Abstract
Objective:
To evaluate sleep disturbances in a diverse, contemporary HIV-positive patient cohort and to identify demographic, clinical, and immune correlates.
Methods:
A convenience sample of 176 patients from a racially and ethnically diverse HIV-positive patient cohort in an urban population. This was a cross-sectional, epidemiologic study. We surveyed participants using multiple standardized instruments to assess depression, sleep quality, and risk for sleep apnea. We analyzed demographic, behavioral, and clinical correlates.
Results:
A total of 56% of participants were female, 75% Black and 64% had heterosexual HIV risk. The median age was 49 years. Poor sleep quality (Pittsburgh Sleep Quality Index > 5) was reported by 73% of patients and 52% met insomnia diagnosis criteria. A single question about self-reported sleep problems predicted a Pittsburgh Sleep Quality Index > 5 with a sensitivity and specificity of 82% and 81%, respectively. Female sex was significantly associated with higher risk of poor sleep quality, depression, and insomnia, whereas higher risk of obstructive sleep apnea was significantly associated with older age, male sex, obesity (body mass index ⩾ 30 kg/m2), and metabolic comorbidities. High risk for obstructive sleep apnea, high rate of depression, and poor sleep hygiene represent treatment targets for sleep problems in HIV patients.
Conclusion:
Sleep disturbances were common in this patient cohort, although largely undiagnosed and untreated. Sleep problems are linked to worse disease progression and increased cardiovascular mortality. Screening for sleep problems with a single question had high sensitivity and specificity. In those patients with self-reported sleep problems, screening for obstructive sleep apnea, depression, and sleep hygiene habits should be part of routine HIV care.
Keywords: HIV, sleep apnea, insomnia, sleep disparities, women’s sleep
Introduction
Sleep disorders are more common in people living with HIV (PLWH) than in the general population, with prevalence ranging from 30% to 100% depending on definition and methodology used.1–5 Although sleep complaints were recognized early in the HIV epidemic,6 and have been described at any stage of the disease, the long-term effects of these disturbances are becoming more important now that highly effective anti-retroviral therapy (ART) have transformed the care of HIV into that of a chronic disease.7 Sleep disturbances have a significant impact in quality of life and are associated with poorer health outcomes, including increase risk of cardiovascular and metabolic diseases as well as impaired cognition.8,9 In HIV patients, impaired sleep quality has been also associated with poor medication adherence, and some have hypothesized that given the regulatory role of sleep in the immune function, sleep disorders could also independently accelerate HIV disease progression.10,11 However, despite a very high prevalence of sleep disturbances in PLWH and significant morbidity, sleep disorders remained largely underdiagnosed and undertreated in this population.
The pathology of sleep disturbances in HIV is not well understood which makes the diagnosis and treatment more challenging. Earlier studies evaluated changes in sleep patterns and architecture, showing increased fragmented sleep, decreased sleep efficiency, and increased sleep latency in patients with HIV compared with controls.4,6,12,13 Abnormal sleep patterns have been attributed to multiple factors including immune dysregulation;14,15 the direct effects of the HIV in the central nervous system,16,17 and more recent studies have focused on the effects of antiretrovirals and lipodystrophy.18–21 Psychosocial factors—including depression, greater perceived stress, substance abuse, and poverty—which are known to significantly impact the quality of sleep, are also more prevalent in PLWH.22,23
Despite a growing body of literature addressing sleep disorders in HIV patients, there is a lack of clinical guidance on how to screen and treat sleep disturbances as part of the routine HIV care. Multiple studies evaluating sleep problems in HIV were done during the early epidemic, before ART was as effective and had lower side effect profile as it is today. Several recent studies have included large patient cohorts but most of them have had participants that do not fully represent the changing demographics of PLWH in America. Women, African American, and Latino communities are now most commonly affected by HIV and also disproportionally affected by sleep problems, which some authors have recognized as “sleep disparity.”24,25 To our knowledge, only a few studies have included a significant proportion of Black and Latina women.26–28 In addition, obstructive sleep apnea (OSA), which can significantly impact sleep quality is underdiagnosed in HIV patients,29 nevertheless most sleep studies have not included assessment of OSA risk is their screening.
Detailed characterization of sleep disorders affecting the diverse communities living and aging with HIV, as well as identification of associated clinical and pathologic factors are indispensable to establish appropriate diagnosis and treatment. The goal of this study was to describe the prevalence, specific characteristics, and clinical correlates of sleep disorders in an urban and diverse HIV-infected cohort. We conducted a comprehensive evaluation that included multiple validated instruments to assess sleep quality, sleep hygiene, risk of OSA, and depression in this population. This is one of the most comprehensive assessments of sleep and comorbid conditions that has been done in an HIV-cohort and is aimed to provide additional clinical guidance on screening and therapeutic targets for sleep disorders affecting HIV patients.
Methods
Participants
This study was a cross-sectional convenience sampling of patients from an outpatient clinic site at Temple University, in Philadelphia; US Patients 18 years old and older, with confirmed HIV diagnosis and presenting for routine HIV care between 2014 and 2015, were offered study participation. The local institutional review board committee approved the study protocol. A total of 176 patients elected to participate in the study and provided written informed consent.
Design
Participants completed five standardized instruments in English or Spanish, to assess sleep quality, insomnia, depression symptoms, daytime sleepiness, and OSA (detailed below). In addition, participants completed a site-generated survey with questions on sleep hygiene habits substance use and self-perceived sleep problems (See sleep habits and hygiene questionnaire in Supplemental Material). We abstracted clinical and demographic data from the electronic medical records (EMR). This clinic site is part of the HIV Outpatient Study (HOPS), an ongoing prospective observational cohort study of HIV-infected adults receiving care at nine HIV clinics in six US cities since 1993. Patient data including demographic characteristics, diagnoses, treatments, and laboratory values are abstracted from medical charts and entered into an electronic database (Discovere©; Cerner Corporation, Kansas City, MO, USA). Among the study participants, there were some that were part of the HOPS database and some that were not. For those patients who were not part of the HOPS database (non-HOPS), similar data were collected from the local EMR (EPIC Systems Corporation, Verona, WI, USA). The de-identified data were combined, reviewed for quality, and analyzed together with the HOPS data.
Instruments and outcome measures
Sleep quality: evaluated using the Pittsburgh Sleep Quality Index (PSQI). A score of >5 indicates poor sleep quality and is the sum of seven measures: sleep duration, disturbance, latency, efficiency, overall quality, required sleep medications, and day dysfunction.30 Complete data for PSQI were available for 173 (98%) of the study participants.
