Abstract
Background:
Acute lung injury (ALI) is the most serious pulmonary complication after lung resection. Although the beneficial effects of low-dose corticosteroids have been demonstrated in patients with postoperative ALI, there are limited data on optimal corticosteroid treatment.
Methods:
We retrospectively analyzed 58 patients who were diagnosed with ALI among 7593 patients who underwent lung cancer surgery between January 2009 and December 2016.
Results:
Of the 58 patients, 42 (72%) received corticosteroid treatment within 72 h (early treatment group) and 16 (28%) received corticosteroid treatment more than 72 h after ALI occurred (late treatment group). The early treatment group demonstrated a higher response to corticosteroid treatment compared with the late treatment group (95% versus 69%, respectively, p = 0.014), had an improved lung injury score (86% versus 63%, p = 0.072), and were more likely to be successfully weaned from the ventilator within 7 days (57% versus 39%, p = 0.332). During corticosteroid treatment, the early treatment group had a lower rate of delirium (24% versus 63%, p = 0.012) compared with the late treatment group. No significant differences in length of stay (30 versus 37 days, p = 0.254) or in-hospital mortality (43% versus 38%, p = 0.773) were observed; however, the early treatment group tended to have a higher rate of successful weaning than the late treatment group (p = 0.098, log-rank test).
Conclusions:
Early initiation of corticosteroid treatment improved lung injury and promoted ventilator weaning in patients with ALI following lung resection for lung cancer.
Keywords: acute lung injury, acute respiratory distress syndrome, glucocorticoid, lung neoplasm, operative procedure
Introduction
Postoperative acute lung injury (ALI), characterized by the acute onset of hypoxemia with radiographic pulmonary infiltrates without a clearly identifiable cause, is a major cause of morbidity and mortality after lung resection surgery.1,2 Many studies have reported that approximately 2–8% of patients develop ALI after lung resection surgery for lung cancer.2–6 Despite its relatively low incidence, the mortality from ALI following lung resection remains high.1,2
The clinical and radiologic characteristics of postoperative ALI are identical to those of acute respiratory distress syndrome (ARDS).7–9 Therefore, treatment of postoperative ALI is based on the management strategies of ARDS, which include general supportive care with lung protective ventilation and restrictive fluid management.1,10 Unfortunately, no pharmacologic therapy for ARDS has been shown to reduce either short-term or long-term mortality;10 however, corticosteroids may improve gas exchange and hasten radiographic improvement in patients with ARDS.11–15 Although a high-dose short course of corticosteroids for early-phase ARDS failed to show improvements in survival,16,17 a recent trial using a low-dose prolonged course of corticosteroids in early ARDS demonstrated improvement in organ dysfunction and a reduction in duration of mechanical ventilation (MV) and length of stay in the intensive care unit (ICU).14 The beneficial effects of early low-dose corticosteroids have been reported in patients with postoperative ALI after lung resection surgery;18 however, there are limited data on the benefit of early administration of corticosteroid in patients with ALI after lung resection surgery.
The objective of this study was to investigate the beneficial effects of early (within 72 h) treatment with corticosteroids in patients with postoperative ALI compared with late treatment. Our hypothesis was that fibroproliferation, which is an early response to lung injury, would be inhibited by early corticosteroid treatment without serious adverse events.
Patients and methods
Study population
Data were collected from all consecutive patients diagnosed with postoperative ALI after lung resection surgery for lung cancer at Samsung Medical Center (a 1989-bed referral hospital in Seoul, South Korea) from January 2009 through December 2016 and retrospectively analyzed. The institutional review board of the Samsung Medical Center approved the review and publication of information obtained from the patients’ records (approval no: 2017-01-018). Informed consent was waived because of the retrospective observational nature of the study. All patient data were anonymized and de-identified by the data coordinator prior to analysis.
