Abstract
Although endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA) has been reported to be a minimally invasive and relatively safe procedure, mediastinitis is a serious complication related to the procedure. The median time of mediastinitis onset is approximately 12 days after EBUS‐TBNA. Here we report two rare cases with mediastinitis onset 40 and 53 days after EBUS‐TBNA. Surgical drainage was performed since systemic treatment with antibiotics was insufficient in both cases. Eikenella corrodens, which is a slow‐growing microorganism, was identified as the causative pathogen in one case. To our knowledge, this is the first report of mediastinitis occurring over a month after EBUS‐TBNA. Clinicians should consider the diagnosis of mediastinitis even if symptoms appear over a month after EBUS‐TBNA.
Keywords: Complication, Eikenella corrodens, endobronchial ultrasound‐guided transbronchial needle aspiration, mediastinitis
Introduction
Endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA) has been widely used and is currently the standard method for the assessment of mediastinal and hilar lymphadenopathy 1. This procedure can be performed for multiple mediastinal and hilar lymph nodes under moderate sedation, even on an outpatient basis 2. Although EBUS‐TBNA has been reported to be a minimally invasive and relatively safe procedure, complications have been gradually reported 3. Some of these complications can be serious, with mediastinitis presenting as an important acute or sub‐acute complication requiring precise diagnosis and treatment 4, 5, 6. The median time of mediastinitis onset after EBUS‐TBNA, estimated by these previous reports, was 12.5 days (interquartile range: 2.75–14.5 days) 4, 7, 8, 9, 10, 11, 12, 13. Here we describe two rare cases in which severe mediastinitis occurred over a month after EBUS‐TBNA.
Case Reports
Case 1
A 67‐year‐old woman with an unremarkable medical history was referred to our hospital for evaluation of a cystic mass with a 55 mm diameter in the middle mediastinum (Fig. 1A). EBUS‐TBNA was performed for diagnosis, and histopathological analysis revealed only mucoid substances without malignancy or bacteria; therefore, no treatment was administered. The patient returned 48 days later with complaints of right‐sided back pain and dysphasia for a week. On admission, she exhibited general malaise, an increased body temperature of 37.5°C, and an oxygen saturation of 97% on room air. Physical examination demonstrated no lymphadenopathy or cardiopulmonary abnormalities. Laboratory examinations revealed an increased white blood cell (WBC) count (10,500/mm3) and C‐reactive protein (CRP) level (8.9 mg/dL). A chest radiograph showed increased mediastinal widening, and chest computed tomography (CT) demonstrated enlargement of the mediastinal mass that compressed the airway (Fig. 1B). Intravenous antibiotic treatment (flomoxef, 2 g/day) was initiated; however, her abnormal laboratory test findings deteriorated. Three days after admission, she underwent right posterolateral thoracotomy for both drainage and release of the airway compression. A cloudy yellow fluid containing Eikenella corrodens, which is normal oral flora, was obtained via drainage. The organism was sensitive to ampicillin, ampicillin/sulbactam, cefotiam, meropenem, levofloxacin, and trimethoprim/sulfamethoxazole, but resistant to tetracycline. The patient recovered and was discharged after two weeks of post‐operative antibiotic therapy (tazobactam/piperacillin, 13.5 g/day).
Figure 1.
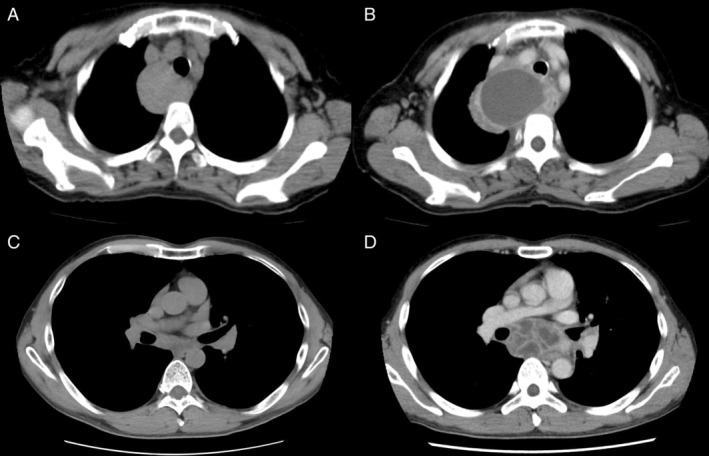
Chest computed tomography (CT) in case 1 (A, B) and in case 2 (C, D) before (A, C) and after (B, D) endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA), respectively.
