Abstract
Background and Purpose
Chronic impairment of the arm and hand is a common consequence of stroke. Animal and human studies indicate that brief bursts of vagus nerve stimulation (VNS) in conjunction with rehabilitative training improves recovery of motor function after stroke. In this study, we tested whether VNS could promote generalization, long-lasting recovery, and structural plasticity in motor networks.
Methods
Rats were trained on a fully automated, quantitative task that measures forelimb supination. Upon task proficiency, unilateral cortical and subcortical ischemic lesions were administered. One week after ischemic lesion, rats were randomly assigned to receive six weeks of rehabilitative training on the supination task with or without VNS. Rats then underwent four weeks of testing on a task assessing forelimb strength to test generalization of recovery. Finally, the durability of VNS benefits was tested on the supination task two months after the cessation of VNS. Following the conclusion of behavioral testing, viral tracing was performed to assess synaptic connectivity in motor networks.
Results
VNS enhances plasticity in corticospinal motor networks to increase synaptic connectivity to musculature of the rehabilitated forelimb. Adding VNS more than doubled the benefit of rehabilitative training, and the improvements lasted months after the end of VNS. Pairing VNS with supination training also significantly improved performance on a similar, but untrained task that emphasized volitional forelimb strength, suggesting generalization of forelimb recovery.
Conclusions
This study provides the first evidence that VNS paired with rehabilitative training after stroke 1) doubles long-lasting recovery on a complex task involving forelimb supination, 2) doubles recovery on a simple motor task that was not paired with VNS and 3) enhances structural plasticity in motor networks.
Keywords: Rehabilitation, Vagal Nerve Stimulation, Ischemic Stroke, Vagus Nerve, Generalization, Neuroplasticity
Subject Terms: Animal Models of Human Disease, Ischemic Stroke, Rehabilitation
Introduction
Neuroplasticity supports recovery after stroke, thus strategies that enhance plasticity in conjunction with rehabilitation hold promise in improving recovery. Recently, vagus nerve stimulation (VNS) paired with motor training has emerged as a strategy to drive robust and long-lasting plasticity1. VNS provides precisely-timed engagement of neuromodulatory systems during rehabilitation to drive plasticity in motor networks2,3. In multiple rat models of stroke, VNS paired with rehabilitative training significantly improved recovery of forelimb movement speed and volitional strength compared to training without VNS1,4–7.
A pilot study in chronic stroke patients reported that pairing VNS with rehabilitative exercises tripled recovery compared to rehabilitative training without VNS. Patients that received VNS paired with standard rehabilitative exercises demonstrated a significant increase in upper extremity Fugl-Meyer (UE-FM) score, even though VNS was never paired with UE-FM testing. These clinical results suggest that the beneficial effects of VNS might generalize to untrained movements. In this study, we tested the hypothesis that VNS-dependent functional improvements would generalize to untrained tasks. Furthermore, we also assessed the durability of the enhanced functional recovery.
The neural mechanism underlying VNS-supported stroke recovery is currently unknown. The corticospinal tract (CST) is the main pathway from cortical motor networks to spinal circuits8, and is a key component in motor dysfunction following stroke9. We used retrograde transneuronal tracing to test the hypothesis that VNS drives plasticity in the CST10.
Materials and Methods
Additional methodological details are included in the Supplemental Information. The data that support the findings of this study are available from the corresponding author upon request.
Subjects
Thirty-one adult female Sprague-Dawley rats (Charles River) weighing approximately 250g throughout the study were used. All rats were maintained above 85% of their average body weight for their specific age. The rats were housed in a 12:12 reversed light cycle environment, and behavioral training was performed during the dark cycle to increase daytime activity levels. All handling, housing, surgical, and behavioral training procedures were approved by the University of Texas at Dallas Institutional Animal Care and Use Committee.
Supination assessment task behavioral testing
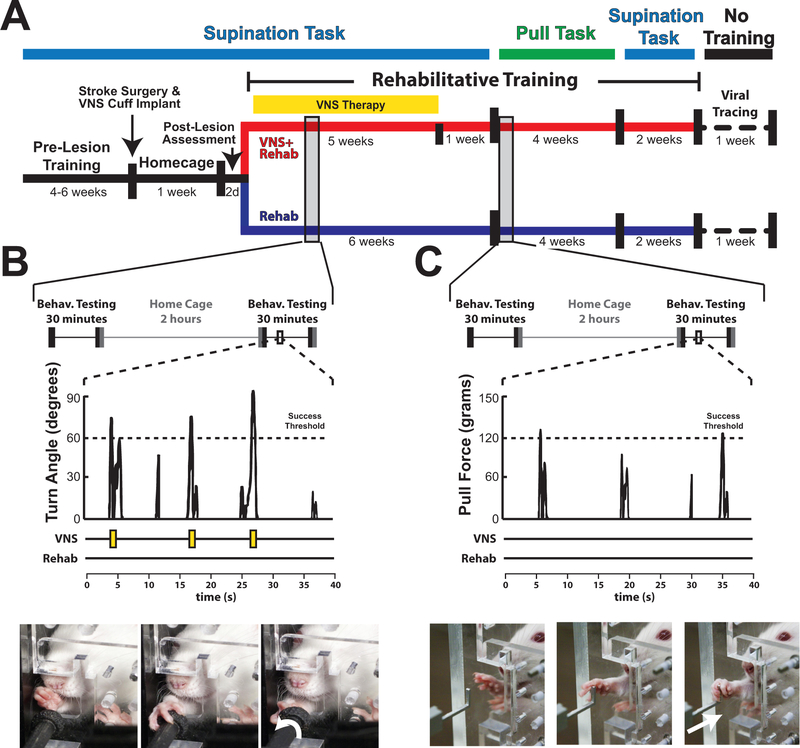
The supination assessment task was performed as previously described11. In brief, animals were trained to reach out of a slot, grasp and rotate a spherical manipulandum clock-wise. The manipulandum was affixed to a rotary encoder that provided turn angle measurements. Control software adaptively scaled the success turn angle thresholds based on the median of the preceding 10 trials to a maximum turn angle threshold of 60 degrees. Training continued until animals achieved a 75% success rate, defined as trials in which the turn angle exceeded 60 degrees, averaged across six consecutive training sessions. All animals then underwent unilateral ischemic lesions.
