Abstract
OBJECTIVES
Minimally invasive surgery is accepted for early-stage lung cancer, but its role in locally advanced disease is controversial, especially using a robotic platform. The aim of this retrospective study was to assess the safety and effectiveness of robot-assisted resection in patients with Stage IIIA non-small-cell lung cancer (NSCLC) or carcinoid tumours in the series as a whole and in different subgroups according to adjuvant treatment.
METHODS
This was a retrospective multicentre study of consecutive patients with clinically evident or occult N2 disease (210 NSCLC and 13 carcinoid) who, in 2007–2016, underwent robot-assisted resection at 7 high-volume centres. Perioperative outcomes, recurrences and overall survival were assessed.
RESULTS
N2 disease was diagnosed preoperatively in 72 (32%) patients and intraoperatively in 151 (68%) patients. Surgical margins were negative in 98.4% of cases with available data. Thirty-four (15.2%) patients received neoadjuvant treatment, 140 (63%) patients received postoperative treatment, and 49 (22%) patients underwent surgery only. There were 22 (9.9%) conversions to thoracotomy, 23 (10.3%) had serious (Grades III–IV) postoperative morbidity and the mean hospital stay was 5.3 days. Complications and outcomes did not differ significantly between treatment groups. Of the 34 patients who were given neoadjuvant chemotherapy, all had R0 resection, 5 (15%) patients required conversion but none required conversion because of bleeding and 4 (12%) patients had Grade III or IV postoperative complications. After a median of 18 (interquartile range 8–33) months, 3-year overall survival in NSCLC patients was 61.2% and 60.3% (P = 0.6) of patients in the subgroup were given induction treatment. However, overall survival was significantly better (P = 0.012) in NSCLC patients with ≤2 positive nodes (vs >2). Nineteen (8.5%) patients developed local recurrence.
CONCLUSIONS
Robot-assisted lobectomy is safe and effective in patients with Stage III NSCLC or carcinoid tumours with low conversions and complications. Among patients with NSCLC, including those who were given induction chemotherapy, survival was similar to that reported for open surgery.
Keywords: Lung cancer, Robotic surgery, Thoracic surgery, Minimally invasive surgery, Stage III lung cancer
INTRODUCTION
Minimally invasive video-assisted thoracoscopic surgery (VATS) lung resection is associated with reduced postoperative pain, reduced cytokine responses, quicker resumption of normal activities and better aesthetic and functional outcomes than thoracotomy [1–6]. Nevertheless, VATS has not been embraced by the majority of thoracic surgeons [7], probably due to its technical challenges, steep learning curve and difficulties in performing complete mediastinal lymphadenectomy, which is standard for lung cancer surgery [8–9], and many thoracic surgeons prefer open thoracotomy for pulmonary lobectomy [7].
Robot-assisted surgery has several advantages over VATS, including improved 3-dimensional visualization, instruments with more degrees of motion freedom and better ergonomics [10–16]. These improvements have allowed surgeons who do not perform VATS to find a minimally invasive platform that they can use. In addition, lymph node dissection is easier and likely to be more complete with robot-assisted surgery than with VATS [15, 16] and is, therefore, suitable for lung cancer cases with mediastinal lymph node involvement, which are usually subjected to thoracotomy. Furthermore, since robot-assisted surgery and VATS are associated with quicker recovery than open surgery, cases with N2 disease not given neoadjuvant chemotherapy have a greater probability of initiating full-dose adjuvant chemotherapy within the 6 weeks after surgery considered optimal.
Minimally invasive lung resections are generally indicated for early-stage (Stages I or II) lung cancer [17], and several groups have reported experience and initial outcomes of robot-assisted lung resection for early disease [11–16]. To our knowledge, however, only 3 studies have described VATS to treat locally advanced non-small-cell lung cancer (NSCLC) [18–20], and another 2 have reported on the use of robotic surgery for locally advanced disease [15, 21]. The aim of this retrospective study was to assess the safety and effectiveness of robot-assisted lung resection in patients with Stage III NSCLC or carcinoid. We were also interested in assessing long-term outcomes in NSCLC patients, particularly those who received induction chemotherapy. The operations were carried out at 7 centres in Europe, North America and Asia Minor.