Diagnosis of postoperative ALI
During the study period, postoperative ALI was diagnosed by (1) sudden onset of respiratory distress within 7 days after surgery; (2) diffuse pulmonary infiltrates on chest computed tomography (CT); (3) impaired oxygenation with partial pressure of arterial oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio (PF ratio) <300 mmHg; (4) symptoms not fully explained by cardiac failure or fluid overload.5 Serum brain-type natriuretic peptide and transthoracic echocardiography were performed to exclude pulmonary edema of cardiac origin. Other causes of respiratory distress such as respiratory/systemic infection were excluded. Severity of ALI was classified by the Berlin definition in patients receiving MV.19
Data collection
The medical records of the patients were reviewed and clinical data were extracted, including demographic characteristics, body mass index, smoking history, American Society of Anesthesiologists physical status, comorbidities (chronic obstructive pulmonary disease, interstitial lung disease, hypertension, diabetes mellitus, and other malignancies), predicted postoperative pulmonary function tests, clinical stage, and the presence of neoadjuvant treatment (radiotherapy or chemotherapy). The following perioperative data were also extracted: side of resection, approach type, type of operation, total operation time, one lung ventilation (OLV) time, peak airway pressure during OLV, tidal volume during OLV, intraoperative volume, transfusion, and bleeding volume. The following variables were measured at the initiation of corticosteroid therapy (day 0): Sequential Organ Failure Assessment (SOFA) score,20 PF ratio, lung injury score (LIS), oxygenation index, MV settings (FiO2, positive end-expiratory pressure, support pressure), monitored tidal volume, serum C-reactive protein (CRP), and arterial blood gas analysis. At day 2 and 7 from the initiation of corticosteroid therapy, the SOFA score, PF ratio, LIS, and oxygenation index were measured. We also extracted data on complications such as newly diagnosed delirium assessed by the Confusion Assessment Method for the ICU (CAM-ICU),21 superimposed infection, and surgical site complications during corticosteroid treatment, and treatment modalities during ICU stays including antibiotics, vasopressor, tracheostomy, continuous renal replacement therapy, and extracorporeal membrane oxygenation.
Corticosteroid treatment and treatment outcomes
During the study period, postoperative ALI was managed by a multidisciplinary team composed of thoracic surgeons, pulmonologists, and intensivists. The team decided the administration of corticosteroid when the diagnosis of postoperative ALI was confirmed, and patients suffered from hypoxemia demonstrating PF ratio of <300 mmHg. We classified patients into two groups according to the time of initiation of corticosteroid treatment: the early treatment group received corticosteroid within 72 h, and the late group received corticosteroid more than 72 h after ALI occurred. A loading dose of methylprednisolone 1–2 mg/kg was followed by infusion of 1 mg/kg/day from day 1 to day 14, 0.5 mg/kg/day from day 15 to day 21, 0.25 mg/kg/day from day 22 to day 25, and 0.125 mg/kg/day from day 26 to day 28.14 Before the protocol of corticosteroid treatment was implemented at our institution, the loading dose was decided at the discretion of the attending physicians. Regarding loading dose, methylprednisolone doses of ⩽2 mg/kg and >2 mg/kg were defined as low-dose and high-dose corticosteroid treatment, respectively.
Response to corticosteroid treatment was defined as weaning from MV within 7 days or improvement in LIS of more than 1 point by day 7. For patients remaining intubated on day 7, improvement in lung function was defined as follows: a reduction in LIS by 1 or more point and a day 7 LIS ⩽2.0 (for study entry LIS ⩽2.9) or ⩽2.5 (for study entry LIS ⩾3.0) as previously described.14 For patients not receiving MV, LIS was calculated using two components: chest X-ray score and hypoxemia score. In addition, we documented outcomes of patients with ALI including length of stay at the ICU and hospital, and 28-day, ICU, and hospital mortality.
Statistical analysis
Data are presented as the median and interquartile range (IQR) for continuous variables and as frequency (percentage) for categorical variables. Data were compared by the Mann–Whitney U test for continuous variables and by the Pearson’s Chi-square test or Fisher’s exact test for categorical variables.22 All tests were two-sided and a p value <0.05 was considered significant. Duration of MV curves for each treatment group were estimated by the Kaplan–Meier method and compared by the log-rank test. Data were analyzed using IBM SPSS Statistics for Windows, version 23.0 (Armonk, NY, USA).