No behavioral testing was conducted for the 7 days following lesion. Following this seven day recovery period, animals were re-assessed on the supination task for 4 sessions with at least 50 trials each session, with this data being used for the “POST” time point in all analyses.
Unilateral ischemic lesions
Unilateral ischemic lesions of primary motor cortex and dorsolateral striatum were administered similar to previously described5,12. Rats were anesthetized with ketamine hydrochloride (50mg/kg, i.p.), xylazine (20 mg/kg, i.p.), and acepromazine (5 mg/kg). A 26-gauge Hamilton Syringe was used to inject Endothelin-1 (Bachem, Torrence, CA, 1mg/mL in saline) at the following cortical locations: anteriorposterior 2.5mm, 1.5mm, 0.5mm, −0.5mm, and mediolateral 2.5mm and 3.5mm relative to bregma, at a depth of 1.8mm. An additional injection site at 3.0mm lateral and 0mm anteriorposterior from bregma at a depth of 6mm was administered to target the dorsolateral striatum. All injections consisted of 1uL endothelin-1 delivered over 2 minutes.
Vagus nerve cuff implant and stimulation delivery
Following ischemic lesions, vagus nerve stimulating cuffs were implanted as previously described6 in all animals. In brief, a stimulating cuff electrode was placed around the left cervical vagus nerve and attached to a connector anchored to the skull. Following the lesion and VNS implant surgery, all animals were administered enrofloxacin (Baytril: 10 mg/kg) and buprenorphine (Buprenex: 0.03 mg/kg). Animals then remained in their homecage for one week.
After the homecage recovery period, rehabilitative training on the supination task commenced for six weeks. All rats received equivalent rehabilitative training, which consisted of freely performing the behavioral task. Rats in the VNS+Rehab group received stimulation paired with successful trials during first five weeks of rehabilitation (Fig. 1B). The software monitoring the rotary encoder provided a trigger signal to the isolated pulse stimulator (AM Systems, Isolated Pulse Stimulator, Sequim, WA) to administer VNS immediately when the rotary encoder crossed the adaptively scaled turn angle threshold (Fig. 1B; Supplementary Video IV). All rats in the Rehab group were similarly connected to the stimulator, but a trigger signal was not sent to the pulse stimulator during training. As in previous studies, stimulation consisted of a 500ms train of pulses at 30 Hz, and each biphasic pulse was 0.8mA in amplitude and 100 ms in pulse duration5.
Figure 1.
Experimental design and methods. (A) Timeline of experiment. (B) Daily rehabilitative training paradigm on the supination task. Subjects in the VNS+Rehab group received VNS immediately upon crossing the turn angle success threshold. An illustration of an animal performing the supination task is shown below. (C) Daily rehabilitative training on the isometric pull task. No VNS was delivered at any point during isometric pull training. Illustration of an animal performing the isometric pull task is shown below.
Treatment group assignment and exclusion criteria
The first rats were randomized into treatment groups (N=6 Rehab; N=8 VNS+Rehab). The remaining rats were dynamically allocated to balanced groups based on maximal post-lesion turn angle to ensure equivalent baselines for comparison (N=4 Rehab; N=5 VNS+Rehab). All behavioral testing was performed by blinded experimenters and all behavioral analysis was automated to eliminate bias. 12 rats were excluded from this study based upon the following exclusion criteria: (1) Did not survive the ischemic lesion and VNS implant (N=4); (2) Did not display at least a 50% reduction in success rate (N=4); (3) Headcap or stimulating cuff failure (N=4). 8 of the 12 exclusions (1&2) were done prior to group assignment and thus could not impact interpretation of results. Data including animals with headcap or cuff failures are included in the supplementary data (Fig. I) as an intent-to-treat analysis, and exclusion had minimal effects on statistical comparisons.