METHODS
We performed a retrospective analysis of prospectively collected data from 7 centres with experience in performing robot-assisted surgery and whose principal surgeons had all attained proficiency in robotic surgery for lung cancer resection. Eligible cases had NSCLC or carcinoid, with pathological (post-surgical) N2 disease (Stage IIIA) diagnosed either before surgery (clinical N2) or encountered intraoperatively (occult N2) and treated by robot-assisted resection with curative intent, before or after chemotherapy or chemoradiation therapy. Patients with clinical N2 but pathological yN0 or yN1 were excluded, as the absence of access to the radiological images made it impossible to confirm preoperative stage. Carcinoid tumours were excluded from the survival analyses. For patients treated from 9 November 2005 to 15 July 2017, information on duration of surgery, extent of lymphadenectomy, perioperative complications according to Clavien–Dindo [22], conversions to open surgery, mortality, length of hospital stay, local and distant recurrences and overall and disease-specific survival were assessed.
Two surgical techniques were used. Surgeons from 4 centres (Milan, New York, Tampa and Istanbul) used a 3- or 4-arm approach, with a utility incision, no CO2 insufflation and anterior-to-posterior hilar dissection. The other 3 North American centres used 3–4 arms, CO2 insufflation, posterior-to-anterior hilar dissection and a specimen extraction incision at the end of the procedure.
Statistical methods
Data are presented for the whole series and by adjuvant treatment group (preoperative, postoperative and none). Categorical data are summarized as numbers and percentages. Continuous data are summarized as means with standard deviations (SDs) or medians and interquartile ranges. Differences between the adjuvant treatment groups were explored using the Kruskal–Wallis non-parametric test for continuous variables or χ2 test for categorical variables. Differences between 2 groups were explored using the Wilcoxon non-parametric test (continuous) or the χ2 test (categorical). Variables with P-value <0.1 were entered into a multivariable logistic regression model, with differences in P-value <0.05 considered significant. Survival was calculated from day of surgery to death or latest contact. Survival curves were constructed by the Kaplan–Meier method. All factors were tested for their influence in survival by the Cox proportional hazards modelling: factors with P-value <0.1 using univariate analysis were entered into a Cox multivariable model. The proportional hazards assumption was evaluated for all factors using Schoenfeld residuals.
RESULTS
A total of 223 patients conformed to our inclusion criteria and were analysed. N2 disease was diagnosed preoperatively in 72 (32%) patients and intraoperatively in 151 (68%) patients. The mean duration of surgery was 194 min (SD 82). Neoadjuvant treatment was given to 34 (15%) patients, adjuvant treatment to 140 (63%) patients and no neoadjuvant/adjuvant treatment to 49 (22%) patients (Table 1). The mean duration of hospital stay was 5.3 days and did not differ (P = 0.64) among the 3 adjuvant treatment groups.
Table 1:
Clinical characteristics and surgical outcomes in patients with N2 lung cancer, treated using robot-assisted surgery, in the whole series and according to adjuvant treatment (preoperative, postoperative and none)
| All cases | Preoperative adjuvant treatment | Postoperative adjuvant treatment | No adjuvant treatment | P-value | |
|---|---|---|---|---|---|
| n (%) | 223 (100) | 34 (15.2) | 140 (62.7) | 49 (21.9) | |
| Age (years), mean (SD) | 66 (10) | 67 (9) | 65 (9) | 69 (13) | 0.036 |