Insomnia assessment: Insomnia Symptoms Questionnaire (ISQ), a 13-item self-report instrument was used to identify insomnia based on the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV) criteria for primary insomnia. Insomnia diagnosis is “present” if three criteria are met: (1) presence of sleep symptoms that occur “frequently” or “always,” (2) for a duration of least 4 weeks, and (3) have significant daytime impairment (“quite a bit” or “extremely”).31
Daytime impairment: Epworth Sleepiness Scale (ESS) with a score >11 indicates “excessive sleepiness,” and is the sum of eight situations where dozing may occur, each scored with 0 (no), 1 (slight), 2 (moderate), or 3 (high) chance of dozing.32
OSA risk: evaluated using “STOP-BANG” screening questionnaire. Each letter stands for a question or demographic characteristic that can be answered as Yes or No: Snoring, Tiredness, Observed apnea, and high blood Pressure, BMI > 35 kg/m, Age > 50 years, Neck circumference [>41 cm in females, 43 cm in males], and male Gender). Survey scores of 0–2, 3–4, and 5–8 indicate low, medium, or high risk of OSA, respectively.33
Depression: Patient Health Questionnaire (PHQ9) scores of 0–9, 10–14, or 15 or greater indicate low, medium, or high risk of depression, and are the sum of nine measures, each of which can be scored from 0 (not at all) to 3 (almost daily).34
Sleep hygiene questionnaire: Contained seven questions, including one about self-perceived sleep problems, caffeine intake, substance use, and sleep environment.
Statistical analyses
Descriptive summaries of the data, univariate and multivariable analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Likelihood ratio, chi-square, or Fisher exact tests were used to compare patient characteristics (binary or class variables) and Kruskal–Wallis or Wilcoxon rank-sum test were used to compare continuous variables. Results with p < 0.05 were considered significant. Univariate and multivariate associations of factors with having poor sleep and medium/high OSA risk in four instruments were identified by logistic regression, reporting odds ratios (ORs) with 95% confidence intervals (CIs). Factors included in the multivariable analysis were determined using backward selection with a cut-off of p < 0.05.
Results
Participants of the study had diverse demographic characteristic, 98 (56%) were female, 132 (75%) were non-Hispanic/Latino Black, 34 (19%) were Hispanic/Latino. Heterosexual HIV risk present in 64% and 88% were publicly insured. Their median age was 49 years old, the median time since HIV diagnosis was 11.9 years. The 70 non-HOPS sleep study participants were similar to the 106 participants in the HOPS database, except regarding insurance status, with participants not enrolled on the HOPS database having a higher percentage of private insurance, and some co-morbid diagnoses including lung disease, anxiety, and Post traumatic stress disorder (PTSD) being more common in the participants not enrolled in HOPS database (See Supplemental Table 1). Compared with the other 599 HOPS patients in the Philadelphia clinic site, the 176 sleep study participants were significantly older, more likely to be female, with longer HIV infection duration, had higher CD4 + cell counts, higher body mass index (BMI), and were less likely to have viral load (VL) > 20 copies/mL (See Supplemental Table 2).
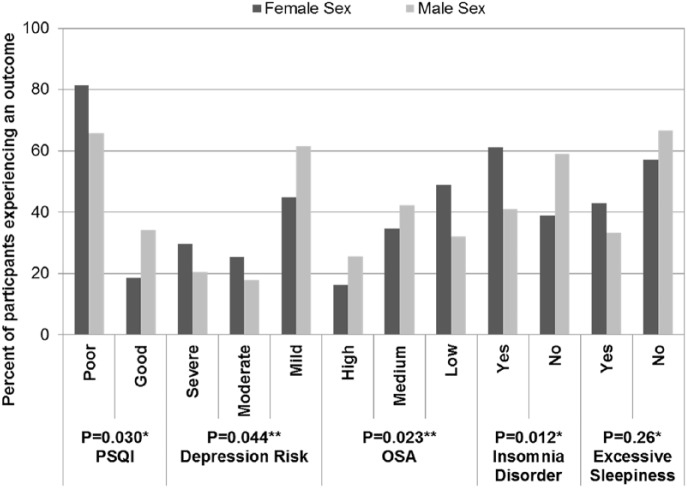
Overall, female participants were more likely to have poor sleep quality, insomnia, daytime impairment, and depression risk, while males were more likely to have a high or moderate chance of OSA and excessive sleepiness (Figure 1). High neck circumference was associated with poor sleep quality, insomnia, OSA, and daytime impairment. Tables 1 and 2 summarize characteristics of the participants and the distribution of sleep measure scores, OSA risk, and depression.
Figure 1.
Sex distribution across survey instruments among Temple University Sleep Study participants (n = 176): 2014–2015.
*Continuity-adjusted chi-square test.
**Cochran-Armitage test for trend.
PSQI: Pittsburgh Sleep Quality Index; OSA: obstructive sleep apnea.
Table 1.
Patient characteristics and distribution of sleep measure scores across participant population (N = 176), Temple University sleep study: 2014–2015.
