Abstract
In this study, we report a case of a 50-year-old Japanese man who had chronic whiplash-associated disorder, hyperlipidaemia, hyperuricacidaemia, and mild liver dysfunction due to excessive alcohol intake. Recently, he developed mild tremor in his left hand. Initiation of clonazepam (0.5 mg once daily before bedtime) helped to gradually ameliorate the tremor. However, 13 days after clonazepam initiation, his liver function and lipid profiles aggravated, and his postprandial glucose level increased to 400 mg/dL. Clonazepam was stopped promptly, and at 7 days after discontinuation, the abnormal triglyceride levels, liver dysfunction, and glycometabolism improved without any other medical intervention. This case may provide information on cautious use of clonazepam. When clonazepam is used for patients with existing hyperlipidaemia and liver dysfunction, it may cause abnormal lipid profile, aggravate liver dysfunction, and lead to remarkable glucose elevation.
Keywords: Clonazepam, drug-induced liver dysfunction, drug-induced glycaemic crisis
Introduction
Clonazepam1,2[5-(2-chlorophenyl)-1,3-dihydro-1-methyl-7-nitro-2H-1,4-benzodiazepin-2-one] is a benzodiazepine structurally related to chlordiazepoxide hydrochloride, diazepam, and nitrazepam. The absolute bioavailability of clonazepam is approximately 90%. Clonazepam is rapidly absorbed after oral ingestion (Tmax = 1–4 h) and is approximately 85% bound to plasma proteins. It is highly metabolized in the liver, with less than 2% unchanged clonazepam being excreted via urine. Cytochrome P450, including CYP3A, may play an important role in clonazepam reduction and oxidation. The elimination half-life of clonazepam is typically 20–40 h. The pharmacokinetics of clonazepam is dose-independent throughout the dosage range. Clonazepam is used to treat typical absence, infantile myoclonic, atypical absence, myoclonic, and akinetic seizures.1 The precise mechanism by which clonazepam exerts its antiseizure and antipanic effects is unknown. However, its mechanism of action is believed to be related to its ability to enhance the activity of gamma aminobutyric acid (GABA), a major inhibitory neurotransmitter in the central nervous system.2 The most common side-effects of clonazepam include drowsiness, dizziness, fatigue, ataxia, depression, and problems with memory.2 Anticonvulsant treatment for epilepsy frequently causes abnormalities in liver function; thus, clonazepam may also cause temporal elevation of liver enzyme levels.2,3 Nevertheless, clonazepam prescribing information does not include hyperlipidaemia or hyperglycaemia as adverse effects.2
In this study, we report a case that showed remarkable aggravation of liver function, lipid profiles, and glycometabolism after initiation of clonazepam. Because these abnormalities improved soon after discontinuation of clonazepam, the drug was the most likely cause of these events.
Case report
A 50-year-old Japanese man, 174 cm tall and weighing 74 kg (body mass index (BMI) = 24.4), had chronic whiplash-associated disorder and was treated with an occasional drip infusion of electrolyte solution (1% glucose and acetated Ringer’s solution). He also had hyperlipidaemia, hyperuricacidaemia, and liver dysfunction partially because of excessive alcohol intake (maximum intake of 350 mL of beer and 500 mL of distilled liquor almost every day) and high-calorie diet. He had been advised to reduce alcohol intake and improve his diet. He was prescribed 160 mg of fenofibrate (Lipidil®; Kaken Pharmaceutical Co., Ltd, Tokyo, Japan) and 10 mg of febuxostat (Feburic®; Teijin Pharma, Tokyo, Japan) for at least 1 year. Recently, he noticed mild tremor in his left hand. Clonazepam (Landsen®; Sumitomo Dainippon Pharma Co., Ltd, Osaka, Japan), a type of benzodiazepine, was initiated at a dose of 0.5 mg once daily before bedtime, and the tremor gradually ameliorated.
