Abstract
Background:
Fiducial markers (FMs) help direct stereotactic body radiation therapy (SBRT) and localization for surgical resection in lung cancer management. We report the safety, accuracy, and practice patterns of FM placement utilizing electromagnetic navigation bronchoscopy (ENB).
Methods:
NAVIGATE is a global, prospective, multicenter, observational cohort study of ENB using the superDimension™ navigation system. This prospectively collected subgroup analysis presents the patient demographics, procedural characteristics, and 1-month outcomes in patients undergoing ENB-guided FM placement. Follow up through 24 months is ongoing.
Results:
Two-hundred fifty-eight patients from 21 centers in the United States were included. General anesthesia was used in 68.2%. Lesion location was confirmed by radial endobronchial ultrasound in 34.5% of procedures. The median ENB procedure time was 31.0 min. Concurrent lung lesion biopsy was conducted in 82.6% (213/258) of patients. A mean of 2.2 ± 1.7 FMs (median 1.0 FMs) were placed per patient and 99.2% were accurately positioned based on subjective operator assessment. Follow-up imaging showed that 94.1% (239/254) of markers remained in place. The procedure-related pneumothorax rate was 5.4% (14/258) overall and 3.1% (8/258) grade ⩾ 2 based on the Common Terminology Criteria for Adverse Events scale. The procedure-related grade ⩾ 4 respiratory failure rate was 1.6% (4/258). There were no bronchopulmonary hemorrhages.
Conclusion:
ENB is an accurate and versatile tool to place FMs for SBRT and localization for surgical resection with low complication rates. The ability to perform a biopsy safely in the same procedure can also increase efficiency. The impact of practice pattern variations on therapeutic effectiveness requires further study.
Trial registration:
ClinicalTrials.gov identifier: NCT02410837.
Keywords: electromagnetic navigation bronchoscopy, fiducial, lung cancer, prospective clinical study, stereotactic body radiation therapy
Introduction
Radiotherapy is an integral component of both palliative and curative-intent therapy for non-small-cell lung cancer (NSCLC). In the last 10–15 years, stereotactic body radiation therapy (SBRT) has been developed and refined to deliver high doses of radiation in fewer fractions compared with conventionally fractionated radiation through improved target localization, motion management, and reduced set-up error.1 SBRT is commonly used to treat early-stage NSCLC and oligometastatic disease, with high local control rates of 83–100%.2 SBRT is typically delivered over one to five fractions within 1–2 weeks. Greater accuracy and precision of treatment allow very high biologically effective radiation doses to the tumor while minimizing toxicity and radiation to surrounding tissues.3 This dose escalation is optimally delivered using computed tomography (CT) simulators and linear accelerators with respiratory motion management. The two basic approaches to motion management during SBRT are gated and nongated treatments. Gated SBRT treatments often depend on fiducial marker (FM) implantation in or near the tumor to localize the corresponding structure and deliver the radiation only when the tumor is in the correct position in the breathing cycle, or during a breath-hold. Nongated SBRT treatments typically minimize motion through mechanical compression of the abdomen to restrict diaphragmatic excursion. Other linear accelerator systems that depend on fiducials for respiratory-gated treatments typically monitor fiducials before, during, and after the radiation beam is turned on to ensure the target center is correctly positioned, maximize accuracy of high-dose radiation treatments to small targets, and afford tight dose conformity to targets that are particularly close to organs at risk.4–6
FMs are also useful for localizing small peripheral lung tumors to aid the surgeon during parenchymal-sparing video-assisted thoracoscopic surgery (VATS) either alone or with pleural dye marking.7–10
FMs can be delivered percutaneously with image guidance11,12 or through a video bronchoscope.8,13,14 One challenge with percutaneous delivery methods is risk of pneumothorax,12 which could be magnified when percutaneous biopsy and FM placement are attempted during the same procedure.15 Cardiac and endovascular embolization have also been rarely reported in the literature after percutaneous fiducial placements.16,17 On the other hand, traditional bronchoscopic methods can be suboptimal for peripheral lesions.18 Electromagnetic navigation bronchoscopy (ENB) is a common method for guiding FM placement.13,19–21 However, most prior studies have been single-center, retrospective analyses that do not allow an evaluation of diversity in practice patterns or safety in a large prospective cohort.
NAVIGATE is the largest prospective study of ENB yet conducted, evaluating ENB-guided lung lesion biopsy, FM placement, pleural dye marking, and lymph node biopsy in a diverse, multicenter setting.22–24 We report the safety, accuracy, and practice patterns of ENB-guided FM placement in NAVIGATE.