| Patient characteristics: |
PSQI poor sleep quality |
PSQI good sleep quality |
p-valuea |
ISQ insomnia diagnosis |
ISQ no insomnia diagnosis |
p-valuea |
ESS excessive sleepiness |
ESS normal sleepiness |
p-valuea |
|---|---|---|---|---|---|---|---|---|---|
| n (%) or median (IQR) | n = 129 | n = 44 | n = 92 | n = 84 | n = 68 | n = 108 | |||
| Age, yearsb | 48 (43–54) | 50 (44–58) | 0.46 | 48 (43–54) | 50 (43–56) | 0.51 | 47 (42–53) | 51 (45–58) | 0.013 |
| Years since HIV diagnosisb | 12.1 (5.7–19.0) | 10.3 (5.1–18.5) | 0.42 | 12.5 (5.6–20.1) | 11.8 (5.6–18.6) | 0.39 | 12.8 (5.3–19.1) | 10.5 (5.9–19.0) | 0.94 |
| Sex | 0.030 | 0.012 | 0.26 | ||||||
| Male | 50 (38.8) | 26 (59.1) | 32 (34.8) | 46 (54.8) | 26 (38.2) | 52 (48.1) | |||
| Female | 79 (61.2) | 18 (40.9) | 60 (65.2) | 38 (45.2) | 42 (61.8) | 56 (51.9) | |||
| Race/ethnicity | 0.21 | 0.07 | 0.22 | ||||||
| White, non-Hispanic/Latino | 7 (5.4) | 1 (2.3) | 8 (8.7) | 1 (1.2) | 2 (2.9) | 7 (6.5) | |||
| Black, non-Hispanic/Latino | 100 (77.5) | 30 (68.2) | 67 (72.8) | 65 (77.4) | 55 (80.9) | 77 (71.3) | |||
| Hispanic/Latino | 21 (16.3) | 13 (29.5) | 16 (17.4) | 18 (21.4) | 10 (14.7) | 24 (22.2) | |||
| HIV risk | 0.08 | 0.024 | 0.23 | ||||||
| IDU | 13 (10.1) | 6 (13.6) | 9 (9.8) | 10 (11.9) | 6 (8.8) | 13 (12.0) | |||
| MSM | 26 (20.2) | 13 (29.5) | 17 (18.5) | 23 (27.4) | 14 (20.6) | 26 (24.1) | |||
| Heterosexual | 88 (68.2) | 22 (50.0) | 66 (71.7) | 46 (54.8) | 48 (70.6) | 64 (59.3) | |||
| Insuranceb | 0.036 | 0.46 | 0.83 | ||||||
| Private | 11 (8.5) | 8 (18.2) | 8 (8.7) | 11 (13.1) | 6 (8.8) | 13 (12.0) | |||
| Public | 117 (90.7) | 34 (77.3) | 83 (90.2) | 71 (84.5) | 61 (89.7) | 93 (86.1) | |||
| Median (IQR) CD4 + cell count/mm3b | 595 (379–891) | 473 (310–835) | 0.09 | 666 (388–1007) | 493 (329–754) | 0.003 | 550 (290–808) | 576 (386–865) | 0.49 |
| Viral load > 20 copies/mLb | 15 (11.6) | 5 (11.4) | 1.00 | 18 (19.6) | 33 (39.3) | 0.007 | 17 (25.0) | 34 (31.5) | |
| Body mass index ⩾ 30 kg/m2 | 56 (43.4) | 15 (34.1) | 0.36 | 45 (48.9) | 27 (32.1) | 0.035 | 32 (47.1) | 40 (37.0) | 0.48 |
| Large neck circumference | 38 (29.5) | 1 (2.3) | < 0.001 | 28 (30.4) | 11 (13.1) | 0.010 | 21 (30.9) | 18 (16.7) | 0.25 |
| Diabetes | 22 (17.1) | 12 (27.3) | 0.21 | 18 (19.6) | 16 (19.0) | 1.00 | 13 (19.1) | 21 (19.4) | 0.043 |
| Hypertension | 69 (53.5) | 20 (45.5) | 0.46 | 48 (52.2) | 43 (51.2) | 1.00 | 35 (51.5) | 56 (51.9) | 1.00 |
| Dyslipidemia | 42 (32.6) | 19 (43.2) | 0.28 | 31 (33.7) | 31 (36.9) | 0.77 | 19 (27.9) | 43 (39.8) | 1.00 |
| Lung disease | 39 (30.2) | 5 (11.4) | 0.023 | 27 (29.3) | 17 (20.2) | 0.22 | 17 (25.0) | 27 (25.0) | 0.15 |
| Depression | 95 (73.6) | 25 (56.8) | 0.06 | 69 (75.0) | 53 (63.1) | 0.12 | 55 (80.9) | 67 (62.0) | 1.00 |
| Any psychiatric condition | 106 (82.2) | 28 (63.6) | 0.020 | 75 (81.5) | 61 (72.6) | 0.22 | 57 (83.8) | 79 (73.1) | 0.013 |
| Sleep apnea | 17 (13.2) | 2 (4.5) | 0.16 | 10 (10.9) | 9 (10.7) | 1.00 | 6 (8.8) | 13 (12.0) | 0.14 |
| Any sleep medication usec | 40 (31.0) | 11 (25.0) | 0.57 | 31 (33.7) | 21 (25.0) | 0.27 | 17 (25.0) | 35 (32.4) | 0.67 |
ESS: Epworth Sleepiness Scale; IDU: intravenous drug use; IQR: interquartile range; ISQ: Insomnia Symptoms Questionnaire; MSM: men who have sex with men; PSQI: Pittsburgh Sleep Quality Index.
A total of 3 of 176 participants had null survey entries and were excluded from the PSQI portion of the table.
Yates-corrected chi-square test or Fisher exact test for categorical variables or Wilcoxon rank-sum test for continuous variables.
At or closest to date of first visit during 2014–2015.
Includes benadryl, diazepam, lorazepam, lunesta, zolpidem, or trazodone.
Table 2.
Patient characteristics and distribution of sleep apnea and depression scores across participant population (N = 176), Temple University Sleep Study: 2014–2015.
| Patient characteristics: |
All study patients |
OSA high risk |
OSA medium risk |
OSA low risk |
p-valuea |
PHQ9 severe/moderately severe depression |
PHQ9 moderate depression |
PHQ9 minimal/mild depression |
p-valuea |
|---|---|---|---|---|---|---|---|---|---|
| n (%) or median (IQR) | n = 176 | n = 36 | n = 67 | n = 73 | n = 45 | n = 39 | n = 92 | ||
| Age, yearsb | 49 (43–55) | 54 (50–60) | 51 (44–57) | 45 (36–49) | < 0.001 | 48 (44–53) | 48 (42–55) | 50 (44–57) | 0.38 |
| Years since HIV diagnosisb | 11.9 (5.6–19.0) | 15.9 (7.7–19.1) | 10.5 (5.3–19.1) | 10.7 (5.8–19.0) | 0.39 | 8.1 (4.6–19.9) | 13.1 (6.6–19.0) | 13.2 (6.2–19.0) | 0.29 |
| Sex | 0.023 | 0.044 | |||||||
| Male | 78 (44.3) | 20 (55.6) | 33 (49.3) | 25 (34.2) | 16 (35.6) | 14 (35.9) | 48 (52.2) | ||
| Female | 98 (55.7) | 16 (44.4) | 34 (50.7) | 48 (65.8) | 29 (64.4) | 25 (64.1) | 44 (47.8) | ||
| Race/ethnicity | 0.47 | 0.76 | |||||||
| White, non-Hispanic/Latino | 9 (5.1) | 4 (11.1) | 2 (3.0) | 3 (4.1) | 2 (4.4) | 2 (5.1) | 5 (5.4) | ||
| Black, non-Hispanic/Latino | 132 (75.0) | 25 (69.4) | 54 (80.6) | 53 (72.6) | 33 (73.3) | 29 (74.4) | 70 (76.1) | ||
| Hispanic/Latino | 34 (19.3) | 7 (19.4) | 11 (16.4) | 16 (21.9) | 10 (22.2) | 7 (17.9) | 17 (18.5) | ||
| HIV risk | 0.91 | 0.75 | |||||||
| IDU | 19 (10.8) | 3 (8.3) | 8 (11.9) | 8 (11.0) | 4 (8.9) | 3 (7.7) | 12 (13.0) | ||
| MSM | 40 (22.7) | 8 (22.2) | 12 (17.9) | 20 (27.4) | 11 (24.4) | 6 (15.4) | 23 (25.0) | ||