| Men, n (%) | 100 (44.8) | 20 (58.8) | 63 (45.0) | 17 (34.7) | 0.096 |
| Stage, n (%) | <0.001 | ||||
| Clinical N2 | 72 (32.3) | 25 (73.5) | 37 (26.4) | 10 (20.4) | |
| Occult N2 | 151 (67.7) | 9 (26.5) | 103 (73.6) | 39 (79.6) | |
| Tumour type, n (%) | 0.073b | ||||
| NSCLC | 210 (94.2%) | 34 (100%) | 139 (99.3%) | 37 (75.5%) | |
| Adenocarcinoma | 158 (70.9) | 28 (82.4) | 105 (75.0) | 25 (51.0) | |
| Squamous cell carcinoma | 27 (12.1) | 3 (8.8) | 14 (10.0) | 10 (20.4) | |
| Other NSCLC | 25 (11.2) | 3 (8.8) | 20 (14.3) | 2 (4.1) | |
| Carcinoid | 13 (5.8) | 0 | 1 (0.7) | 12 (24.5) | |
| Lymph nodes removed, mean (SD) | 15.4 (7.9) | 14.5 (8.7) | 15.3 (7.2) | 16.5 (9.2) | 0.350 |
| Positive nodes, mean (SD) | 4.3 (4.9) | 3.7 (5.4) | 4.8 (5.1) | 3.2 (3.5) | 0.007 |
| N1 nodes removed, mean (SD)c | 7.0 (4.1) | 6.8 (4.0) | 6.9 (3.9) | 7.6 (4.6) | 0.721 |
| N1 nodes positive, mean (SD)c | 2.0 (2.5) | 1.2 (1.6) | 2.3 (2.6) | 1.7 (2.9) | 0.016 |
| N2 nodes removed, mean (SD)c | 8.3 (6.1) | 8.1 (7.6) | 8.5 (5.7) | 8.1 (6.1) | 0.579 |
| N2 nodes positive, mean (SD)c | 2.5 (3.4) | 2.6 (4.6) | 2.7 (3.4) | 1.9 (1.8) | 0.333 |
| Tumour size (mm), mean (SD) | 3.36 (1.9) | 2.91 (1.7) | 3.44 (1.9) | 3.44 (2.0) | 0.340 |
| Conversions, n (%) | 22 (9.9) | 5 (14.7) | 11 (7.9) | 6 (12.2) | 0.354 |
| Reason for conversion, n (%) | |||||
| Anatomical | 7 (31.8) | 2 (40.0) | 4 (36.4) | 1 (16.7) | |
| Bleeding | 6 (27.3) | 0 | 3 (27.3) | 3 (50.0) | |
| Oncological | 7 (31.8) | 3 (60.0) | 2 (18.2) | 2 (33.3) | |
| Technical | 2 (9.1) | 0 | 2 (18.2%) | 0 | |
| 3-Year overall survivalb | 61.2% | 60.3% | 63.5% | 56.2% | 0.110a |
| 3-Year progression-free survival | 37.7% | 25.8% | 38.4% | 57.1% | 0.262a |
| Local recurrences, n (%)d | 19 (8.5) | 4 (11.8) | 12 (8.6) | 3 (6.1) | 0.647 |
| Distant recurrences, n (%)d | 56 (25.1) | 13 (38.2) | 37 (26.4) | 6 (12.2) | 0.021 |
| Lung recurrences, n (%)d | 19 (8.5) | 4 (11.8) | 15 (10.7) | 0 | 0.019 |
| Postoperative stay (days), mean (SD) | 5.13 (4.7) | 5.09 (3.4) | 4.71 (3.4) | 6.35 (7.6) | 0.641 |
| Duration of surgery (min), mean (SD) | 194 (82) | 221 (101) | 188 (75) | 191 (85) | 0.303 |
| Postoperative complications, n (%) | 0.139 | ||||
| Complications | 148 (66.4) | 19 (55.8) | 101 (72.1) | 28 (57.1) | |
| Grades I–II | 52 (23.3) | 11 (32.4) | 28 (20.0) | 13 (26.5) | |
| Grades III–V | 23 (10.3) | 4 (11.8) | 11 (7.9) | 8 (16.3) | |
| 30-Day mortalitye | 4/209 (1.9) | 0 | 0 | 4/45 (8.9) | 0.049 |
| 90-Day mortalitye | 8/198 (4.0) | 0 | 4/128 (3.1) | 4/39 (10.3) | 0.079 |
The log-rank test.
Excluding carcinoids.
Data missing for 36 cases.
Not mutually exclusive (1 case local + lung, 9 cases lung + distant and 3 cases distant + local).
Excluding patients lost to follow-up within 30 days (14 patients) or within 90 days (25 patients).
NSCLC: non-small-cell lung cancer; SD: standard deviation.
Severe (Clavien–Dindo Grade III–V) postoperative complications occurred in 23 (10.3%) cases, with no difference (P = 0.14) between those who received neoadjuvant chemotherapy or surgery as first treatment. Twenty-two (9.9%) cases were converted to thoracotomy, with no difference between the treatment groups (P = 0.35). Causes of conversion were oncological in 7 (including 3 with unforeseen mediastinal nodal involvement), bleeding in 6, anatomical in 7 (including tight adhesions in 5) and technical in 2 (including 1 intraoperative airway complication). Factors significantly associated with conversion were large tumour size and >2 positive lymph nodes but only using univariable analysis (Table 2). No factor was significantly associated with conversion using multivariable analysis.