Isometric pull task behavioral testing
Behavioral testing on the isometric pull task commenced on Week 7 after the completion of rehabilitative training on the supination task. Isometric pull testing was performed as previously described13. Animals were trained to reach through a slot in the behavioral chamber and pull on an aluminum pull handle affixed to a force transducer. The adaptive thresholding algorithm used the median maximal pull force of the preceding 10 trials, with a 10-gram minimum and 120-gram maximum adaptive threshold bounds. Success rate was defined as the percentage of trials greater than the maximum threshold (120 grams).
Pseudorabies virus injections and analysis
Retrograde transneuronal tracer injections using pseudorabies virus (PRV-152), kindly provided by Dr. Lynn Enquist (Princeton University, Princeton, NJ; Virus Center Grant P40OD010996), were performed in a subset of rats (Rehab N=5; VNS N=5: Two subjects died during PRV surgical procedure resulting in group sizes of Rehab N=4; VNS N=4) after the conclusion of behavioral testing on week 13 post-lesion (Fig. 1A). Animals were deeply anesthetized and an incision was made over the medial face of the radius and ulna of the trained right forelimb to expose the flexor digitorum profundus (FDP) and palmaris longus (PL), the main extrinsic forelimb grasping muscles in the rat. 15 μL of PRV-152 was injected into the belly of each muscle in three separate injections of 5 μL. The skin was then sutured, rinsed with saline, and treated with antibiotic ointment. The PRV-152 used in this study was ~8.5 × 108 plaque-forming units (PFU). At 144 hours post-injection, rats were transcardially perfused with 4% paraformaldehyde in 0.1 M PBS (pH 7.5). The brain and spinal cord were removed, post-fixed overnight, then cryoprotected in 30% sucrose.
The brain was blocked and frozen at −80°C in Shandon M1 embedding matrix (Thermo Fisher Scientific; Waltham, MA). Forebrain blocks were cryosectioned at 35 μm and immediately slide-mounted. Slides were coverslipped then scanned at 10× magnification using the Virtual Slide Microscope VS120 (Olympus Life Sciences Solutions; Waltham, MA). PRV-152 neurons were visually identified by an experimenter blinded to treatment group and counted on every other forebrain section restricted to layer V sensorimotor cortex. All experimenters processing the tissue and analyzing cell counts were blinded to the group of the animal.
Statistics
All data are represented as mean ± SEM. All comparisons were planned in the experimental design a priori, and significant differences were determined using unpaired t-tests (Fig. 2D; Fig. 3C) and two-way repeated measures ANOVAs followed by unpaired t-tests (Fig. 2A&B). Confidence intervals for all comparisons are provided in the text and online supplement. Alpha level was set to 0.05 for single comparisons. Based on an effect size of 1.342 from a previous study6, 10 rats per group would yield a power of 0.95 at an alpha of 0.05. In all figures, * indicates p<0.05, ** indicates p<0.01, and *** indicates p<0.001. Error bars indicate mean ± SEM in all figures.
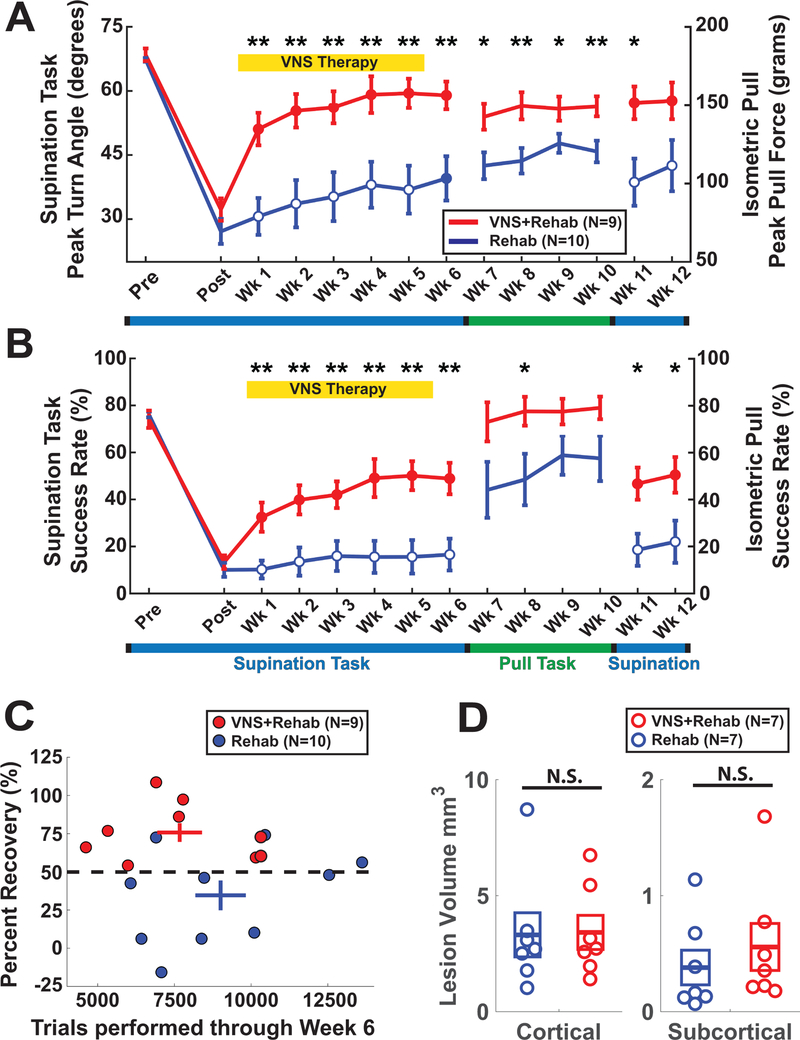
Figure 2.