Methods
In the NAVIGATE study [ClinicalTrials.gov identifier: NCT02410837], 1390 patients were enrolled at 37 sites in the United States and Europe. Currently, 2-year follow up is in progress. The full study design, an interim analysis of safety and usage patterns in the first 1000 patients enrolled, and diagnostic yield outcomes in the United States cohort have been previously published.22–24 The clinical study protocol prespecified an evaluation of the FM-placement accuracy as a secondary endpoint, as well as subgroup assessments of FM usage and outcomes.24 In the current subgroup analysis, patient demographics, procedural characteristics, and outcomes were prospectively collected in NAVIGATE patients from the full United States cohort who had FMs placed.
Adult patients undergoing an elective ENB-guided FM placement were eligible for enrollment. The ENB-guided FM placement and SBRT procedures were conducted according to the manufacturer’s instructions and institutional practices and were not specified per protocol in this observational study.
Procedure-related pneumothorax, bronchopulmonary hemorrhage, and respiratory failure were defined according to the validated Common Terminology Criteria for Adverse Events (CTCAE) scale and adjudicated by an independent medical monitor.24 At least 20% of the data were verified against source files by the sponsor using risk-based monitoring.24
No sample size calculations were conducted for this single-arm, observational subgroup analysis. Analyses were performed using SAS® Version 9.4 (SAS Inc., Cary, NC). Data were summarized by descriptive statistics, including frequency distributions and cross-tabulations for discrete variables and mean, standard deviation, median, minimum, and maximum values for continuous variables.
This study is being conducted in accordance with the Declaration of Helsinki and all local regulatory requirements. The clinical study protocol, which prespecified the current FM subgroup analysis, was approved by the institutional review board of all participating clinical sites. Written informed consent was obtained from all patients, including use of study data for publication purposes.
Results
Participants
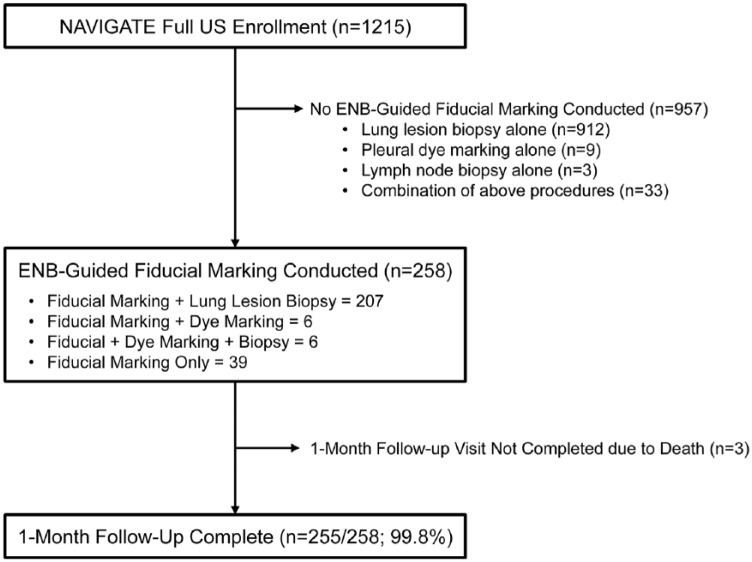
From 1 May 2015 to 31 August 2016, 258 patients underwent ENB-guided FM placement at 21 centers (29 operators) in the United States. A 1-month follow up was completed in 99.8% (255/258) of patients (Figure 1). Patient demographics are shown in Table 1.
Figure 1.
Patients included in the analysis.
Table 1.
Patient demographics.
| Age at consent, years [(mean ± SD) range] | 72.0 [(71.0 ± 9.8)
41.0–93.0] n = 258 |
|---|---|
| Female/male | 53.5/46.5% |
| Race | |
| White | 84.1% (217/258) |
| Black or African American | 15.1% (39/258) |
| Unknown | 0.8% (2/258) |
| Ethnicity | |
| Hispanic or Latino | 0.4% (1/258) |
| Not Hispanic or Latino | 98.8% (255/258) |
| Unknown | 0.8% (2/258) |
| Tobacco history (current or former) | 89.5% (231/258) |
| Current | 33.3% (77/231) |
| Former | 66.7% (154/231) |
| Chronic obstructive pulmonary disease | 54.7% (141/258) |
| Asthma | 14.7% (38/258) |
| FEV1 (% of predicted) | 75.0 ([72.3±28.7] 21.0–127.0) n=80 |
| DLCO (% of predicted) | 59.5 ([62.6±26.3] 11.0–128.0) n=62 |
| Personal history of cancer | 56.6% (146/258) |
Data are presented as % (n/total n), or median [(mean±SD) range] n.
DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 s; SD, standard deviation.
Procedural characteristics
Among the 258 patients undergoing ENB-guided FM placement, most had lung lesion biopsy or pleural dye marking in the same procedure (Figure 1). There were 39 patients who had FM placement alone.