Table 2:
Univariable and multivariable analyses of factors influencing conversion to open surgery
| Conversion |
P-value univariate | P-value multivariable | ||
|---|---|---|---|---|
| No | Yes | |||
| n | 201 | 22 | ||
| Males, n (%) | 90 (44.8) | 10 (45.5) | 1.000 | |
| Age (years), mean (SD) | 66 (11) | 66 (9) | 0.953 | |
| Clinical N2, n (%) | 62 (30.9) | 10 (45.5) | 0.228 | |
| Preoperative treatment, possibly also postoperative, n (%) | 29 (14.4) | 5 (22.7) | 0.345 | |
| FEV1%, mean (SD) | 86.6 (19.2) | 85.4 (11.8) | 0.588 | |
| Tumour type, n (%) | 0.152 | |||
| Adenocarcinoma | 145 (72.14) | 13 (59.09) | ||
| Squamous cell carcinoma | 22 (10.95) | 5 (22.73) | ||
| Other | 21 (10.45) | 4 (18.18) | ||
| Carcinoids | 13 (6.47) | 0 | ||
| N lymph nodes removed, mean (SD) | 15.3 (8.1) | 16.6 (6.5) | 0.271 | |
| N positive lymph nodes removed, mean (SD) | 4.0 (4.6) | 6.8 (6.2) | 0.002 | 0.384 |
| Tumour size (mm), mean (SD) | 3.25 (1.8) | 4.35 (2.5) | 0.025 | 0.141 |
| Cases with skip metastases,an (%) | 57/164 (34.8) | 3/22 (13.6) | 0.053 | 0.159 |
Thirty-seven cases had no recorded data on sites of involved lymph nodes.
SD: standard deviation.
Of the 34 patients given neoadjuvant chemotherapy, 8 also received preoperative radiotherapy. All patients had negative surgical margins. Five (15%) required conversion, and none because of bleeding. None had a blood transfusion. Four (12%) patients had Grade III or IV postoperative complications. The mean postoperative hospital stay was 5 days. None died within 30 or 90 days, although 2 were lost to follow-up at 30 days and 3 at 90 days.
Negative surgical margins were obtained in 185 (98.4%) of the 188 cases with available data. Of the 189 patients not given neoadjuvant therapy, 47 (25%) patients had suspected or proven clinical N2 disease but were intentionally operated on before chemotherapy, whereas 142 (75%) patients had nodal disease discovered only during surgery. Using univariable analysis (Table 3), no variable was significantly associated with complications. A mean of 15.4 (SD 7.9) lymph nodes was removed, and 4.3 (SD 4.9) of which were positive; a mean of 7.0 (SD 4.1) lymph nodes were removed from Station N1 and a mean of 8.3 (SD 6.1) lymph nodes were removed from Station N2; 203 (91.0% of 223) patients had more than 6 lymph nodes removed.
Table 3:
Univariable analysis of factors influencing complications
| Complications |
P-value | ||
|---|---|---|---|
| No | Yes | ||
| n | 147 | 76 | |
| Male versus female, n (%) | 64 (43.5) | 36 (47.4) | 0.670 |
| Age (years), mean (SD) (continuous) | 65 (11) | 67 (9) | 0.448 |
| Clinical N2 versus occult N2, n (%) | 42 (28.6) | 30 (39.5) | 0.130 |
| Preoperative treatment, possibly also postoperative, n (%) | 19 (12.9) | 15 (19.8) | 0.238 |
| FEV1%, mean (SD) (continuous) | 87.93 (19.1) | 83.60 (17.3) | 0.138 |
| Tumour type, n (%) | 0.466 | ||
| Adenocarcinoma | 109 (74.2) | 49 (64.5) | |
| Squamous cell carcinoma | 16 (10.9) | 11 (14.5) | |
| Other | 14 (9.5) | 11 (14.5) | |
| Carcinoids | 8 (5.4) | 5 (6.6) | |
| N lymph nodes removed, mean (SD) (continuous) | 15.5 (8.1) | 15.2 (7.7) | 0.973 |
| N nodes positive, mean (SD) (continuous) | 4.4 (5.2) | 4.1 (4.1) | 0.776 |
| Tumour size (mm), mean (SD) (continuous) | 3.18 (1.8) | 3.72 (2.1) | 0.052 |
| Cases with skip metastases, n (%) (continuous) | 34 (29.1) | 26 (37.7) | 0.257 |
| Operating time (min), mean (SD) (continuous) | 186 (76) | 208 (91) | 0.107 |
| Conversions, n (%) (continuous) | 16 (10.9) | 6 (7.9) | 0.637 |
SD: standard deviation.
Overall, 56 (25%) patients had distant relapse, whereas local or lung recurrence occurred in 37 (16.6%) patients: in 6 cases (2 carcinoids) in hilar or mediastinal lymph nodes and in 19 (8.5%) cases, the recurrence was described as in the lung, but it was not possible to distinguish between second primary, metastasis and local relapse from the records.