VNS paired with rehabilitative training improves forelimb function after stroke. (A) VNS improves recovery of supination turn angle during task-oriented rehabilitative training (Post-Wk6). The beneficial effects of VNS delivered on the supination task transfer to the isometric pull task although no VNS is delivered during this time (Wk7-Wk10). Furthermore, when subjects are retested on the supination task, the benefits of VNS were maintained 7 weeks following the cessation of stimulation (Wk11 & 12). (B) Similar effects were observed for success rate. (C) All subjects (9 of 9) in the VNS+Rehab group achieved at least 50% recovery of function, compared to only 3 of 10 Rehab subjects. No differences in trials performed through Week 6 were observed across groups. (D) No significant difference in cortical or subcortical lesion volume was observed. Asterisks denote: *p<0.05, **p<0.01, ***p<0.001 between the VNS+Rehab and Rehab groups. Filled circles denote significant difference compared to Post timepoint.
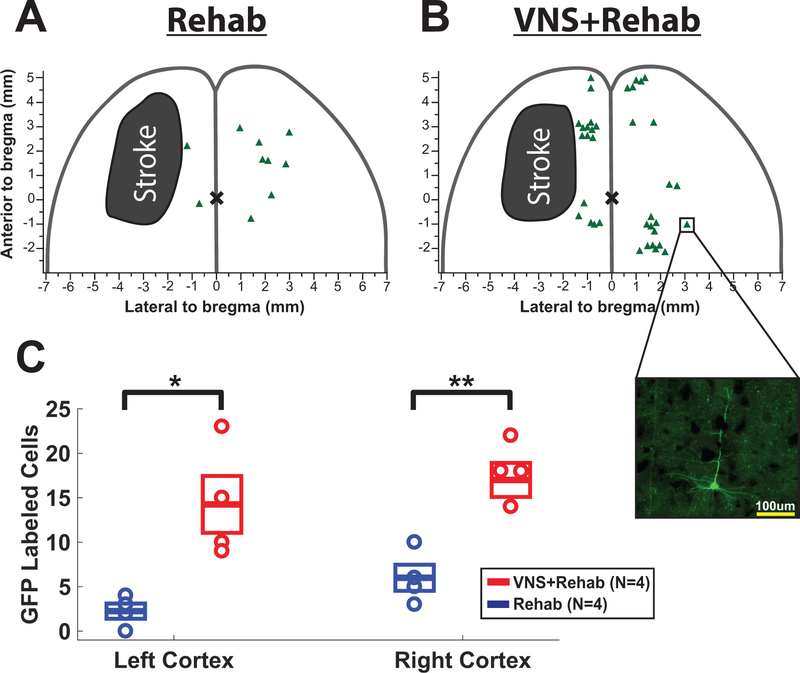
Figure 3.
VNS paired with rehabilitative training increases synaptic connectivity in motor networks. (A) Representative cortical lesion location and PRV labeled cells from a Rehab subject and (B) a VNS subject. Inset illustrates a PRV-positive cell. (C) VNS paired with rehabilitative training increases cortical PRV labeling in both the left and right sensorimotor cortices.
Results
Stroke impairs forelimb supination
Rats were trained on the supination task and reached proficiency within 21 ± 3 days. Both groups of animals were highly proficient at the task and no significant differences between groups were observed (Fig. 2A&B; Supplemental Video I).
Ischemic lesions substantially reduced performance on the supination task (Supplemental Video II). One week post-lesion, both groups exhibited significant reductions in peak turn angle (Fig. 2A; Rehab: paired t-test POST vs. PRE, p=9.5 × 10−8; VNS+Rehab: paired t-test POST vs. PRE, p=1.85 × 10−6) and success rate (Fig. 2B; Rehab: paired t-test POST vs. PRE, p=9.5 × 10−9; VNS+Rehab: paired t-test POST vs. PRE, p=7.17 × 10−7). No difference was observed between groups (Fig. 2; Post: Peak Turn Angle: unpaired t-test, p=0.21; Success Rate: unpaired t-test, p=0.45).
VNS paired with rehabilitative training improves forelimb rotational function
First we tested whether VNS paired with rehabilitative supination training improves recovery of forelimb rotational function. Animals in the VNS+Rehab group underwent identical rehabilitative training as the Rehab group (Fig. 2C), but brief 500 ms trains of VNS were delivered coincident with successful trials (Fig. 1B; Supplemental Video III & IV).