General anesthesia was used in 68.2% (176/258) of patients (Table 2). Lesion visualization by radial endobronchial ultrasound was used in 34.5% (89/258) of ENB procedures. Among all 258 FM cases, the procedure time was a median of 57.0 min overall (bronchoscope in to bronchoscope out), including 31.0 min specifically for the ENB procedure (as measured by the entry and exit of the locatable guide or extended working channel; Table 2). Among the 39 patients with only FM placement and no lung lesion biopsy or dye marking [all 39 used the SuperLock (Medtronic, Minneapolis, MN) nitinol coil FM], the median total and ENB-specific procedure times were 31.0 min and 20.0 min, respectively.
Table 2.
Procedural characteristics.
| General anesthesia, % (n) | 68.2% (176/258) |
|---|---|
| Moderate sedation | 31.8% (82/258) |
| ENB software version | |
| Version 6 | 11.6% (30/258) |
| Version 7 | 88.4% (228/258) |
| Radial EBUS used during ENB procedure* | 34.5% (89/258) |
| Cone–beam CT used | 4.7% (12/258) |
| Total procedure time, min [bronchoscope in/out (range)], | 57.0 [41.0 (34.0–75.0)] |
| ENB procedure time, min [locatable guide in/out (range)] | 31.0 [29.0 (18.0–47.0)] |
| Fiducial marker type$ | |
| SuperLock nitinol coil fiducial marker | 80.6% (208/258): 447 markers |
| superDimension coil fiducial marker | 3.1% (8/258): 8 markers |
| superDimension 3-band fiducial marker | 1.6% (4/258): 4 markers |
| superDimension 2-band fiducial marker | 2.7% (7/258): 7 markers |
| VortX Diamond-18 | 12.4% (32/258): 72 markers |
| VortX Diamond-35 | 7.4% (19/258): 24 markers |
| VISICOIL™§ image markers | 0.4% (1/258): 1 marker |
Data are presented as % (n/total n) or median (interquartile range, Q1–Q3)].
Other than for lymph node biopsy but including all biopsy, fiducial, and pleural dye marking procedures.
A total of 563 fiducial markers were placed in 258 patients (average 2.2 ± 1.7 markers per patient). Individual patient numbers do not sum to totals because each patient may be included in more than one category.
VISICOIL™§ image markers, IBA Dosimetry, Louvain-La-Neuve, Belgium
CT, computed tomography; EBUS, endobronchial ultrasound; ENB, electromagnetic navigational bronchoscopy; Q, quartile.
SuperLock nitinol coil FMs were used in 80.6% (208/258) of patients, followed by VortX Diamond-18 markers (Boston Scientific, Marlborough, MA) in 12.4% (32/258) and VortX Diamond-35 markers (Boston Scientific) in 7.4% (19/258).
Outcomes
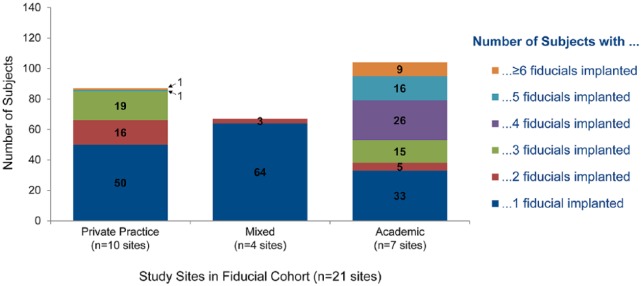
A total of 563 FMs were placed in 258 patients. An average of 2.2 ± 1.7 FMs (median 1.0, range 1–12) were placed per Patient (Table 3), most receiving 1 to 5 FMs. Academic centers were more likely to place at least four FMs than private practice or mixed academic/private centers (Figure 2). Based on subjective operator assessment, 99.2% (256/258) of FMs were accurately placed. Follow-up imaging occurred an average of 8.1 days postprocedure and a median of 0.0 days, with 81.8% (211/258) of patients having follow-up imaging on the procedure day. Based on follow-up imaging, 94.1% (239/254) of markers remained in place.
Table 3.
Outcomes of fiducial marker placement procedures.
| Number of fiducial markers placed per patient | 2.2 ± 1.7 [1.0
(1.0–12.0)] n = 258 |
|---|---|
| Days between ENB procedure and follow-up imaging | 8.1 ± 33.1 [0.0 (0.0–341.0)] n = 253 |
| Purpose of placing fiducial markers | |
| Localization only | 19.4% (50/258) |
| SBRT only | 76.0% (196/258) |
| Localization and SBRT | 4.7% (12/258) |
| Fiducial marker accurately placed (based on subjective operator assessment) | 99.2% (256/258) |
| Fiducial marker still present at follow-up imaging | 94.1% (239/254*) |
Data are presented as % (n/total n) or mean ± SD [median (range)].