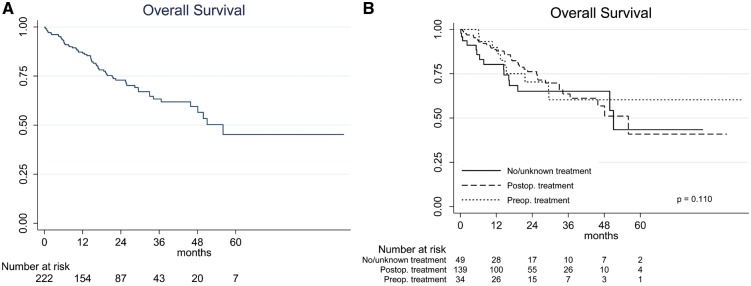
After a median follow-up of 18 (interquartile range 9–32) months, median survival for the 210 NSCLC patients was 51 months, with 3-year overall survival (OS) estimated at 61.2% (95% confidence interval 51.7–69.4%). OS was similar (log-rank P = 0.134) in the 3 adjuvant treatment groups (Fig. 1). Large tumour size, the presence of postoperative complications, advanced age and male sex were significantly associated with worse OS in the univariable analyses. Only large tumour size, the presence of postoperative complications and advanced age were associated with worse survival in the multivariable model (Table 4). Figure 2 shows that OS was worse (log rank P = 0.010) in patients with >2 involved lymph nodes when compared with those with ≤2 involved nodes, whereas OS did not differ between cases with clinically evident versus occult N2 disease or between cases with and without skip metastases.
Figure 1:
The Kaplan–Meier overall survival (OS) curves for non-small-cell lung cancer (NSCLC) patients. (A) OS for the entire NSCLC cohort. (B) OS according to whether patients received preoperative chemotherapy (which could also be associated with postoperative treatment), postoperative treatment only or no or unknown adjuvant treatment.
Table 4:
Univariable and multivariable analyses of overall survival for NSCLC
| Univariate analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Male versus female | 1.59 (0.95–2.66) | 0.076 | 1.65 (0.98–2.76) | 0.059 |
| Age (years) (continuous) | 1.04 (1.01–1.07) | 0.001 | 1.05 (1.02–1.08) | <0.001 |
| Clinical N2 versus occult N2 | 1.27 (0.72–2.09) | 0.455 | ||
| Adjuvant treatment | ||||
| Preoperative, possibly also postoperative | 1 | |||
| Postoperative only | 0.99 (0.49–1.99) | 0.967 | ||
| None | 1.90 (0.83–4.35) | 0.130 | ||
| FEV1% (continuous) | 0.995 (0.98–1.01) | 0.494 | ||
| Postoperative complication (yes versus no) | 1.89 (1.13–3.16) | 0.015 | 1. 17 (1.03–2.85) | 0.038 |
| Tumour type | ||||
| Adenocarcinoma | 1 | |||
| Squamous cell carcinoma | 1.15 (0.52–2.57) | 0.724 | ||
| Other | 1.33 (0.62–2.83) | 0.460 | ||
| N lymph nodes removed (continuous) | 1.01 (0.98–1.04) | 0.559 | ||
| N nodes positive (continuous) | 1.04 (1.01–1.08) | 0.026 | 1.03 (0.99–1.08) | 0.127 |
| Tumour size (continuous) | 1.21 (1.08–1.36) | 0.001 | 1.24 (1.10–1.40) | <0.001 |
| Skip metastases (yes versus no) | 0.87 (0.46–1.66) | 0.681 | ||
| Conversion (yes versus no) | 0.93 (0.44–1.97) | 0.852 | ||
CI: confidence interval; NSCLC: non-small-cell lung cancer; OR: odds ratio.
Figure 2:
The Kaplan–Meier overall survival (OS) curves for non-small-cell lung cancer (NSCLC) patients according to lymph node involvement. (A) OS according to the presence of clinically evident or occult lymph node metastasis. (B) OS according to the number of positive lymph nodes (≤2 and >2). (C) OS according to the presence or absence of skip metastases.
DISCUSSION
In this retrospective multicentre study of 223 patients with clinically evident or occult N2 lung cancer who underwent robot-assisted lung resection, we found that perioperative outcomes were highly encouraging. Only 2.7% (95% confidence interval 0.99–5.76%) of cases were converted to open surgery due to bleeding. A similar figure (vascular injuries in 2.9% and leading to conversion in 2.2%) was reported for VATS resections of 3076 lung cancer cases (all stages) archived in the European Society of Thoracic Surgeons database [23]. A somewhat lower proportion of bleeding complications (1.7% for both VATS and robot-assisted surgery) was reported for major lung resections archived in the US national database [24]. In the same database, the conversion rate did not differ significantly between patients who underwent surgery after neoadjuvant treatment versus those who underwent surgery before adjuvant treatment. In our series, large tumour size and >2 positive lymph nodes were associated with conversion to thoracotomy (univariate analysis only), suggesting that patients with more advanced disease should be informed of the greater risk of conversion to open surgery. However, only 3 of the 151 patients with unforeseen mediastinal nodal invasion required conversion to open surgery. This is an acceptable figure and suggests that unforeseen mediastinal lymph node involvement is not a major cause of conversion in robot-assisted surgery. A large retrospective analysis of lung cancers treated by VATS had suggested unforeseen mediastinal lymphadenopathy as a potential cause of conversion [22].