VNS+Rehab significantly improved recovery of peak turn angle compared to Rehab, consistent with an enhanced recovery of forelimb supination (Fig. 2B; Two-way repeated measures ANOVA, Wk1–6, F[6, 102] = 4.60, p=3.61 × 10−4). Significantly improved performance was observed during all 6 weeks of therapy (VNS+Rehab vs. Rehab, unpaired t-test, p<0.01 for Weeks 1–6). Additionally, VNS+Rehab improved success rate compared to Rehab alone (Fig. 2B; Two-way repeated measures ANOVA (Interaction effect of time × group), F[6, 102] = 5.12, p=1.22 × 10−4). Post hoc tests revealed significant improvements during all weeks of therapy (VNS+Rehab vs. Rehab, unpaired t-test, p<0.01 for Weeks 1–6). The number of trials performed did not differ between groups (Fig. 2C; Wk1-Wk6; Rehab: 9006 ± 824 trials (CI: ±1865); VNS: 7673 ± 730 trials (CI: ±1683); unpaired t-test, p=0.38), suggesting that the VNS improvement cannot be accounted for by training intensity. Taken together, these results indicate that VNS paired with rehabilitative training significantly improves forelimb rotational function compared to rehabilitative training alone.
Benefits of VNS therapy generalize to untrained tasks
To test if the benefits of VNS generalize to a similar, untrained task, we next evaluated forelimb strength of all subjects using the isometric pull task. The isometric pull task requires similar reach and grasp motion as the supination task, but emphasizes volitional forelimb strength rather than forelimb supination (Fig. 1B&C). Neither group received VNS during this phase of testing (Fig. 1C).
Animals that previously received VNS paired with rehabilitative training on the supination task performed significantly better on the isometric pull task compared to animals that underwent rehabilitative training without VNS (Fig. 2A; Two-way repeated measures ANOVA, Weeks 7–10; F[1, 17] = 8.44, p=9.86 × 10−3). Post-hoc comparisons revealed significantly improved peak pull force during Weeks 7–10 (Rehab vs. VNS+Rehab, unpaired t-test, p < 0.05 during Weeks 7–10). Similar improvements were observed in success rate (Fig. 2B; Two-way repeated measures ANOVA; F[1, 17] = 4.45, p=0.04; unpaired t-test, p=0.03 during Week 7). These results indicate that previous VNS-dependent benefits generalize to similar untrained tasks.
Benefits of VNS therapy are maintained for multiple weeks
After the completion of generalization testing during Weeks 7–10, animals returned to the supination assessment task for an additional two weeks of testing (Fig. 1A). No reduction in peak turn angle or success rate was observed in the VNS+Rehab group (Fig. 2; Weeks 5–6 & 11–12, One-way repeated measures ANOVA, Peak Turn Angle: F[3, 24] = 0.40, p=0.84; Success Rate: F[3, 24] = 0.28, p=0.84). Subjects in the VNS+Rehab group remain significantly improved compared to the Rehab group for both turn angle (Fig. 2A; Weeks 11&12, Two-way repeated measures ANOVA, F[1, 17] = 10.90, p=4.21 × 10−3) and success rate (Fig. 2B; Weeks 11&12, Two-way repeated measures ANOVA, F[1,17] = 12.76, p=2.34 × 10−3). These results indicate that the benefits of VNS are maintained for up to 7 weeks following the cessation of stimulation.
VNS does not affect lesion size
Consistent with previous studies4–6, no significant difference in cortical (Fig. 2D; Rehab: 3.31 ± 0.95 mm3 (CI: ±2.32); VNS+Rehab: 3.41 ± 0.73 mm3 (CI: ±1.80); unpaired t-test, p=0.93) or subcortical lesion size (Fig. 2D; Rehab: 0.38 ± 0.15 mm3 (CI: ±0.37); VNS+Rehab: 0.56 ± 0.20 mm3 (CI: ±0.50); unpaired t-test, p=0.49) was observed between groups. Improved recovery in the absence of a reduction in lesion extent is consistent with the notion that VNS improves recovery by enhancing plasticity in motor networks1.
VNS alters cortical synaptic connectivity to musculature of the trained forelimb
Plasticity in descending motor networks controlling task-relevant forelimb muscles is associated with recovery after stroke10,14. Therefore, we next tested the hypothesis that VNS paired with rehabilitative training improves recovery by increasing synaptic connectivity within motor networks controlling the grasping muscles of the forelimb. We injected the retrograde transsynaptic tracer pseudorabies virus (PRV-152) into the extrinsic digit flexors of the rehabilitated (right) forelimb and counted labeled cortical neurons six days later. VNS resulted in a six-fold increase in labeled sensorimotor cortical neurons in the lesioned (left) hemisphere (Fig. 3C; VNS+Rehab, 14.25 ± 3.20 PRV-positive cells (CI: ±10.18); Rehab, 2.25 ± 0.85 PRV-positive cells (CI: ±2.72); unpaired t-test, p=0.011), and a three-fold increase in the unlesioned (right) hemisphere (Fig. 3C; VNS+Rehab, 17.0 ± 1.91 PRV-positive cells (CI: ±6.09); Rehab, 6.0 ± 1.47 PRV-positive cells (CI: ±4.68); unpaired t-test, p=0.004). These results demonstrate that VNS paired with rehabilitative training enhances synaptic connectivity in descending motor circuits after stroke.