As of the database snapshot date, four patients did not have follow-up imaging completed.
ENB, electromagnetic navigational bronchoscopy; SBRT, stereotactic body radiation therapy; SD, standard deviation.
Figure 2.
Number of fiducial markers implanted per patient.
Among all 258 patients undergoing FM placement (with or without concurrent lung lesion biopsy, dye marking, or lymph node sampling), pneumothorax rated CTCAE grade ⩾ 2 was observed in 3.1% (8/258) while any grade pneumothorax was observed in 5.4% (14/258). There were no bronchopulmonary hemorrhages. Four respiratory failure events were observed (1.6%; 4/258), all in patients undergoing general anesthesia. One grade 5 respiratory failure led to an anesthesia-related death, 9 days postprocedure, in a patient with cirrhosis, hepatocellular carcinoma, small-cell carcinoma, and ovarian cancer, as previously reported.23 The other three respiratory failures were all grade 4, in patients with chronic obstructive pulmonary disease and tobacco history; all three patients were reintubated and successfully extubated. There were no deaths related to the ENB system, FM placement, or other associated tools.
As shown in Table 4, the majority of complications occurred in the 219 patients who had ENB-guided lung lesion biopsy in addition to ENB-guided fiducial placement. Among the 39 patients who had FM placement alone, without concurrent lung lesion biopsy or dye marking, there was one respiratory failure event (this was the patient with respiratory failure and death described above) and no pneumothoraces or bronchopulmonary hemorrhages.
Table 4.
Adverse events related to the ENB index procedure or devices as of 1-month follow-up*.
| Patients with fiducial markers placed, in addition to other procedures (n = 219)$ | Patients with fiducial placement only (n = 39) | |
|---|---|---|
| Pneumothorax | ||
| CTCAE grade 2 or higher | 3.7% (8/219) | 0.0% (0/39) |
| All grades | 6.4% (14/219) | 0.0% (0/39) |
| Bronchopulmonary hemorrhage, all grades | 0.0% (0/219) | 0.0% (0/39) |
| Respiratory failure, CTCAE grade 4 or higher | 1.4% (3/219) | 2.6% (1/39) |
| Death (anesthesia-related respiratory failure 9 days post-ENB) | 0.0% (0/219) | 2.6% (1/39) |
Data are presented as % (n/total n).
Other than expected observations associated with anesthesia (e.g. common or expected postprocedure pain, transient nausea, transient emesis, postprocedure constipation).
Includes 207 patients with ENB-guided fiducial marker placement plus ENB-guided lung lesion biopsy, 6 patients with ENB-guided fiducial marker placement plus ENB-guided pleural dye marking, and 6 patients with all 3 procedures (ENB-guided fiducial marker placement, ENB-guided lung lesion biopsy, and ENB-guided pleural dye marking).
CTCAE, Common Terminology Criteria for Adverse Events; ENB, electromagnetic navigational bronchoscopy.
Additional details regarding complication rates and outcomes in patients undergoing lung lesion biopsy in NAVIGATE have been previously published.22,23
Discussion
To our knowledge, this is the largest study of FM placement by any method for SBRT or surgical localization and the only prospective, multicenter study of FM placement yet conducted. Most studies report placing markers by bronchoscopic or percutaneous image-guided approaches with sample sizes ranging from 6 to 112 (18–245 FMs placed).12,13,19,20
Our cohort demonstrated that ENB-guided FM placement is associated with low complication rates overall (7.0%), with pneumothorax in 5.4% (3.1% grade ⩾ 2), occurring less often compared with reports of percutaneous FM placement (5–67%).12 Pneumothorax rates in this FM-placement substudy were similar to those published for the entire United States cohort (including ENB-guided lung biopsy, FM placement, and dye marking) at 4.3% overall and 2.9% for grade ⩾ 2.22 In the 39 patients who had FM placement without biopsy, there were no pneumothoraces or significant bleeding events. Thus, with or without concurrent biopsy, ENB-guided FM placement has low complication rates.
The scope of this multicenter investigation highlighted several interesting practice patterns not previously available in single-center studies. First, ENB is a versatile tool for evaluating thoracic malignancies, allowing for FM placement, tissue sampling, and pleural dye marking in the same anesthetic event. Of the 258 patients undergoing ENB-guided FM placement, 82.6% (213/258) also had lung lesion biopsy and 4.7% (12/258) also had dye marking (including six patients with all three procedures). Complication rates were similar for patients that had FM placement alone versus FM placement with other concurrent procedures. The flexibility to conduct multiple tasks under one anesthetic agent has the potential to be more convenient for the patient and reduce time from biopsy to therapy compared with conducting multiple procedures at different times. Further study of the impact of ENB in the evaluation of thoracic malignancies is needed.