We also found that estimated 3-year OS (excluding carcinoids) was good at 61.2%. Patients with Stage III NSCLC constitute a heterogeneous group, and outcomes vary considerably. Factors reported to predict better survival include limited mediastinal lymph node disease, good response to induction therapy and the absence of comorbidities [25]. The favourable outcomes in our NSCLC patients are likely to be in part due to the fact that two-thirds had clinically occult nodal involvement, with relatively good prognosis; furthermore, all underwent preoperative PET and a large proportion probably had routine brain imaging.
Only a small proportion (15%) of our series received preoperative chemotherapy, because a large proportion (142/223, 63%) had occult N2 disease, which was only detected intraoperatively. However, a further 47 cases had suspected or proven N2 disease but were intentionally operated on before chemotherapy, in accordance with the treatment policy of one of the participating centres (Moffitt Cancer Center, Tampa, FL, USA). Because our study was retrospective, we did not have reliable data on how many N2 cases had microscopic involvement and how many were truly locally advanced with involvement of nearby structures.
To our knowledge, 3 articles have presented data on the use of VATS to treat locally advanced NSCLC [18–20], and 2 articles have described robot-assisted surgery for locally advanced disease [15, 21]. Data on these 5 studies, in comparison with the findings of this study, are summarized in Table 5. The study by Petersen et al. [18] reported on the feasibility and safety of VATS lobectomy in 12 patients who received preoperative chemotherapy and/or radiotherapy for locally advanced disease. One case was converted to open surgery. The authors concluded that VATS was safe, that outcomes were relatively good and that length of hospital stay (∼2 days) was shorter than for the 85 thoracotomy cases. However, the small size of the VATS group and smaller average tumour size in this group were study limitations.
Table 5:
Publications on locally advanced lung cancer treated using minimally invasive surgery
| Publication | Year | VATS/robot/ open (n) | Stage III (n) | Preoperative chemotherapy (n) | Duration of surgery (min) | N lymph nodes removed (median) | Postoperative complications (%) | Length of stay (days), mean | Mortality (%) | 3-Year OS |
|---|---|---|---|---|---|---|---|---|---|---|
| Petersen et al. [19] | 2006 | 12/0/85 | 3 | 12 | NA | 6.1 | 16.6 | 3.5 | 0 (30-day) | 27 monthsa |
| Nakanishi et al. [20] | 2014 | 76/0/0 | 33 | 12 | 327 | 16 | 35 | 14 | 2.6% (hospital) | 36.2% |
| Hennon et al. [21] | 2011 | 125/0/0 | 16 | 18 | 231 | NA | 35 | 4 | 1% (hospital) | 62% |
| Park et al. [15] | 2016 | 14/17/397 | 11 | All | NA | 5 (stations) | 32 | 4 | NA | 48% |
| Dylewski et al. [22] | 2011 | 0/13/58 | 15 | 15 | 121 | 6 (stations) | NA | 3 | NA | NA |
| This article | 2018 | 0/223/0 | 223 | 34 | 198 | 13 | 33 | 5 | 1.9% (30-day) | 63% |
Median survival.
NA: not available (not reported); OS: overall survival.
Nakanishi et al. [19] retrospectively reviewed data on 76 patients with Stage II or Stage III NSCLC treated by VATS. Induction treatment was given to 12 patients. Mean duration of surgery was 327 min, but 36% of cases underwent resection of adjacent structures. A mean of 16 lymph nodes was removed. In-hospital mortality was 2.6%. In the 33 cases with Stage III disease, 3-year survival was 36.2%.
Hennon et al. [20] reported on 16 Stage III patients treated using induction chemotherapy and VATS. Perioperative outcomes were good, and 3-year OS was around 62%, which is similar to the OS in our series.
The 2016 article by Park et al. [21] retrospectively assessed 428 patients with locally advanced NSCLC, with 397 treated by thoracotomy, 17 by robot-assisted surgery and 14 by VATS. Oncological outcomes were similar in the open and minimally invasive groups, but the latter had a significantly shorter length of hospital stay.
The 2011 article by Dylewski et al. [15] reported on optimal perioperative outcomes with robot-assisted surgery to treatment 15 patients with locally advanced NSCLC pretreated using chemotherapy and/or chemoradiation therapy.
