Abstract
Patient: Male, 56
Final Diagnosis: ANCA associated vasculitis
Symptoms: Dyspnea
Medication: —
Clinical Procedure: —
Specialty: Cardiology
Objective:
Rare disease
Background:
Granulomatosis with polyangiitis (GPA)/Wegener’s granulomatosis (WG) and eosinophilic granulomatosis with polyangiitis (EGPA)/Churg-Strauss’ syndrome (CSS) are ANCA (antineutrophil cytoplasmic antibodies) associated vasculitides that can affect the heart, predominantly the myocardium. Valvular affection is rare and is described anecdotally. The purpose of this case report was to present aortic valve affection of an ANCA positive vasculitis.
Case Report:
We present the case with a 56-year-old male diagnosed with ANCA associated vasculitis, who began experiencing respiratory symptoms primarily thought to be respiratory tract affection. These symptoms worsened, and an echocardiography revealed heart failure with decreased left ventricular ejections fraction (EF=30–35%) and a severe insufficiency of the aortic valve. The patient underwent aortic valve replacement with symptomatic relief. Pathological examination of aortic valve resectates revealed inflammation and thickening of the aortic cusps.
Conclusions:
Patients with ANCA associated vasculitis can rarely present with valvular inflammation causing severe regurgitation. The aortic valve can be involved, although cases have also described mitral valve involvement and both valves simultaneously. In patients with ANCA associated vasculitis a severe worsening of dyspnea can be caused by exacerbation of pulmonary involvement, but severe valvular disease should also be considered.
MeSH Keywords: Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis, Aortic Valve Insufficiency, Heart Failure
Background
Eosinophilic granulomatosis with polyangiitis, former named Churg-Strauss syndrome, EGPA/CSS, is a usually ANCA (antineutrophil cytoplasmic antibodies) positive, systemic, small vessel vasculitis condition occurring usually in males in their fourth decade with manifestations of rhinitis and asthma, eosinophilia and systemic vasculitis of small and medium-sized arteries and veins. Beside vasculitis, the condition is characterized by eosinophilic infiltration, high blood eosinophil count, and extra-vascular granulomas, typically seen in skin, lungs, and peripheral nerves. This results in fibrinoid necrosis, thrombosis, and aneurism formation. Corticosteroids usually are successful in treating this ailment. About half of patients with EGPA/CSS have cardiac involvement. Among those electrophysiological alterations are observed ranging from asymptomatic electrocardiographic changes, complete heart block [1] and sudden cardiac death [2]. Granulomatous eosinophilic infiltration of the myocardium and coronary artery vasculitis leads to severe congestive heart failure and coronary artery disease. Pericardial effusions and pericardial fibrosis might also occur. One case with aortic valve involvement has been described with aortic valve biopsy containing necrotizing granulomatous inflammation with an eosinophil-rich lymphoplasmacytic inflammatory infiltrate causing aortic regurgitation [3].
Granulomatous with polyangiitis (GPA), former named Wegener’s granulomatosis, is another important primary systemic small-vessel vasculitis. GPA is a systemic necrotizing vasculitis of unknown etiology characterized by granulomatous lesions of the respiratory tract (i.e., nose, sinuses, and lungs) and renal glomerular disease. ANCA is present in the majority of patients. The lesions are characterized by parenchymal necrosis, vasculitis and a granulomatous inflammation composed of neutrophils, lymphocytes, plasma cells, macrophages, and eosinophils [4]. Microscopic polyangiitis (MPA), also an ANCA-associated vasculitis is similar to GPA/WG except it only affects small blood vessels in the lungs or kidneys.
Cardiac involvement in EGPA/CSS has been reported in 6% to 44% of cases including constrictive pericarditis and fibrinous hemorrhagic pericarditis. Valvular involvement with severe aortic regurgitation as evidenced by histology revealing myxoid degeneration, focal fibrosis and thickening of the cusps and neovascularization, granulomas with central necrosis has been described [5,6] as well as mitral valve stenosis and regurgitation due to granulomatous infiltration of mitral valve leaflets [7]. Direct perforation of the aortic and mitral valve has also been reported [8]
Cardiac valvular involvement associated with GPA/WG has also been described albeit uncommon. Both aortic and mitral valves have been involved [9–11]. Importantly, aortic valve histopathology has been reported to reveal myxoid degeneration indicating previously active vasculitis affecting the vessels of the aortic wall and valvular necrosis with subsequent progressive degeneration of the cusps [12].