Discussion
In this study we evaluated whether VNS paired with rehabilitative training could improve recovery of forelimb rotational function and if the functional benefits generalize to an untrained forelimb task. We find that VNS paired with rehabilitative training more than doubles recovery of supination function compared to extensive rehabilitative training alone. Furthermore, subjects that received VNS during training on a task emphasizing forelimb supination displayed significantly improved performance on a separate task measuring forelimb strength compared to subjects undergoing training without VNS. Recovery of supination function persists for at least 7 weeks following cessation of VNS, indicative of robust and long-lasting improvements of forelimb function. Moreover, we provide the first evidence that VNS paired with rehabilitative training promotes plasticity after stroke. These findings support VNS therapy as an effective post-stroke intervention and provide insight into the neural mechanisms that may subserve recovery.
The ability to supinate the forearm is essential for dexterous hand function. Loss of supination ability is a major contributor to post-stroke disability, and recovery is limited even after rehabilitation in both rodents15,16 and humans17,18. The development of strategies to improve recovery of forearm rotational function is critical to reduce post-stroke disability. Previous studies demonstrate that VNS improves some metrics of recovery after stroke4–6, but it is unknown if VNS could improve recovery of fine movements, like supination, that are generally resistant to rehabilitation. Consistent with previous literature, the present study shows that intensive task-oriented rehabilitation yields only modest recovery of forelimb supination function11,19. Adding VNS to rehabilitative training more than doubled recovery. These results suggest that pairing VNS with a range of rehabilitative exercises that incorporate rotational movements may be beneficial for patients.
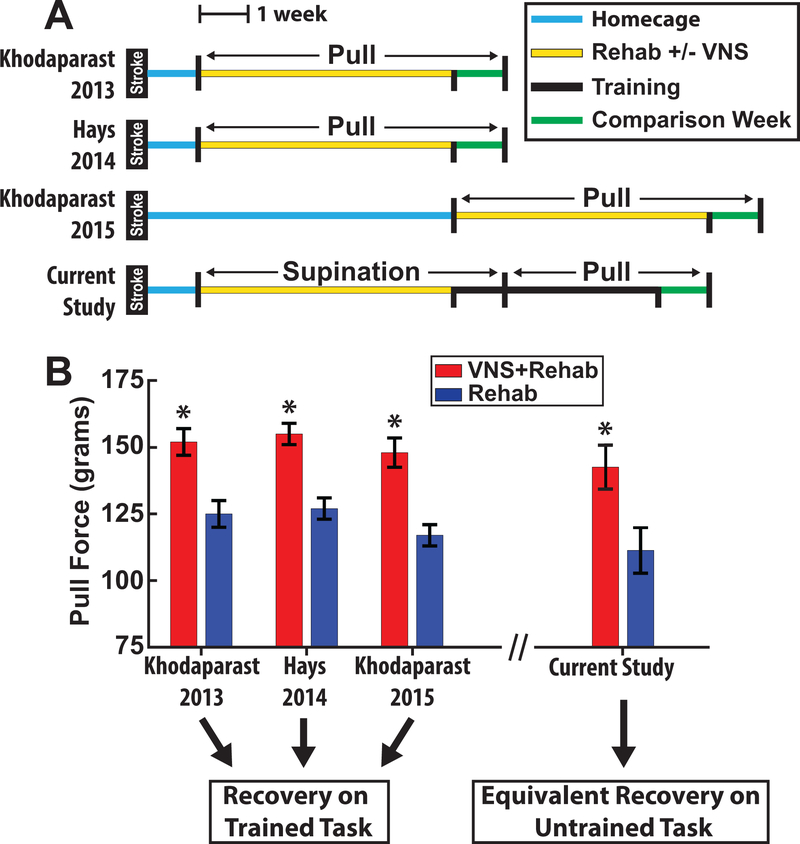
Generalization of recovery to non-rehabilitated tasks is an important consideration for translating potential rehabilitative therapies to clinical use. In practical terms, assuming a fixed amount of time with a physical therapist, it would be useful to determine whether a patient should receive a greater number of VNS pairings on a more restricted set of exercises or a greater breadth of rehabilitative exercises with fewer VNS pairings on each. Generalization of stroke recovery in previous studies have been mixed, with some studies demonstrating that task oriented recovery compromises performance on untrained tasks20,21, while other studies demonstrate a transfer of benefits22,23. VNS-dependent plasticity is highly specific to the paired event1; therefore, it was unclear if enhancement of recovery would be restricted to the trained exercise. We find that VNS paired with the supination task substantially improved subsequent performance on the isometric pull task. Importantly, no animals received VNS during pull task training, indicating that the VNS-mediated improvements are likely attributed to improved forelimb motor control. In the context of previous work, animals in this study that received VNS with supination training recovered to similar levels on the isometric pull task as animals that received VNS during rehabilitation on the isometric pull task (Fig. 4), providing strong evidence of generalization. The improved function driven by VNS in the absence of task-directed training on the pull task is consistent with the notion that VNS augments spontaneous biological recovery (SBR)24. SBR strongly predicts recovery after stroke25, and the influence of VNS on SBR may suggest that benefits would generalize beyond specifically rehabilitated tasks in stroke patients. It is important to note that both the supination and isometric pull tasks involve engagement of the distal forelimb, thus the degree of generalization to particularly dissimilar tasks (e.g., ladder rung, gait analyses) remains unclear. Despite these limitations, these results provide insight for the development of rehabilitative paradigms for future clinical trials.
