Abstract
The Drosophila olfactory system is an attractive model for exploring the wiring logic of complex neural circuits. Remarkably, olfactory local interneurons exhibit high diversity and variability in their morphologies and intrinsic properties. Although olfactory sensory and projection neurons have been extensively studied of development and wiring; the development, mechanisms for establishing diversity, and integration of olfactory local interneurons into the developing circuit remain largely undescribed. In this review, we discuss some challenges and recent advances in the study of Drosophila olfactory interneurons.
Keywords: Neuronal diversity, interneuron, adult neurogenesis, neurodegeneration, pruning, neuronal cell death, olfactory system
The wiring logic of the Drosophila olfactory system shares some common features with vertebrate olfactory systems, such as those found in zebrafish, mice, rats, and humans. In the fly and mouse olfactory circuits, olfactory sensory neurons (OSNs) detect environmental odorants and relay the information to the projection neurons (PNs) (or mitral/tufted cells in mouse) in the first olfactory information processing centers, either the fly antennal lobe (AL) or the olfactory bulb (OB) in mouse (Figure 1A).1-6 In the AL or OB, different types of interneurons form extensive synaptic connections with PNs (mitral/tufted cells), OSNs, and/or other interneurons, creating complicated neural networks that modulate olfactory information.2,7-10 In studying the olfactory network, the application of powerful fly genetic tools to the handful of resident neurons has allowed researchers to extensively explore cell fate determination, development, axon/dendrite targeting, and synaptic formation and connections within OSNs and PNs.11-25 However, our understanding of the development and wiring logic of fly olfactory local interneurons (LNs) remains poor. This lack of knowledge is at least partly due to the high morphological and electrophysiological diversity of LNs,7,18,26-30 as well as their variability and plasticity, which can also complicate experimental design and data interpretation.7,29,31,32 Here, we discuss several aspects of LN research, including major challenges, recent findings regarding LN development and the underlying cellular mechanisms, methodological advances in phenotype analysis, and potential directions for future study.
Figure 1.
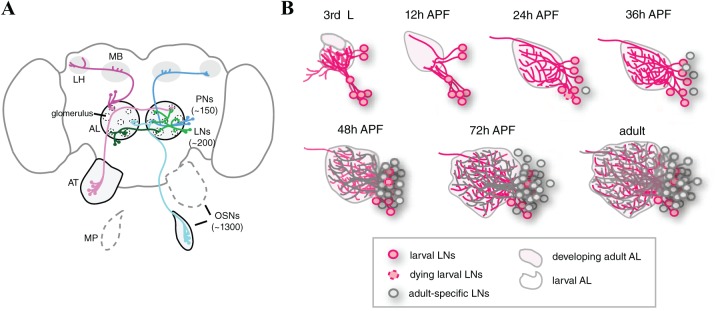
The development of fly olfactory local interneurons: (A) Schematic of Drosophila olfactory system. The cell bodies of olfactory sensory neurons (OSNs, pink and cyan) are in the antennae (AT) or maxillary palps (MPs). Olfactory sensory neurons sense odorants and transduce information to second-order projection neurons (PNs, magenta and blue) at the first central brain information processing center, the antennal lobe (AL). Projection neurons, in turn, relay information to neurons in higher brain centers, including the mushroom body (MB) and lateral horn (LH). Local interneurons (LNs, green and dark green) form extensive synapses with OSNs, PNs, and other LNs in the AL. (B) The development of fly olfactory local interneurons. See text for detail.