| Heterosexual | 112 (63.6) | 24 (66.7) | 45 (67.2) | 43 (58.9) | 29 (64.4) | 29 (74.4) | 54 (58.7) | ||
| Insuranceb | 0.051 | 0.28 | |||||||
| Private | 19 (10.8) | 3 (8.3) | 6 (9.0) | 10 (13.7) | 2 (4.4) | 6 (15.4) | 11 (12.0) | ||
| Public | 154 (87.5) | 30 (83.3) | 61 (91.0) | 63 (86.3) | 43 (95.6) | 33 (84.6) | 78 (84.8) | ||
| Median (IQR) CD4 + cell count/mm3b | 559 (359–848) | 564 (394–847) | 616 (359–936) | 541 (335–793) | 0.27 | 585 (254–934) | 554 (376–1010) | 554 (386–798) | 0.81 |
| Viral load > 20 copies/mLb | 51 (29.0) | 3 (8.3) | 9 (13.4) | 8 (11.0) | 0.80 | 13 (28.9) | 13 (33.3) | 25 (27.2) | 0.75 |
| Body mass index ⩾ 30 kg/m2 | 72 (40.9) | 27 (75.0) | 29 (43.3) | 16 (21.9) | < 0.001 | 21 (46.7) | 16 (41.0) | 35 (38.0) | 0.63 |
| Large neck circumference | 39 (22.2) | 13 (36.1) | 16 (23.9) | 10 (13.7) | 0.007 | 14 (31.1) | 11 (28.2) | 14 (15.2) | 0.025 |
| Diabetes | 34 (19.3) | 13 (36.1) | 16 (23.9) | 5 (6.8) | < 0.001 | 8 (17.8) | 8 (20.5) | 18 (19.6) | 0.83 |
| Hypertension | 91 (51.7) | 30 (83.3) | 43 (64.2) | 18 (24.7) | < 0.001 | 18 (40.0) | 22 (56.4) | 51 (55.4) | 0.12 |
| Dyslipidemia | 62 (35.2) | 16 (44.4) | 27 (40.3) | 19 (26.0) | 0.037 | 11 (24.4) | 15 (38.5) | 36 (39.1) | 0.11 |
| Lung disease | 44 (25.0) | 9 (25.0) | 18 (26.9) | 17 (23.3) | 0.77 | 20 (44.4) | 9 (23.1) | 15 (16.3) | < 0.001 |
| Depression | 122 (69.3) | 22 (61.1) | 45 (67.2) | 55 (75.3) | 0.11 | 38 (84.4) | 31 (79.5) | 53 (57.6) | < 0.001 |
| Any psychiatric condition | 136 (77.3) | 24 (66.7) | 53 (79.1) | 59 (80.8) | 0.13 | 41 (91.1) | 32 (82.1) | 63 (68.5) | 0.002 |
| Sleep apnea | 19 (10.8) | 9 (25.0) | 6 (9.0) | 4 (5.5) | 0.004 | 3 (6.7) | 4 (10.3) | 12 (13.0) | 0.26 |
| Any sleep medication usec | 52 (29.5) | 11 (30.6) | 22 (32.8) | 19 (26.0) | 0.52 | 14 (31.1) | 11 (28.2) | 27 (29.3) | 0.86 |
IDU: intravenous drug use; IQR: interquartile range; MSM: men who have sex with men; OSA: obstructive sleep apnea; PHQ9: Patient health questionnaire for depression.
OSA low risk is a score of 0–2, medium risk is a score of 3–4, and high risk is a score of 5–8; PHQ9 minimal/mild depression risk is defined as a score of 0–9, moderate depression risk is defined as a score of 10–14, and severe/moderately severe depression risk is defined as a score of 15 or higher.
Cochran-Armitage trend test for binary variables, Yates-corrected chi-square test or Fisher exact test for categorical variables, or Kruskal–Wallis test for continuous variables.
At or closest to date of first visit during 2014–2015.
Includes benadryl, diazepam, lorazepam, lunesta, zolpidem, or trazodone.
Sleep quality, insomnia, and self-reported sleep disturbance
In our study, 75% of all participants reported poor sleep quality (PSQI > 5) and 52% met insomnia diagnosis criteria based on ISQ. Patients with poor sleep quality as well as those with insomnia diagnosis were more likely to be female (61% vs 41% and 65% vs 45%, respectively) and have larger neck circumference (30% vs 2% and 30% vs 13%, respectively) (See Table 1). Poor sleep quality was additionally associated with being publicly insured (91% vs 77%), having lung disease (30% vs 11%), and any psychiatric diagnosis (82% vs 64%). Patients with insomnia diagnosis were more likely to have heterosexual HIV risk (72% vs 55%), a BMI ⩾ 30 kg/m2 (49% vs 32%), or use an over the counter sleep aid (12% vs 2%) (Table 1).
Self-reported sleep problems were present in 66.5% of the participants, as assessed with a single question “Do you consider that you have sleep problems?.” Interestingly, self-reported sleep problems were significantly associated with both poor sleep quality (82.9% vs 18.2%, p < 0.001) and insomnia diagnosis (92.4 vs 38.1, p < 0.001). Therefore, for our sample the sensitivity and specificity of this single question in predicting a PSQI > 5 was 82% and 81%, respectively, while the sensitivity and specificity for predicting insomnia diagnosis was 92% and 61%, respectively.
Daytime impairment (ESS)
Daytime sleepiness, measured as ESS > 10, was present in 39% of participants. Those with excessive sleepiness were more likely to be younger (median 47 vs 51 years), have larger neck circumference (31% vs 17%), depression (81% vs 62%), and self-reported sleep problems (85% vs 55%) (Table 1).
Risk of OSA
In the “STOP-BANG” OSA Survey instrument, 36 (20%), 67 (38%), and 73 (41%) had high, medium, or low OSA risk, respectively. Increased OSA risk was associated with older median age (54 vs 51 vs 45), male sex (56% vs 49% vs 34%), having BMI ⩾ 30 kg/m2 (75% vs 43% vs 22%), large neck circumference (36% vs 24% vs 14%), and metabolic comorbid conditions including diabetes (36% vs 24% vs 7%), hypertension (83% vs 64% vs 25%), dyslipidemia (44% vs 40% vs 26%), preexisting diagnosis of sleep apnea (25% vs 9%) (Table 2), or self-reported sleep problems (78% vs 70% vs 58%).
Depression assessment (PHQ9)
Almost half of participants (48%) had either severe/moderately severe or moderate, depression based on PHQ9. Increased depression risk was associated with: female sex (64% vs 64% vs 48%), large neck circumference (31% vs 28% vs 15%), lung disease (44% vs 23% vs 16%), previous depression diagnosis (84% vs 80% vs 58%), any psychiatric diagnosis (91% vs 82% vs 69%), and self-reported sleep problems (89% vs 85% vs 48%) (Table 2).
