (1.43 to 2.80) | 26 | 2.05 (1.34 to 3.01) |
| Pheterogeneity | .45 | .62 | .69 | .63 | .008 | .43 | ||||||
| Latency, y | ||||||||||||
| <5 | 193 | 2.18 (1.88 to 2.51) | 75 | 1.23 (0.97 to 1.54) | 69 | 1.37 (1.07 to 1.74) | 37 | 1.62 (1.14 to 2.23) | 56 | 1.14 (0.86 to 1.48) | 33 | 1.93 (1.33 to 2.72) |
| ≥5 | 94 | 1.64 (1.32 to 2.00) | 53 | 1.22 (0.91 to 1.59) | 50 | 1.25 (0.93 to 1.64) | 12 | 0.76 (0.39 to 1.33) | 35 | 1.82 (1.27 to 2.53) | 26 | 1.57 (1.02 to 2.29) |
| Pheterogeneity | .007 | .84 | .52 | .01 | .11 | .31 | ||||||
| Geographic region | ||||||||||||
| Northern | 89 | 1.57 (1.26 to 1.94) | 37 | 1.03 (0.72 to 1.42) | 30 | 0.94 (0.63 to 1.34) | 16 | 1.11 (0.63 to 1.80) | 30 | 1.21 (0.82 to 1.73) | 18 | 1.43 (0.85 to 2.26) |
| Central | 71 | 2.05 (1.60 to 2.59) | 35 | 1.32 (0.92 to 1.84) | 27 | 1.24 (0.82 to 1.81) | 9 | 1.06 (0.48 to 2.01) | 24 | 1.48 (0.95 to 2.20) | 17 | 2.25 (1.31 to 3.60) |
| Southern | 127 | 2.31 (1.93 to 2.75) | 56 | 1.32 (1.00 to 1.72) | 62 | 1.69 (1.30 to 2.17) | 24 | 1.53 (0.98 to 2.28) | 37 | 1.35 (0.95 to 1.86) | 24 | 1.77 (1.14 to 2.64) |
| Ptrend | 0.006 | .26 | .006 | .29 | .67 | .53 | ||||||
| CM location | ||||||||||||
| Head and neck | 78 | 1.97 (1.56 to 2.46) | 36 | 1.37 (0.96 to 1.89) | 42 | 1.95 (1.40 to 2.63) | 14 | 1.47 (0. 80 to 2.47) | 22 | 1.24 (0.78 to 1.88) | 9 | 1.37 (0.63 to 2.61) |
| Trunk | 99 | 2.18 (1.77 to 2.65) | 43 | 1.32 (0.95 to 1.77) | 32 | 1.13 (0.78 to 1.60) | 18 | 1.56 (0.92 to 2.46) | 25 | 1.17 (0.76 to 1.73) | 19 | 1.62 (0.97 to 2.53) |
| Limb | 101 | 1.85 (1.51 to 2.25) | 44 | 1.07 (0.78 to 1.43) | 39 | 1.06 (0.75 to 1.45) | 15 | 0.94 (0.53 to 1.56) | 39 | 1.49 (1.06 to 2.03) | 27 | 1.92 (1.26 to 2.79) |
| Other | 9 | 1.38 (0.63 to 2.62) | 5 | 1.10 (0.36 to 2.57) | 6 | 1.55 (0.57 to 3.38) | <5 | 1.20 (0.15 to 4.34) | 5 | 1.66 (0.54 to 3.87) | <5 | 3.09 (0.84 to 7.91) |
| Pheterogeneity | .66 | .34 | .009 | .20 | .85 | .81 | ||||||
| CM stage‖ | ||||||||||||
| Localized and ≤1.0 mm thick | 155 | 1.86 (1.58 to 2.17) | 73 | 1.20 (0.94 to 1.51) | 73 | 1.37 (1.08 to 1.73) | 22 | 0.98 (0.61 to 1.48) | 50 | 1.27 (0.94 to 1.67) | 34 | 1.63 (1.13 to 2.28) |
| Localized and >1.0 mm thick | 69 | 2.23 (1.73 to 2.82) | 21 | 0.97 (0.60 to 1.48) | 26 | 1.42 (0.93 to 2.08) | 17 | 2.12 (1.24 to 3.40) | 19 | 1.34 (0.81 to 2.09) | 13 | 2.11 (1.12 to 3.60) |
| Regional/ distant | 48 | 2.34 (1.73 to 3.10) | 24 | 1.66 (1.07 to 2.47) | 15 | 1.22 (0.68 to 2.02) | 7 | 1.33 (0.53 to 2.74) | 14 | 1.47 (0.81 to 2.47) | 10 | 2.31 (1.11 to 4.25) |
| Missing stage or missing thickness for localized disease | 10 | 1.75 (0.84 to 3.22) | <5 | 1.00 (0.27 to 2.57) | <5 | 0.59 (0.07 to 2.13) | <5 | 2.05 (0.42 to 5.99) | <5 | 0.76 (0.09 to 2.73) | 0 | − |
| Missing | 5 | 0.92 (0.30 to 2.14) | 6 | 1.56 (0.57 to 3.40) | <5 | 0.91 (0.19 to 2.67) | 0 | − | 6 | 2.37 (0.87 to 5.17) | <5 | 1.76 (0.21 to 6.37) |