However, 13 days after clonazepam initiation, his regular blood test revealed remarkable increases in the levels of triglycerides, liver enzymes, and markers for glycometabolism (Table 1), compared to those at 9 days before clonazepam initiation. He was not feverish and had no skin rash. The result of abdominal computed tomography (CT) scan performed on that day showed remarkable fatty liver, which was not observed in the CT scan result at 8 months ago. Because specific antibodies for viral hepatitis or specific immunological parameters in autoimmune liver diseases were absent (Table 1), viral hepatitis or autoimmune liver diseases were unlikely to be the cause of the abrupt liver dysfunction. Levels of blood glucose and HbA1c abruptly increased to 400 mg/dL and 6.6% from 108 mg/dL and 5.4%, respectively, compared to those at 9 days before clonazepam initiation. High glycoalbumin (GA) level (20.4%) also suggested recent robust increase in glucose level. Because the patient showed negative result in anti-glutamic acid decarboxylase (GAD) antibody test and sufficient postprandial C-peptide level (8.27 ng/mL), it was unlikely that he had developed type 1 diabetes. There had been no drastic changes in the patient’s daily life in this period (including food and alcohol intake), except clonazepam usage. Therefore, we suspected clonazepam as the cause of the sudden emergence of abnormalities and discontinued the drug.
Table 1.
Laboratory results at 13 days after clonazepam initiation.
| CBC | Glycometabolism tests | ||
| WBC | 8400/mL | Plasma glucose | 400 mg/dL |
| RBC | 504 × 104/mL | HbA1c | 6.6% |
| Hb | 17.0 g/dL | Glycoalbumin | 20.4% |
| Ht | 48.3% | CPR | 8.27 ng/mL |
| Platelet | 26.9 × 104/mL | Anti-GAD antibody | <5.0 U/mL |
| Biochemical tests | Auto-antibody tests | ||
| T-bilirubin | 1.0 mg/dL | ANA | <40 |
| AST | 305 U/L | IgG | 1079 mg/dL |
| ALT | 225 U/L | IgM | 100 mg/dL |
| γ-GTP | 591 U/L | AMA-M2 | <1.5 |
| Amylase | 41 U/L | Virus infections | |
| CK | 57 U/L | Anti-HAV-IgM | <0.4 |
| BUN | 13.8 mg/dL | HBsAg | 0.449 |
| Creatinine | 1.04 mg/dL | Anti-HBc-IgM | 0.07 |
| e-GFR | 60.5 ml/min/1.73m2 | Anti-HCV | Negative |
| UA | 3.3 mg/dL | Cytomegalo IgG | 27.5 |
| Na | 134 mEq/L | Cytomegalo IgM | 0.47 |
| K | 4.8 mEq/L | EB VCA IgG | 5.8 |
| Cl | 93 mEq/L | EB VCA IgM | 0.2 |
| Triglyceride | 2370 mg/dL | Tumour markers | |
| LDL-C | 46 mg/dL | CEA | 2.7 ng/mL |
| HDL-C | 17 mg/dL | CA19-9 | 9.5 U/mL |
| DLST | Negative | ||
CBC: complete blood count; WBC: white blood cell count; RBC: red blood cell count; ANA: antinuclear antibodies; AST: aspartate transaminase; ALT: alanine transaminase; GTP: Glutamyl transpeptidase; AMA: antimitochondrial antibodies; CK: creatine kinase; BUN: blood urea nitrogen; HAV: hepatitis A virus; IgM: immunoglobulin M; IgG: immunoglobulin G; e-GFR: estimated glomerular filtration rate; HCV: hepatitis C virus; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; DLST: drug lymphocyte-stimulation test.