Immune correlates
There was no correlation between poor sleep quality and the CD4 + cell count or VL. Patients with insomnia diagnosis had higher median CD4 + cell counts (666 vs 493 cells/mm3) and were less likely to have a VL > 20 cells/mL (20% vs 29%).
Sleep hygiene, substance use, and sleeping medications
A large percentage of patients (63.1%) reported sleeping with lights/TV on sometimes, mostly, or always. Significant difference among groups was noted for those with high daytime impairment (ESS > 10), most of whom reported sleeping with lights/TV on (75% vs 56%, P = 0.015). Caffeine intake was excessive (> 5 cups) in 17% of the patients, with no significant differences among groups.
Cigarette smoking was present in 40% of participants. Those with higher risk of OSA were less likely to use cannabis (3% vs 8% vs 22%, P = 0.002) and less likely to smoke cigarettes (39% vs 27% vs 52%, P = 0.009), while people with depression were more likely to smoke cigarettes (56% vs 41% vs 32%, P = 0.026).
Only 7% of the patients reported using over the counter sleep aid, but 31% of patients with poor sleep quality and 25% of those with good sleep quality reported using hypnotic/sedative medications, the most popular being Trazodone, followed by Zolpidem. (See Supplemental Tables 3a and 3b)
Combined 4-variable measure (sleep problems and elevated OSA risk)
We performed univariate and multivariable logistic regression analyses of factors associated with having poor sleep and medium/high OSA risk in four assessment instruments (PHQ9 results were excluded). Poor sleep outcomes in all four sleep score measurements were seen in 31 (17%) of 176 participants. Univariate analysis showed statistically significant correlation with non-Hispanic/Latino Black race, higher CD4 + cell count, BMI ⩾ 30 kg/m2 and large neck circumference. In a multivariable analysis, factors associated with poor sleep were non-Hispanic/Latino Black race (adjusted OR (aOR) 6.60; 95% CI 1.47–29.69) and BMI ⩾ 30 kg/m2 (aOR 4.98; 95% CI 2.09–11.85) (Table 3).
Table 3.
Logistic regression analyses of factors associated with having poor sleep and medium/high OSA risk in all four sleep instruments, Temple University Sleep Study: 2014–2015.
| Patient characteristics | Poor sleep and medium/high OSA risk (31/176) | |||||
|---|---|---|---|---|---|---|
| Univariate |
Initial multivariablea |
multivariableb |
||||
| OR (95% CI) | p-value | aOR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Age, per 10 years | 0.92 (0.64–1.33) | 0.67 | ||||
| Years since HIV diagnosis | 1.01 (0.97–1.06) | 0.60 | ||||
| Sex | ||||||
| Male | referent | |||||
| Female | 1.32 (0.60–2.92) | 0.49 | ||||
| Race/ethnicity | ||||||
| Black, non-Hispanic/Latino | 5.91 (1.35–25.9) | 0.018 | 6.23 (1.35–28.64) | 0.019 | 6.60 (1.47–29.69) | 0.014 |
| All other race/ethnicities | referent | referent | ||||
| HIV risk | ||||||
| Heterosexual | 2.22 (0.90–5.49) | 0.08 | 1.12 (0.39–3.20) | 0.83 | ||
| All other HIV risk categories | referent | referent | ||||
| Insuranceb | ||||||
| Public/none/other | 0.78 (0.24–2.53) | 0.68 | ||||
| Private | referent | |||||
| CD4 + cell count per 100 cells/mm3b | 1.09 (1.00–1.19) | 0.042 | 1.05 (0.96–1.16) | 0.29 | ||
| Viral load > 20 copies/mLb | 0.67 (0.27–1.67) | 0.39 | ||||
| Viral load > 200 copies/mLb | 0.71 (0.23–2.21) | 0.56 | ||||
| Body mass index ⩾ 30 kg/m2 | 4.64 (1.99–10.8) | < 0.001 | 3.75 (1.39–10.07) | 0.009 | 4.98 (2.09–11.85) | < 0.001 |
| Neck circumference > 43 cm. (male) or > 37 cm. (female) | 3.31 (1.44–7.58) | 0.005 | 1.26 (0.45–3.56) | 0.66 | ||
| Diabetes | 1.59 (0.64–3.95) | 0.32 | ||||
| Hypertension | 1.90 (0.85–4.24) | 0.12 | 1.20 (0.50–2.90) | 0.69 | ||
| Dyslipidemia | 0.71 (0.31–1.66) | 0.43 | ||||
| Lung disease | 1.29 (0.54–3.05) | 0.57 | ||||
| Anxiety | 0.53 (0.21–1.39) | 0.20 | ||||
| Bipolar disorder | 1.21 (0.45–3.27) | 0.71 | ||||
| Depression | 1.10 (0.47–2.58) | 0.83 | ||||
| Psychosis | 1.67 (0.56–4.99) | 0.36 | ||||
| Any psychiatric condition | 1.01 (0.40–2.55) | 0.98 | ||||
| Sleep apnea | 0.86 (0.24–3.17) | 0.83 | ||||
| Any sleep medication use‡ | 1.17 (0.51–2.69) | 0.72 | ||||
aOR: adjusted odds ratio; CI: confidence interval; OR: odds ratio; OSA: obstructive sleep apnea.
Factors included in the initial multivariable analysis were those with univariate p-values < 0.20.
Multivariable analysis results obtained using backwards selection.
diphenhydramine, doxylamine, diazepam, alprazolam, trazodone, zolpidem and Eszopiclone.
Discussion
In this study, we found that the prevalence of poor sleep quality and insomnia in a diverse urban cohort of HIV-infected patients was high, 75% and 52%, respectively. This prevalence is higher than estimates in the general population of 30% and 10%, respectively.35 Associated risk factors included female sex, Black race, large neck circumference, depression, and BMI > 30 kg/m2. Our study represents one of the most comprehensive epidemiologic reports of sleep disturbances in a diverse, contemporary HIV-cohort. To our knowledge, we are the first group that included a broad array of instruments to assess sleep and also screened for OSA. Our findings largely corroborate those from prior studies and add to the body of evidence that sleep disturbances in urban, low-income PLWH contribute to the burden of disease of these patients who are aging with a significant burden of comorbid medical and psychological disorders. All of which are likely exacerbating health disparities in these populations. We were surprised to find that a single question about self-reported sleep problems could identify, with good sensitivity and specificity, patients that have poor sleep quality and insomnia diagnosis.
Several authors have used poor sleep quality (PSQI ⩾ 5) as an interchangeable term with insomnia,3,5 which might account in part for the differences in prevalence reported previously. Nevertheless, our results support the notion that instruments like the ISQ, which include currently accepted insomnia diagnosis criteria based on DSM-IV are more specific than PSQI, which overestimates the prevalence of insomnia.