Figure 4.
VNS delivered on supination training results in equivalent recovery on the isometric pull task compared to previous studies in which VNS was delivered during pull training. (A) Experimental timelines of previous VNS stroke studies and the current study. (B) Pull forces of the VNS+Rehab and Rehab groups. Previous studies demonstrate recovery on the isometric pull task in which VNS was delivered. The current study extends these findings by showing equivalent recovery on the isometric pull task despite that VNS was delivered only during supination training.
The durability of improvements following cessation of VNS is a key consideration in translating VNS therapy for stroke recovery to the clinic. Previous studies in multiple models of neurological injury demonstrate that VNS therapy improves recovery of forelimb function for up to a week after the cessation of stimulation5,7,26. The present study extends these findings and observed no detectable decline in performance up to 7 weeks after the cessation of stimulation.
The mechanisms that underlie VNS-dependent recovery are unknown. Previous studies have demonstrated an association between reorganization of corticospinal tract (CST) projections and recovery after stroke10,14,27–29. PRV enables multisynaptic tracing of motor networks connected to the injected muscles, providing specific labeling throughout neural hierarchical chains30. Previous studies have shown connectivity changes within the CST in the first month after stroke10; therefore, we performed PRV tracing 2 months following the cessation of VNS to assess CST connectivity when the lesion induced plasticity cascades have subsided24,31. Our findings demonstrate that VNS increases synaptic connectivity in CST motor networks to rehabilitated musculature. Furthermore, the VNS-dependent reorganization of synaptic connectivity in descending motor circuits is not labile, but robust and enduring. These findings reveal the first evidence of VNS-dependent enhancement of plasticity after stroke and are consistent with role of CST reorganization in post-stroke recovery.
Restoration of arm and hand function in stroke patients is a key priority for long-term quality of life, and current treatment options are limited32,33. A recent open active comparator pilot trial demonstrates that delivery of VNS paired with rehabilitation is safe and feasible in chronic stroke patients. Moreover, patients who received VNS paired with rehabilitation exhibited a clinically significant 3-fold increase in Upper Extremity Fugl-Meyer scores compared to those patients receiving rehabilitation alone, providing preliminary evidence that VNS therapy may be efficacious34. Here we report that VNS therapy yields significant, lasting improvements in forelimb supination function and that VNS-dependent enhancement of recovery generalizes to a similar, untrained task. Moreover, we demonstrate that VNS paired with rehabilitative training enhances neuroplasticity in corticospinal motor networks to task-relevant musculature, which may provide insight into the neural changes that subserve VNS-dependent improvement of recovery. Taken together, the findings from this study extend the viability of VNS as a safe, effective, and robust therapeutic strategy to improve motor dysfunction following stroke.
Supplementary Material
Acknowledgements:
The authors thank Andrew Sloan and David Pruitt for engineering assistance, Kimiya Rahebi for electronics construction, and Nicole Robertson for assistance with surgeries. In addition, they thank Katherine Adcock, Tania Kader, Preston D’Souza, Sanketh Kichena, Sania Khan, Sina Kashef, Jennifer Putman, Hansaim Jeong, Shreya Permanaki, Ivan Rahman, Ami Shah, Nimit Kasliwal, Sharanya Ajaykumar, and Seong Park for help with behavioral testing.
Sources of Funding:
This work was supported by NIH R01NS085167, R01NS094384, NIH Virus Center P40 OD010996, and the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Electrical Prescriptions (ElectRx) program under the auspices of Dr. Doug Weber and Eric Van Gieson through the Space and Naval Warfare Systems Center, Pacific Cooperative Agreement No. HR0011-15-2-0017 and N66001-15-2-4057 and the DARPA BTO Targeted Neuroplasticity Training (TNT) program under the auspices of Dr. Bradley Ringeisen through the Space and Naval Warfare Systems Center, Pacific Grant/Contract No. N66001-17-2-4011.
Footnotes
Conflicts of Interest / Disclosures
MPK is a consultant for and shareholder in MicroTransponder, Inc., which is developing VNS for stroke and tinnitus.