Case Report
A 56-year-old male at the age of 49 years developed a cerebral stroke which resolved spontaneously within a few days leaving no permanent damage and a magnetic resonance (MR)-scan was not performed. The patient had no cardiovascular risk factors apart from family history of ischemic heart disease.
Ten weeks later, the patient presented with third degree atrioventricular block (AV)-block initially thought to be associated with an acute Borrelia infection. However, the AV-block persisted despite antibiotic therapy and a dual-chamber pacemaker was implanted.
During hospitalization, the patient complained of fatigue, weight-loss, and night sweating; blood analyses demonstrated a sedimentation rate >100 mm/hr and anemia with a hemoglobin of 5.0 g/mL, thrombocytosis of 615×109 cells/L and C-reactive protein (CRP) of 95. There was no leukocytosis. Gastrointestinal hemorrhage was not suspected due to lack of symptoms from the gastrointestinal tract. The patient was 4 months later diagnosed with an ANCA positive vasculitis due to malaise, low grade fewer, arthritis, sinusitis (biopsy with acute and chronic inflammation, but no necrosis, granulomas or vasculitis signs), interstitial lung disease (ground-glass phenomenon in both lungs on high resolution computed tomography (HRCT) scan (biopsy was not performed) and myeloperoxidaseantineutrophil cytoplasmic antibody (MPO-ANCA) positivity. There were no signs of mononeuritis multiplex, asthma, involvement of skin, kidneys, brain, or gastrointestinal canal. The Birmingham Vasculitis Activity Score (BVAS) score was 15.
The patient underwent treatment with steroids (for 6 months) and methotrexate, and the vasculitis remission was achieved including normalization of MPO-ANCA levels. After 2 years methotrexate monotherapy was discontinued.
After 1 year, the patient presented with respiratory tract symptoms including dyspnea and increasing MPO-ANCA levels. Consequently, steroid and methotrexate therapy was resumed. However, the patient did not improve in respiratory symptoms and the patient was referred for a multidisciplinary examination, including a cardiac, pulmonary, and rheumatic evaluation. The cardiac evaluation included a normal echocardiographic examination with a preserved left ventricular (LV) function, no signs of pulmonary hypertension and a mild-to-moderate aortic regurgitation determined by transthoracic echocardiogram (TTE).
A treadmill exercise test was performed demonstrating severely reduced functional capacity with a peak exercise of 113W (52% of expected) and an adequate chronotropic response. A pulmonary functional test was performed demonstrating severe obstructive pulmonary disease with forced expiratory volume in one second (FEV1) of 1.1 L (29% of predicted), forced vital capacity (FVC) 3.0 L (63% of predicted), a diffusion capacity of 66% and a Tiffeneau-Pinelli index of 37%. A ventilation/perfusion lung scan was performed demonstrating bilateral pulmonary embolisms. In addition, an HRCT was performed demonstrating ground glass phenomenon in both lungs. Testing for thrombophilia and antiphospholipid antibodies revealed no abnormality. BVAS score was 13. Anticoagulant therapy (rivaroxaban) was added to the treatment with methylprednisolone (3 pulses), prednisolone, methotrexate and bronchodilators (LABA/LAMA combination).
During the following 12 weeks, the patient’s dyspnea worsened, and a new echocardiographic examination demonstrated a dilated left ventricle with reduced LV function with an ejection fraction (EF)=30% to 35%; and on this basis, the dual-chamber pacemaker (PM) was upgraded to a cardiac resynchronization therapy (CRT)-PM. As a result of symptomatic improvement of dyspnea after dual-chamber implantation, aortic valve replacement was not relevant.
The patient was closely monitored at the Pulmonology Center for interstitial lung disease, at the Rheumatology Center for connective tissue diseases and at the Department of Cardiology. A development of pneumonia was treated successfully with antibiotics.