Rubenstein and Selwyn5 reported a prevalence of 73% poor sleep quality, close to the 75% observed by us. Newer studies, like that by Crum-Cianflone et al., documented a prevalence of poor sleep quality of 46% in a US military cohort.3 Another recent French study of 1354 HIV-patients reported a similar rate of poor sleep quality.36 Sociodemo-graphic characteristics probably account for the higher rates observed in our study compared with other contemporary studies. Before the 2000s, most reports of sleep disorders in HIV included predominantly male and Caucasian participants. The article by Reid and Dwyer4 presents an overview of the demographic characteristics of patients included in early HIV-related sleep disorders studies. Table 4 offers a comparison of our cohort and results with some representative studies in the field in the last 10 years. This table does not seek to be a comprehensive review of the literature but aims to illustrate the spectrum of sleep disorders among different HIV-infected cohorts.
Table 4.
Comparison of our results with the most representative epidemiologic studies of sleep disorders in HIV-infected patients in the last 10 years.
| Study | Location | Study design | n | Mean age (range) | Sex % male race/ethnicity | Obesity rate or BMI | CD4 count | Assessment instruments | Depression rate | Sleep outcome(s) | Major findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crum-Cianflone et al.3 | Multi-center, USA | CS | 193 HIV+, 50 matched controls | 36 (18–54) | 95% male, 50% White | Mean BMI kg/m2 (SD) 27.5 (4.5) |
CD4 count mean (SD) 587 (230) |
PSQI, ESS | 7% (HIV+) and 0% (Control) | Insomnia (PSQI > 5), daytime drowsiness (ESS ⩾ 10) | 46% insomnia in HIV-patients vs 38% (p = 0.3) in controls. Depression (OR:16.8), increased in waist size (OR:2.7), and fewer years of education (OR:0.8) were associated with insomnia |
| Jean-Louis et al.28 | Brooklyn, USA | CS | 1161 HIV+, 521 controls | 20–70 years | 0% male, 63% Black, 24% Hispanic | Obesity rate (BMI > 30) 40% |
CD4 < 500 cells/mL (44% vs 46%, control vs HIV+) |
insomnia survey, CES-D | 27.6% vs 9.6% in patients with insomnia vs no insomnia | Insomnia diagnosis | Prevalence of insomnia symptoms did not vary significantly by HIV status except in younger women. Depression was the most significant predictor for insomnia. |
| Avellana et al.36 | “Pays de la Loire,” France | CS | 1354 HIV+ | 47 (40–54) | 73.5% male, race not reporteda | Mean BMI kg/m2
(range) 23.5 (21–26.1) |
CD4 count mean (range) 604 (434–784) |
PHQI, BDI-II, WHO QOL | 19.70% | Poor sleep quality (PSQI > 5) |
47% poor sleep quality, poor sleep was associated with depression, male gender, active smoking, nevirapine, or efavirenz in ART |
| Byun et al.11 | San Francisco, USA | CS | 268 HIV+ | 44.8 (27.8–61.5) | 67% male, 42% White | Mean BMI kg/m2
(range) 27 (21.4–32.6) |
CD4 count ⩾ 200 (83%) | PSQI, MOS, LFS, Actigraphy | b | Poor sleep quality (PSQI > 5), TST, WASO | 63% poor sleep quality. Lower self-reported cognitive functional scores were associated with poorer sleep quality, total sleep time (low or high), and greater fatigue |
| Gutierrez et al.47 | Philadelphia, USA | CS | 176 HIV+ | 49 (43–55) | 44% male, 75% Black, 12% Hispanic | Mean BMI kg/m2 (range) 29.5 (16.9–55.2) |
CD4 count mean (range) 559 (359–848) |
PSQI, ESS, ISQ, PHQ-9, STOP-BANG and sleep hygiene survey | 48% | Poor sleep quality (PSQI > 5), Insomnia diagnosis, OSA risk |
73% poor sleep quality, 52% met insomnia diagnosis, 59% mod-high risk of OSA. Self-reported sleep disturbances, Black race, and obesity were associated with poor sleep. |
n: number of patients; SD: standard deviation; BMI: body mass index; CS: cross-sectional; PSQI: Pittsburgh Sleep Quality Index; ESS: Epworth Sleepiness Scale; OR: odds ratios; CES-D: Center for Epidemiological Studies Depression; ART: Anti-Retroviral Therapy; BDI-II: Beck Depression Inventory-II; WHO QOL: World Health Organization quality of life assessment HIV BREF questionnaire; MOS: Medical Outcome Study Cognitive Functional Scale; LFS: Lee Fatigue Scale; TST: Actigraphy-based total sleep time; WASO: wake after sleep onset; ISQ: Insomnia Symptoms Questionnaire; PHQ9: Patient Health Questionnaire for depression; OSA: obstructive sleep apnea.
race/ethnicity were not reported. Country of birth was France for 82.9%, Africa 14.7%.
Depression rate not reported. Antidepressant rate use was 35%. Participants were excluded if reported schizophrenia, bipolar disorder, or dementia.
The predominance of women could have certainly contributed to the higher prevalence of insomnia and poor sleep observed in our sample. Women are 1.4–2 times more likely to report sleep disturbances, particularly insomnia compared with men,25 and to develop chronic sleep problems which could be due to higher psychosocial distress or emotional reactivity.37 Similarly, race and ethnicity may play a role. Previous studies have suggested that racial/ethnic minorities are more likely to experience poor sleep quality.24,38
Depression goes hand-in-hand with sleep disturbances. Sleep disorders are known to precede episodes of depression and predispose to recurrence, although it is difficult to establish a cause-effect association.39 We reported a higher rate of moderate to severe depression compared with the French cohort36 and with the US military cohort (Table 4). Although the US military cohort had a low rate of depression, it found that depression was the strongest factor associated with insomnia with 89% of the patients with moderate-severe depression (PHQ9) reporting problems with sleep. In our study, depression was more common in women, as previously reported.28,40 Identifying and treating depression might have an impact on quality of sleep, and similarly addressing sleep problems might help in the management of depression and prevention of relapses.
More than half of the participants in our study had moderate to high risk for OSA, which was also associated with more metabolic correlates including hypertension, diabetes, and higher BMI. OSA is associated with co-morbid conditions such as hypertension, cardiovascular disease, metabolic syndrome, obesity, and aging.8 Our findings are highly relevant as we are seeing a shift in the leading causes of death in PLWH to include cardiovascular diseases.41 Although the prevalence of OSA in PLWH is not known, recent studies suggest that patients with HIV are less likely to be diagnosed with OSA even if they report symptoms.29 OSA was more common in patients with other pulmonary diagnoses, which is similar to what others have reported in non-HIV populations.42,43 Interestingly, although high risk for OSA was more common in males, as expected, women of this cohort were also more likely to screen positive (44%) than in the general population (10–27%).44,45 Although our OSA screening data were not verified with polysomnography, our results suggest the possibility of under-diagnosis of OSA in this cohort. It is worth noting that the United States Preventive Services Task Force (USPSTF) recommends against routine screening for OSA in asymptomatic patients. Nevertheless, we propose that all HIV-infected patients reporting sleep problems should be considered symptomatic and be screened for OSA.