Large central tumours, multistation lymph node involvement and previous irradiation render the surgical management of locally advanced lung cancers highly challenging; as a result, outcomes are variable. Nevertheless, in selected patients with Stage III NSCLC and good prognostic factors, minimally invasive surgery has been reported as safe and effective [19]. Our experience with the N2 cases of this series, particularly the 34 patients who received preoperative chemotherapy, further supports this conclusion. Among these 34 patients, 8 of whom also received preoperative radiotherapy, an R0 resection was achieved in all, none had a blood transfusion or required conversion for bleeding and only 12% had Grade III–IV postoperative complications. However, we do not know the proportion of N2 patients (treated at the same time) who received preoperative chemotherapy plus radiotherapy and proceeded directly to open surgery, emphasizing the highly selected nature of our series. It should also be noted that we have excluded cN2 patients who were downstaged to ypN1 or ypN0, so as to study a homogenous cohort of pN2 cases: ypN1 and ypN0 cases may have had better outcomes.
Limitations
The main limitations of our study arise from its retrospective nature. Thus, the proportion of R0 resections carried out according to the International Association for the Study of Lung Cancer (IASLC) specifications [26] could not be reconstructed because data on lymph nodes removed in fragments, the highest station was involved and the presence/absence of extracapsular invasion in positive nodes were not available. We were also unable to assess the proportion of patients with NSCLC in the left upper lobe in whom Station 4L was removed. Nevertheless, in 97% of our series, at least 6 lymph nodes were removed in accordance with the European Society of Thoracic Surgeons (ESTS)/IASLC guidelines, although we were unable to ascertain whether at least 3 nodes were removed from mediastinal stations. This contrasts with a recent article on nodal staging during surgical resection of NSCLC [27] in which at least 6 lymph nodes were removed in only 53% of cases.
The strengths of our study are that it investigated a consecutive series of patients with sufficiently long follow-up (median 18 months) with all patients being staged preoperatively with PET and all receiving complete thoracic node dissection.
To our knowledge, this is the largest series of patients with pN2 disease treated robotically although some patients are also reported on in a recent article on long-term survival in NSCLC that places considerable emphasis on N2 cases [27]. The low conversion rate for bleeding, low 30-day mortality and 90-day mortality (zero for neoadjuvant cases) and good 3-year OS suggest that the robotic approach to locally advanced lung cancer is safe and effective.
ACKNOWLEDGEMENTS
We thank Don Ward for help with the English and the Umberto Veronesi Foundation for fellowships to Pierluigi Novellis and Elisa Dieci.
Conflict of interest: Giulia Veronesi has received honoraria from Ab Medica SpA, Medtronic and Verb Medical. Bernard Park was the past proctor for Intuitive Surgical and has received honoraria from Bard Medical, Baxter and Covidien. Robert Cerfolio is a teacher for Intuitive Surgical, C-SATS, Bovie, Ethicon, Covidien, Myriad, Community Health Services and Bard Medical. Mark Dylewski is a clinical educator for Intuitive Surgical and has received honoraria from Verb Medical, Ethicon and Bard Corp. Eric M. Toloza is a consultant for Medtronic and an observation site for Intuitive Surgical. All other authors have declared no conflict of interest.
Footnotes
Presented at the 25th European Conference on General Thoracic Surgery, Innsbruck, Austria, 28–31 May 2017.