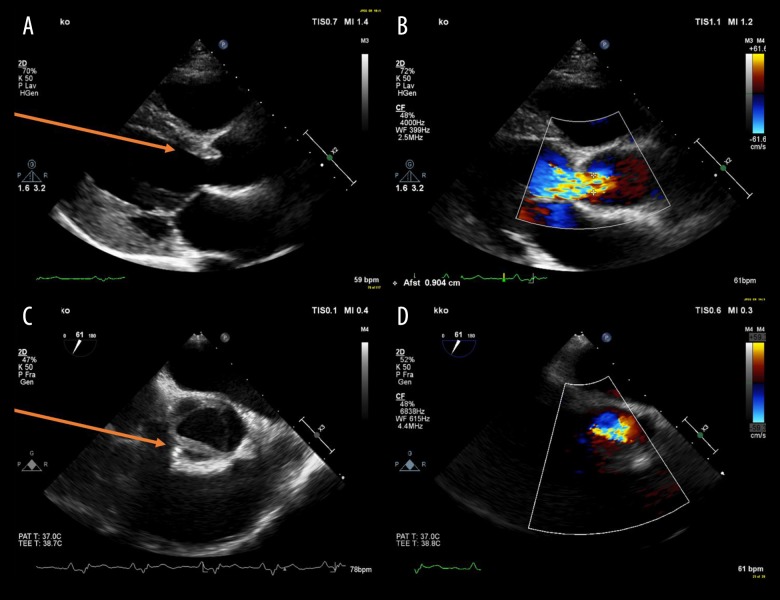
After 12 weeks, a new cardiac examination was performed. Transthoracic echocardiography showed a dilated left ventricle and a transesophageal echocardiography showed a tricuspid aortic valve in which the right coronary cusp was immobile/fixed and edematous causing a severe insufficiency with a vena contracta between 8 mm and 9 mm (Figure 1).
Figure 1.
Transthoracic and transesophageal echocardiography. (A, B) Transthoracic echocardiography, parasternal long axis view. (C, D) Transesophageal echocardiography set at 61 degrees. (A, C) Red arrows point at thickened and fixed right coronary cusp leaflet of the aortic valve. (B, D) Color Doppler reveals severe regurgitation of the aortic valve.
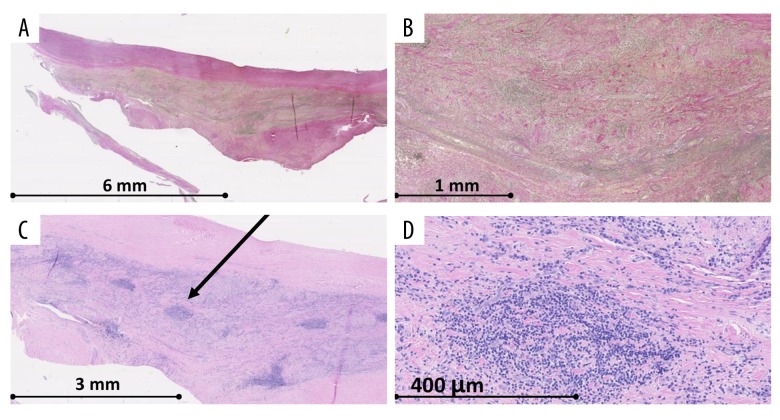
We assessed that the substantial cause of dyspnea was due to severe aortic regurgitation. On this basis, the patient was assigned for thoracotomy and implantation of an aortic valve bioprosthesis. Histological analysis of the resectates of aortic cusps showed that 2 of these cusps contained fibrosis and myxoid degeneration. The last of these cusps contained inflammatory alterations, fibrosis, and thickening. An abundance of lymphocytes and plasma cells were evident as well as thickened vessels and granulomatous structures (Figure 2).
Figure 2.
Microscopy of resectates of right coronary cusp. (A, B) Verhoeff’s elastic stain. (C, D) Hematoxylin eosin stain. The microscopy pictures show fibrosis and chronical inflammation with lymphocytes and plasma cells (arrow).
After uncomplicated surgery, an intermittent fast atrial flutter was diagnosed. After amiodarone loading, beta blocker initiation, and reprogramming of the pacemaker to DDI mode, ventricular frequency was normalized, and improvement of dyspnea was seen.
Discussion
First of all, a distinction between EGPA/CSS and GPA/WG can be challenging as serology can be unspecific, even if MPOANCA usually is found in EGPA/CSS [13] and PR3-ANCA is associated with GPA/WG. In the present case, semiquantitative p-ANCA measurement was strongly positive with MPO-ANCA being positive (>200×103 IU/L). Semiquantitative c-ANCA was mildly positive, whereas PR3-ANCA IgG antibody was negative. PR3-ANCA are detectable in the vast majority of patients with severe active GPA/WG [4]. Although a negative result for PR3 ANCA reduces the likelihood of active GPA/WG, 20% of patients with milder GPA/WG test negative for PR3-ANCA. Moreover, successful treatment of GPA/WG may lead to decline in the levels of PR3-ANCA [14]. Due to the difficulty diagnostically distinguishing between the 2 vasculitides, and due to overlapping treatment regimens, the term ANCA-associated vasculitis is often applied.