Daytime impairment and sleepiness were associated with younger age and sleeping with lights or TV on frequently or always. This finding points toward the broader problem of deficiencies in sleep hygiene in the current society. The National Sleep Foundation has noted that the prevalence of daytime sleepiness and sleep complaints is increasing with the use of technology such as cell phones or computers during the hour before sleep.46 We did not assess the role of technology in sleep, but our results point toward opportunities to impact sleep quality by providing sleep hygiene education.
We performed multivariable analysis to explore associated characteristics of patients with poor outcomes in all assessment instruments, patients who are presumably the most symptomatic. Some of the primary correlates, such as BMI ⩾ 30 kg/m2 and self-reported sleep problems, could be integrated as a routine part of HIV care and help identify patients at higher risk for sleep disturbances. Diagnosing and treating sleep disorders could also influence HIV disease management. In a cohort of HIV-infected patients, poor sleep quality was associated with reduced medication adherence, decreased cognitive function, and lower quality of life.10
Our study had several limitations. Sleep disturbances were diagnosed based on questionnaire data rather than polysomnography data. However, we used standardized instruments like most of the other studies in this filed, and some experts have suggested that self-reported data may be more representative of sleep issues.4 Similarly, the instruments used for depression and risk for OSA assessment are non-diagnostic and the self-reported data should be validated by a clinician or by objective data before a diagnosis is firmly established. Nevertheless, these instruments have been widely used in the literature and accepted as an appropriate method for epidemiologic studies. Participation bias is a possibility as patient with sleep problems may have been more likely to volunteer for the study. Finally, our research was limited to a single site and lacked a comparison group of HIV-uninfected patients.
Our study has several strengths. We conducted research in an HIV population enriched in women, persons of Black, and non-Hispanic race/ethnicity at a site receiving Ryan White care funding support, which provides a unique glimpse into sleep problems among patients who represent the changing demographics of PLWH in the United States and are often underrepresented in HIV clinical research. We conducted a comprehensive analysis of the types of sleep disorders, sleep hygiene habits, and other comorbid conditions including depression and OSA. There has not been another sleep study in HIV patients that take into account this many variables. In addition, we provided specific strategies to screen for both sleep disorders and then for specific comorbid conditions like OSA and depression that represent appropriate treatment targets. Our study contributes to start bridging the gap between the high prevalence in sleep disorders but very low treatment targets strategies for sleep disorders among HIV patients.
In conclusion, sleep disturbances are highly prevalent in urban PLWH. Routine clinical management of HIV-patients should include screening for self-reported sleep problems, depression, sleep apnea and measuring neck circumference. Addressing modifiable risk factors such as obesity, sleep hygiene, and treating depression may have a measurable impact in sleep and quality of life of PLWH. In addition, as the cohorts of PLWH age, the medical morbidities related to sleep disturbances can have a significant effect on mortality. The progress in effective life prolonging anti-retroviral therapy may be undermined by the development of metabolic, cardiovascular and psychological complications from poor sleep health.
Supplemental Material
Supplemental material, Gutierrez_et_al_Supplemental_Material_revision20190224 for Sleep disturbances in HIV-infected patients associated with depression and high risk of obstructive sleep apnea by Jeydith Gutierrez, Ellen M Tedaldi, Carl Armon, Vaidahi Patel, Rachel Hart and Kate Buchacz in SAGE Open Medicine
Acknowledgments
The authors thank Dr Fredric Jaffe for his contributions regarding sleep disorders and the development of methods for this project.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Ethical approval: Ethical approval for this study was obtained from the Temple University Institutional Review Board (Approval Number: 21858).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Mental Health (NIMH) grant number (P30NH092177) through the Temple Comprehensive NeuroAIDS center, also by the HIV Outpatient Study, Centers for Disease Control and Prevention (contract number 200-2015-63931).
Informed consent: Written informed consent was obtained from all subjects before the study.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Jeydith Gutierrez  https://orcid.org/0000-0001-7596-1779
https://orcid.org/0000-0001-7596-1779
References
- 1. National Sleep Foundation. 2010 sleep in America poll: summary of findings. Washington, DC: National Sleep Foundation, 2010. [Google Scholar]
- 2. Lee KA, Gay C, Portillo CJ, et al. Types of sleep problems in adults living with HIV/AIDS. J Clin Sleep Med 2012; 8(1): 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crum-Cianflone NF, Roediger MP, Moore DJ, et al. Prevalence and factors associated with sleep disturbances among early-treated HIV-infected persons. Clin Infect Dis 2012; 54(10): 1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reid S, Dwyer J. Insomnia in HIV infection: a systematic review of prevalence, correlates, and management. Psychosom Med 2005; 67(2): 260–269. [DOI] [PubMed] [Google Scholar]
- 5. Rubinstein ML, Selwyn PA. High prevalence of insomnia in an outpatient population with HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 19(3): 260–265. [DOI] [PubMed] [Google Scholar]
- 6. Norman SE, Resnick L, Cohn MA, et al. Sleep disturbances in HIV-seropositive patients. JAMA 1988; 260(7): 922. [PubMed] [Google Scholar]
- 7. Miles SA. HIV infection and AIDS: new biology, therapeutic advances, and clinical implications. Introduction. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 16(Suppl 1): S1–S2. [PubMed] [Google Scholar]
- 8. Institute of Medicine Committee on Sleep Medicine and Research. Sleep disorders and sleep deprivation: an unmet public health problem (ed HR Colten and BM Altevogt). Washington, DC: National Academies Press, 2006. [PubMed] [Google Scholar]
- 9. Institute of Medicine Committee on Sleep Medicine and Research. Extent and health consequences of chronic sleep loss and sleep disorders. In: Colten HR, Altevogt BM. (eds) Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: National Academies Press, 2006. [PubMed] [Google Scholar]
- 10. Babson KA, Heinz AJ, Bonn-Miller MO. HIV medication adherence and HIV symptom severity: the roles of sleep quality and memory. AIDS Patient Care STDS 2013; 27(10): 544–552. [DOI] [PubMed] [Google Scholar]
