Abstract
Objective
The purpose of this study was to examine the interaction between the endogenous opioid and endocannabinoid (eCB) systems in a pain modulatory process known as exercise-induced hypoalgesia (EIH).
Design
Randomized controlled trial.
Setting
Clinical research unit in a hospital.
Subjects
Fifty-eight healthy men and women (mean age = 21 ± 3 years) participated in this study.
Methods
Participants were administered (randomized, double-blind, counterbalanced procedure) an opioid antagonist (i.e., naltrexone) and a placebo prior to performing pain testing and isometric exercise.
Results
Results indicated that 2-arachidonoylglycerol (2-AG) and 2-oleoylglycerol (2-OG) increased significantly (P < 0.05) following exercise in both placebo and naltrexone conditions. In comparison, N-arachidonylethanolamine (AEA) and oleoylethanolamine (OEA) increased significantly (P < 0.05) following exercise in the placebo condition but not the naltrexone condition. There were no significant (P > 0.05) differences in palmitolethanolamine (PEA) between the placebo and naltrexone conditions.
Conclusions
As reductions in pain (i.e., EIH) were observed following both conditions, these results suggest that the opioid system may not be the primary system involved in exercise-induced hypoalgesia and that 2-AG and 2-OG could contribute to nonopioid exercise-induced hypoalgesia. Moreover, as exercise-induced increases in AEA and OEA were blocked by naltrexone pretreatment, this suggests that the opioid system may be involved in the increase of AEA and OEA following exercise.
Keywords: EIH Mechanisms, Pain Modulation, Isometric Exercise, 2-AG, AEA
Introduction
The perception of pain is modulated by multiple endogenous systems involving both opioid and nonopioid systems. One nonopioid system that is involved in pain modulation is the endocannabinoid (eCB) system [1]. The eCB system is a complex neuromodulatory system primarily composed of cannabinoid receptors (CB1R and CB2R) and endocannabinoids (eCBs; N-arachidonylethanolamine [AEA] and 2-arachidonoylglycerol [2-AG]) that bind to CB1 and CB2 receptors. Several other related biogenic lipids (oleoylethanolamine [OEA], palmitoylethanolamine [PEA], 2-oleoylglycerol [2-OG]) are often examined alongside eCBs (AEA, 2-AG) due to the fact that they are all fatty acid derivatives formed via similar enzymatic pathways involving the same precursors: N-acylated ethanolamine phospholipids (AEA, OEA, and PEA) and diacylglycerol (2-AG and 2-OG) [2,3].
Support for the involvement of the eCB system in pain modulatory processes stems from reports demonstrating that CB1 receptors are highly localized at pain processing areas of the brain and spinal cord [4–6], and exogenous stimulation of these receptors has been shown to produce antinociception [7]. Further, there is evidence of an interplay between eCB and opioid systems such that the activation of one system is mediated by the other [8–10]. Preclinical and human studies involving exogenous administration of cannabinoids and opiates have indicated significant interactions between opioid and eCB systems in pain responses [11]. However, the interaction between these two systems in specific endogenous pain modulatory processes such as exercise-induced hypoalgesia has not been examined. Exercise-induced hypoalgesia (EIH) occurs when a noxious stimulus is perceived as less painful after a bout of aerobic, resistance, or isometric exercise [12,13]. Exercise requires muscular contractions that stimulate group III (A-delta) and group IV (C) nociceptive fibers, and this stimulation has been shown to result in activation of endogenous analgesia mechanisms [14]. Therapeutic exercise programs are an important element in the treatment of many chronic pain syndromes. Furthermore, exercise has been effective in situations where pain medications have not provided optimal relief [15]. Although numerous studies have indicated a reduction in pain following exercise [12], the mechanisms responsible for EIH are poorly understood. Results from preclinical research suggest that both opioid and eCB mechanisms play a role in EIH; however, these systems have typically been examined in isolation of one another. There is a need for additional research exploring the interaction between these systems in endogenous pain modulation because of its importance to furthering our understanding of combination therapies for pain relief [12,16]. Therefore, the purpose of the current study was to examine opioid and endocannabinoid interactions in an EIH paradigm by measuring plasma concentrations of eCBs in the presence of an opioid antagonist or placebo, before and after experimental pain testing and exercise in healthy young adults.
Methods
Procedures and results presented here are part of a larger study designed to examine the mechanisms of EIH with some of the previously published results [13]. It was decided after further reflection upon the published results and the availability of additional funding to examine the interaction between opioid and endocannabinoid systems; thus, novel unpublished results of the endocannabinoid responses in the naltrexone (i.e., opioid antagonist) condition are presented in this article.