Secondly, differential diagnosis in general was particularly difficult in this patient case due to a multitude of medical conditions potentially causing dyspnea. This case clearly emphasizes the challenges of establishing an exact diagnosis when several co-morbidities exist. The patient presented with a stroke months prior to the fulminant conglomerate of cardiac symptoms and general malaise. Thus, it is possible that the stroke was an undiagnosed manifestation of the vasculitis. Unfortunately, an MRI of the cerebrum was not conducted as this could have revealed whether the stroke was secondary to vasculitis. Pulmonary involvement with an obstructive pattern would be compatible with EGPA/CSS, although asthma was never diagnosed and bilateral pulmonary embolisms with reduced diffusion capacity could equally represent manifestations of GPA/WG. Furthermore, dilated cardiomyopathy might lead to pulmonary congestion exacerbating pulmonary symptoms. The patient was diagnosed with bilateral pulmonary embolisms and was on relevant novel oral anticoagulants (NOAC) therapy (rivaroxaban) for more than half a year; however, this would make it less likely to account for worsening of dyspnea. Also, the patient was known to have heart failure with an EF of 35%, and due to a third-degree AV block, the patient had a CRT-PM implanted. The latest echocardiography did not show a decrease in EF. Although progression of symptomatic congestive heart failure could not be completely ruled out, it was less likely to significantly contribute to worsening of dyspnea as the implantation of the CRT-PM increased the EF due to synchronization the systolic LV contraction. Indeed, the patient had mentioned a transient improvement after CRT-PM implantation, which was why valvular surgery was deferred at the time. Moreover, due to vasculitis, corticosteroid treatment was initiated, which led to the assumption that these treatment strategies were sufficient in targeting the patient’s dyspnea. Another autoimmune disease, causing involvement of valves, has been seen in the cardiac manifestation of the autoimmune disease systemic lupus erythematosus, known as Libman-Sacks endocarditis (verrucous, marantic, nonbacterial thrombotic endocarditis) with accumulations of immune complexes and mononuclear cells on valves causing diffuse leaflet thickening. Valvular regurgitation is seen most commonly in patients due to leaflet thickening. However, thrombophilia testing, including antiphospholipid titers, were negative in our patient, deeming this ailment improbable.
Appropriate therapy management of vasculitides, as well as close monitoring due to the chronic relapsing nature of these inflammatory diseases, requires regular use of validated disease activity and damage measurements. In terms of measurement of disease activity in vasculitis, the BVAS provides a checklist of symptoms and clinical findings occurring in patients with active systemic vasculitis is widely used in clinical trials and is recommended for clinical practice [15,16]. Stratifying patients based on prognosis in order to direct more aggressive therapy than those with a better prognosis, the Five Factor Score (FFS) has been validated in patients with small and medium vessel vasculitis based on the extent of organ involvement [17–19]. The FFS is based on 5 clinical items, each accorded 1 point for a maximum score of 5: renal insufficiency, proteinuria, central nervous system involvement, cardiomyopathy, and severe gastrointestinal involvement. At FFS=0 the 5-year survival is 88%. When FFS=1, the survival rate is 74%. When FFS >2, the rate is 54%. However, it is not validated for Wegener’s granulomatosis (WG) and it is not certain whether it can be used as a prognostic tool during a disease flare. The FFS is not valuable in assessment of general disease activity. BVAS also provides prognostic information; high BVAS at diagnosis predicts increased mortality, but it also predicts responsiveness to therapy [20,21]. Overall, the treatment recommendation algorithm is such that upon new diagnosis of ANCA-positive vasculitis, a differentiation should be performed based on organ damage. If no organs are threatened, methotrexate or mycophenolate mofetil with glucocorticoid is recommended. If organ affection is at hand or life-threatening disease, cyclophosphamide or rituximab with glucocorticoid should be administered. If remission, then azathioprine or methotrexate or rituximab should be considered. In cases of rapidly progressive renal failure or pulmonary hemorrhage, plasma exchange should be considered [22].