Case 2
A 47‐year‐old man presented at our hospital for further examination of bilateral hilar and mediastinal lymphadenopathy (Fig. 1C). He underwent EBUS‐TBNA for the subcarinal lymph nodes (#7), and histopathological analysis showed epithelioid granulomas without caseous necrosis, which was consistent with a diagnosis of sarcoidosis. The patient developed high fever, chest pain, and headache 53 days after EBUS‐TBNA. Oral antibiotic therapy (levofloxacin, 500 mg/day) was prescribed by a general physician, but his symptoms persisted, and he was readmitted to our hospital. On examination, he exhibited general malaise, an increased body temperature of 37.1°C, and an oxygen saturation of 98% on room air. Pulmonary and cardiovascular physical examinations showed normal findings. Laboratory tests showed an increased WBC count (21,200/mm3) and CRP level (11.9 mg/dL), and chest CT showed enlargement of the subcarinal lymph node with a low‐density area (Fig. 1D). Intravenous antibiotic therapy (tazobactam/piperacillin, 18 g/day) was initiated and surgical drainage was performed on day 2 of hospitalization. The abscess was drained via right mediastinotomy, and the drained fluid exhibited white purulent material. No bacteria were detected. His post‐operative course was uneventful, and he was discharged 14 days after hospitalization.
Discussion
In this report, we have described, to the best of our knowledge, the first two cases of mediastinitis that occurred over a month after EBUS‐TBNA. The two cases reported here convey two important messages. First, mediastinitis can occur over a month after EBUS‐TBNA. Second, E. corrodens must be considered as one of the pathogenic bacteria that can cause this complication.
EBUS‐TBNA is becoming increasingly popular and has almost replaced mediastinoscopy as a diagnostic and staging modality for both benign and malignant mediastinal and lung masses or adenopathy 14. This is because its diagnostic sensitivity ranges from 85% to 100%, similar to that of mediastinoscopy 15. Moreover, the complication rate for EBUS‐TBNA is 1.02%, which is considerably lower than that for mediastinoscopy (2%–3%) 3, 16. Common complications of EBUS‐TBNA include infection, haemorrhage, pneumothorax, and respiratory failure 3. In Japan, the reported incidence of infectious complications after EBUS‐TBNA was 0.19%, with mediastinitis accounting for 0.10% cases 17. Mediastinitis is a rare but important complication because it can occur as a progressive and occasionally life‐threatening condition requiring surgical intervention 4, 5.
We searched the PubMed database for reported cases of mediastinitis after EBUS‐TBNA and retrieved a total of 13 cases 4, 7, 8, 9, 10, 11, 12, 13. In addition to the two cases reported here, the patient characteristics, clinical courses, pathogens, and treatments for these 13 cases and a case treated at our hospital are shown in Table 1 (n = 16). The median time of mediastinitis onset after EBUS‐TBNA, estimated by these previous reports, was 12.5 days (interquartile range: 2.75–14.5 days) 4, 7, 8, 9, 10, 11, 12, 13. The most common pathogens were Streptococcus spp. (four of nine cases), which are considered normal oral flora, suggesting that infection is caused via the contaminated needle of TBNA. Surgical intervention was required for 10 of 16 cases in whom systemic antibiotic therapy proved inadequate.
Table 1.
Patients characteristics, clinical course, pathogens, and treatments for previous cases of mediastinitis after EBUS‐TBNA.
| Age/sex | Antibiotic treatment before/after EBUS‐TBNA | Final diagnosis by EBUS‐TBNA | Number of days after EBUS‐TBNA | Treatment | Pathogen | Reference |
|---|---|---|---|---|---|---|
| 72/M | None | Necrotic tissue | 2 days | Surgery | Group C Streptococcus | 7 |
| 68/M | None | Lung cancer (SCLC) | 2 days | Antibiotic therapy | NR | 8 |
| 58/F | None | Lung cancer (SQ) | 2 days | Antibiotic therapy | NR | 9 |
| 33/F | None | Cyst | 3 days | Surgery | Staphylococcus epdermidis | 10 |
| 51/M | None | Pneumoconiosis | 3 days | Antibiotic therapy | Gamella morbillorum | 11 |
| 51/M | None | Pneumoconiosis | 3 days | Antibiotic therapy | Gamella morbillorum | 11 |
| 73/M | None | Lung cancer (SQ) | 11 days | Antibiotic therapy | NR | 12 |
| 70/F | None | Lung cancer (ADC) | 14 days | Surgery | Strepctococcus intermedius | Our hospital case |
| 64/M | None | Metastatic lung cancer(colon cancer) | 14 days | Surgery | NR | 13 |
| 49/M | None | Granuloma | 14 days | Surgery | Gemella mobillorum | 13 |
| 36/M | None | Sarcoidosis | 16 days | Surgery |
Prevotella buccae, Streptococcus anginosus, Actinomyces |
13 |
| 50/M | None | Lung cancer (ADC) | 19 days | Surgery |
Actinomyces odontolyticus, Streptococcus mutans |
9 |
| 48/M | None | NR | 21 days | Surgery | Klebsiella pneumoniae | 4 |
| 67/F | None | Bronchogenic cyst | 40 days | Surgery | Eikenella corrodens | Case 1 |
| 47/M | None | Sarcoidosis | 53 days | Surgery | None | Case 2 |
| 59/M | NR | NR | NR | Antibiotic therapy | NR | 18 |
| 67/M | NR | NR | NR | Antibiotic therapy | NR | 18 |
Abbreviations: ADC, adenocarcinoma; CLDM, clindamycin; EBUS‐TBNA, endobronchial ultrasound‐guided transbronchial needle aspiration; NR, not reported; SCLC, small cell lung carcinoma; SQ, squamous cell carcinoma.