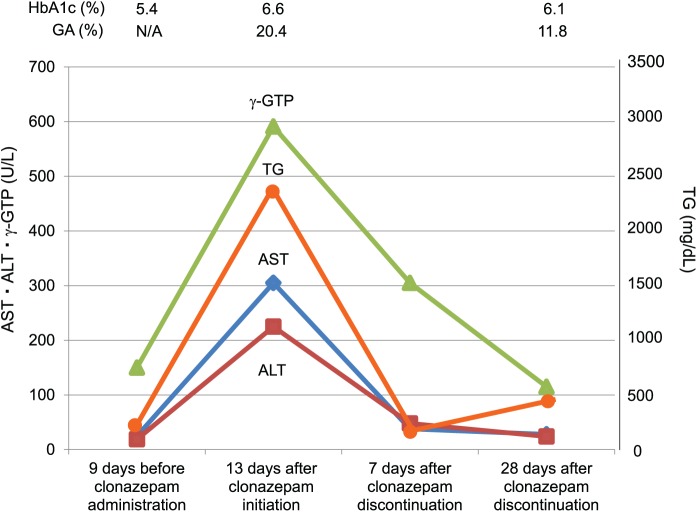
Seven days after clonazepam discontinuation, the abnormal levels of triglycerides, liver dysfunction, and glycometabolism improved without any other medical intervention. Twenty-eight days after discontinuation, the levels of fasting triglycerides and γ-GTP were still high but had decreased to levels similar to those before clonazepam initiation. The levels of markers for glycometabolism, such as fasting glucose, HbA1c, and GA, decreased to 107 mg/dL, 6.1%, and 11.8%, respectively (Figure 1), and did not increase thereafter. Fasting C-peptide level at 28 days after drug discontinuation was 2.41 ng/mL, indicating that the patient’s insulin secretion had not depleted. The result of CT scan performed 10 months after clonazepam discontinuation still showed a certain degree of fatty liver, and thus, continuous monitoring was recommended.
Figure 1.
Changes in liver function, triglycerides levels, and glycometabolism over the time of clonazepam administration and discontinuation.
Discussion
Drug-induced liver injury (DILI) is defined as liver injury caused by various medications, herbs, or xenobiotics that results in abnormalities in liver tests.4,5 DILI is a potentially serious adverse reaction; however, it is difficult to predict. The diagnosis is challenging because it is a clinical diagnosis of exclusion that requires demonstration of a close correlation between a patient’s history of drug administration and clinical data.4–7 Common diseases to be excluded are chronic liver diseases, such as alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), and non-alcoholic steatohepatitis (NASH), and acute or chronic viral infections.4 The known risk factors for DILI include genetic susceptibility, co-medication, alcohol use, pre-existing chronic liver diseases, drug lipophilicity, extensive metabolic rates, and high daily doses of drugs,4–8 although some of these risk factors are controversial.8
The Roussel Uclaf Causality Assessment Method (RUCAM) is a well-established tool commonly used to quantitatively assess causality in cases of suspected DILI.9–11 Among seven items in RUCAM, our patient satisfied the following five items: (1) time to onset from the beginning of the drug: 5–90 days (score +2); (2) course of ALT after cessation of the drug: ⩾50% decrease within 8 days (score +3); (3) risk factors: alcohol use, currently >3 drinks/day (score +1); (4) search for alternative causes: reasonably ruled out (score +2); and (5) previous hepatotoxicity of the drug: reaction labelled in the product characteristics (score +2). The resulting causality is graded by the total score as follows: ⩽0, excluded; 1–2, unlikely; 3–5, possible; 6–8, probable; and ⩾9, highly probable. Therefore, because the total score of our patient reached 10, it was considered ‘highly probable’ that clonazepam was the causal drug for DILI in our patient.
Although the patient showed negative results in drug lymphocyte-stimulation test (DLST), clonazepam was not ruled out as a suspected drug. DLST is problematic because of a lack of standardization and reproducibility in suspected DILI.4
The patient had been taking fenofibrate and febuxostat regularly, which are potential risk factors for liver dysfunction.12,13 Although these drugs may have partially contributed to chronic liver dysfunction in our patient, they are unlikely to be the sole cause for the event because they were started at least 1 year ago and their dosages were not changed during that period. The patient had been receiving occasional drip infusion of electrolyte solution for his chronic whiplash-associated disorder. However, considering the content of the solution and the timing of his last drip infusion before the events, which was approximately 5 months ago, the drip infusion was also unlikely to cause the events. Based on results of pancreatic function-related blood parameters and repeated CT scans, pancreatic disease was unlikely to have contributed to the events. Because γ-GTP levels increased at the same time as ALT levels, we suspected that the patient binged on food and alcohol shortly before we examined him. Although he denied it and this suspicion would not necessarily explain the remarkable glucose elevation, the possibility of a recent binge should be considered for a patient such as ours.