This case undoubtedly not only emphasizes the importance of a multidisciplinary team approach in identifying the etiology of a particular symptom in patients with several co-morbidities, but also emphasizes the need for the management and follow-up of vasculitis to base on an exhaustive evaluation of organ failures, assisted by clinical scores that aid physicians in their clinical work. One month after surgery, our patient underwent clinical control in the cardiology outpatient clinic. The patient expressed a substantial improvement in dyspnea, interpreted as New York Heart Association (NYHA) II as compared to the outset classification of NYHA III and 2 month after the operation, it was noted in the pulmonary outpatient clinic that the patient expressed a substantial improvement in physical capabilities. Prior to the operation, he was able to walk 500 m before experiencing fatigue. Now he was able to walk several kilometers a day. Pulmonary function testing (spirometry and diffusion analysis) showed no lung capacity improvement compared to the test prior to surgery. The patient had frequent visits to the heart failure clinic and was meticulously up-titrated in beta blockers and angiotensin-converting enzyme (ACE)-inhibitors. Echocardiography one year after surgery exhibited a near-normalization of the systolic function (EF was 50% to 55%). Therefore, a substantial symptomatic improvement in dyspnea was due to aortic valve implantation. In retrospect, there is no doubt that performing an MRI would have obtained invaluable information in regard to myocardial affection of the ANCA-associated vasculitis in assessing the primary etiology behind the heart failure [23–33].
Conclusions
Although the phenomenon of valvular involvement of ANCA-associated vasculitides is not novel, it is still rare. Patients with the vasculitides EPA/CSS and/or GPA/WG rarely present with valvular inflammation that causes severe regurgitation. The aortic valve can be involved, although cases have also described mitral valve involvement and both valves involved simultaneously.
Patients with vasculitis presenting with worsening of dyspnea should not only be suspected to have an exacerbation of pulmonary involvement, but also severe valvular involvement should be considered. Due to the multi-organ affection of ANCA-positive vasculitides, the clinical implication of this case report is that these patients should undergo a regular multidisciplinary assessment especially in clinical situations when vasculitides respond poorly on treatment or in case of disease flare-ups. A systematic evaluation must be obtained when exploring the cause of dyspnea, including evaluation of cardiac valves. In general, it is important to stress that proper management of vasculitides depends on a solid primary rheumatological evaluation of the patient, supported by clinical score systems. Follow-ups should be based on the first evaluation, followed by annual evaluation or in case of flare-ups, where tests are repeated according to sign and symptoms.
Acknowledgments
Microscopy pictures were kindly made available by Professor Niels Marcussen, DMedSc, Department of Clinical Pathology, Odense University Hospital.
Abbreviations
- ANCA
anti-neutrophil cytoplasmic antibody;
- CSS
Churg-Strauss’ syndrome = EPA (compound abbreviation is EPA/CSS);
- c-ANCA
PR3-ANCA or cytoplasmic antineutrophil cytoplasmic antibodies;
- CRT-PM
cardiac resynchronization therapy pacemaker;
- EF
ejection fraction;
- EGPA
eosinophilic granulomatosis with polyangiitis = CSS (compound abbreviation is EGPA/CSS);
- FEV1
forced expiratory volume in 1 second;
- FVC
forced vital capacity;
- GPA
granulomatosis with polyangiitis = WG (compound abbreviation is GPA/WG);
- HRCT
high-resolution computed tomography;
- IgG
immunoglobulin G;
- LABA
long-acting beta-adrenoceptor agonist;
- LAMA
long-acting muscarinic antagonist;
- MPA
microscopic polyangiitis;
- MPO-ANCA
p-ANCA or perinuclear anti-neutro-phil cytoplasmic antibodies;
- NOAC
novel oral anticoagulants;
- PR3-ANCA
see c-ANCA;
- WG
Wegener’s granulomatosis = GPA (compound abbreviation is GPA/WG)
Footnotes
Department and Institution where work was done
Department of Cardiology, Odense University Hospital, Denmark.