| Ptrend | .10 | .31 | .80 | .10 | .55 | .23 | ||||||
| Initial course of treatment | ||||||||||||
| Any treatment | 75 | 1.92 (1.51 to 2.41) | 117 | 1.20 (0.99 to 1.44) | 104 | 1.36 (1.11 to 1.64) | 38 | 1.35 (0.96 to 1.85) | 74 | 1.50 (1.18 to 1.89) | 58 | 1.84 (1.40 to 2.37) |
| No/unknown treatment | 212 | 1.98 (1.72 to 2.27) | 11 | 1.54 (0.77 to 2.75) | 15 | 1.10 (0.61 to 1.81) | 11 | 1.05 (0.52 to 1.88) | 17 | 0.89 (0.52 to 1.42) | <5 | 0.48 (0.01 to 2.66) |
| Pheterogeneity | .75 | .40 | .44 | .46 | .05 | .11 | ||||||
| Chemotherapy | ||||||||||||
| Any chemotherapy | 53 | 1.88 (1.41 to 2.46) | 105 | 1.18 (0.97 to 1.43) | 67 | 1.34 (1.04 to 1.70) | 20 | 1.62 (0.99 to 2.50) | 65 | 1.51 (1.16 to 1.92) | 53 | 1.92 (1.44 to 2.51) |
| No/unknown chemotherapy | 234 | 1.98 (1.74 to 2.26) | 23 | 1.45 (0.92 to 2.17) | 52 | 1.29 (0.96 to 1.69) | 29 | 1.11 (0.74 to 1.59) | 26 | 1.03 (0.67 to 1.51) | 6 | 0.99 (0.36 to 2.15) |
| Pheterogeneity | .62 | .35 | .89 | .21 | .12 | .12 | ||||||
| Radiation | ||||||||||||
| Any radiation | <5 | 1.11 (0.13 to 4.00) | 39 | 1.34 (0.95 to 1.83) | 21 | 1.24 (0.77 to 1.89) | 9 | 0.97 (0.44 to 1.83) | 19 | 1.52 (0.91 to 2.37) | 27 | 2.09 (1.37 to 3.03) |
| No/unknown radiation | 285 | 1.98 (1.75 to 2.22) | 89 | 1.18 (0.95 to 1.45) | 98 | 1.33 (1.08 to 1.63) | 40 | 1.37 (0.98 to 1.86) | 72 | 1.29 (1.01 to 1.62) | 32 | 1.54 (1.06 to 2.18) |
| Pheterogeneity | .38 | .62 | .81 | .29 | .58 | .33 | ||||||
SIRs and exact, Poisson-based 95% confidence intervals compared the number of observed cases with that expected in the general population (see the Methods for further details). P values to test differences in the SIRs were computed using a likelihood ratio test derived from Poisson regression models stratified by age at first primary lymphoid neoplasm, sex, and latency, with expected numbers of cases included as an offset. Exact numbers of cases are not reported for categories with fewer than five observed cases to maintain patient confidentiality. Tests for trend do not include missing stage or thickness. All statistical tests were two-sided. – = not applicable; CI = confidence interval; CLL/SLL = chronic lymphocytic leukemia/small lymphocytic leukemia; CM = cutaneous melanoma; DLBCL = diffuse large B-cell lymphoma; FL = follicular lymphoma; HL = Hodgkin lymphoma; MZL = marginal zone lymphoma; O = observed; PCN = plasma cell neoplasm; SEER = Surveillance, Epidemiology, and End Results; SIR = standardized incidence ratio.
Includes age 20–49 years.
Includes age 70–83 years.
Includes age 60–83 years.
Regional/distant includes cases with unknown stage who have “no mass found” for thickness. Among cases with missing information on melanoma thickness, 75% had unspecified histology (morphology code: 8720). Thickness was missing for 17% of all regressing malignant melanomas (morphology code: 8723).
Table 3.