Olfactory LNs are Sequentially Recruited to the Developing Circuit
Because interneurons are highly diverse, it is well accepted that combinations of multiple signatures, including morphology, connectivity, molecular identity (markers and neurotransmitters), and intrinsic electrophysiological properties, are required to fully define the subtypes of interneurons.33 Thus, a prerequisite step for disentangling LN diversity and variability is to develop a set of drivers that allow researchers to unambiguously label subsets of LNs. The use of such drivers for fluorescent protein expression allows the labeled LNs to be identified according to both molecular and morphological features. In a large-scale screen of 1058 InSITE GAL4 enhancer trap lines, 25 GAL4 drivers that label distinct or partially overlapping LN subsets were identified.34 Systematic examination of the innervation patterns of labeled LNs (beginning at the late third instar larval stage, proceeding through the pupal stage and concluding in adults) revealed that adult LNs are sequentially recruited to the developing olfactory circuit in the AL (Figure 1B).34 LNs that initially function in the larval olfactory system and those that solely function in the adult olfactory system are defined as larval and adult-specific LNs, respectively. The first wave of LNs that are recruited to the developing adult olfactory circuit includes larval LNs. These cells receive ecdysone signal and undergo pruning at 0 to 6 hours after puparium formation (APF), followed by re-extension of neurites to the developing AL at around 12 to 24 hours APF (Figure 2A). Distinct subsets of larval LNs differentially integrate into the circuit within a few hours. The second wave is comprised of adult-specific LNs that emerge and innervate the AL at about 24 to 48 hours APF. The synapses between cognate OSN axons and PN dendrites mature at around 48 to 50 hours APF.35 By this time, the functional subunits of the AL (glomeruli) are easily observed, and it is well accepted that the wiring of the olfactory circuit can be considered to be mature.35 Therefore, any neurons integrating into the AL after 48 hours APF may reshape the existing synapses and/or circuit connections. Interestingly, a third wave of LNs, also adult-specific LNs, emerges and integrates into the olfactory circuit at ~60 to 72 hours APF, 1 day before eclosion. Therefore, LNs in the adult olfactory circuit include both larval and adult-specific LNs. Because the LNs integrate into the circuit at different stages, it is possible that the different types of LNs may have timing-dependent contributions to the developing olfactory circuit. In addition, it is likely that different cellular and molecular mechanisms are used to establish the individual types of LNs. Such an idea is supported by the observation that different types of larval LNs differentially elaborate neurites to form distinct innervation patterns during the pupal stage.34 Granule cells are continuously generated and integrate into the adult mouse OB,9,36 demonstrating that inhibitory interneurons also sequentially integrate into the mouse olfactory circuit. It will be interesting for future studies to compare the similarities and differences of olfactory interneuron development in Drosophila and mouse.
Figure 2.
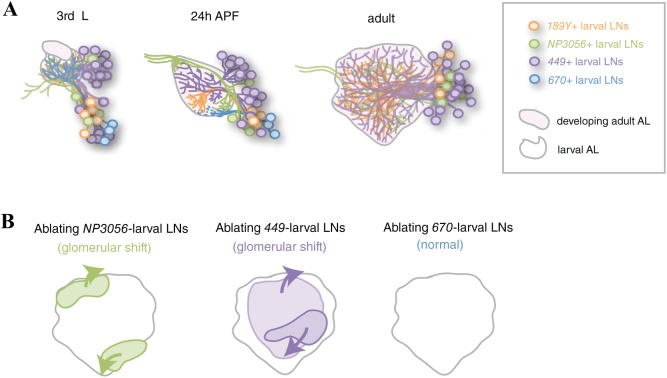
Larval LNs shape the geometry of developing adult antennal lobe. (A) 4 different subsets of larval LNs are visualized by distinct GAL4 drivers: 189Y, NP3056, 449, and 670. 3 subsets of larval LNs undergo pruning and re-innervate the AL at the pupal stage, whereas 670-positive larval LNs die. The processes of these larval LNs occupy different domains of the 24 hours APF AL. Adult-specific LNs are not shown. (B) Summary of glomerular shift phenotypes observed in the AL with ablated larval LNs. The lighter magenta area indicates a group of posterior glomeruli in the AL with ablated 449-positive larval LNs.