Results
During the study period, a total of 7593 patients underwent lung resection surgery for lung cancer and 58 (0.8%) patients developed ALI. Preoperative and perioperative characteristics are summarized in Table 1. Among the 58 patients, 53 (91%) were male and the median age of all patients was 70 years (IQR, 62–72 years). A total of 11 (19%) patients received neoadjuvant treatment before operation. Overall, 36 (62%) patients received a right-side operation. Regarding the type of operation, 7 (12%) patients underwent pneumonectomy, 4 (7%) underwent bilobectomy, 43 (74%) underwent lobectomy, and 4 (7%) underwent wedge resection. The early treatment group contained 42 (72%) patients and the late treatment group contained 16 (28%). There were no significant differences in preoperative and perioperative characteristics between the two groups.
Table 1.
Preoperative and perioperative data for patients with acute lung injury after pulmonary resection.
| Total (n = 58) |
Early treatment (n = 42) |
Late treatment (n = 16) |
p value | |
|---|---|---|---|---|
| Age, years | 70 (62–72) | 70 (61–72) | 71 (63–74) | 0.508 |
| Male | 53 (91) | 38 (91) | 15 (94) | 1.0 |
| BMI, kg/m2 | 22.3 (20.2–24.5) | 23.1 (20.2–25.3) | 22.0 (19.3–23.3) | 0.175 |
| Smoking history | 0.905 | |||
| Ex-smoker | 32 (55) | 22 (52) | 10 (63) | |
| Current smoker | 21 (36) | 16 (38) | 5 (31) | |
| ASA physical status | 0.120 | |||
| 1 | 5 (9) | 4 (10) | 1 (6) | |
| 2 | 41 (71) | 32 (76) | 9 (56) | |
| ⩾3 | 12 (21) | 6 (14) | 6 (38) | |
| Comorbidities | ||||
| COPD | 30 (52) | 19 (45) | 11 (69) | 0.146 |
| Interstitial lung disease | 10 (17) | 6 (14) | 4 (25) | 0.439 |
| Hypertension | 24 (41) | 16 (38) | 8 (50) | 0.552 |
| Diabetes mellitus | 18 (31) | 15 (36) | 3 (19) | 0.342 |
| Other malignancies | 10 (17) | 7 (17) | 3 (19) | 1.0 |
| Pulmonary function test | ||||
| FEV1 % predicted postoperative | 63 (54–75) | 65 (55–75) | 60 (51–75) | 0.596 |
| DLCO % predicted postoperative | 51 (44–61) | 53 (46–63) | 48 (38–51) | 0.070 |
| Clinical stage | 0.465 | |||
| I | 22 (38) | 18 (43) | 4 (25) | |
| II | 21 (36) | 14 (33) | 7 (44) | |
| III | 15 (26) | 10 (24) | 5 (31) | |
| Neoadjuvant treatment | 11 (19) | 5 (12) | 6 (38) | 0.055 |
| Side of operation | 0.877 | |||
| Right | 36 (62) | 25 (60) | 11 (69) | |
| Left | 20 (35) | 15 (36) | 5 (31) | |
| Approach type | 0.217 | |||
| Open thoracotomy | 39 (67) | 26 (62) | 13 (81) | |
| Minimal invasive surgery | 19 (33) | 16 (38) | 3 (19) | |
| Type of operation | 0.767 | |||
| Partial resection | 4 (7) | 3 (7) | 1 (6) | |
| Lobectomy | 43 (74) | 32 (76) | 11 (69) | |
| Bilobectomy | 4 (7) | 2 (5) | 2 (13) | |
| Pneumonectomy | 7 (12) | 5 (12) | 2 (13) | |
| Total operation time, min | 254 (180–318) | 236 (178–337) | 265 (221–300) | 0.626 |
| One lung ventilation time, min | 134 (100–199) | 133 (100–199) | 150 (113–229) | 0.565 |
| Peak airway pressure, cmH2O | 21 (18–23) | 21 (18–23) | 21 (17–25) | 0.643 |
| Tidal volume, ml | 398 (364–424) | 400 (378–427) | 368 (344–421) | 0.095 |
| Intraoperative volume infusion, ml | 1750 (1238–2108) | 1725 (1250–2025) | 1750 (965–2200) | 0.931 |
| Transfusion | 6 (10) | 4 (10) | 2 (13) | 0.664 |
| Bleeding, ml | 300 (200–400) | 300 (200–400) | 300 (163–400) | 0.854 |
Data are presented as number (percentage) or as median (interquartile range).