- 11. Byun E, Gay CL, Lee KA. Sleep, fatigue, and problems with cognitive function in adults living with HIV. J Assoc Nurses AIDS Care 2016; 27(1): 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiegand M, Moller AA, Schreiber W, et al. Nocturnal sleep EEG in patients with HIV infection. Eur Arch Psychiatry Clin Neurosci 1991; 240(3): 153–158. [DOI] [PubMed] [Google Scholar]
- 13. White JL, Darko DF, Brown SJ, et al. Early central nervous system response to HIV infection: sleep distortion and cognitive-motor decrements. AIDS 1995; 9(9): 1043–1050. [DOI] [PubMed] [Google Scholar]
- 14. Moeller AA, Oechsner M, Backmund HC, et al. Self-reported sleep quality in HIV infection: correlation to the stage of infection and zidovudine therapy. J Acquir Immune Defic Syndr 1991; 4(10): 1000–1003. [PubMed] [Google Scholar]
- 15. Cruess DG, Antoni MH, Gonzalez J, et al. Sleep disturbance mediates the association between psychological distress and immune status among HIV-positive men and women on combination antiretroviral therapy. J Psychosom Res 2003; 54(3): 185–189. [DOI] [PubMed] [Google Scholar]
- 16. Norman SE, Chediak AD, Kiel M, et al. Sleep disturbances in HIV-infected homosexual men. AIDS 1990; 4(8): 775–781. [DOI] [PubMed] [Google Scholar]
- 17. Wang T, Jiang Z, Hou W, et al. HIV Tat protein affects circadian rhythmicity by interfering with the circadian system. HIV Med 2014; 15(9): 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Decloedt EH, Maartens G. Neuronal toxicity of efavirenz: a systematic review. Expert Opin Drug Saf 2013; 12(6): 841–846. [DOI] [PubMed] [Google Scholar]
- 19. de Boer MG, van den Berk GE, van Holten N, et al. Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. AIDS 2016; 30(18): 2831–2834. [DOI] [PubMed] [Google Scholar]
- 20. Fumaz CR, Tuldra A, Ferrer MJ, et al. Quality of life, emotional status, and adherence of HIV-1-infected patients treated with efavirenz versus protease inhibitor-containing regimens. J Acquir Immune Defic Syndr 2002; 29(3): 244–253. [DOI] [PubMed] [Google Scholar]
- 21. Dorey-Stein Z, Amorosa VK, Kostman JR, et al. Severe weight gain, lipodystrophy, dyslipidemia, and obstructive sleep apnea in a human immunodeficiency virus-infected patient following highly active antiretroviral therapy. J Cardiometab Syndr 2008; 3(2): 111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gamaldo CE, Gamaldo A, Creighton J, et al. Evaluating sleep and cognition in HIV. J Acquir Immune Defic Syndr 2013; 63(5): 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Low Y, Preud’homme X, Goforth HW, et al. The association of fatigue with depression and insomnia in HIV-seropositive patients: a pilot study. Sleep 2011; 34(12): 1723–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel NP, Grandner MA, Xie D, et al. “Sleep disparity” in the population: poor sleep quality is strongly associated with poverty and ethnicity. BMC Public Health 2010; 10: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davidson JR. Insomnia treatment options for women. Obstet Gynecol Clin North Am 2009; 36(4): 831–846, x–xi. [DOI] [PubMed] [Google Scholar]
- 26. Fekete EM, Seay J, Antoni MH, et al. Oxytocin, social support, and sleep quality in low-income minority women living with HIV. Behav Sleep Med 2014; 12(3): 207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marion I, Antoni M, Pereira D, et al. Distress, sleep difficulty, and fatigue in women co-infected with HIV and HPV. Behav Sleep Med 2009; 7(3): 180–193. [DOI] [PubMed] [Google Scholar]
- 28. Jean-Louis G, Weber KM, Aouizerat BE, et al. Insomnia symptoms and HIV infection among participants in the Women’s Interagency HIV Study. Sleep 2012; 35(1): 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kunisaki KM, Akgun KM, Fiellin DA, et al. Prevalence and correlates of obstructive sleep apnoea among patients with and without HIV infection. HIV Med 2015; 16(2): 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 31. Okun ML, Kravitz HM, Sowers MF, et al. Psychometric evaluation of the Insomnia Symptom Questionnaire: a self-report measure to identify chronic insomnia. J Clin Sleep Med 2009; 5(1): 41–51. [PMC free article] [PubMed] [Google Scholar]
- 32. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14(6): 540–545. [DOI] [PubMed] [Google Scholar]
- 33. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest 2016; 149(3): 631–638. [DOI] [PubMed] [Google Scholar]
- 34. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16(9): 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. National Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13–15, 2005. Sleep 2005; 28(9): 1049–1057. [DOI] [PubMed] [Google Scholar]
- 36. Allavena C, Guimard T, Billaud E, et al. Prevalence and risk factors of sleep disturbance in a large HIV-infected adult population. AIDS Behav 2016; 20(2): 339–344. [DOI] [PubMed] [Google Scholar]
- 37. Troxel WM. It’s more than sex: exploring the dyadic nature of sleep and implications for health. Psychosom Med 2010; 72(6): 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grandner MA, Williams NJ, Knutson KL, et al. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Med 2016; 18: 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci 2008; 10(4): 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Junqueira P, Bellucci S, Rossini S, et al. Women living with HIV/AIDS: sleep impairment, anxiety and depression symptoms. Arq Neuropsiquiatr 2008; 66(4): 817–820. [DOI] [PubMed] [Google Scholar]
- 41. Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a a multicohort collaboration. Lancet 2014; 384: 241–248. [DOI] [PubMed] [Google Scholar]
- 42. Tsai SC. Chronic obstructive pulmonary disease and sleep related disorders. Curr Opin Pulm Med 2017; 23: 124–128. [DOI] [PubMed] [Google Scholar]
- 43. Margaritopoulos GA, Antoniou KM, Wells AU. Comorbidities in interstitial lung diseases. Eur Respir Rev 2017; 26: 160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kapsimalis F, Kryger M. Sleep breathing disorders in the U.S. female population. J Womens Health 2009; 18(8): 1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kang K, Seo JG, Seo SH, et al. Prevalence and related factors for high-risk of obstructive sleep apnea in a large korean population: results of a questionnaire-based study. J Clin Neurol 2014; 10(1): 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gradisar M, Wolfson AR, Harvey AG, et al. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med 2013; 9(12): 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gutierrez, et al. Sleep disturbances in HIV-infected patients associated with depression and high risk of obstructive sleep apnea. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Gutierrez_et_al_Supplemental_Material_revision20190224 for Sleep disturbances in HIV-infected patients associated with depression and high risk of obstructive sleep apnea by Jeydith Gutierrez, Ellen M Tedaldi, Carl Armon, Vaidahi Patel, Rachel Hart and Kate Buchacz in SAGE Open Medicine