References
- 1.Hays SA. Enhancing Rehabilitative Therapies with Vagus Nerve Stimulation. Neurotherapeutics. 2016;13:382–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp. Neurol 2017;289:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hulsey DR, Hays SA, Khodaparast N, Ruiz A, Das P, Rennaker RL, et al. Reorganization of Motor Cortex by Vagus Nerve Stimulation Requires Cholinergic Innervation. Brain Stimul. 2016;9:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khodaparast N, Hays SA, Sloan AM, Fayyaz T, Hulsey DR, Rennaker RL, et al. Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke. Neurorehabil. Neural Repair 2014;28:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, Ganzer PD, et al. Vagus Nerve Stimulation During Rehabilitative Training Improves Forelimb Recovery After Chronic Ischemic Stroke in Rats. Neurorehabil. Neural Repair 2016;30:676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, et al. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol. Dis 2013;60:80–8. [DOI] [PubMed] [Google Scholar]
- 7.Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL, et al. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke. 2014;45:3097–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemon RN. Descending pathways in motor control. Annu. Rev. Neurosci 2008;31:195–218. [DOI] [PubMed] [Google Scholar]
- 9.Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Zhang RL, Li Y, Cui Y, Chopp M. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke. 2009;40:2546–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyers E, Sindhurakar A, Choi R, Solorzano R, Martinez T, Sloan A, et al. The supination assessment task: An automated method for quantifying forelimb rotational function in rats. J. Neurosci. Methods 2016;266:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang P, Barbay S, Plautz EJ, Hoover E, Strittmatter SM, Nudo RJ. Combination of NEP 1–40 treatment and motor training enhances behavioral recovery after a focal cortical infarct in rats. Stroke. 2010;41:544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyers EC, Granja R, Solorzano BR, Romero-Ortega M, Kilgard MP, Rennaker RL, et al. Median and ulnar nerve injuries reduce volitional forelimb strength in rats. Muscle Nerve. 2017;12:133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindau NT, Bänninger BJ, Gullo M, Good NA, Bachmann LC, Starkey ML, et al. Rewiring of the corticospinal tract in the adult rat after unilateral stroke and anti-Nogo-A therapy. Brain. 2014;137:739–56. [DOI] [PubMed] [Google Scholar]
- 15.Alaverdashvili M, Whishaw IQ. Compensation aids skilled reaching in aging and in recovery from forelimb motor cortex stroke in the rat. Neuroscience. 2010;167:21–30. [DOI] [PubMed] [Google Scholar]
- 16.Farr TD, Whishaw IQ. Quantitative and qualitative impairments in skilled reaching in the mouse (Mus musculus) after a focal motor cortex stroke. Stroke. 2002;33:1869–1875. [DOI] [PubMed] [Google Scholar]
- 17.Lambercy O, Dovat L, Yun H, Wee SK, Kuah CWK, Chua KSG, et al. Effects of a robot-assisted training of grasp and pronation/supination in chronic stroke: a pilot study. J. Neuroeng. Rehabil 2011;8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot-based hand motor therapy after stroke. Brain. 2008;131:425–37. [DOI] [PubMed] [Google Scholar]
- 19.Sindhurakar A, Butensky SD, Meyers E, Santos J, Bethea T, Khalili A, et al. An Automated Test of Rat Forelimb Supination Quantifies Motor Function Loss and Recovery After Corticospinal Injury. Neurorehabil. Neural Repair 2017;31:122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girgis J, Merrett D, Kirkland S, Metz GAS, Verge V, Fouad K. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain. 2007;130:2993–3003. [DOI] [PubMed] [Google Scholar]
- 21.García-Alías G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat. Neurosci 2009;12:1145–1151. [DOI] [PubMed] [Google Scholar]
- 22.Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schröter A, et al. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344:1250–5. [DOI] [PubMed] [Google Scholar]
- 23.El Amki M, Baumgartner P, Bracko O, Luft AR, Wegener S. Task-Specific Motor Rehabilitation Therapy After Stroke Improves Performance in a Different Motor Task: Translational Evidence. Transl. Stroke Res 2017;8:347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeiler SR, Krakauer JW. The interaction between training and plasticity in the poststroke brain. Curr. Opin. Neurol 2013;26:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krakauer JW, Marshall RS. The proportional recovery rule for stroke revisited. Ann. Neurol 2015;78:845–7. [DOI] [PubMed] [Google Scholar]
- 26.Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, et al. Vagus Nerve Stimulation Delivered with Motor Training Enhances Recovery of Function after Traumatic Brain Injury. J. Neurotrauma 2016;33:871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao S, Zhao M, Xiao T, Jolkkonen J, Zhao C. Constraint-induced movement therapy overcomes the intrinsic axonal growth-inhibitory signals in stroke rats. Stroke. 2013;44:1698–1705. [DOI] [PubMed] [Google Scholar]
- 28.Bachmann LC, Lindau NT, Felder P, Schwab ME. Sprouting of brainstem-spinal tracts in response to unilateral motor cortex stroke in mice. J. Neurosci 2014;34:3378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z, Li Y, Zhang RL, Cui Y, Chopp M. Bone marrow stromal cells promote skilled motor recovery and enhance contralesional axonal connections after ischemic stroke in adult mice. Stroke. 2011;42:740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, et al. Pseudorabies virus expressing enhanced green fluorescent protein: A tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc. Natl. Acad. Sci. U. S. A 2000;97:9264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat. Rev. Neurosci 2009;10:861–872. [DOI] [PubMed] [Google Scholar]
- 32.Cramer SC, Wolf SL, Adams HP, Chen D, Dromerick AW, Dunning K, et al. Stroke Recovery and Rehabilitation Research: Issues, Opportunities, and the National Institutes of Health StrokeNet. Stroke. 2017;48:813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J, et al. Interventions for improving upper limb function after stroke. Cochrane database Syst. Rev 2014;CD010820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, et al. Safety, Feasibility, and Efficacy of Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation After Ischemic Stroke. Stroke. 2016;47:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.