Second, 99% of FMs were accurately placed under ENB guidance based on the operator’s subjective assessment. Accuracy of marker placement for SBRT has been described in some studies as the ability of various tracking systems to track at least three FMs a certain distance and angle apart in six dimensions [X (pitch), Y (row), Z (yaw) directions and rotation around the X/Y/Z axes].5,6 Target tracking errors may result in inaccurate dosing and damage to uninvolved surrounding tissue.19,20,25 Unfortunately, details on interfiducial marker distance, degree of angles between FMs, tracking accuracy, and SBRT system type were not captured in NAVIGATE. These questions require further study.
FM position and stability are critical for optimal SBRT with linear accelerator systems using a gated technique. In the current study, the type of FM used and number placed per patient varied between operators, ranging from 1–12 with a mean of 2.2 (the patient with 12 FMs placed had 2 lung lesions). This practice pattern variation is very interesting given that most markers placed were for SBRT therapy. If respiratory gating is utilized, the general recommendation is to place at least three FMs per lesion, although this is not universally accepted.5,6 However, some NAVIGATE centers routinely placed only two markers. ENB-guided FM placement may also be employed if FMs previously placed by percutaneous methods were inadequate in number or location. The range observed in NAVIGATE likely reflects practice pattern variation between centers, as well as differences between private practice and academic centers (which may be more likely to treat two or more tumors with SBRT and therefore place more FM per patient overall). Interestingly, this practice pattern variation has also been documented in survey studies of radiation oncologists across the United States.26
The SuperLock nitinol coil FM was the most common (80.6%) FM used, possibly due to the exclusive use of that FM at the highest enrolling center of the cohort. Concern about marker migration or better tactile ability for surgical localization may influence choice of FM type. There is sparse information comparing the performance of various types of FMs. In one study of 15 patients using SuperLock nitinol coil FMs who received SBRT, 100% of patients had retention of FMs from implantation. The authors also observed minimal interfractional fiducial migration during the course of radiation treatment.27 In another study, the VortX coil FM had a retention rate of 96.7% (from pretreatment radiation planning CT scan to the first day of therapy) compared to two-band (72%) and the gold-seed (69%) markers.19 The VortX FM was the second most common marker utilized in the NAVIGATE study. Regardless of the type of FM used, 94% were present on follow-up imaging. For those patients that ultimately received radiation therapy, data on whether the FMs were maintained through radiation treatment and whether there was significant interfractional FM migration is unavailable. Factors affecting physician choice of FM type needs further exploration.
While most operators utilized general anesthesia (69%) for FM placement, a significant number also employed moderate sedation in concordance with published literature.28–30 The effect of sedation method on ENB-guided FM placement has not been specifically addressed. One report demonstrated no significant difference in procedural complications between general anesthesia and moderate sedation with ENB-guided biopsies, including 23 patients who underwent ENB solely for FM placement.30
The median duration of the procedure was 57 min overall and 31 min for the ENB procedure, similar to the overall NAVIGATE study (52 min and 25 min, respectively)22 and ENB literature reports ranging from 20 to 70 min.31,32 Procedure times were even shorter in those patients with only FM placement and no lung lesion biopsy. Additionally, ENB guidance was aided by cone–beam CT in 4% and rEBUS in 37%. rEBUS is commonly used to confirm appropriate positioning for biopsy or FM placement.33,34 These complimentary technologies help to confirm appropriate positioning for FM placement or biopsy,35 and to correct any divergence between the data obtained preoperatively by CT scan and data obtained during bronchoscopy.
A total of 62 patients had FM placed prior to lung surgery (50 for localization only and 12 for both SBRT and localization). Data were not collected on whether the markers were useful during the surgery, or the type of surgical approach (i.e. wedge resection followed by lobectomy). Additionally, the type of FM preferred by the surgeons or reasoning behind the FM choice was not recorded. Interestingly, there are only two prior, retrospective, single-center reports of ENB-guided FM placement in four patients to aid in wedge resection of peripheral pulmonary nodules.7,9 Successful CT-guided percutaneous localization with FM, hook wire, and pleural dye is well established for small peripheral nodules.36–39 However, percutaneous placement has been associated with multiple complications, including pneumothorax, hemorrhage, pleural reaction, and dislodgement, which can lead to localization failure.40,41 Further studies will clarify the optimal method and indication for preoperative marker placement to localize deeper nodules for surgical resection. The growing interest in robotic surgery for segmental and subsegmental resection of ground glass opacities and subsolid nodules will likely require improved methods of localization, such as dye or FM placement.