Larval Interneurons Undergo Pruning or Cell Death
Axon or dendritic pruning has been observed in a wide variety of species, ranging from Caenorhabditis elegans to humans, where the process is used to shape neural circuits in developing nervous systems.37-39 At the same time, developmental programmed cell death occurs in developing brains to fine-tune the number of cells that will be integrated into circuits or to remove cells already integrated into circuits.40-42 Drosophila develops both larval and adult olfactory systems during its life cycle. All larval mushroom body (MB) γ neurons and almost all larval PNs undergo pruning and reintegration into the developing adult olfactory circuit.43,44 In contrast, all larval OSNs die at the early pupal stage.22 Interestingly, a subset of larval LNs undergoes pruning (eg, 189Y-, NP3056-, and 449-positive larval LNs; Figure 2A), whereas others die during the early pupal stage (eg, 670-positive larval LNs; Figure 2A).34
The steroid molting hormone, ecdysone, is required to initiate the pruning of MB γ neurons, PNs, and peripheral dendritic arborization (da) neurons in Drosophila.39 The pruned larval LNs were also found to express ecdysone receptor EcRB1, and their pruning is controlled by ecdysone signaling.34 Whether the ecdysone signal triggers similar downstream events to induce pruning in larval LNs as those that induce pruning in MB γ neurons, PNs, and/or da neurons remains unclear. Unlike larval LNs that undergo pruning, dying larval LNs express cleaved Caspase 3, a key marker of apoptosis. Such programmed cell death can be blocked by ectopic expression of an anti-apoptotic viral protein, p35.34 Notably, the cell bodies of pruning and dying larval LNs exist in the same neural cluster (Figure 2A).34 It will be interesting to determine how these two different cell fates are dictated in larval LNs.
Pruning Larval LNs Shape the Global Axes of Developing ALs
The set of pruning larval LNs can be further divided into 3 non-overlapping, molecularly distinct subsets based on the expression of drivers: 189Y-, NP3056-, and 449-positive larval LNs. Notably, the re-extended processes of these 3 groups of LNs respectively occupy the medial, lateroventral, and central regions of the 24 hours APF AL. The degenerating processes of dying 670 larval LNs occupy the ventral region of the AL (Figure 2A). Degenerating larval OSNs are known to provide Semaphorin 2a/b, which instructs the dendrite targeting of developing Semaphorin 1a-positive PNs at the early pupal stage.15,22 Therefore, the distinct regional occupation of neurites among the 4 subsets of larval LNs implies these cells may provide regional cues to instruct the targeting of OSN axons and/or PN dendrites in the AL. However, premature ablation of 449-, NP3056-, or 670-positive larval LNs at late larval stage did not lead to dendrite mistargeting in examined PNs.34
Despite the lack of PN mistargeting, this early elimination of pruning larval LNs results in a shift in the global arrangement of glomeruli in developing adult ALs (Figure 2B).34 Interestingly, elimination of NP3056- and 449-positive LNs affects distinct groups of glomeruli (Figure 2B). Killing NP3056-positive larval LNs causes a dorso-medial group of glomeruli to shift dorso-posteriorly and a ventral group of glomeruli to shift ventro-anteriorly, whereas killing 449-positive larval LNs causes an anterior group of glomeruli to shift antero-ventrally and the posterior glomeruli to shift dorso-posteriorly. In contrast to killing the pruning larval LNs, prematurely ablating 670-positive LNs at late larval stage only causes very mild changes in the distances between any 2 glomeruli in the AL (Figure 2B). It remains unclear whether the observed changes are mild because the ablation of dying larval LNs is not early enough, the function of ablated dying larval LNs is compensated by pruning larval LNs, or if dying larval LNs indeed have less impact on developing adult ALs. Nevertheless, these observations support the idea that re-extended larval LN processes are likely to serve as a guiding framework in the developing AL, providing relative positional cues for individual glomeruli. This hypothesis can be tested in future studies by killing 189Y-positive larval LNs. In addition, identifying the positional cues (molecules) that are derived from different subgroups of larval LNs will help to explain how individual glomeruli acquire their stereotypic positions during AL development.