ASA, American Society of Anesthesiologists; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in 1 second.
Clinical characteristics at the time of ALI diagnosis are presented in Table 2. The median LIS and PF ratio of all patients were 2.0 (1.5–2.5) and 161 (142–207), respectively. A total of 43 (74%) patients required MV support, and 38 (66%) and 2 (3%) were classified as moderate and severe ARDS by the Berlin definition, respectively. There was no significant difference in the severity of ALI, laboratory findings except for CRP level, and treatments between the two groups (Table 2).
Table 2.
Severity of acute lung injury and treatment modalities in the intensive care unit.
| Total (n = 58) |
Early treatment (n = 42) |
Late treatment (n = 16) |
p value | |
|---|---|---|---|---|
| SOFA score | 4 (2–6) | 4 (2–6) | 5 (2–6) | 0.464 |
| Severity of ALI | ||||
| LIS | 2.0 (1.5–2.5) | 2.0 (1.5–2.5) | 2.0 (1.6–2.3) | 0.833 |
| OI | 10.2 (6.9–14.2) | 10.9 (6.9–13.9) | 9.0 (6.3–15.9) | 0.768 |
| PaO2/FiO2 ratio | 161 (142–207) | 160 (136–215) | 167 (145–201) | 0.770 |
| Laboratory data | ||||
| pH | 7.42 (7.38–7.45) | 7.41 (7.37–7.45) | 7.42 (7.38–7.44) | 0.520 |
| PaO2, mmHg | 77 (66–88) | 76 (65–88) | 79 (68–89) | 0.558 |
| PaCO2, mmHg | 38 (33–42) | 37 (33–42) | 38 (32–45) | 0.902 |
| HCO3, mmol/L | 25 (22–26) | 25 (22–26) | 26 (24–27) | 0.167 |
| CRP, mg/dL | 19.9 (13.7–24.8) | 21.4 (14.4–26.1) | 17.5 (7.9–21.2) | 0.013 |
| Mechanical ventilation | 43 (74) | 30 (71) | 13 (81) | 0.522 |
| FiO2 | 0.5 (0.4–0.6) | 0.5 (0.4–0.6) | 0.5 (0.4–0.6) | 0.571 |
| PEEP, cmH2O | 6 (5–8) | 6 (5–8) | 5 (5–9) | 0.858 |
| Above-PEEP, cmH2O | 14 (12-–15) | 14 (12–15) | 12 (12–16) | 0.337 |
| Tidal volume, ml | 370 (330–490) | 350 (295–460) | 440 (353–570) | 0.112 |
| Corticosteroid | 0.375 | |||
| Low dose | 33 (57) | 22 (52) | 11 (69) | |
| High dose | 25 (43) | 20 (48) | 5 (31) | |
| Tracheostomy | 19 (33) | 12 (29) | 7 (44) | 0.351 |
| CRRT | 8 (14) | 5 (12) | 3 (19) | 0.672 |
| ECMO | 10 (17) | 7 (17) | 3 (19) | 1.0 |
| Vasopressor | 28 (48) | 18 (43) | 10 (63) | 0.243 |
| Antibiotics | 57 (98) | 42 (100) | 15 (94) | 0.276 |
Data are presented as number (percentage) or as median (interquartile range).