Human Subjects
All procedures were approved by the University of Wisconsin Health Sciences Institutional Review Board, and informed consent was obtained from each participant prior to data collection. A power analysis was performed to estimate an optimal sample size for detecting a potential difference between men and women in the effects of naltrexone (NAL) on EIH using a repeated measures design, with an alpha of 0.05, a power of 0.80, and a medium effect. Results from the analysis indicated that 44 participants (22 women and 22 men) would be needed for the study; however, the sample size was increased in anticipation of potential subject attrition.
Sixty healthy adults (30 women and 30 men) between age 18 and 40 years without a history of major medical conditions (e.g., clinical pain, hypertension, cancer) were recruited for participation in the study. Two participants did not complete testing: One woman experienced blood draw complications, and one man had trouble swallowing the capsule. Thus, the final sample consisted of 29 men and 29 women. Participants were asked to refrain from taking caffeine, nicotine, alcohol, or participating in vigorous exercise for at least four hours prior to arrival, as well as to refrain from taking over-the-counter pain medicine for 24 hours prior to arrival.
Experimental Sessions
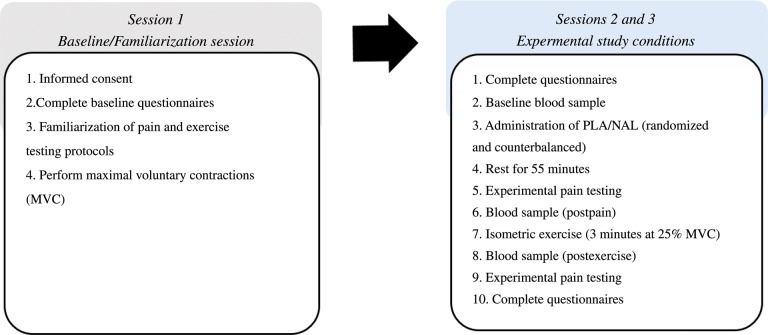
Participants completed a total of three sessions (Figure 1 contains a schematic of the experiemental procedures). The first session was a familiarization session in which participants completed informed consent and questionnaires and were introduced to the experimental pain and exercise protocols. Pain testing protocols involved inducing pain by delivering noxious pressure and thermal heat stimuli to the dominant hand. The isometric exercise intensity for the experimental sessions was determined by having participants perform two maximal voluntary contractions (MVCs) of the dominant arm by squeezing a hand-grip dynamometer for five seconds at maximum strength. The average of the two contractions was used as an estimated maximum when calculating the workload for the experimental sessions.
Figure 1.
Schematic of experimental procedures. NAL = naltrexone; PLA = placebo.
The following experimental sessions were conducted on two different days separated by at least 48 hours (mean time between visits = 56 hours). During these sessions, blood was drawn from the nondominant arm at baseline to examine eCB levels prior to completing baseline experimental pain testing. Next, participants were administered an opioid antagonist (50 mg naltrexone) or a placebo via a randomized, double-blind, counterbalanced procedure. Participants then sat quietly for 55 minutes before completing pain testing prior to exercise. Blood was immediately drawn after pain testing to examine eCB responses to pain testing (postpain). Participants then engaged in isometric handgrip exercise for three minutes at 25% MVC. Blood was drawn within five minutes after isometric exercise to examine changes in eCBs following exercise (postexercise). Experimental pain testing was conducted following the blood draw.
Statistics
All statistical analyses were conducted using IBM SPSS Statistics, version 22.0 (IBM Corp, Armonk, NY, USA). Descriptive statistics were computed for both the placebo and naltrexone conditions. Endocannabinoid (AEA, 2AG) and related biogenic lipid (OEA, PEA, 2OG) concentrations were analyzed with 2 (conditions: placebo, naltrexone) × 2 (time: % change from baseline to postpain testing, % change from postpain to postexercise) repeated measures analyses of variance (ANOVAs). ECB data were positively skewed and therefore did not meet the normality assumption (i.e., Shapiro-Wilk test). Therefore, a log-transformation (log10) of the eCB data was performed so that our data met the normality assumption required for conducting statistical analyses (ANOVA). For a more detailed description of the methods, see Koltyn et al. (2014) [13].