Methodologies Toward Quantifying Axon and Dendrite Targeting and Glomerular Geometry
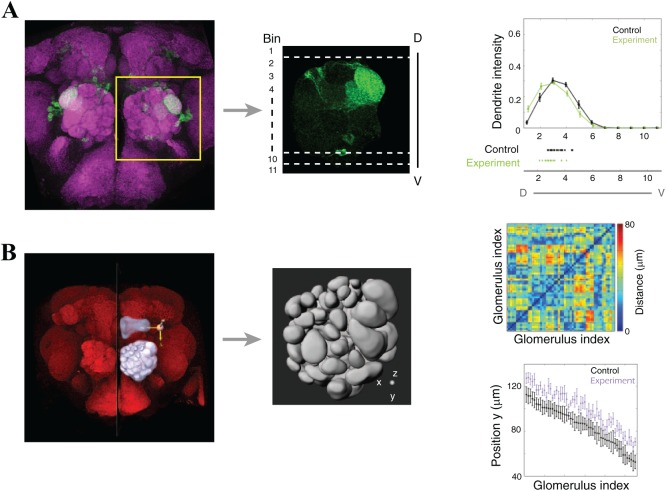
To examine possible phenotypes of axons or dendrites, neurons and their processes are often monitored by expression of fluorescent proteins. The axon and multiple dendrites of a neuron are distributed within the 3-dimensional context of a neuropil. Thus, quantifying the distribution or targeting area of a given neuron is a powerful method for deciphering the functional effects of experimentally altered molecules (mutated genes) or cells on that neuron. One such quantification method is to analyze digital images to extract features that describe the distribution of cellular processes.15 In this method, confocal stacks of an AL may be projected along the anteroposterior axis and compressed into a 2-dimensional image. This 2-dimensional image of the entire AL is then subdivided into several bins along one axis, eg, dorsoventral or mediolateral axes (Figure 3A, middle panel). The mean fluorescence intensity in each bin is calculated to quantify the dendrite (or axon) distribution. This binning method provides quantitative comparisons of neurite distributions in control and experimental brains; however, the neurite distribution along one dimension (antero-posterior axis in this case) and the anatomical distortion of glomeruli and ALs may be lost through image projection.
Figure 3.
Quantifying dendrite or axon mistargeting and structural changes of glomeruli. (A) A confocal stack through the entire antennal lobe was projected into a 2-dimensional image. Neuropil staining (magenta) was used to adjust the orientation of ALs (square outline), and only the signal from innervated PN dendrites or OSN axons was retained. Processed images were subjected to fluorescence intensity analysis. Each AL was divided into 11 bins with bin one being the most dorsal, in this case. Projection neuron dendrite intensity was quantified along the dorsal-ventral axis of control and experimental ALs. Beneath the horizontal axis, scatter plots of the mean dendrite intensity for each AL are shown. (B) The confocal stack of a brain was subjected to analysis in Imaris. Individual glomeruli, the AL, and the second neuropil, mushroom body, were manually reconstructed. The x-, y-, and z-axes were manually determined based on the midline of the brain and the peduncle of the mushroom body (left panel). Anatomical information for individual glomeruli and the entire AL were calculated by implemented functions within Imaris software. Such digital information can be represented by many different parameters, including the relative distance changes between any 2 glomeruli in ALs (top right panel) or the positional shift of individual glomeruli along the y-axis (bottom right panel).