References:
- 1.Yu L, Zhao N, Zhu Y. [Allergic granulomatous angiitis] Zhonghua Nei Ke Za Zhi. 1993;32(10):685–87. [in Chinese] [PubMed] [Google Scholar]
- 2.Kozak M, Gill E, Green L. The Churg Strauss syndrome. A case report with angiographically documented coronary involvement and a review of the literature. Chest. 1995;107:578–80. doi: 10.1378/chest.107.2.578. [DOI] [PubMed] [Google Scholar]
- 3.Doherty L, Kumar P, Bexton R, et al. Aortic regurgitation and Churg-Strauss syndrome. QJM. 2005;98(10):772–73. doi: 10.1093/qjmed/hci125. [DOI] [PubMed] [Google Scholar]
- 4.Finkielman J, Lee A, Hummel A, et al. ANCA are detectable in nearly all patients with active severe Wegener’s granulomatosis. Am J Med. 2007;120(7):643.e9–14. doi: 10.1016/j.amjmed.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Vervloet D, De Backer T, van Dorpe J. Granulomatosis with polyangiitis (Wegener’s) hidden in the aortic valve. Int J Cardiovasc Imaging. 2016;32(5):753–55. doi: 10.1007/s10554-016-0835-y. [DOI] [PubMed] [Google Scholar]
- 6.Korantzopoulos P, Papaioannides D, Siogas K. The heart in Wegener’s granulomatosis. Cardiology. 2004;102(1):7–10. doi: 10.1159/000076995. [DOI] [PubMed] [Google Scholar]
- 7.Koyalakonda S, Krishnan U, Hobbs W. A rare instance of multiple valvular lesions in a patient with Wegener’s granulomatosis. Cardiology. 2010;117(1):28–30. doi: 10.1159/000319603. [DOI] [PubMed] [Google Scholar]
- 8.Castellanos D, Travelli F, Reyhan I, et al. kAcute aortic and mitral valve perforations caused by granulomatosis with polyangiitis. Circulation. 2015;131(24):e527–29. doi: 10.1161/CIRCULATIONAHA.114.014304. [DOI] [PubMed] [Google Scholar]
- 9.Greidinger E, Lemes V, Hellmann D. Cardiac valve disease in Wegener’s granulomatosis. J Rheumatol. 1996;23:1485–87. [PubMed] [Google Scholar]
- 10.Leff R, Hellman R, Mullany C. Acute aortic insufficiency associated with Wegener granulomatosis. Mayo Clin Proc. 1999;74(9):897–99. doi: 10.4065/74.9.897. [DOI] [PubMed] [Google Scholar]
- 11.Nallasivan M, Clewes A. Acute aortic valvular regurgitation with pulmonary haemorrhage in Wegener’s granulomatosis presenting as dyspnoea: A rare presentation. BMJ Case Rep. 2010;2010 doi: 10.1136/bcr.11.2009.2474. pii: bcr1120092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davenport A, Goodfellow J, Goel S, et al. Aortic-valve disease in patients with Wegener’s granulomatosis. Am J Kidney Dis. 1994;24:205–8. doi: 10.1016/s0272-6386(12)80182-x. [DOI] [PubMed] [Google Scholar]
- 13.Yoshihara K, Arimura Y, Kobayashi O, et al. [Clinical study on five myeloperoxidase specific anti-neutrophil cytoplasmic antibody (MPO-ANCA) positive Churg-Strauss syndrome cases] Ryumachi. 1998;38(5):696–704. [in Japanese] [PubMed] [Google Scholar]
- 14.Russell K, Wiegert E, Schroeder D, et al. Detection of anti-neutrophil cytoplasmic antibodies under actual clinical testing conditions. Clin Immunol. 2002;103(2):196–203. doi: 10.1006/clim.2001.5200. [DOI] [PubMed] [Google Scholar]
- 15.Mukhtyar C, Guillevin L, Cid M, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis. 2009;68(3):310–17. doi: 10.1136/ard.2008.088096. [DOI] [PubMed] [Google Scholar]
- 16.Luqmani R, Bacon P, Moots R, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87(11):671–78. [PubMed] [Google Scholar]
- 17.Guillevin L, Le Thi Huong D, Godeau P, et al. Clinical findings and prognosis of polyarteritis nodosa and Churg-Strauss angiitis: A study in 165 patients. Br J Rheumatol. 1988;27(4):258–64. doi: 10.1093/rheumatology/27.4.258. [DOI] [PubMed] [Google Scholar]
- 18.Guillevin L, Lhote F, Gayraud M, et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore) 1996;75(1):17–28. doi: 10.1097/00005792-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Guillevin L, Pagnoux C, Seror R, et al. The Five-Factor Score revisited: Assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 2011;90(1):19–27. doi: 10.1097/MD.0b013e318205a4c6. [DOI] [PubMed] [Google Scholar]