Limitations
NAVIGATE is an observational cohort study collecting data on ENB procedures in a large and diverse study population. This pragmatic design provides a broad picture of ENB usage patterns and outcomes in a generalizable, unrestricted setting. However, it also poses some limitations with regard to important data not mandated by the intentionally flexible study protocol. First, defining placement accuracy based on the operator’s subjective assessment may not be the most clinically appropriate indicator for SBRT success. Distance from the marker to the lesion, position of markers, and migration were not recorded. The ability to successfully track FM in three or more dimensions for those patients receiving gated radiation treatments may have been a more relevant definition. Second, the type of SBRT system used per operator was not specified. Given the heterogeneity of radiation systems employed to treat patients with lung SBRT, it is unclear whether the FMs were used to deliver gated treatments or whether they were simply used to improve target localization for nongated treatments. Third, only the number of FMs placed per patient, not per lesion, was recorded. The number of fiducials used was left to the discretion of the bronchoscopist, presumably based on feedback from the radiation oncologist or local physicist. This reflects widely variable SBRT clinical practice patterns.26 Finally, lesion characteristics data (such as location and size) were not collected for the FM subgroup.
Conclusion
NAVIGATE is the largest prospective study of ENB yet conducted and, to our knowledge, this substudy is the only multicenter study of ENB-guided FM placement. This NAVIGATE cohort demonstrates that ENB-guided FM placement for SBRT and localization for surgery is versatile and accurate, with low complication rates. Further research is necessary to understand physician practice patterns, in terms of optimizing FM marker placement utilizing ENB guidance.
Acknowledgments
This work was previously presented at the IASLC 18th World Conference on Lung Cancer, 18 October 2017 (Yokohama, Japan).43
Footnotes
Funding: This study was sponsored and funded by Medtronic.
Conflict of interest statement: MRB, EEF, SJK, WSK, GL, MAN, MP, and TW: consultants for Medtronic; WSK: part-time employee of Medtronic (began after enrollment completion) with intellectual property rights; EEF: consultant (Boston Scientific and Olympus); CWT: travel reimbursement (Medtronic). Biostatistical analysis was provided by Haiying Lin of Medtronic. Technical and editorial assistance was provided by Kristin L. Hood PhD, of Medtronic, in accordance with Good Publication Practice (GPP3) guidelines.42 The first and final drafts were written by the lead author (MRB).
Data availability: Compiled NAVIGATE data will be made publicly available on [ClinicalTrials.gov identifier NCT02410837] after the completion of the 2-year follow up. The interim data on which this paper is based are available from the corresponding author upon reasonable request.
ORCID iD: Mark R. Bowling  https://orcid.org/0000-0002-3490-5647
https://orcid.org/0000-0002-3490-5647
Contributor Information
Mark R. Bowling, Department of Internal Medicine, Division of Pulmonary, Critical Care and Sleep Medicine, Brody School of Medicine, East Carolina University, 521a Moye Boulevard, Greenville, NC 27834, USA.
Erik E. Folch, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
Sandeep J. Khandhar, Inova Health System, Virginia Cancer Specialists, Fairfax, VA, USA
Jordan Kazakov, University Hospitals Cleveland Medical Center and Case Western Reserve School of Medicine, Cleveland, OH, USA.
William S. Krimsky, Medstar Franklin Square Hospital Center, Baltimore, MD, USA
Gregory P. LeMense, Blount Memorial Physicians Group, Maryville, TN, USA
Philip A. Linden, University Hospitals Cleveland Medical Center and Case Western Reserve School of Medicine, Cleveland, OH, USA
Boris A. Murillo, Providence Health Center, Waco, TX, USA
Michael A. Nead, University of Rochester Medical Center, Rochester NY, USA
Michael A. Pritchett, Pulmonary Department, Pinehurst Medical Clinic and FirstHealth Moore Regional Hospital, Pinehurst, NC, USA
Catalina V. Teba, University Hospitals Cleveland Medical Center and Case Western Reserve School of Medicine, Cleveland, OH, USA
Christopher W. Towe, University Hospitals Cleveland Medical Center and Case Western Reserve School of Medicine, Cleveland, OH, USA
Terence Williams, Department of Radiation Oncology, Ohio State University Wexner Medical Center, Columbus OH, USA Brigham and Women’s Hospital, Boston, MA, USA.