Standardized incidence ratios for second primary lymphoid neoplasm incidence by age, sex, latency, and stage among ≥1-year Caucasian adult survivors of first primary cutaneous melanoma, 17 SEER Program registries, 2000–2014*
| Characteristic | Second primary lymphoid neoplasms |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLL/SLL |
DLBCL |
FL |
MZL |
PCN |
HL |
|||||||
| O | SIR (95% CI) | O | SIR (95% CI) | O | SIR (95% CI) | O | SIR (95% CI) | O | SIR (95% CI) | O | SIR (95% CI) | |
| Overall | 197 | 1.44 (1.25 to 1.66) | 120 | 0.85 (0.71 to 1.02) | 114 | 1.47 (1.21 to 1.77) | 39 | 0.98 (0.70 to 1.34) | 149 | 1.25 (1.06 to 1.47) | 28 | 1.01 (0.67 to 1.47) |
| Age at first primary CM, y | ||||||||||||
| 20–49 | 21 | 2.11 (1.31 to 3.23) | 8 | 0.49 (0.21 to 0.97) | 16 | 1.37 (0.78 to 2.22) | 7 | 1.58 (0.63 to 3.25) | 13 | 1.35 (0.72 to 2.31) | 8 | 0.87 (0.37 to 1.71) |
| 50–59 | 38 | 1.33 (0.94 to 1.82) | 19 | 0.66 (0.39 to 1.02) | 32 | 1.63 (1.12 to 2.30) | 11 | 1.25 (0.62 to 2.24) | 28 | 1.13 (0.75 to 1.64) | 10 | 1.68 (0.80 to 3.08) |
| 60–69 | 72 | 1.53 (1.20 to 1.93) | 28 | 0.63 (0.42 to 0.91) | 38 | 1.53 (1.08 to 2.10) | 14 | 1.06 (0.58 to 1.78) | 49 | 1.20 (0.89 to 1.58) | 10‡ | 0.80 (0.39 to 1.48) |
| 70–79 | 54 | 1.19 (0.89 to 1.55) | 59 | 1.30 (0.99 to 1.68) | 28† | 1.31 (0.87 to 1.89) | 7† | 0.53 (0.21 to 1.09) | 59† | 1.35 (1.03 to 1.74) | ||
| 80–83 | 12 | 2.07 (1.07 to 3.61) | 6 | 1.03 (0.38 to 2.24) | ||||||||
| Ptrend | .14 | .002 | .37 | .06 | .85 | .66 | ||||||
| Sex | ||||||||||||
| Male | 148 | 1.47 (1.24 to 1.73) | 77 | 0.81 (0.64 to 1.02) | 76 | 1.59 (1.25 to 1.99) | 18 | 0.76 (0.45 to 1.20) | 100 | 1.20 (0.97 to 1.45) | 19 | 1.09 (0.65 to 1.70) |
| Female | 49 | 1.35 (1.00 to 1.79) | 43 | 0.93 (0.68 to 1.26) | 38 | 1.28 (0.91 to 1.76) | 21 | 1.31 (0.81 to 2.00) | 49 | 1.39 (1.03 to 1.83) | 9 | 0.89 (0.41 to 1.69) |
| Pheterogeneity | .55 | .38 | .29 | .13 | .39 | .68 | ||||||
| Latency, y | ||||||||||||
| <5 | 122 | 1.58 (1.32 to 1.89) | 76 | 0.98 (0.77 to 1.22) | 74 | 1.71 (1.34 to 2.15) | 19 | 0.88 (0.53 to 1.37) | 91 | 1.39 (1.12 to 1.71) | 18 | 1.13 (0.67 to 1.79) |
| ≥5 | 75 | 1.25 (0.99 to 1.57) | 44 | 0.70 (0.51 to 0.93) | 40 | 1.17 (0.83 to 1.59) | 20 | 1.11 (0.68 to 1.72) | 58 | 1.08 (0.82 to 1.40) | 10 | 0.86 (0.41 to 1.58) |
| Pheterogeneity | .08 | .26 | .03 | .80 | .15 | .41 | ||||||
| Lymphoid neoplasm stage | ||||||||||||
| I | – | − | 33 | 0.90 (0.62 to 1.26) | 43 | 2.00 (1.45 to 2.69) | 17 | 1.11 (0.65 to 1.78) | – | − | 9 | 1.74 (0.80 to 3.31) |
| II | – | − | 17 | 0.66 (0.39 to 1.06) | 16 | 1.32 (0.76 to 2.15) | 6 | 1.57 (0.57 to 3.41) | – | − | 8 | 0.98 (0.42 to 1.93) |
| III | – | − | 19 | 0.79 (0.47 to 1.23) | 28 | 1.52 (1.01 to 2.19) | 7§ | 0.46 (0.18 to 0.95) | – | − | 8§ | 0.64 (0.28 to 1.26) |
| IV | – | − | 47 | 1.00 (0.74 to 1.33) | 24 | 1.19 (0.76 to 1.77) | – | − | ||||
| Unspecified | – | − | <5 | 0.54 (0.15 to 1.39) | <5 | 0.57 (0.12 to 1.65) | 9 | 1.68 (0.77 to 3.20) | – | − | <5 | 1.75 (0.36 to 5.12) |
| Ptrend | – | .47 | .05 | .08 | .04 | |||||||
| CLL/SLL stage‖ | ||||||||||||
| Early stage | 149 | 1.47 (1.24 to 1.72) | – | − | – | − | – | − | – | − | – | − |
| Advanced stage | 48 | 1.36 (1.00 to 1.81) | – | − | – | − | – | − | – | − | – | − |
| Pheterogeneity | .58 | − | − | − | − | − | ||||||
SIRs and exact, Poisson-based 95% confidence intervals compared the number of observed cases with that expected in the general population (see the Methods for further details). P values to test differences in the SIRs were computed using a likelihood ratio test derived from Poisson regression models stratified by age at first primary melanoma, sex, and latency, with expected numbers of cases included as an offset. Exact numbers of cases are not reported for categories with fewer than five observed cases to maintain patient confidentiality. Tests for trend do not include unspecified stage. All statistical tests were two-sided. – = not applicable; CI = confidence interval; CLL/SLL = chronic lymphocytic leukemia/small lymphocytic leukemia; CM = cutaneous melanoma; DLBCL = diffuse large B-cell lymphoma; FL = follicular lymphoma; HL = Hodgkin lymphoma; MZL = marginal zone lymphoma; O = observed; PCN = plasma cell neoplasm; SEER = Surveillance, Epidemiology, and End Results; SIR = standardized incidence ratio.
Includes age 70–83 years.