Because our patient consumed excessive alcohol regularly, ALDs, such as previously unrecognized alcoholic fatty liver disease, could be a possible reason for liver dysfunction, and this could have triggered and aggravated DILI.
Idiosyncratic DILI is classified as ‘hypersensitivity’ or ‘metabolic’ DILI.14 Hypersensitivity DILI is often accompanied by immunoallergic symptoms, such as skin rashes, eosinophilia, or lymphadenopathy; however, none of these were observed in our patient. Therefore, we considered that the DILI in our patient was metabolic rather than allergic or hypersensitive. Regarding pharmacological risk factors for DILI, the drugs metabolized by cytochrome P450 enzymes have nearly four times higher likelihood of causing DILI.6 Because clonazepam is mainly metabolized in the liver, primarily by cytochrome P450 isoenzyme 3A4,2,15 underlying liver diseases might have been associated with decreased hepatic drug clearance and resulted in drug accumulation. This could have eventually caused DILI in our patient.
After clonazepam initiation, the patient showed exacerbation of dyslipidaemia and developed remarkable fatty liver. We consider this as drug-induced steatosis (DIS) or steatohepatitis (DISH). DISH is a rare form of DILI that is manifested through the characteristic pathological patterns of intracellular accumulation of lipids in hepatocytes, often accompanied by oxidative stress and hepatic inflammation.7,15,16 Pre-existing steatosis could possibly render the hepatocytes vulnerable to drug insults, alter hepatic drug metabolism, and eventually exacerbate existing damage.7 Therefore, DISH is increasingly important, given the high background of metabolic disease in the general population.16 Because our patient had a long medical history of hypertriglyceridaemia before clonazepam initiation, he could have had a potential risk of developing DISH due to the suspected drug.
In addition to DILI and DISH, clonazepam was considered a direct or indirect cause of the hyperglycaemic crisis in our patient owing to the medical time course of the initiation and discontinuation of the drug. Broad classes of commonly used drugs, such as the drugs used in hormonal therapy, mTOR inhibitors, tyrosine kinase inhibitors, antihypertensive agents, statins, antipsychotics, antiretroviral agents, and interferon, are known to cause diabetes.17 Drugs may induce hyperglycaemia through various mechanisms, including alterations in insulin secretion and sensitivity, direct cytotoxic effects on pancreatic cells, and increases in glucose production.18 Therefore, we suggest that clonazepam directly caused hyperglycaemia. However, we should also consider the effect of DISH on glycometabolism because fatty acids (FAs) are known to induce a pleiotropic effect on beta-cell function, including changes in cell signalling, insulin secretion, mitochondrial metabolism, and membrane composition.19 Although we did not examine FAs in the patient, high levels of FAs could have severely impaired glucose-stimulated insulin secretion; thus, DISH and hyperglycaemia may have developed simultaneously or hyperglycaemia may have developed shortly after DISH.
Another possible explanation for the effect on glycometabolism is mitochondrial dysfunction due to the drug. Mitochondria plays a significant role in regulating insulin secretion in beta-cells by regulating adenosine triphosphate (ATP) levels responsible for facilitating membrane depolarization and insulin granule exocytosis.20 The major mechanisms of mitochondrial toxicity involve the inhibition of FA beta-oxidation, oxidative phosphorylation, and mitochondrial respiration.15 Clinically, impaired insulin secretion is observed in mitochondrial diabetes, a hereditary disorder of mitochondrial function.21 DISH development is largely due to off-target therapeutic effects that cause mitochondrial damage.15 Therefore, we speculated that a certain level of mitochondrial damage serious enough to disturb both lipid and glucose metabolism occurred in our patient.