- 20.Gayraud M, Guillevin L, le Toumelin P, et al. Long-term followup of polyarteritis nodosa, microscopic polyangiitis, and Churg-Strauss syndrome: Analysis of four prospective trials including 278 patients. Arthritis Rheum. 2001;44(3):666–75. doi: 10.1002/1529-0131(200103)44:3<666::AID-ANR116>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 21.Koldingsnes W, Nossent H. Predictors of survival and organ damage in Wegener’s granulomatosis. Rheumatol. 2002;41(5):572–81. doi: 10.1093/rheumatology/41.5.572. [DOI] [PubMed] [Google Scholar]
- 22.Yates M, Watts R, Bajema I, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75(9):1583–94. doi: 10.1136/annrheumdis-2016-209133. [DOI] [PubMed] [Google Scholar]
- 23.Cereda A, Pedrotti P, De Capitani L, et al. Comprehensive evaluation of cardiac involvement in eosinophilic granulomatosis with polyangiitis (EGPA) with cardiac magnetic resonance. Eur J Intern Med. 2017;39:51–56. doi: 10.1016/j.ejim.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Dennert R, van Paassen P, Schalla S, et al. Cardiac involvement in Churg-Strauss syndrome. Arthritis Rheum. 2010;62(2):627–34. doi: 10.1002/art.27263. [DOI] [PubMed] [Google Scholar]
- 25.Dunogué B, Terrier B, Cohen P, et al. Impact of cardiac magnetic resonance imaging on eosinophilic granulomatosis with polyangiitis outcomes: A long-term retrospective study on 42 patients. Autoimmun Rev. 2015;14(9):774–80. doi: 10.1016/j.autrev.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Fijolek J, Wiatr E, Gawryluk D, et al. The significance of cardiac magnetic resonance imaging in detection and monitoring of the treatment efficacy of heart involvement in eosinophilic granulomatosis with polyangiitis patients. Sarcoidosis Vasc Diffus Lung Dis. 2016;33(1):51–58. [PubMed] [Google Scholar]
- 27.Hazebroek M, Kemna M, Schalla S, et al. Prevalence and prognostic relevance of cardiac involvement in ANCA-associated vasculitis: Eosinophilic granulomatosis with polyangiitis and granulomatosis with polyangiitis. Int J Cardiol. 2015;199:170–79. doi: 10.1016/j.ijcard.2015.06.087. [DOI] [PubMed] [Google Scholar]
- 28.Marmursztejn J, Vignaux O, Cohen P, et al. Impact of cardiac magnetic resonance imaging for assessment of Churg-Strauss syndrome: A cross-sectional study in 20 patients. Clin Exp Rheumatol. 2009;27(1 Suppl. 52):S70–76. [PubMed] [Google Scholar]
- 29.Mavrogeni S, Karabela G, Gialafos E, et al. Cardiac involvement in ANCA (+) and ANCA (−) Churg-Strauss syndrome evaluated by cardiovascular magnetic resonance. Inflamm Allergy Drug Targets. 2013;12(5):322–27. doi: 10.2174/18715281113129990054. [DOI] [PubMed] [Google Scholar]
- 30.Neumann T, Manger B, Schmid M, et al. Cardiac involvement in Churg-Strauss syndrome: impact of endomyocarditis. Medicine (Baltimore) 2009;88(4):236–43. doi: 10.1097/MD.0b013e3181af35a5. [DOI] [PubMed] [Google Scholar]
- 31.Pugnet G, Gouya H, Puéchal X, et al. Cardiac involvement in granulomatosis with polyangiitis: A magnetic resonance imaging study of 31 consecutive patients. Rheumatology (Oxford) 2017;56(6):947–56. doi: 10.1093/rheumatology/kew490. [DOI] [PubMed] [Google Scholar]
- 32.Wassmuth R, Göbel U, Natusch A, et al. Cardiovascular magnetic resonance imaging detects cardiac involvement in Churg-Strauss syndrome. J Card Fail. 2008;14(10):856–60. doi: 10.1016/j.cardfail.2008.07.227. [DOI] [PubMed] [Google Scholar]
- 33.Yune S, Choi D, Lee B, et al. Detecting cardiac involvement with magnetic resonance in patients with active eosinophilic granulomatosis with polyangiitis. Int J Cardiovasc Imaging. 2016;32(Suppl. 1):155–62. doi: 10.1007/s10554-016-0843-y. [DOI] [PubMed] [Google Scholar]