Results
Baseline Demographics
A total of 58 men and women, 21 ± 3 (mean ± SD) years of age, participated in three sessions and were included in the final analyses. Maximum grip strength (kg) and body mass index (BMI) were the only significant sex differences, with men having a higher average grip strength and higher BMI compared with women (see Table 1 for a demographic summary of the sample). Exercise-induced hypoalgesia (EIH), as previously reported [13], was found to occur in both placebo and naltrexone conditions, as evident by significant (P < 0.05) reductions in pain ratings (thermal and pressure) and significant (P < 0.05) increases in pain thresholds following exercise. These changes did not differ (P > 0.05) between men and women or between the placebo and naltrexone conditions.
Table 1.
Demographic characteristics of study sample
| Men | Women | |
|---|---|---|
| No. | 29 | 29 |
| Age, y | 21 ± 3 | 21 ± 3 |
| African American | 4 | 6 |
| Asian American | 6 | 2 |
| Latino | 2 | 4 |
| American Indian | 0 | 1 |
| Caucasian | 17 | 16 |
| Max. grip, kg | 38 ± 10 | 22 ± 7* |
| Body mass index, kg/m2 | 26.1 ± 4.9 | 22.2 ± 3.2* |
Values for age, max grip, and body mass index variables are listed as mean (SD). Values for race/ethnicity groups are listed as a frequency.
BMI = body mass index.
Indicates a significant sex difference in scores (P < 0.05).
eCB Responses Following Placebo and Naltrexone Conditions
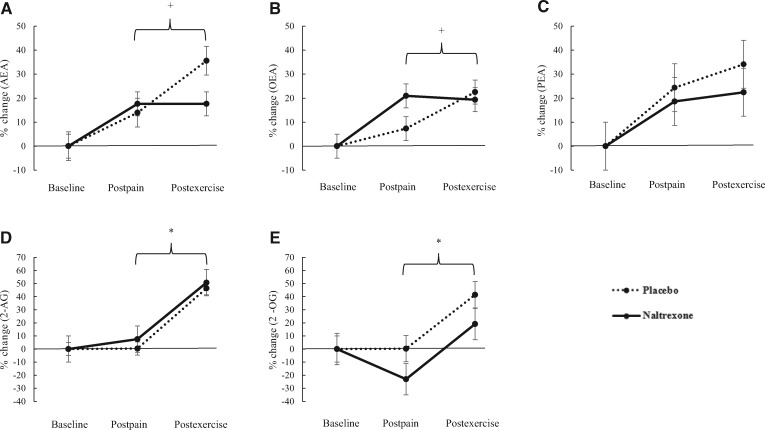
Results indicated significant main effects for time for 2-AG (P = 0.008) and 2-OG (P = 0.001), but the main effects for condition and the condition × time interactions were not significant (P > 0.05). Both 2-AG and 2-OG were found to increase significantly following exercise in both the placebo and naltrexone conditions. In comparison, there were significant condition × time interaction effects for AEA (P = 0.05) and OEA (P = 0.009), and post hoc testing revealed significant differences between placebo and naltrexone conditions in response to exercise for AEA (P = 0.001) and OEA (P = 0.01). There were significant increases in AEA and OEA following exercise in the placebo condition but not the naltrexone condition. There were no significant time, condition, or interaction effects (P > 0.05) for PEA (see Figure 2).
Figure 2.
Percent change in circulating concentrations of eCB and related lipids before and after pain testing and exercise in placebo and naltrexone conditions. Postpain % change values refer to the eCB response to pain testing as a percentage change from baseline plasma concentrations. Postexercise % change values refer to the eCB response to exercise as a percentage change from postpain testing plasma concentrations. A) Significant condition × time interaction effect (P < 0.05; denoted +) for AEA. B) Significant condition × time interaction effect (P < 0.05; denoted +) for OEA. C) No significant main effect for time or condition or significant condition × time interaction found for PEA. D) Significant main effect for time (P < 0.05, denoted *) found for 2-AG. E) Significant main effect for time (P < 0.05; denoted *) found for 2-OG. Error bars are ± SEM. 2AG = 2-arachidonoylglycerol; 2OG = 2-oleoylglycerol; AEA = N-arachidonylethanolamine; OEA = oleoylethanolamine; PEA = palmitoylethanolamine.