ALI, acute lung injury; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; FiO2, fraction of inspired oxygen; LIS, lung injury score; OI, oxygenation index; PaO2, partial pressure of oxygen in the arterial blood; PEEP, positive end-expiratory pressure; SOFA, sequential organ failure assessment.
Treatment outcomes of patients are summarized in Table 3. Over the study period, 43 (74%) patients received MV support and 28 patients were weaned from MV. Of the 15 patients who failed to wean from MV, 13 (22%) died and 2 (4%) were transferred to other hospitals. Overall in-hospital mortality was 41%. In comparisons of outcomes between the two groups, the overall treatment response to corticosteroid was higher in the early treatment group compared with the late treatment group (95% versus 69%, p = 0.014). In detail, the early treatment group showed an improved LIS (86% versus 63%, p = 0.072) and were more likely to be successfully weaned from MV within 7 days (57% versus 39%, p = 0.332) compared with the late treatment group. Although successful weaning from MV in patients receiving MV support was not significantly different between the two groups, there was a trend toward less time to weaning from MV in the early treatment group compared with the late treatment group (p = 0.098 by the log-rank test; Figure 1). Nonetheless, mortalities in the ICU and hospital were not different.
Table 3.
Treatment outcomes of patients with acute lung injury after pulmonary resection.
| Total (n = 58) |
Early treatment (n = 42) |
Late treatment (n = 16) |
p value | |
|---|---|---|---|---|
| Response to corticosteroid treatmenta | 51 (88) | 40 (95) | 11 (69) | 0.014 |
| Weaning from MV within 7 days | 22 (51) | 17 (57) | 5 (39) | 0.332 |
| Improvement in LIS | 46 (79) | 36 (86) | 10 (63) | 0.072 |
| Weaning from MV | 28 (65) | 21 (70) | 7 (54) | 0.305 |
| Complications during corticosteroid treatment | ||||
| Arrhythmia | 17 (29) | 11 (26) | 6 (38) | 0.520 |
| Delirium | 20 (35) | 10 (24) | 10 (63) | 0.012 |
| Superimposed infection | 25 (43) | 18 (43) | 7 (44) | 1.0 |
| Surgical site complications | 0.356 | |||
| Persistent air leakage | 18 (31) | 13 (31) | 5 (31) | |
| Bleeding | 1 (2) | 0 | 1 (6) | |
| ICU mortality | 21 (36) | 15 (36) | 6 (38) | 1.0 |
| Length of ICU stay, days | 9 (4–28) | 8 (4–29) | 17 (8–27) | 0.247 |
| 28-day mortality | 7 (12) | 6 (14) | 1 (6) | 0.660 |
| In-hospital mortality | 24 (41) | 18 (43) | 6 (38) | 0.773 |
| Length of hospital stay, days | 33 (21–46) | 30 (21–44) | 37 (21–54) | 0.254 |
Data are presented as number (percentage) or as median (interquartile range).
ICU, intensive care unit; LIS, lung injury score; MV, mechanical ventilation.
Defined as weaning from MV within 7 days or improvement in LIS, which was defined as follows: (1) a reduction in LIS of 1 point or more by study day 7 and (2) a day 7 LIS ⩽2.0 (for study entry LIS ⩽2.9) or ⩽2.5 (for study entry LIS ⩾3.0).
Figure 1.