Includes age 60–83 years.
Includes stages III and IV.
No initial treatment for CLL/SLL was used as a proxy for early stage, and any initial treatment for CLL/SLL was used as a proxy for advanced disease.
Among survivors of first primary LN, the risks for second primary CM described above were largely consistent across patient subgroups based on multivariable Poisson regression analyses (Table 2). There was, however, some limited evidence of heterogeneity. After CLL/SLL and MZL, second primary CM risk was higher in the early follow-up period (CLL/SLL Pheterogeneity = .007; MZL Pheterogeneity = .01). After first primary PCN, SIRs for CM decreased with increasing age (Ptrend = .02), were higher for females than males (Pheterogeneity = .008), and varied by receipt of initial treatment for PCN (Pheterogeneity = .05). After CLL/SLL and FL, second primary CM risk was highest for LN survivors residing in southern regions (Ptrend = .006 and .006, respectively), and after FL, SIRs were highest for CMs occurring on the head and neck (Pheterogeneity = .009).
Among survivors of first primary CM, there was little heterogeneity in risk across patient subgroups (Table 3). However, SIRs for second primary DLBCLs increased statistically significantly with increasing age at CM diagnosis (Ptrend = .002). For FL and HL, elevated risks for were more pronounced for early-stage disease (FL Ptrend = .05; HL Ptrend = .04).
Survival
Development of second primary CM was associated with increased risk of mortality from any cause after first primary CLL/SLL (HR = 1.52, 95% CI = 1.25 to 1.85), DLBCL (HR = 1.82, 95% CI = 1.30 to 2.55), FL (HR = 1.58, 95% CI = 1.11 to 2.27), or HL (HR = 2.46, 95% CI = 1.45 to 4.16) but not after MZL (HR = 1.19, 95% CI = 0.57 to 2.50) or PCN (HR = 1.04, 95% CI = 0.73 to 1.48) (Table 4). Risks were higher for regional/distant CM occurring after CLL/SLL (HR = 5.00, 95% CI = 3.53 to 7.07), DLBCL (HR = 7.87, 95% CI = 4.96 to 12.51), and FL (HR = 5.30, 95% CI = 2.65 to 10.61) than for localized CM (Supplementary Table 1, available online). Among CM survivors, development of second primary LN was associated with increased risk of mortality from any cause, with the highest risks observed after second primary PCN (HR = 6.28, 95% CI = 5.00 to 7.88) and DLBCL (HR = 5.06, 95% CI = 3.84 to 6.66) and more modest risks for FL (HR = 1.75, 95% CI = 1.15 to 2.65) and HL (HR = 3.64, 95% CI = 1.89 to 6.99).
Table 4.
Risk of death due to any cause among ≥1-year Caucasian adult survivors who developed a second primary malignancy of interest in comparison with the risk of death among those who did not develop a second primary malignancy of interest by lymphoid neoplasm subtype, 17 SEER Program registries, 2000–2014*
| Lymphoid neoplasm subtype | First primary lymphoid neoplasm |
First primary cutaneous melanoma |
||||
|---|---|---|---|---|---|---|
| Second primary cutaneous melanoma |
Second primary lymphoid neoplasm |
|||||
| Alive | Dead | HR (95% CI) | Alive | Dead | HR (95% CI) | |
| Chronic lymphocytic leukemia/small lymphocytic leukemia | ||||||
| No second primary of interest | 26 108 | 10 389 | Ref | 127 228 | 20 911 | Ref |
| Second primary | 187 | 100 | 1.52 (1.25 to 1.85) | 139 | 58 | 2.68 (2.07 to 3.46) |
| Diffuse large B-cell lymphoma | ||||||
| No second primary of interest | 24 358 | 8957 | Ref | 127 298 | 20 918 | Ref |
| Second primary | 94 | 34 | 1.82 (1.30 to 2.55) | 69 | 51 | 5.06 (3.84 to 6.66) |
| Follicular lymphoma | ||||||
| No second primary of interest | 20 735 | 5358 | Ref | 127 275 | 20 947 | Ref |
| Second primary | 89 | 30 | 1.58 (1.11 to 2.27) | 92 | 22 | 1.75 (1.15 to 2.65) |
| Marginal zone lymphoma | ||||||
| No second primary of interest | 9297 | 2060 | Ref | 127 331 | 20 966 | Ref |
| Second primary | 42 | 7 | 1.19 (0.57 to 2.50) | 36 | <5† | − |
| Plasma cell neoplasm | ||||||
| No second primary of interest | 13 078 | 13 380 | Ref | 127 293 | 20 894 | Ref |
| Second primary | 60 | 31 | 1.04 (0.73 to 1.48) | 74 | 75 | 6.28 (5.00 to 7.88) |
| Hodgkin lymphoma | ||||||
| No second primary of interest | 15 262 | 2235 | Ref | 127 348 | 20 960 | Ref |
| Second primary | 45 | 14 | 2.46 (1.45 to 4.16) | 19 | 9 | 3.64 (1.89 to 6.99) |
Hazard ratios were estimated from multivariable Cox regression models using age as the underlying time scale and adjusting for sex and year of first primary diagnosis (2000–2004, 2005–2009, 2010–2013). Diagnosis of a second primary malignancy was modeled as a time-dependent variable. In order to protect patient confidentially, Surveillance, Epidemiology, and End Results does not provide exact day of diagnosis, which resulted in survival dates slightly different from the SIR analysis and the following missing cases: chronic lymphocytic leukemia/small lymphocytic leukemia (n = 4), diffuse large B-cell lymphoma (n = 1), follicular lymphoma (n = 1), plasma cell neoplasm (n = 16), Hodgkin lymphoma (n = 1), and melanoma (n = 2). – = not applicable; CI = confidence interval; HR = hazard ratio; SEER = Surveillance, Epidemiology, and End Results.