In the present cases, mediastinitis developed 40 and 53 days after EBUS‐TBNA. This indicates that mediastinitis must be considered even if patients present with symptoms over a month after EBUS‐TBNA. Although the precise reason for the late onset is unclear, one hypothesis is that the inflammation in the patients was restricted (e.g. cyst); hence, a systemic inflammatory response was not evoked. Another hypothesis is that the condition was caused by a low‐virulence pathogen, which resulted in slow disease development. In particular, E. corrodens detected in case 1, which can lead to pneumonia or empyema even in immunocompetent patients, is a slow‐growing microorganism that appears to be indolent 19. Although the characteristics of the pathogen may be related to the time of onset of mediastinitis, further investigation is necessary to confirm such an association.
Currently, there is no consensus regarding prophylactic measures for infectious complications after EBUS‐TBNA. Randomized studies have revealed that the use of prophylactic antibiotics after conventional diagnostic bronchoscopy did not alter the frequency of infectious events 20, 21. Therefore, the British Thoracic Society Guidelines for diagnostic and therapeutic bronchoscopy does not recommend antibiotic prophylaxis for the prevention of infection. While we have previously reported that antibiotic prophylaxis was unrelated to the development of fever after the procedure even in EBUS‐TBNA 22, the preventive effects of prophylactic antibiotics against the infectious complications like a mediastinitis associated with EBUS‐TBNA remain uncertain. Some possible factors associated with the development of mediastinitis after EBUS‐TBNA have been reported as follows: number of times the needle is passed into the lesion, number of samples obtained per lesion, operator expertise, presence of necrotic or cystic lesions, and bacterial contamination of the working channel 13. Although there is no strong evidence supporting these factors, there are some reports of mediastinitis after EBUS‐TBNA of cystic lesions 7, 10. Considering case 1 and other reports, we no longer perform EBUS‐TBNA for cystic lesions. Additionally, to identify whether the lesion is cystic, magnetic resonance imaging or contrast enhanced CT may be considered prior to the examination. Additional investigation to detect the risk factors involving procedures and comorbidity may contribute to prevention of infectious complications after EBUS‐TBNA.
In conclusion, we described two cases of severe mediastinitis with delayed onset after EBUS‐TBNA that were successfully treated using a surgical approach. The findings from these cases suggest that early diagnosis is essential for appropriate treatment. Clinicians should consider mediastinitis even if symptoms appear over a month after EBUS‐TBNA. Moreover, identification of the pathogenic organism is important for appropriate treatment.
Disclosure Statement
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Acknowledgments
We thank Hiroaki Motomura, Kimiko Horikoshi, Yasuhito Sekimoto, Shoichi Okamoto, and Haruhi Takagi for their assistance.