Carlos J. Anciano, East Carolina University, Greenville, NC, USA
References
- 1. Caillet V, Booth JT, Keall P. IGRT and motion management during lung SBRT delivery. Phys Med 2017; 44: 113–22. [DOI] [PubMed] [Google Scholar]
- 2. Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e278S–e313S. [DOI] [PubMed] [Google Scholar]
- 3. Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: executive summary of an ASTRO evidence-based guideline. Pract Radiat Oncol 2017; 7: 295–301. [DOI] [PubMed] [Google Scholar]
- 4. Hagmeyer L, Priegnitz C, Kocher M, et al. Fiducial marker placement via conventional or electromagnetic navigation bronchoscopy (ENB): an interdisciplinary approach to the curative management of lung cancer. Clin Respir J 2016; 10: 291–297. [DOI] [PubMed] [Google Scholar]
- 5. Murphy MJ. Fiducial-based targeting accuracy for external-beam radiotherapy. Med Phys 2002; 29: 334–344. [DOI] [PubMed] [Google Scholar]
- 6. Van der Voort van Zyp NC, Prevost JB, Hoogeman MS, et al. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: clinical outcome. Radiother Oncol 2009; 91: 296–300. [DOI] [PubMed] [Google Scholar]
- 7. Andrade RS. Electromagnetic navigation bronchoscopy-guided thoracoscopic wedge resection of small pulmonary nodules. Semin Thorac Cardiovasc Surg 2010; 22: 262–265. [DOI] [PubMed] [Google Scholar]
- 8. Zhao ZR, Lau RW, Ng CS. Hybrid theatre and alternative localization techniques in conventional and single-port video-assisted thoracoscopic surgery. J Thorac Dis 2016; 8: S319-S327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abbas A, Kadakia S, Ambur V, et al. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg 2017; 153: 1581–1590. [DOI] [PubMed] [Google Scholar]
- 10. Ng CSH, Chu CM, Lo CK, et al. Hybrid operating room Dyna-computed tomography combined image-guided electromagnetic navigation bronchoscopy dye marking and hookwire localization video-assisted thoracic surgery metastasectomy. Interact Cardiovasc Thorac Surg 2018; 26: 338–340. [DOI] [PubMed] [Google Scholar]
- 11. Ohta K, Shimohira M, Murai T, et al. Percutaneous fiducial marker placement prior to stereotactic body radiotherapy for malignant liver tumors: an initial experience. J Radiat Res 2016; 57: 174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trumm CG, Haussler SM, Muacevic A, et al. CT fluoroscopy-guided percutaneous fiducial marker placement for CyberKnife stereotactic radiosurgery: technical results and complications in 222 consecutive procedures. J Vasc Interv Radiol 2014; 25: 760–768. [DOI] [PubMed] [Google Scholar]
- 13. Kular H, Mudambi L, Lazarus DR, Cornwell L, et al. Safety and feasibility of prolonged bronchoscopy involving diagnosis of lung cancer, systematic nodal staging, and fiducial marker placement in a high-risk population. J Thorac Dis 2016; 8: 1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris K, Gomez J, Dhillon SS, et al. Convex probe endobronchial ultrasound placement of fiducial markers for central lung nodule (with video). Endosc Ultrasound 2015; 4: 156–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yousefi S, Collins BT, Reichner CA, et al. Complications of thoracic computed tomography-guided fiducial placement for the purpose of stereotactic body radiation therapy. Clin Lung Cancer 2007; 8: 252–256. [DOI] [PubMed] [Google Scholar]
- 16. Farkas EA, Stoeckel DA, Nassif AS, et al. Intracoronary fiducial embolization after percutaneous placement for stereotactic radiosurgery. Ann Thorac Surg 2012; 93: 1715-1717. [DOI] [PubMed] [Google Scholar]
- 17. Hennessey H, Valenti D, Cabrera T, et al. Cardiac embolization of an implanted fiducial marker for hepatic stereotactic body radiotherapy: a case report. J Med Case Rep 2009; 3: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e142S–e65S. [DOI] [PubMed] [Google Scholar]
- 19. Minnich DJ, Bryant AS, Wei B, et al. Retention rate of electromagnetic navigation bronchoscopic placed fiducial markers for lung radiosurgery. Ann Thorac Surg 2015; 100: 1163–1166. [DOI] [PubMed] [Google Scholar]
- 20. Schroeder C, Hejal R, Linden PA. Coil spring fiducial markers placed safely using navigation bronchoscopy in inoperable patients allows accurate delivery of CyberKnife stereotactic radiosurgery. J Thorac Cardiovasc Surg 2010; 140: 1137–1142. [DOI] [PubMed] [Google Scholar]
- 21. Belanger AR, Burks AC, Chambers DM, et al. Peripheral lung nodule diagnosis and fiducial marker placement using a novel tip-tracked electromagnetic navigation bronchoscopy system. J Bronchology Interv Pulmonol 2019; 26: 41–48. [DOI] [PubMed] [Google Scholar]