HRs are not presented when the number of deceased cases was less than five.
Discussion
In this large, population-based study among Caucasian US adults, we show for the first time that the association between CM and LN varies substantially among the six most common LN subtypes. Specifically, we observed mutually increased risks for second primary CM after initial diagnoses of CLL/SLL, FL, and PCN and for these same subtypes occurring as second cancers after a first primary diagnosis of CM. In contrast, CM risk was elevated after DLBCL and HL, but risks of second DLBCL and HL were not statistically significantly increased after first primary CM, and no statistically significant association was observed between CM and MZL. For nearly all survivors, development of a second primary CM or LN was associated with statistically significantly higher risk of death, highlighting the clinical impact of developing second primary malignancies.
Our observations are consistent with previous studies that have reported elevated risks for CM after first primary CLL/SLL in SEER (13) and other settings (22,23) and increased risks of CLL/SLL after initial CM diagnosis (8,24,25). One earlier study has also reported mutually elevated risks for CLL/SLL and CM (26). Our results are also consistent with previous SEER-based studies showing elevated risk of CM after FL (13) and PCN (27), whereas our findings of elevated risk for CM after DLBCL and PCN after CM differ from previous population-based reports, including SEER, which showed no statistically significant association (13,28). To our knowledge, this is the first study to assess risk of CM after MZL and risks of DLBCL, FL, and MZL after CM. The heterogeneity of associations between CM and specific types of LN provides insight into the etiology of these malignancies. In particular, mutually elevated risks of CM and CLL/SLL, FL, and PCN may be suggestive of shared etiologic factors (29).
An immune link has long been thought to underlie mutually elevated risks of CM and LN (30). Populations that experience prolonged broad immunosuppression, such as solid organ transplant recipients and individuals with HIV/AIDS, have moderately increased risk for melanoma and strikingly increased risk for LN, particularly DLBCL and MZL (16,31–35). However, whereas we observed mutually elevated risks for CM with CLL/SLL, FL, and PCN, there was no evidence for increased risk of DLBCL and MZL after CM. Several lines of evidence point specifically to T-cell dysfunction as a plausible explanation for the mutually elevated risks we observed, with the strongest data for CM after CLL/SLL. Following a diagnosis of CLL/SLL, patients typically experience a relapsing/remitting disease course characterized by progressive immunosuppression and elevated risk for infection (36–38). Investigations of specific immune defects in CLL/SLL describe a complex immunomodulatory effect of malignant leukemia cells that results in defects in certain T-cell populations, leading to an overall decrease in helper activity and increase in regulatory (immunosuppressive) activity (30,39,40). Consistent with this hypothesis, one study of CLL/SLL survivors demonstrated increased risk of CM associated with receipt of fludarabine, which is known to deplete T-helper cells, and history of T-cell-activating autoimmune conditions, such as Graves’ disease, psoriasis, chronic rheumatic heart disease, localized scleroderma/psoriasis, and asthma (41). Additionally, the predominantly T-cell inflammatory infiltrate at the base of CMs is important prognostically (42). Less clear is whether T-cell dysfunction could explain the risk of CLL/SLL after CM or the mutually increased risk of CM with PCN and FL, although immune dysfunction also has been reported after a diagnosis of PCN, FL, and CM (27,43–45). Additional research is therefore needed to understand whether specific T-cell defects may underlie the shared etiology of CM and CLL/SLL, FL, and PCN.
Other potential shared etiologic factors to consider include ultraviolet radiation (UVR) and genetic susceptibility. Although UVR is an important risk factor for CM, epidemiologic studies have demonstrated an inverse association for UVR with HL, PCN, and most NHL subtypes (46–49). The similarity of the UVR association among LNs as well as the inverse nature of risk argue against UVR as an explanation for the mutually elevated risks we observed for CM and CLL/SLL, FL, and PCN but not DLBCL, MZL or HL. With respect to shared inherited susceptibility, although both common and rare genetic variants have been identified separately for CM (50–57) and LN (58–62), shared genetic factors between LN subtypes and CM have not been identified.
We observed statistically significantly elevated risk for CM after DLBCL and HL but not for DLBCL or HL after CM; MZL followed a similar pattern, but risk for second primary CM was not statistically significant. The new finding for an increased risk of CM after DLBCL may stem from changes in DLBCL treatment over time because we only included patients diagnosed since 2000, whereas previous studies (13) included patients treated in earlier calendar periods when five-year relative survival was lower, prior to the introduction of rituximab. Previous studies of HL survivors have suggested that the intensive systemic therapy typically used for HL may introduce long-term immune dysfunction (63,64), which may increase risk for subsequent CM. However, no study has evaluated associations for specific agents, and detailed chemotherapy and radiation data are not available in SEER. Thus, future studies evaluating CM risk after HL should include data on treatment and markers of immune dysfunction, if possible.