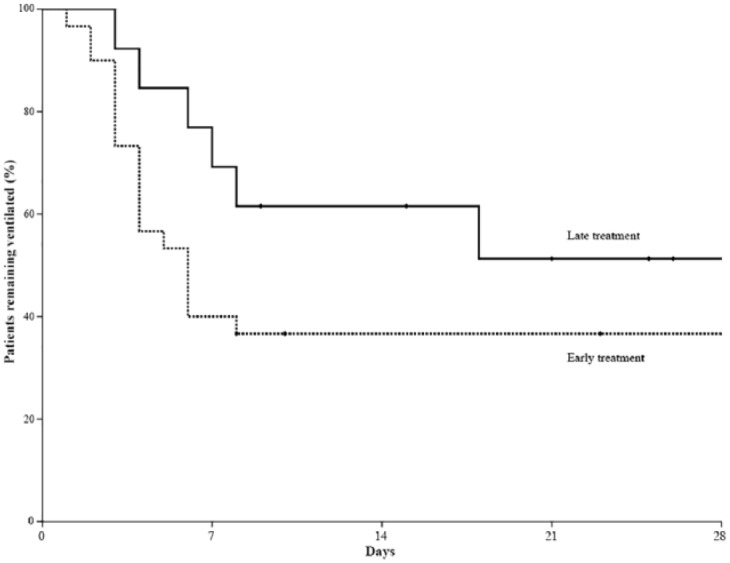
Kaplan–Meier curves of the probability of weaning from mechanical ventilation in patients who received low-dose corticosteroid treatment within 72 h after development of acute lung injury following lung resection (early treatment group; dotted line) and those who received corticosteroids more than 72 h after development of acute lung injury (late treatment group; solid line).
Regarding complications during corticosteroid treatment, delirium was significantly less common in the early treatment group compared with the late treatment group (24% versus 63%, p = 0.012). However, there was no difference in the development of infection and surgical site complications including persistent air leakage and bleeding between the two groups (Table 3).
Discussion
In this observational study, we demonstrated the beneficial effect of early administration of corticosteroid in patients with ALI after lung resection surgery for lung cancer. Corticosteroid treatment within 72 h after development of postoperative ALI was associated with a greater improvement of LIS than treatment after 72 h. In addition, there was a trend toward less time to weaning from MV in the early treatment group than in the late treatment group. Finally, there was no significant difference in the incidence of surgical site infection between the two groups.
During the study period, the overall prevalence rate of ALI was 0.8%, which is relatively low compared with previous studies reporting rates of 2.6–7% in pneumonectomy and 1–3% in lobectomy.2–6 We ascribe our lower prevalence rate in part to our lung protective ventilation strategy during surgery.23 A large tidal volume and high airway pressure during OLV is associated with an increased risk of postoperative ALI.24,25 This is supported by a recent meta-analysis showing that the use of low tidal volume resulted in a lower incidence of ARDS in patients undergoing OLV.26
The role of corticosteroid treatment in the management of ARDS has been systematically studied.11–15 In a small randomized controlled trial,14 early infusion (⩽72 h after onset of ARDS) of low-dose methylprednisolone was associated with significant improvement in pulmonary and extrapulmonary organ dysfunction. A recent analysis of individual patient data from four randomized trials combined with a trial-level meta-analysis of the updated literature demonstrated that early and prolonged corticosteroid treatment accelerated resolution of ARDS and decreased hospital mortality and healthcare utilization without increasing the risk of infection.27 The beneficial effects of corticosteroids in ARDS are consistent with the hypothesis that fibroproliferation is an early response to lung injury that is inhibited by early low-dose corticosteroid treatment.28 Despite many studies investigating corticosteroid therapy in ARDS, there is limited information on the therapy in ALI after lung resection, although Lee and colleagues18 demonstrated a possible benefit of using low-dose corticosteroids in patients with ARDS after thoracotomy in a small observational study. In the present study, early initiation of corticosteroid demonstrated a higher ratio of improvement in LIS and a trend toward more successful weaning from MV, although the early treatment group showed a significantly higher level of CRP than the late treatment group, indicating more severe inflammation. More severe inflammatory markers might have led clinicians to initiate corticosteroid treatment in the early treatment group of this study. Despite more severe inflammation, patients of the early treatment group demonstrated a higher response to corticosteroid treatment and more successful weaning from MV compared with patients of the late treatment group. However, there was no significant difference in mortality between the early and late treatment group in this study. Our results, combined with the findings of other recent study not showing any beneficial effects of corticosteroid on mortality in patients with ARDS,29 suggest that more studies are needed to prove its mortality benefit in ARDS.