REFERENCES
- 1. Demmy TL, Curtis JJ.. Minimally invasive lobectomy directed toward frail and high-risk patients a case-control study. Ann Thorac Surg 1999;68:194–200. [DOI] [PubMed] [Google Scholar]
- 2. Hoksch B, Ablassmaier B, Walter M, Muller JM.. Complication rate after thoracoscopic and conventional lobectomy. Zentralblatt Fur Chirurgie 2003;128:106–10. [DOI] [PubMed] [Google Scholar]
- 3. Nakata M, Saeki H, Yokoyama N, Kurita A, Takiyama W, Takashima S.. Pulmonary function after lobectomy video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2000;70:938–41. [DOI] [PubMed] [Google Scholar]
- 4. Nomori H, Ohtsuka T, Horio H, Naruke T, Suemasu K.. Difference in the impairment of vital capacity and 6-minute walking after a lobectomy performed by thoracoscopic surgery, an anterior limited thoracotomy, an anteroaxillary thoracotomy, and a posterolateral thoracotomy. Surg Today 2003;33:7–12. [DOI] [PubMed] [Google Scholar]
- 5. Yim AP, Wan S, Lee TW, Arifi AA.. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 2000;70:243–7. [DOI] [PubMed] [Google Scholar]
- 6. Li WW, Lee RL, Lee TW, Ng CS, Sihoe AD, Wan IY. et al. The impact of thoracic surgical access on early shoulder function video-assisted thoracic surgery versus posterolateral thoracotomy. Eur J Cardiothorac Surg 2003;23:390–6. [DOI] [PubMed] [Google Scholar]
- 7. Seder CW, Salati M, Kozower BD, Wright CD, Falcoz PE, Brunelli A. et al. Variation in pulmonary resection practices between the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons General Thoracic Surgery Databases. Ann Thorac Surg 2016;101:2077–84. [DOI] [PubMed] [Google Scholar]
- 8. McKenna RJ Jr, Wolf RK, Brenner M, Fischel RJ, Wurnig P.. Is VATS lobectomy an adequate cancer operation? Ann Thorac Surg 1998;66:1903–8. [DOI] [PubMed] [Google Scholar]
- 9. Leschber G, Holinka G, Linder A. . Video-assisted mediastinoscopic lymphadenectomy (VAMLA)—a method for systematic mediastinal lymph node dissection. Eur J Cardiothorac Surg 2003;24:192–5. [DOI] [PubMed] [Google Scholar]
- 10. Park BJ, Flores RM, Rusch VW.. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54–9. [DOI] [PubMed] [Google Scholar]
- 11. Veronesi G, Galetta D, Maisonneuve P, Melfi F, Schmid RA, Borri A. et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19–25. [DOI] [PubMed] [Google Scholar]
- 12. Cerfolio RJ, Bryant AS, Skylizard L, Minnich DJ.. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740–6. [DOI] [PubMed] [Google Scholar]
- 13. Melfi FM, Mussi A.. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 2008;18:289–95, vi–vii. [DOI] [PubMed] [Google Scholar]
- 14. Giulianotti PC, Buchs NC, Caravaglios G, Bianco FM.. Robot-assisted lung resection: outcomes and technical details. Interact CardioVasc Thorac Surg 2010;11:388–92. [DOI] [PubMed] [Google Scholar]
- 15. Dylewski MR, Ohaeto AC, Pereira JF.. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36–42. [DOI] [PubMed] [Google Scholar]
- 16. Louie BE, Farivar AS, Aye RW, Vallières E.. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598–604. [DOI] [PubMed] [Google Scholar]
- 17. Yan TD, Cao C, D'Amico TA, Demmy TL, He J, Hansen H. et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633–9. [DOI] [PubMed] [Google Scholar]
- 18. Petersen RP, Pham D, Toloza EM, Burfeind WR, Harpole DH Jr, Hanish SI. et al. Thoracoscopic lobectomy: a safe and effective strategy for patients receiving induction therapy for non-small cell lung cancer. Ann Thorac Surg 2006;82:214–18. [DOI] [PubMed] [Google Scholar]
- 19. Nakanishi R, Fujino Y, Yamashita T, Shinohara S, Oyama T.. Thoracoscopic anatomic pulmonary resection for locally advanced non-small cell lung cancer. Ann Thorac Surg 2014;97:980–5. [DOI] [PubMed] [Google Scholar]
- 20. Hennon MW, Demmy TL.. Video-assisted thoracoscopic surgery (VATS) for locally advanced lung cancer. Ann Cardiothorac Surg 2012;1:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park BJ, Yang HX, Woo KM, Sima CS.. Minimally invasive (robotic assisted thoracic surgery and video-assisted thoracic surgery) lobectomy for the treatment of locally advanced non-small cell lung cancer. J Thorac Dis 2016;8:S406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dindo D, Demartines N, Clavien PA.. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Decaluwe H, Petersen RH, Hansen H, Piwkowski C, Augustin F, Brunelli A, et al. ; ESTS Minimally Invasive Thoracic Surgery Interest Group (MITIG). Major intraoperative complications during video-assisted thoracoscopic anatomical lung resections: an intention-to-treat analysis. Eur J Cardiothorac Surg 2015;48:588–98. [DOI] [PubMed] [Google Scholar]
- 24. Kent M, Wang T, Whyte R, Curran T, Flores R, Gangadharan S.. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236–42. [DOI] [PubMed] [Google Scholar]
- 25. Yoon SM, Shaikh T, Hallman M.. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol 2017;8:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edwards T, Balata H, Elshafi M, Foden P, Bishop P, Fontaine E. et al. Adequacy of intraoperative nodal staging during surgical resection of NSCLC: influencing factors and its relationship to survival. J Thorac Oncol 2017;12:1845–50. [DOI] [PubMed] [Google Scholar]
- 27. Cerfolio RJ, Ghanim AF, Dylewski M, Veronesi G, Spaggiari L, Park BJ.. The long-term survival of robotic lobectomy for non-small cell lung cancer: a multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]