In addition to etiologic insights, several findings merit comment from a clinical perspective. Overall, SIRs within LN subtypes were fairly consistent among patient subgroups defined by age, sex, calendar year, and time since diagnosis. Additionally, among LN survivors of a given subtype, a majority of the second CMs were diagnosed as localized disease (≤1.0 mm thick), and risks of CM were generally consistent across CM stage, suggesting that LN survivors are at increased risk of both early and more advanced-stage CMs. This finding contrasts with previous literature, which suggested that more advanced CMs tend to develop after LNs (10,11). Nevertheless, we found that a diagnosis of second primary CM was associated with 1.5- to more than twofold higher risk of death among CLL/SLL, DLBCL, FL, and HL survivors and a fivefold or higher risk of death among CLL/SLL, DLBCL, and FL survivors who developed advanced CM. Notably, development of second primary CM was associated with the highest risk of death among survivors of DLBCL and HL. Among first primary CM survivors, development of second primary LNs statistically significantly increased risk of death, with the highest mortality observed after DLBCL and PCN. These results resemble LN mortality in the general population, which is higher after DLBCL and PCN as compared with the other LN subtypes (65).
The major strength of this study is the use of large-scale population registry-based data to systematically assess specific LN subtypes diagnosed since 2000, leveraging both the expansion of SEER and the introduction of the WHO classification for LN, which improved classification of specific disease subtypes (14). Despite this large sample size, however, we were unable to investigate the association between CM and other less common LNs to investigate long-term risks (>10 years) or include non-Caucasian populations. Lack of detailed treatment and other clinical data precluded investigation of specific risk factors that may partly explain the observed associations. The lack of detailed clinical staging data for CM may have limited our ability to detect differences in the SIRs by stage at CM.
In conclusion, we present a comprehensive analysis demonstrating that the association between CM and LN differs by LN subtype among Caucasian adults and that the development of second primary CM or LN substantially reduces survival. Mutually increased risks were observed for CM and three subtypes of LNs: CLL/SLL, FL, and PCN. In contrast, CM risk was elevated after DLBCL and HL, but there was no increase in the opposite direction. Further research should seek to include treatment data for first and second neoplasms and characterize immune function in patients with subtype-specific LNs and CM to elucidate a potential role for specific immune perturbations in the etiology of these malignancies. Our observation that the development of second primary CM or LNs is associated with statistically significantly reduced survival underscores the importance of understanding the etiology of these malignancies to ultimately devise prevention, surveillance, and/or targeted treatment strategies.
Funding
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Notes
Affiliation of authors: Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD.
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors indicate no potential conflicts of interest.
Supplementary Material
References
- 1. Gunz FW, Angus HB.. Leukemia and cancer in the same patient. Cancer. 1965;18(2):145–152. [DOI] [PubMed] [Google Scholar]
- 2. Berg JW. The incidence of multiple primary cancers. I. Development of further cancers in patients with lymphomas, leukemias, and myeloma. J Natl Cancer Inst. 1967;38(5):741–752. [PubMed] [Google Scholar]
- 3. Greene MH, Hoover RN, Fraumeni JF Jr.. Subsequent cancer in patients with chronic lymphocytic leukemia—a possible immunologic mechanism. J Natl Cancer Inst. 1978;61(2):337–340. [PubMed] [Google Scholar]
- 4. Tashima C. Association of malignant melanoma and malignant lymphoma. Lancet. 1973;302(7823):266. [DOI] [PubMed] [Google Scholar]
- 5. Goggins WB, Finkelstein DM, Tsao H.. Evidence for an association between cutaneous melanoma and non‐Hodgkin lymphoma. Cancer. 2001;91(4):874–880. [DOI] [PubMed] [Google Scholar]
- 6. Lens M, Newton-Bishop J.. An association between cutaneous melanoma and non-Hodgkin's lymphoma: Pooled analysis of published data with a review. Ann Oncol. 2005;16(3):460–465. [DOI] [PubMed] [Google Scholar]
- 7. Levi F, Randimbison L, Te VC, et al. Non-Hodgkin's lymphomas, chronic lymphocytic leukaemias and skin cancers. Br J Cancer. 1996;74(11):1847–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: A population-based study. J Am Acad Dermatol. 2010;62(5):757–767. [DOI] [PubMed] [Google Scholar]
- 9. Royle JA, Baade PD, Joske D, et al. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: A population-based study. Br J Cancer. 2011;105(7):1076–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Famenini S, Martires KJ, Zhou H, et al. Melanoma in patients with chronic lymphocytic leukemia and non-Hodgkin lymphoma. J Am Acad Dermatol. 2015;72(1):78–84. [DOI] [PubMed] [Google Scholar]