Despite the beneficial effects of early low-dose corticosteroid treatment in ARDS, the suppressant effect of corticosteroids on wound healing and the immune response raises concern in patients undergoing lung resection. However, recent trials investigating low-dose corticosteroids in ARDS have not reported an increased rate of nosocomial infections.27,30 In addition, neither wound infection nor anastomosis dehiscence was reported in an observational study of patients with postoperative ALI.18 In the present study, prolonged air leakage was the main surgical site complication in patients receiving corticosteroid treatment, which is similar to the previous report by Lee and colleagues.18
There are several limitations to our study that should be acknowledged. The limitations of this study are attributed primarily to univariate analysis with a relatively small sample size of the two groups, which were unable to perform additional statistical analyses including propensity matching or severity stratification of ALI. Another major limitation is the fact that we did not systematically screen patients with acute hypoxemic respiratory failure within 7 days after lung resection surgery. More severely ill patients might not have undergone chest CT scanning even if they were strongly suspected to have ALI. Data regarding how many patients with suspicion of ALI refused further evaluation could not be extracted from the medical records during the study period. In addition, given the retrospective nature of our study, there is the inherent possibility that selection bias may have influenced the significance of our findings. Furthermore, since our study did not compare patients receiving corticosteroids with those not receiving corticosteroids directly, we could not make a conclusion confirming its efficacy in postoperative ALI. Additional studies that are performed in larger, well-defined prospective cohorts are warranted to address the issue. Finally, our study was from a single institution with the largest number of lung cancer surgeries performed in Korea for the last 10 years, which limits the generalization of our findings to other institutions.
In summary, early administration of corticosteroid in patients with ALI after lung resection surgery for lung cancer was associated with greater improvement of lung injury and reduced time to weaning from MV, without affecting operative wound healing. However, further evaluation with a prospective, randomized, controlled study is needed to confirm these observations.
Footnotes
Funding: This work was supported by a Samsung Medical Center grant (OTA1602901).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Kyeongman Jeon  https://orcid.org/0000-0002-4822-1772
https://orcid.org/0000-0002-4822-1772
Contributor Information
Hayoung Choi, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, Seoul, South Korea.
Beomsu Shin, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea Department of Pulmonology, Wonju Severance Christian Hospital, Yonsei Wonju College of Medicine, Wonju, South Korea.
Hongseok Yoo, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea.
Gee Young Suh, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea Department of Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea.
Jong Ho Cho, Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea.
Hong Kwan Kim, Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea.
Yong Soo Choi, Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea.
Jhingook Kim, Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea.
Jae Ill Zo, Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea.
Young Mog Shim, Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea.
Kyeongman Jeon, Division of Pulmonary and Critical Care Medicine, Department of Medicine and Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, South Korea.