Two attempts were recently made to partially recover global anatomical information describing ALs. The first method begins with drawing a vector that connects the centers of 2 adjacent glomeruli in the projected AL image.17 The angle between this vector and a given axis of the AL (eg, dorsoventral axis) is then estimated. The angular changes between control and experimental ALs reportedly reflect the relative position changes between 2 glomeruli. Because the projected 2-dimensional images are used, possible position changes along the z-axis (anteroposterior axis in this case) cannot be observed. The second method involves reconstructing all glomeruli in the AL to extract multiple anatomical features, including the relative positions of any 2 glomeruli (Figure 3B).34 Briefly, the AL and individual glomeruli are manually segmented through single confocal sections and then reconstructed by the software Imaris (BITPLANE, Oxford Instruments, Zurich, Switzerland). A nearby neuropil (the MB in this case) within the same brain is also reconstructed to serve as an internal reference. This method offers extensive information about the 3-dimensional anatomical features of the glomeruli and the AL. Such information includes the size and shape of individual glomeruli and AL, relative distances between glomeruli, and the relative positions of individual glomeruli and the AL in the brain. In addition, the dendrite mass or axon density in a given glomerulus and its 3-dimensional distribution in the AL are available. However, a major limitation of this method is that it is highly time-consuming and requires an expert who can unambiguously assign the identities of individual glomeruli, especially in distorted ALs.
Several automatic segmentation and annotation methods have been developed to systematically extract digital information from fly brains, including BrainAligner,45 3D fly brain atlas,46 Braincode,47 and NBLAST.48 These methods solve the issue of long analysis times. However, the production of whole brain confocal stacks is prerequisite data for this type of analysis. In addition, as the positions and sizes of some glomeruli are variable across ALs, precise segmentation and annotation of glomeruli, especially those in distorted mutant ALs, remain challenging.
Conclusions
The mechanisms that establish fly olfactory LN diversity during development are not well described. Research in this area has been mainly limited by a lack of appropriate reagents to unambiguously track different subsets of LNs. Recent research identified a collection of GAL4 drivers that label distinct subsets of LNs and shed light on the development of these cells in the adult brain. LNs in the adult olfactory system are composed of larval and adult-specific LNs. Some larval LNs undergo pruning and re-integrate into the developing AL, whereas others die. Distinct subsets of pruning larval LNs collectively shape the global geometry of the developing AL. Moreover, the identification of pruning and dying larval LNs provides an opportunity to elucidate the underlying mechanism(s) that dictate developmental neuronal cell death and neuronal pruning, possibly offering critical insights into neural protection. In addition, the third wave of LNs integrates into the mature circuit at a late pupal stage and may serve as a model system to address how neurons can properly integrate into a mature circuit in adult stages. Thus, the insights offered by studying Drosophila olfactory LNs continue to make the model system an attractive platform for exploring interneuron development, function, death, and diversity.
Acknowledgments
We thank Dr Marcus Calkins for critical readings and comments on the manuscript. K.-T.T. is an Academia Sinica Regular Postdoctoral Scholar.
Footnotes
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Career Development Award (AS-102-CDA-L02) and a MOST grant (104-2311-B-001-033-MY3) to Y.-H.C.
Author Contributions: Y-HC supervised the project. C-JY wrote the “LNs undergo pruning or cell death” section. N-FL wrote the “LNs shape the global Axes of developing ALs” section. K-TT wrote the “Methodologies toward quantification” section. Y-HC wrote the rest of manuscript and prepared Figure 1 and Figure 2. K-TT and N-FL prepared Figure 3.