- 11. Brewer JD, Shanafelt TD, Otley CC, et al. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignant melanoma and merkel cell carcinoma in a SEER population-based study. J Clin Oncol. 2012;30(8):843–849. [DOI] [PubMed] [Google Scholar]
- 12. Frankenthaler A, Sullivan RJ, Wang W, et al. Impact of concomitant immunosuppression on the presentation and prognosis of patients with melanoma. Melanoma Res. 2010;20(6):496–500. [DOI] [PubMed] [Google Scholar]
- 13. Morton LM, Curtis RE, Linet MS, et al. Second malignancy risks after non-Hodgkin's lymphoma and chronic lymphocytic leukemia: Differences by lymphoma subtype. J Clin Oncol. 2010;28(33):4935–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jaffe ES, Harris NL, Stein H, et al. Pathology and Genetics: Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2001. [Google Scholar]
- 15. Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014. Bethesda, MD: National Cancer Institute; 2007. https://seer.cancer.gov/csr/1975_2014/. Accessed March 23, 2018. [Google Scholar]
- 16. Olsen CM, Knight LL, Green AC.. Risk of melanoma in people with HIV/AIDS in the pre- and post-HAART eras: A systematic review and meta-analysis of cohort studies. PLoS One. 2014;9(4):e95096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization. International Classification of Diseases for Oncology. Third ed. First Revision. Geneva: World Health Organization; 2013. [Google Scholar]
- 18. Morton LM, Turner JJ, Cerhan JR, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). Blood. 2007;110(2):695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carbone PP, Kaplan HS, Musshoff K, et al. Report of the committee on Hodgkin's disease staging classification. Cancer Res. 1971;31(11):1860–1861. [PubMed] [Google Scholar]
- 20. Preston D, Lubin J, Pierce D, et al. Epicure Risk Regression and Person-Year Computation Software: Command Summary and User Guide. Ottawa: Risk Sciences International; 2015. [Google Scholar]
- 21. Yasui Y, Liu Y, Neglia JP, et al. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158(11):1108–1113. [DOI] [PubMed] [Google Scholar]
- 22. Schöllkopf C, Rosendahl D, Rostgaard K, et al. Risk of second cancer after chronic lymphocytic leukemia. Int J Cancer. 2007;121(1):151–156. [DOI] [PubMed] [Google Scholar]
- 23. Brewer JD, Shanafelt TD, Call TG, et al. Increased incidence of malignant melanoma and other rare cutaneous cancers in the setting of chronic lymphocytic leukemia. Int J Dermatol. 2015;54(8):e287–e293. [DOI] [PubMed] [Google Scholar]
- 24. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5 Suppl 1):S69–S77. [DOI] [PubMed] [Google Scholar]
- 25. Bradford PT, Freedman D, Goldstein AM, et al. Increased risk of second primary cancers after a diagnosis of melanoma. Arch Dermatol. 2010;146(3):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKenna D, Stockton D, Brewster D, et al. Evidence for an association between cutaneous malignant melanoma and lymphoid malignancy: A population-based retrospective cohort study in Scotland. Br J Cancer. 2003;88(1):74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Razavi P, Rand K, Cozen W, et al. Patterns of second primary malignancy risk in multiple myeloma patients before and after the introduction of novel therapeutics. Blood Cancer J. 2013;3(6):e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crocetti E, Guzzinati S, Paci E, et al. The risk of developing a second, different, cancer among 14 560 survivors of malignant cutaneous melanoma: A study by AIRTUM (the Italian Network of Cancer Registries). Melanoma Res. 2008;18(3):230–234. [DOI] [PubMed] [Google Scholar]
- 29. Curtis RE, Freedman DM, Ron E, et al. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. Bethesda, MD: National Institute of Health; 2006. [Google Scholar]
- 30. Brewer JD, Christenson LJ, Weenig RH, et al. Effects of chronic lymphocytic leukemia on the development and progression of malignant melanoma. Derm Surg. 2010;36(3):368–376. [DOI] [PubMed] [Google Scholar]
- 31. Engels EA, Pfeiffer RM, Fraumeni JF, et al. Spectrum of cancer risk among us solid organ transplant recipients. JAMA. 2011;306(17):1891–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robbins HA, Clarke CA, Arron ST, et al. Melanoma risk and survival among organ transplant recipients. J Invest Dermatol. 2015;135(11):2657–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Friedberg JW. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22(5):941–952, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clarke C, Morton L, Lynch C, et al. Risk of lymphoma subtypes after solid organ transplantation in the United States. Br J Cancer. 2013;109(1):280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187–194. [DOI] [PubMed] [Google Scholar]
- 36. Forconi F, Moss P.. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573–581. [DOI] [PubMed] [Google Scholar]
- 37. Christopoulos P, Pfeifer D, Bartholome K, et al. Definition and characterization of the systemic T-cell dysregulation in untreated indolent B-cell lymphoma and very early CLL. Blood. 2011;117(14):3836–3846. [DOI] [PubMed] [Google Scholar]
- 38. Riches JC, Gribben JG.. Immunomodulation and immune reconstitution in chronic lymphocytic leukemia. Semin Hematol. 2014;51(3):228–234. [DOI] [PubMed] [Google Scholar]