Discussion
The results from this research support the hypothesis that exercise produces EIH via an eCB mechanism. Also in support of this hypothesis, Galdino and colleagues conducted preclinical research and found that both aerobic [17] and resistance exercise [18] produced significant increases in pressure and thermal nociceptive thresholds, as well as significant increases in AEA, 2-AG, PEA, and OEA. In addition, systemic and central pretreatment with eCB-metabolizing enzyme inhibitors prolonged the antinociceptive response, while pretreatment with CB1 and CB2 receptor antagonists blocked the antinociceptive response following exercise. Importantly, both aerobic and resistance exercise produced increases in the expression and activation of CB1 receptors in the dorsolateral and ventrolateral periaqueductal brain regions of the exercised rats. Moreover, Fuss and colleagues examined eCB mechanisms by injecting mice with eCB or opioid antagonists before wheel running. Exercise-induced antinociception was found to still be evident following administration of the opioid antagonist but not following administration of central and peripheral CB1 and CB2 receptor antagonists. Thus, exercise-induced antinociception appeared to be mediated by central and peripheral CB1 and CB2 receptors [19].
The results from the present study extend the findings from the previous research by examining the interaction between eCB and opioid systems. The results indicated differential eCB responses to exercise following administration of an opioid antagonist. In the placebo condition, AEA and 2-AG were found to increase significantly following exercise; however, in the naltrexone condition, 2-AG increased significantly following exercise whereas AEA did not. It is interesting that exercise-induced increases in AEA were blocked by pretreatment with naltrexone, a nonspecific opiate receptor antagonist. These results suggest that opiate receptor activation during exercise elevates AEA concentrations. There are no direct reports that opiate receptor activity can regulate AEA, though there are several studies in preclinical models that could be interpreted as reflecting mu opiate receptor–induced recruitment of AEA-mediated signaling [20,21]. Our current hypothesis is that the period and intensity of exercise employed in this study elevated AEA concentrations a small amount subsequent to opiate receptor activation. However, the increase in AEA was not sufficient to induce EIH. On the other hand, 2-AG concentrations are elevated by isometric exercise through a mechanism that does not involve opiate receptors (as it was not affected by naltrexone), and 2-AG concentrations rise high enough to produce EIH. Further studies are required to test these hypotheses.
As EIH occurred in both the placebo and naltrexone conditions, these results suggest a nonopioid mechanism of EIH following isometric exercise. 2-AG (and 2-OG) concentrations increased by nearly twofold and were unaffected by opioid antagonist pretreatment, suggesting that 2-AG in particular is the eCB involved in this model of EIH. 2-OG and 2-AG have overlapping mechanisms of synthesis and degradation, such that their concentrations often increase and decrease together [22], and results from this study confirm this relationship. The same is true for the N-acylethanolamines (NAEs) with regards to overlapping mechanisms of synthesis and degradation, and results from this study indicated AEA and OEA responded in a similar manner but PEA did not. The regulation of NAE synthesis is not completely understood, but some suggested mechanisms demonstrate specificity for acyl chain saturation [23]. Thus, it is our current hypothesis that one of the opiate receptors that is activated during exercise couples to the synthesis of AEA and OEA via a mechanism that is selective for the synthesis of unsaturated NAEs such as OEA and AEA over saturated NAEs like PEA.
There are several limitations associated with this study. First, the focus of this study was on eCB and opioid mechanisms of EIH. This does not rule out other potential mechanisms such as other neurotransmitter systems, nitric oxide, and psychosocial mechanisms [24–27]. Second, the current study focused on healthy, young adults and one session of isometric exercise, so the results cannot be generalized to chronic pain patients or to an exercise training program (i.e., multiple exercise sessions over time). Finally, the sample size of this study (N = 58) may have been limited but was large enough to be able to detect moderate to large changes in the outcome variables.
In sum, this study offers insight regarding the interactions that may exist between neurobiological systems involved in endogenous pain modulation and suggests that future research should be designed to tease out the nuances of these systems in relation to one another rather than studying potential mechanisms in isolation. Further examination of these systems and differential eCB responses to exercise is necessary to help clarify eCB and opioid system interactions so that future therapeutic treatments can offer effective pain relief through optimal engagement of both of these systems.