ORCID iDs: Chi-Jen Yang  https://orcid.org/0000-0002-0940-3901
https://orcid.org/0000-0002-0940-3901
Nan-Fu Liou  https://orcid.org/0000-0002-5353-5477
https://orcid.org/0000-0002-5353-5477
Ya-Hui Chou  https://orcid.org/0000-0001-6552-8728
https://orcid.org/0000-0001-6552-8728
References
- 1. Axel R. The molecular logic of smell. Sci Am. 1995;273:154–159. [DOI] [PubMed] [Google Scholar]
- 2. Friedrich RW, Wiechert MT. Neuronal circuits and computations: pattern decorrelation in the olfactory bulb. FEBS Lett. 2014;588:2504–2513. [DOI] [PubMed] [Google Scholar]
- 3. Jefferis GS, Hummel T. Wiring specificity in the olfactory system. Semin Cell Dev Biol. 2006;17:50–65. [DOI] [PubMed] [Google Scholar]
- 4. Su CY, Menuz K, Carlson JR. Olfactory perception: receptors, cells, and circuits. Cell. 2009;139:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takeuchi H, Sakano H. Neural map formation in the mouse olfactory system. Cell Mol Life Sci. 2014;71:3049–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. [DOI] [PubMed] [Google Scholar]
- 7. Chou YH, Spletter ML, Yaksi E, Leong JC, Wilson RI, Luo L. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat Neurosci. 2010;13:439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kosaka T, Kosaka K. Neuronal organization of the main olfactory bulb revisited. Anat Sci Int. 2016;91:115–127. [DOI] [PubMed] [Google Scholar]
- 9. Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 2008;31:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson RI. Early olfactory processing in Drosophila: mechanisms and principles. Annu Rev Neurosci. 2013;36:217–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chou YH, Zheng X, Beachy PA, Luo L. Patterning axon targeting of olfactory receptor neurons by coupled hedgehog signaling at two distinct steps. Cell. 2010;142:954–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Endo K, Aoki T, Yoda Y, Kimura K, Hama C. Notch signal organizes the Drosophila olfactory circuitry by diversifying the sensory neuronal lineages. Nat Neurosci. 2007;10:153–160. [DOI] [PubMed] [Google Scholar]
- 14. Hong W, Zhu H, Potter CJ, et al. Leucine-rich repeat transmembrane proteins instruct discrete dendrite targeting in an olfactory map. Nat Neurosci. 2009;12:1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Komiyama T, Sweeney LB, Schuldiner O, Garcia KC, Luo L. Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell. 2007;128:399–410. [DOI] [PubMed] [Google Scholar]
- 16. Lattemann M, Zierau A, Schulte C, Seidl S, Kuhlmann B, Hummel T. Semaphorin-1a controls receptor neuron-specific axonal convergence in the primary olfactory center of Drosophila. Neuron. 2007;53:169–184. [DOI] [PubMed] [Google Scholar]
- 17. Li H, Horns F, Wu B, et al. Classifying Drosophila olfactory projection neuron subtypes by single-cell RNA sequencing. Cell. 2017;171:1206.e22–1220.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin S, Kao CF, Yu HH, Huang Y, Lee T. Lineage analysis of Drosophila lateral antennal lobe neurons reveals notch-dependent binary temporal fate decisions. PLoS Biol. 2012;10:e1001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mosca TJ, Luo L. Synaptic organization of the Drosophila antennal lobe and its regulation by the Teneurins. Elife. 2014;3:e03726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ray A, van Naters WG, Shiraiwa T, Carlson JR. Mechanisms of odor receptor gene choice in Drosophila. Neuron. 2007;53:353–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sekine SU, Haraguchi S, Chao K, et al. Meigo governs dendrite targeting specificity by modulating Ephrin level and N-glycosylation. Nat Neurosci. 2013;16:683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sweeney LB, Chou YH, Wu Z, et al. Secreted semaphorins from degenerating larval ORN axons direct adult projection neuron dendrite targeting. Neuron. 2011;72:734–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sweeney LB, Couto A, Chou YH, et al. Temporal target restriction of olfactory receptor neurons by Semaphorin-1a/PlexinA-mediated axon-axon interactions. Neuron. 2007;53:185–200. [DOI] [PubMed] [Google Scholar]