Kurokawa, K , Asao, T , Ko, R , Nagaoka, T , Suzuki, K , Takahashi, K . (2019) Severe mediastinitis over a month after endobronchial ultrasound‐guided transbronchial needle aspiration. Respirology Case Reports, 7(5), ;e00426. 10.1002/rcr2.426
Associate Editor: Arata Azuma
References
- 1. Yasufuku K, Chiyo M, Sekine Y, et al. 2004. Real‐time endobronchial ultrasound‐guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 126:122–128. [DOI] [PubMed] [Google Scholar]
- 2. Navani N, Nankivell M, Lawrence DR, et al. 2015. Lung cancer diagnosis and staging with endobronchial ultrasound‐guided transbronchial needle aspiration compared with conventional approaches: an open‐label, pragmatic, randomised controlled trial. Lancet Respir. Med. 3:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaidya PJ, Munavvar M, Leuppi JD, et al. 2017. Endobronchial ultrasound‐guided transbronchial needle aspiration: safe as it sounds. Respirology 22:1093–1101. [DOI] [PubMed] [Google Scholar]
- 4. Parker KL, Bizekis CS, and Zervos MD. 2010. Severe mediastinal infection with abscess formation after endobronchial ultrasound‐guided transbrochial needle aspiration. Ann. Thorac. Surg. 89:1271–1272. [DOI] [PubMed] [Google Scholar]
- 5. Moffatt‐Bruce SD, and Ross P Jr. 2010. Mediastinal abscess after endobronchial ultrasound with transbronchial needle aspiration: a case report. J. Cardiothorac. Surg. 5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yokoyama Y, Nakagomi T, Shikata D, et al. 2017. Surgical treatment for mediastinal abscess induced by endobronchial ultrasound‐guided transbronchial needle aspiration: a case report and literature review. World J. Surg. Oncol. 15:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsuoka K, Ito A, Murata Y, et al. 2015. Severe mediastinitis and pericarditis after transbronchial needle aspiration. Ann. Thorac. Surg. 100:1881–1883. [DOI] [PubMed] [Google Scholar]
- 8. Aerts JG, Kloover J, Los J, et al. 2008. EUS‐FNA of enlarged necrotic lymph nodes may cause infectious mediastinitis. J. Thorac. Oncol. 3:1191–1193. [DOI] [PubMed] [Google Scholar]
- 9. Haas AR. 2009. Infectious complications from full extension endobronchial ultrasound transbronchial needle aspiration. Eur. Respir. J. 33:935–938. [DOI] [PubMed] [Google Scholar]
- 10. Mogal R, Banerjee N, Yung B, et al. 2016. A young woman with severe chest pain after undergoing endobronchial ultrasound‐guided transbronchial needle aspiration for a large mediastinal mass. J. Bronchology Interv. Pulmonol. 23:236–238. [DOI] [PubMed] [Google Scholar]
- 11. Sanchez‐Font A, Alvarez L, Ledesma G, et al. 2015. Localized subcarinal adenitis following endobronchial ultrasound‐guided transbronchial needle aspiration. Respiration 90:329–331. [DOI] [PubMed] [Google Scholar]
- 12. Fukunaga K, Kawashima S, Seto R, et al. 2015. Mediastinitis and pericarditis after endobronchial ultrasound‐guided transbronchial needle aspiration. Respirol. Case Rep. 3:16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Voldby N, Folkersen BH, and Rasmussen TR. 2017. Mediastinitis: a serious complication of endobronchial ultrasound‐guided transbronchial needle aspiration. J. Bronchology Interv. Pulmonol. 24:75–79. [DOI] [PubMed] [Google Scholar]
- 14. Vaidya PJ, Kate AH, and Chhajed PN. 2013. Endobronchial ultrasound‐guided transbronchial needle aspiration: the standard of care for evaluation of mediastinal and hilar lymphadenopathy. J. Cancer Res. Ther. 9:549–551. [DOI] [PubMed] [Google Scholar]
- 15. Varela‐Lema L, Fernandez‐Villar A, and Ruano‐Ravina A. 2009. Effectiveness and safety of endobronchial ultrasound‐transbronchial needle aspiration: a systematic review. Eur. Respir. J. 33:1156–1164. [DOI] [PubMed] [Google Scholar]
- 16. Hammoud ZT, Anderson RC, Meyers BF, et al. 1999. The current role of mediastinoscopy in the evaluation of thoracic disease. J. Thorac. Cardiovasc. Surg. 118:894–899. [DOI] [PubMed] [Google Scholar]
- 17. Asano F, Aoe M, Ohsaki Y, et al. 2013. Complications associated with endobronchial ultrasound‐guided transbronchial needle aspiration: a nationwide survey by the Japan Society for Respiratory Endoscopy. Respir. Res. 14:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kayawake H, Chen‐Yoshikawa TF, Oda H, et al. 2017. Complications of endobronchial ultrasound‐guided transbronchial needle aspiration. Ann. Thorac. Surg. 104:e363–e365. [DOI] [PubMed] [Google Scholar]
- 19. Sheng WS, Hsueh PR, Hung CC, et al. 2001. Clinical features of patients with invasive Eikenella corrodens infections and microbiological characteristics of the causative isolates. Eur. J. Clin. Microbiol. Infect. Dis. 20:231–236. [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto M, Nagano T, Okuno K, et al. 2012. An open‐label, prospective clinical study to evaluate the efficacy of prophylactic antibiotics after diagnostic bronchoscopy. Kobe J. Med. Sci. 58:E110–E118. [PubMed] [Google Scholar]
- 21. Park JS, Lee CH, Yim JJ, et al. 2011. Impact of antibiotic prophylaxis on postbronchoscopy fever: a randomised controlled study. Int. J. Tuberc. Lung Dis. 15:528–535. [DOI] [PubMed] [Google Scholar]
- 22. Takagi H, Nagaoka T, Ando K, et al. 2017. Efficacy of antibiotic prophylaxis after endobronchial ultrasound‐guided transbronchial needle aspiration: a preliminary prospective study. J. Pulm. Respir. Med. 7: 416. [Google Scholar]