Conclusion
This case provided insights into the risks of DILI, DISH, and remarkable hyperglycaemia possibly triggered by clonazepam. In addition, our findings suggested that caution should be exercised when any drug is initiated in patients with pre-existing metabolic abnormality. Further investigation on the relationship between DISH and hyperglycaemia in patients will improve our understanding of how lipid and glycaemic metabolism are linked.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and the Helsinki Declaration of 1975, as revised in 2000 and 2008. Ethical approval to report this case was obtained from Ethics Committee of Sanno Hospital (25 August 2018, approval number: 18-S-13).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient for his anonymized information to be published in this article.
ORCID iD: Miyako Kishimoto  https://orcid.org/0000-0003-3043-2720
https://orcid.org/0000-0003-3043-2720
References
- 1. Browne TR. Clonazepam. N Engl J Med 1978; 299: 812–816. [DOI] [PubMed] [Google Scholar]
- 2. KLONOPIN TABLETS (clonazepam), https://www.gene.com/download/pdf/klonopin_prescribing.pdf (2017, accessed 6 March 2019).
- 3. Olsson R, Zettergren L. Anticonvulsant-induced liver damage. Am J Gastroenterol 1988; 83: 576–577. [PubMed] [Google Scholar]
- 4. Teschke R, Schulze J, Eickhoff A, et al. Drug induced liver injury: can biomarkers assist RUCAM in causality assessment. Int J Mol Sci 2017; 18(4): 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katarey D, Verma S. Drug-induced liver injury. Clin Med 2016; 16: s104–s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarges P, Steinberg JM, Lewis JH. Drug-induced liver injury: highlights from a review of the 2015 literature. Drug Saf 2016; 39(9): 801–821. [DOI] [PubMed] [Google Scholar]
- 7. Rabinowich L, Shibolet O. Drug induced steatohepatitis: an uncommon culprit of a common disease. Biomed Res Int 2015; 2015: 168905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teschke R, Danan G. Drug-induced liver injury: is chronic liver disease a risk factor and a clinical issue. Expert Opin Drug Metab Toxicol 2017; 13(4): 425–438. [DOI] [PubMed] [Google Scholar]
- 9. Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci 2015; 17(1): E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Danan G, Benichou C. Causality assessment of adverse reactions to drugs – I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 1993; 46(11): 1323–1330. [DOI] [PubMed] [Google Scholar]
- 11. Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs – II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol 1993; 46(11): 1331–1336. [DOI] [PubMed] [Google Scholar]
- 12. Product Information Lipidil, http://www.medicines.org.au/files/goplipid.pdf (accessed 6 March 2019).
- 13. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. FEBUXOSTAT, https://livertox.nih.gov/Febuxostat.htm#insert (accessed 6 March 2019). [PubMed]
- 14. Devarbhavi H. An update on drug-induced liver injury. J Clin Exp Hepatol 2012; 2: 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schumacher JD, Guo GL. Mechanistic review of drug-induced steatohepatitis. Toxicol Appl Pharmacol 2015; 289(1): 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dash A, Figler RA, Sanyal AJ, et al. Drug-induced steatohepatitis. Expert Opin Drug Metab Toxicol 2017; 13: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jain V, Patel RK, Kapadia Z, et al. Drugs and hyperglycemia: a practical guide. Maturitas 2017; 104: 80–83. [DOI] [PubMed] [Google Scholar]
- 18. Fathallah N, Slim R, Larif S, et al. Drug-induced hyperglycaemia and diabetes. Drug Saf 2015; 38: 1153–1168. [DOI] [PubMed] [Google Scholar]
- 19. Janikiewicz J, Hanzelka K, Kozinski K, et al. Islet β-cell failure in type 2 diabetes – within the network of toxic lipids. Biochem Biophys Res Commun 2015; 460: 491–496. [DOI] [PubMed] [Google Scholar]
- 20. Stroh M, Swerdlow RH, Zhu H. Common defects of mitochondria and iron in neurodegeneration and diabetes (MIND): a paradigm worth exploring. Biochem Pharmacol 2014; 88(4): 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kishimoto M, Hashiramoto M, Araki S, et al. Diabetes mellitus carrying a mutation in the mitochondrial tRNA(Leu(UUR)) gene. Diabetologia 1995; 38(2): 193–200. [DOI] [PubMed] [Google Scholar]