- 24. Ward A, Hong W, Favaloro V, Luo L. Toll receptors instruct axon and dendrite targeting and participate in synaptic partner matching in a Drosophila olfactory circuit. Neuron. 2015;85:1013–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu B, Li J, Chou YH, Luginbuhl D, Luo L. Fibroblast growth factor signaling instructs ensheathing glia wrapping of Drosophila olfactory glomeruli. Proc Natl Acad Sci U S A. 2017;114:7505–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Das A, Sen S, Lichtneckert R, et al. Drosophila olfactory local interneurons and projection neurons derive from a common neuroblast lineage specified by the empty spiracles gene. Neural Dev. 2008;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okada R, Awasaki T, Ito K. Gamma-aminobutyric acid (GABA)-mediated neural connections in the Drosophila antennal lobe. J Comp Neurol. 2009;514:74–91. [DOI] [PubMed] [Google Scholar]
- 28. Seki Y, Rybak J, Wicher D, Sachse S, Hansson BS. Physiological and morphological characterization of local interneurons in the Drosophila antennal lobe. J Neurophysiol. 2010;104:1007–1019. [DOI] [PubMed] [Google Scholar]
- 29. Thum AS, Leisibach B, Gendre N, Selcho M, Stocker RF. Diversity, variability, and suboesophageal connectivity of antennal lobe neurons in D. melanogaster larvae. J Comp Neurol. 2011;519:3415–3432. [DOI] [PubMed] [Google Scholar]
- 30. Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Das S, Sadanandappa MK, Dervan A, et al. Plasticity of local GABAergic interneurons drives olfactory habituation. Proc Natl Acad Sci U S A. 2011;108:E646–E654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sachse S, Rueckert E, Keller A, et al. Activity-dependent plasticity in an olfactory circuit. Neuron. 2007;56:838–850. [DOI] [PubMed] [Google Scholar]
- 33. Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liou NF, Lin SH, Chen YJ, et al. Diverse populations of local interneurons integrate into the Drosophila adult olfactory circuit. Nat Commun. 2018;9:2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jefferis GS, Vyas RM, Berdnik D, et al. Developmental origin of wiring specificity in the olfactory system of Drosophila. Development. 2004;131:117–130. [DOI] [PubMed] [Google Scholar]
- 36. Sakamoto M, Ieki N, Miyoshi G, et al. Continuous postnatal neurogenesis contributes to formation of the olfactory bulb neural circuits and flexible olfactory associative learning. J Neurosci. 2014;34:5788–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luo L, O’Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. [DOI] [PubMed] [Google Scholar]
- 38. Riccomagno MM, Kolodkin AL. Sculpting neural circuits by axon and dendrite pruning. Annu Rev Cell Dev Biol. 2015;31:779–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu F, Schuldiner O. Axon and dendrite pruning in Drosophila. Curr Opin Neurobiol. 2014;27:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kimura K, Ote M, Tazawa T, Yamamoto D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438:229–233. [DOI] [PubMed] [Google Scholar]
- 41. Marzban H, Del Bigio MR, Alizadeh J, Ghavami S, Zachariah RM, Rastegar M. Cellular commitment in the developing cerebellum. Front Cell Neurosci. 2014;8:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wright LL, Cunningham TJ, Smolen AJ. Developmental neuron death in the rat superior cervical sympathetic ganglion: cell counts and ultrastructure. J Neurocytol. 1983;12:727–738. [DOI] [PubMed] [Google Scholar]
- 43. Lee T, Marticke S, Sung C, Robinow S, Luo L. Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron. 2000;28:807–818. [DOI] [PubMed] [Google Scholar]
- 44. Marin EC, Watts RJ, Tanaka NK, Ito K, Luo L. Developmentally programmed remodeling of the Drosophila olfactory circuit. Development. 2005;132:725–737. [DOI] [PubMed] [Google Scholar]
- 45. Peng H, Chung P, Long F, et al. BrainAligner: 3D registration atlases of Drosophila brains. Nat Methods. 2011;8:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shao HC, Wu CC, Chen GY, Chang HM, Chiang AS, Chen YC. Developing a stereotypical Drosophila brain atlas. IEEE Trans Biomed Eng. 2014;61:2848–2858. [DOI] [PubMed] [Google Scholar]
- 47. Panser K, Tirian L, Schulze F, et al. Automatic segmentation of Drosophila neural compartments using GAL4 expression data reveals novel visual pathways. Curr Biol. 2016;26:1943–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Costa M, Manton JD, Ostrovsky AD, Prohaska S, Jefferis GS. NBLAST: rapid, sensitive comparison of neuronal structure and construction of neuron family databases. Neuron. 2016;91:293–311. [DOI] [PMC free article] [PubMed] [Google Scholar]