- 22. Folch EE, Pritchett MA, Nead MA, et al. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective, multicenter NAVIGATE study. J Thorac Oncol 2019; 14: 445–458. [DOI] [PubMed] [Google Scholar]
- 23. Khandhar SJ, Bowling MR, Flandes J, et al. Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: first results of the prospective, multicenter NAVIGATE study. BMC Pulm Med 2017; 17: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Folch EE, Bowling MR, Gildea TR, et al. Design of a prospective, multicenter, global, cohort study of electromagnetic navigation bronchoscopy. BMC Pulm Med 2016; 16: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura M, Takamiya M, Akimoto M, et al. Target localization errors from fiducial markers implanted around a lung tumor for dynamic tumor tracking. Phys Med 2015; 31: 934–941. [DOI] [PubMed] [Google Scholar]
- 26. Pan H, Rose BS, Simpson DR, et al. Clinical practice patterns of lung stereotactic body radiation therapy in the United States: a secondary analysis. Am J Clin Oncol 2013; 36: 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rong Y, Bazan JG, Sekhon A, et al. Minimal inter-fractional fiducial migration during image-guided lung stereotactic body radiotherapy using Superlock nitinol coil fiducial markers. PLoS One 2015; 10: e0131945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bolton WD, Richey J, Ben-Or S, Hale AL, et al. Electromagnetic navigational bronchoscopy: a safe and effective method for fiducial marker placement in lung cancer patients. Am Surg 2015; 81: 659–662. [PubMed] [Google Scholar]
- 29. Jackson P, Steinfort DP, Kron T, et al. Practical assessment of bronchoscopically inserted fiducial markers for image guidance in stereotactic lung radiotherapy. J Thorac Oncol 2016; 11: 1363–1368. [DOI] [PubMed] [Google Scholar]
- 30. Bowling MR, Kohan MW, Walker P, Efird J, Ben Or S. The effect of general anesthesia versus intravenous sedation on diagnostic yield and success in electromagnetic navigation bronchoscopy. J Bronchology Interv Pulmonol 2015; 22: 5–13. [DOI] [PubMed] [Google Scholar]
- 31. Pearlstein DP, Quinn CC, Burtis CC, Ahn KW, Katch AJ. Electromagnetic navigation bronchoscopy performed by thoracic surgeons: one center’s early success. Ann Thorac Surg 2012; 93: 944–949; discussion 9–50. [DOI] [PubMed] [Google Scholar]
- 32. Ozgul G, Cetinkaya E, Ozgul MA, et al. Efficacy and safety of electromagnetic navigation bronchoscopy with or without radial endobronchial ultrasound for peripheral lung lesions. Endosc Ultrasound 2016; 5: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ng CS, Yu SC, Lau RW, et al. Hybrid DynaCT-guided electromagnetic navigational bronchoscopic biopsy. Eur J Cardiothorac Surg 2016; 49(Suppl 1): i87–i8. [DOI] [PubMed] [Google Scholar]
- 34. Hsia DW, Jensen KW, Curran-Everett D, et al. Diagnosis of lung nodules with peripheral/radial endobronchial ultrasound-guided transbronchial biopsy. J Bronchology Interv Pulmonol 2012; 19: 5–11. [DOI] [PubMed] [Google Scholar]
- 35. Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007; 176: 36–41. [DOI] [PubMed] [Google Scholar]
- 36. Finley RJ, Mayo JR, Grant K, et al. Preoperative computed tomography-guided microcoil localization of small peripheral pulmonary nodules: a prospective randomized controlled trial. J Thorac Cardiovasc Surg 2015; 149: 26–31. [DOI] [PubMed] [Google Scholar]
- 37. Lee NK, Park CM, Kang CH, et al. CT-guided percutaneous transthoracic localization of pulmonary nodules prior to video-assisted thoracoscopic surgery using barium suspension. Korean J Radiol 2012; 13: 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Su TH, Fan YF, Jin L, et al. CT-guided localization of small pulmonary nodules using adjacent microcoil implantation prior to video-assisted thoracoscopic surgical resection. Eur Radiol 2015; 25: 2627–2633. [DOI] [PubMed] [Google Scholar]
- 39. Klinkenberg TJ, Dinjens L, Wolf RFE, et al. CT-guided percutaneous hookwire localization increases the efficacy and safety of VATS for pulmonary nodules. J Surg Oncol 2017; 115: 898–904. [DOI] [PubMed] [Google Scholar]
- 40. Ciriaco P, Negri G, Puglisi A, Nicoletti R, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004; 25: 429–433. [DOI] [PubMed] [Google Scholar]
- 41. Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011; 25: 1723–1729. [DOI] [PubMed] [Google Scholar]
- 42. Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med 2015; 163: 461–464. [DOI] [PubMed] [Google Scholar]
- 43. Bowling M, Folch E, Khandhar S, et al. Fiducial Marker Placement Using Electromagnetic Navigation Bronchoscopy in the Prospective, Multicenter NAVIGATE Study. J Thorac Oncol. 2017; 12: S1886–S1887. [DOI] [PMC free article] [PubMed] [Google Scholar]