References
- 1. Kometani T, Okamoto T, Yoshida S, et al. Acute respiratory distress syndrome after pulmonary resection. Gen Thorac Cardiovasc Surg 2013; 61: 504–512. [DOI] [PubMed] [Google Scholar]
- 2. Alam N, Park BJ, Wilton A, et al. Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg 2007; 84: 1085–1091; discussion 1091. [DOI] [PubMed] [Google Scholar]
- 3. Kutlu CA, Williams EA, Evans TW, et al. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Ann Thorac Surg 2000; 69: 376–380. [DOI] [PubMed] [Google Scholar]
- 4. Ruffini E, Parola A, Papalia E, et al. Frequency and mortality of acute lung injury and acute respiratory distress syndrome after pulmonary resection for bronchogenic carcinoma. Eur J Cardiothorac Surg 2001; 20: 30–36, discussion 36–37. [DOI] [PubMed] [Google Scholar]
- 5. Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 2003; 97: 1558–1565. [DOI] [PubMed] [Google Scholar]
- 6. Dulu A, Pastores SM, Park B, et al. Prevalence and mortality of acute lung injury and ARDS after lung resection. Chest 2006; 130: 73–78. [DOI] [PubMed] [Google Scholar]
- 7. Jordan S, Mitchell JA, Quinlan GJ, et al. The pathogenesis of lung injury following pulmonary resection. Eur Respir J 2000; 15: 790–799. [DOI] [PubMed] [Google Scholar]
- 8. Beddow E, Goldstraw P. The pulmonary physician in critical care * Illustrative case 8: acute respiratory failure following lung resection. Thorax 2003; 58: 820–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Villeneuve PJ, Sundaresan S. Complications of pulmonary resection: postpneumonectomy pulmonary edema and postpneumonectomy syndrome. Thorac Surg Clin 2006; 16: 223–234. [DOI] [PubMed] [Google Scholar]
- 10. Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017; 377: 562–572. [DOI] [PubMed] [Google Scholar]
- 11. Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA 1998; 280: 159–165. [DOI] [PubMed] [Google Scholar]
- 12. Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006; 354: 1671–1684. [DOI] [PubMed] [Google Scholar]
- 13. Annane D, Sebille V, Bellissant E, et al. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med 2006; 34: 22–30. [DOI] [PubMed] [Google Scholar]
- 14. Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 2007; 131: 954–963. [DOI] [PubMed] [Google Scholar]
- 15. Meduri GU, Marik PE, Chrousos GP, et al. Steroid treatment in ARDS: a critical appraisal of the ARDS network trial and the recent literature. Intensive Care Med 2008; 34: 61–69. [DOI] [PubMed] [Google Scholar]
- 16. Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med 1987; 317: 1565–1570. [DOI] [PubMed] [Google Scholar]
- 17. Luce JM, Montgomery AB, Marks JD, et al. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis 1988; 138: 62–68. [DOI] [PubMed] [Google Scholar]
- 18. Lee HS, Lee JM, Kim MS, et al. Low-dose steroid therapy at an early phase of postoperative acute respiratory distress syndrome. Ann Thorac Surg 2005; 79: 405–410. [DOI] [PubMed] [Google Scholar]
- 19. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 20. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 21. Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 2001; 29: 1370–1379. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med 2016; 4: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang M, Ahn HJ, Kim K, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery? A randomized controlled trial. Chest 2011; 139: 530–537. [DOI] [PubMed] [Google Scholar]
- 24. Fernandez-Perez ER, Keegan MT, Brown DR, et al. Intraoperative tidal volume as a risk factor for respiratory failure after pneumonectomy. Anesthesiology 2006; 105: 14–18. [DOI] [PubMed] [Google Scholar]
- 25. Jeon K, Yoon JW, Suh GY, et al. Risk factors for post-pneumonectomy acute lung injury/acute respiratory distress syndrome in primary lung cancer patients. Anaesth Intensive Care 2009; 37: 14–19. [DOI] [PubMed] [Google Scholar]
- 26. El Tahan MR, Pasin L, Marczin N, et al. Impact of low tidal volumes during one-lung ventilation. a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth 2017; 31: 1767–1773. [DOI] [PubMed] [Google Scholar]
- 27. Meduri GU, Bridges L, Shih MC, et al. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients’ data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med 2016; 42: 829–840. [DOI] [PubMed] [Google Scholar]
- 28. Meduri GU, Muthiah MP, Carratu P, et al. Nuclear factor-kappaB- and glucocorticoid receptor alpha-mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids. Neuroimmunomodulation 2005; 12: 321–338. [DOI] [PubMed] [Google Scholar]
- 29. Zhang Z, Chen L, Ni H. The effectiveness of corticosteroids on mortality in patients with acute respiratory distress syndrome or acute lung injury: a secondary analysis. Sci Rep 2015; 5: 17654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marik PE, Meduri GU, Rocco PR, et al. Glucocorticoid treatment in acute lung injury and acute respiratory distress syndrome. Crit Care Clin 2011; 27: 589–607. [DOI] [PubMed] [Google Scholar]