- 39. Aslakson CJ, Lee G, Boomer JS, et al. Expression of regeneration and tolerance factor on B cell chronic lymphocytic leukemias: A possible mechanism for escaping immune surveillance. Am J Hematol. 1999;61(1):46–52. [DOI] [PubMed] [Google Scholar]
- 40. Kipps TJ, Stevenson FK, Wu CJ, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3:16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lam CJK, Curtis RE, Dores GM, et al. Risk factors for melanoma among survivors of non-Hodgkin lymphoma. J Clin Oncol. 2015;33(28):3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mihm MC, Mulé JJ.. Reflections on the histopathology of tumor-infiltrating lymphocytes in melanoma and the host immune response. Cancer Immunol Res. 2015;3(8):827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pratt G, Goodyear O, Moss P.. Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol. 2007;138(5):563–579. [DOI] [PubMed] [Google Scholar]
- 44. Yang ZZ, Ansell SM.. The tumor microenvironment in follicular lymphoma. Clin Adv Hematol Oncol. 2012;10(12):810–818. [PubMed] [Google Scholar]
- 45. Critchley-Thorne RJ, Simons DL, Yan N, et al. Impaired interferon signaling is a common immune defect in human cancer. Proc Natl Acad Sci U S A. 2009;106(22):9010–9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morton LM, Slager SL, Cerhan JR, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr. 2014;(48):130–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Monnereau A, Glaser SL, Schupp CW, et al. Exposure to UV radiation and risk of Hodgkin lymphoma: A pooled analysis. Blood. 2013;122(20):3492–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chang ET, Canchola AJ, Cockburn M, et al. Adulthood residential ultraviolet radiation, sun sensitivity, dietary vitamin D, and risk of lymphoid malignancies in the California Teachers Study. Blood. 2011;118(6):1591–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kricker A, Armstrong BK, Hughes AM, et al. Personal sun exposure and risk of non Hodgkin lymphoma: A pooled analysis from the Interlymph Consortium. Int J Cancer. 2008;122(1):144–154. [DOI] [PubMed] [Google Scholar]
- 50. Van den Oord J, Vandeghinste N, De Ley M, et al. Bcl-2 expression in human melanocytes and melanocytic tumors. Am J Pathol. 1994;145(2):294. [PMC free article] [PubMed] [Google Scholar]
- 51. Saenz‐Santamaría M, Reed JA, Scott McNutt N, et al. Immunohistochemical expression of BCL‐2 in melanomas and intradermal nevi. J Cutan Pathol. 1994;21(5):393–397. [DOI] [PubMed] [Google Scholar]
- 52. Ramsay JA, From L, Kahn HJ.. Bcl-2 protein expression in melanocytic neoplasms of the skin. Mod Pathol. 1995;8(2):150–154. [PubMed] [Google Scholar]
- 53. Kanitakis J, Baldassini S, Lora V, et al. BRAF mutations in melanocytic tumors (nevi and melanomas) from organ transplant recipients. Eur J Dermatol. 2010;20(2):167–171. [DOI] [PubMed] [Google Scholar]
- 54. Healy E, Belgaid CE, Takata M, et al. Allelotypes of primary cutaneous melanoma and benign melanocytic nevi. Cancer Res. 1996;56(3):589–593. [PubMed] [Google Scholar]
- 55. Hussussian CJ, Struewing JP, Goldstein AM, et al. Germline p16 mutations in familial melanoma. Nat Genet. 1994;8(1):15–21. [DOI] [PubMed] [Google Scholar]
- 56. Zuo L, Weger J, Yang Q, et al. Germline mutations in the p16INK4a binding domain of CDK4 in familial melanoma. Nat Genet. 1996;12(1):97–99. [DOI] [PubMed] [Google Scholar]
- 57. Peris K, Keller G, Chimenti S, et al. Microsatellite instability and loss of heterozygosity in melanoma. J Invest Dermatol. 1995;105(4):625–628. [DOI] [PubMed] [Google Scholar]
- 58. Ngan BY, Chen-Levy Z, Weiss LM, et al. Expression in non-Hodgkin's lymphoma of the bcl-2 protein associated with the t(14;18) chromosomal translocation. N Engl J Med. 1988;318(25):1638–1644. [DOI] [PubMed] [Google Scholar]
- 59. Vaux DL, Cory S, Adams JM.. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335(6189):440–442. [DOI] [PubMed] [Google Scholar]
- 60. Gahn B, Schafer C, Neef J, et al. Detection of trisomy 12 and Rb-deletion in CD34+ cells of patients with B-cell chronic lymphocytic leukemia. Blood. 1997;89(12):4275–4281. [PubMed] [Google Scholar]
- 61. Gahrton G, Robert KH, Friberg K, et al. Nonrandom chromosomal aberrations in chronic lymphocytic leukemia revealed by polyclonal B-cell-mitogen stimulation. Blood. 1980;56(4):640–647. [PubMed] [Google Scholar]
- 62. Juliusson G, Oscier DG, Fitchett M, et al. Prognostic subgroups in B-cell chronic lymphocytic leukemia defined by specific chromosomal abnormalities. N Engl J Med. 1990;323(11):720–724. [DOI] [PubMed] [Google Scholar]
- 63. Fisher RI, DeVita VT Jr., Bostick F, et al. Persistent immunologic abnormalities in long-term survivors of advanced Hodgkin's disease. Ann Intern Med. 1980;92(5):595–599. [DOI] [PubMed] [Google Scholar]
- 64. Hancock SL, Hoppe RT.. Long-term complications of treatment and causes of mortality after Hodgkin's disease. Semin Radiat Oncol. 1996;6(3):225–242. [DOI] [PubMed] [Google Scholar]
- 65. Teras LR, DeSantis CE, Cerhan JR, et al. 2016US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016 Sep 12. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.