References
- 1. Hohmann AG, Suplita RL.. Endocannabinoid mechanisms of pain modulation. AAPS J 2006;84:E693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hill MN, Gorzalka BB.. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol Disord Drug Target 2009;86:451–8. [DOI] [PubMed] [Google Scholar]
- 3. Katona I, Freund TF.. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci 2012;35:529–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herkenham M, Lynn AB, Johnson MR, et al. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci 1991;112:563–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM.. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 1998;832:393–411. [DOI] [PubMed] [Google Scholar]
- 6. Guindon J, Beaulieu P.. The role of the endogenous cannabinoid system in peripheral analgesia. Curr Mol Pharmacol 2009;21:134–9. [DOI] [PubMed] [Google Scholar]
- 7. Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol 2001;635:569–611. [DOI] [PubMed] [Google Scholar]
- 8. Reis GM, Pacheco D, Perez AC, et al. Opioid receptor and NO/cGMP pathway as a mechanism of peripheral antinociceptive action of the cannabinoid receptor agonist anadamide. Life Sci 2009;859:351–6. [DOI] [PubMed] [Google Scholar]
- 9. Pachocho D, Klein A, Perez AC, et al. Central antinociception induced by mu-opioid receptor agonist morphine, but not delta- or kappa-, is mediated by cannabinoid CB1 receptor. Br J Pharmacol 2009;1581:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pachoco D, Klein A, Perez A, et al. The µ-opioid receptor agonist morphine, but not agonists at gabba- or delta-opioid receptors, induces peripheral antinociception mediated by cannabinoid receptors. Br J Pharmacol 2008;1545:1143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Welch SP. Interaction of the cannabinoid and opioid systems in the modulation of nociception. Int Rev Psychiatry 2009;212:143–51. [DOI] [PubMed] [Google Scholar]
- 12. Koltyn KF. Analgesia following exercise: A review. Sports Med 2000;292:85–98. [DOI] [PubMed] [Google Scholar]
- 13. Koltyn KF, Brellenthin AG, Cook DB, Sehgal N, Hillard CJ.. Mechanisms of exercise-induced hypoalgesia. J Pain 2014;1512:1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thoren P, Floras JS, Hoffmann P, Seals DR.. Endorphins and exercise: Physiological mechanisms and clinical implications. Med Sci Sports Exer 1990;224:417–28. [PubMed] [Google Scholar]
- 15. van Middelkoop M, Rubinstein SM, Kuipers T, et al. A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur Spine J 2011;201:19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haller VL, Stevens DL, Welch SP.. Modulation of opioids via protection on anandamide degradation by fatty acid hydrolase. Eur J Pharmacol 2008;6001:50–8. [DOI] [PubMed] [Google Scholar]
- 17. Galdino G, Romero TR, Silva JF, et al. The endocannabinoid system mediates aerobic exercise-induced antinociception in rats. Neuropharmacology 2014;77:313–24. [DOI] [PubMed] [Google Scholar]
- 18. Galdino G, Romero T, Silva JF, et al. Acute resistance exercise induces antinociception by activation of the endocannabinoid system in rats. Anesth Analg 2014;1193:702–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fuss J, Steinle J, Bindila L, et al. A runner’s high depends on cannabinoid receptors in mice. Proc Natl Acad Sci U S A 2015;11242:13105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Butler RK, Rea K, Lang Y, Gavin AM, Finn DP.. Endocannabinoid-mediated enhancement of fear-conditioned analgesia in rats: Opioid receptor dependancy and molecular correlates. Pain 2008;1403:491–500. [DOI] [PubMed] [Google Scholar]
- 21. Jhaveri MD, Richardson D, Kendall DA, Barrett DA, Chapman V.. Analgesic effects of fatty acid amide hydrolase inhibition in a rat model of neuropathic pain. J Neurosci 2006;2651:13318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hillard CJ. Endocannabinoids and vascular function. J Pharmacol Exp Ther 2000;2941:27–32. [PubMed] [Google Scholar]
- 23. Simon GM, Cravatt BF.. Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo. Mol Biosyst 2010;68:1411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Souza GG, Duarte ID, de Castro Perez A.. Differential involvement of central and peripheral alpha-2 adrenoreceptors in the antinocicpetion induced by aerobic and resistance exercise. Anesth Analg 2013;1163:703–11. [DOI] [PubMed] [Google Scholar]
- 25. Mazzardo-Martins L, Martins DF, Marcon R, et al. High-intensity extended swimming exercise reduces pain-related behavior in mice: Involvement of endogenous opioids and the serotonergic system. J Pain 2010;1112:1384–93. [DOI] [PubMed] [Google Scholar]
- 26. Galdino GS, Cortes SF, Duarte ID, Perez AC.. Involvement of the nitric oxide/(C)GMP/K(ATP) pathway in antinociception induced by exercise in rats. Life Sci 2010;8613:505–9. [DOI] [PubMed] [Google Scholar]
- 27. Brellenthin AG, Crombie KM, Cook DB, Sehgal N, Koltyn KF.. Psychosocial influences on exercise-induced hypoalgesia. Pain Med 2017;183:538–50. [DOI] [PMC free article] [PubMed] [Google Scholar]