Abstract
Introduction
To assess the cost-effectiveness of stent-retriever mechanical thrombectomy and intravenous tissue plasminogen activator compared with intravenous tissue plasminogen activator alone in patients with acute ischaemic stroke due to large vessel occlusions in Spain.
Materials and methods
Clinical data were taken from the SWIFT PRIME clinical trial. A lifetime Markov state transition model defined by the modified Rankin Scale score was developed to estimate costs and health outcomes (life years gained and quality adjusted life years). A Spanish National Health System perspective (direct medical costs) was considered. Resource utilisation and utilities were obtained from available published data and endorsed by an expert panel. Costs (€, 2016) were obtained from various Spanish sources. Deterministic and probabilistic sensitivity analyses were performed.
Results
Stent-retriever thrombectomy after intravenous tissue plasminogen activator was associated with better outcomes (1.17 life years gained and 2.51 quality adjusted life years) and savings of €44,378, resulting in a dominant therapy over intravenous tissue plasminogen activator alone. A net monetary benefit of €119,744 was obtained considering a willingness-to-pay threshold of €30,000/quality adjusted life year gained. The combined therapy was also dominant in all sensitivity analyses, deterministic and probabilistic.
Discussion
The results were consistent with a previously published cost-effectiveness analysis and reinforce the likeliness of the selection of stent-retriever mechanical thrombectomy plus intravenous tissue plasminogen activator over intravenous tissue plasminogen activator alone.
Conclusion
Stent-retriever thrombectomy after intravenous tissue plasminogen activator is a dominant alternative over intravenous tissue plasminogen activator alone (more effective and less costly) for the treatment of acute ischaemic stroke patients with large vessel occlusions in the Spanish setting.
Keywords: Acute ischaemic stroke, cost-effectiveness, mechanical thrombectomy, stent retriever, thrombolytic therapy, tissue plasminogen activator
Introduction
Global stroke burden is increasing, and it is one of the largest contributors to disability and mortality worldwide.1 In 2014, stroke was the most common cause of death in women and the third most common cause in men in Spain,2 and it is related to a substantial economic burden as a result of high hospitalisation costs and long-term care due to stroke-related disability. A recent study showed that the one-year follow-up direct healthcare cost per patient with ischaemic stroke admitted to stroke units in Spain was €8,623, out of an overall cost of €27,597, mostly due to informal care.3 Another study estimated the cost of acute management and rehabilitation of cardioembolic stroke patients in €13,139, with the highest costs related to hospital stay and rehabilitation therapies.4
For the last 20 years, the only evidence-based therapy for acute ischaemic stroke was intravenous tissue plasminogen activator (IV t-PA), administered within 3 h or up to 4.5 h after ischaemic stroke.5,6 Recent randomised clinical trials demonstrated the efficacy of adding mechanical thrombectomy to IV t-PA,7–11 which led European scientific societies to recommend mechanical thrombectomy, primarily with stent retrievers, after intravenous thrombolysis within 4.5 h if eligible, for the treatment of acute stroke patients with large artery occlusions in the anterior circulation within 6 h12 or even up to 8 h after symptom onset.11
The Solitaire Revascularisation Device is a stent retriever for mechanical thrombectomy in acute stroke treatment, which was the only or the most used device in four of these recent clinical trials.8–11 Recent meta-analyses assessed studies in which stent retrievers (mostly Solitaire) were used and showed that acute ischaemic stroke patients treated with a stent retriever after IV t-PA experienced significant improvement in independent functional outcomes (modified Rankin Scale [mRS] 0–2) at 90 days13,14 and an increased likelihood of complete recanalisation/reperfusion in comparison with IV t-PA alone.15
Apart from clinical evidence, it is also necessary to assess cost-effectiveness so that decision-makers have complete information to include new therapies in our health system. The aim of this analysis was to evaluate the cost-effectiveness of mechanical thrombectomy with a stent-retriever device (Solitaire) after IV t-PA compared to IV t-PA alone in acute ischaemic stroke patients with confirmed occlusions in the proximal anterior intracranial circulation and absence of large ischaemic core lesions in Spain.
Material and methods
Model structure
A Markov model was designed to represent the evolution of acute ischaemic stroke patients according to the treatment received: mechanical thrombectomy with Solitaire stent retriever after IV t-PA and IV t-PA alone. It was based on the pragmatic review of previous models in stroke care in consultation with clinical experts, with a similar structure to the model used in the United Kingdom technology appraisal of alteplase.16
The model included seven health states describing the different degrees of disability defined by the mRS scores (0, no symptoms; 6, death). During each cycle, in a fixed period of time during the follow-up, patients transited from one health state to another or remained in the same state. The model had a two-phase structure: an acute phase and a rest-of-life phase (Figure 1). The acute phase described patients’ management and outcomes from stroke onset to 90 days. All treatment effects were assumed to occur within this phase. Patients were then assigned a mRS score at 90 days after stroke. To allow for half-cycle correction, patients were also previously assigned a mRS score at 7–10 days after stroke or at discharge. A half-cycle correction is often used to compensate for patients’ transitions from one health state to another, which usually occurs at the beginning or end of a cycle, whereas transitions actually occur in the middle of each cycle on average.17 The rest-of-life phase directly followed the acute phase from 91 days after stroke to the end of patients’ life. This phase included two different cycle lengths: from day 91 to a year after stroke and one-year period afterwards.
Figure 1.
Markov model structure.
During the acute phase, it was assumed that patients were at no risk of recurrent stroke, whereas in the rest-of-life phase, a patient remained in the same mRS health state as that at 90 days until either a recurrent stroke or death occurred.18 If a recurrence occurred, patients entered the acute phase again. In this case, transitions to health states were restricted to mRS scores equal to or greater than the previous mRS score. Risk of recurrent stroke was the same across all mRS scores with a maximum of one recurrent stroke per cycle.
Patient population
The acute ischaemic stroke patients included in the model had confirmed occlusions in the proximal anterior intracranial circulation and absence of large ischaemic core lesions and had to be able to undergo mechanical thrombectomy within 6 h from symptom onset. Patients’ characteristics were based on the SWIFT PRIME trial10 population and a starting age of 66.
Clinical effectiveness
Clinical effectiveness, assessed by mRS scores at 7–9 days after stroke or at discharge and at 90 days, was obtained from the SWIFT PRIME clinical trial, in which both treatment options were compared.10 During the acute phase, patients could be at risk of various adverse events: symptomatic intracranial haemorrhage and malignant cerebral oedema requiring craniectomy. Adverse event rates were obtained from a study on Spanish patients with acute ischaemic stroke treated with thrombectomy (REVASCAT study).11 In the rest-of-life phase, an annual recurrence rate of 4.91% was considered in the period comprising 90 days to one year after stroke and a 2.01% for the following years.19 Age-specific mortality was applied to patients in the rest-of-life phase one year after stroke, according to Spanish mortality tables.20 Other-cause mortality was adjusted to reflect the higher rates observed in stroke survivors, by the use of relative risks of dying by mRS.21 Efficacy data considered in the model can be found in Table 1.
Table 1.
Clinical efficacy data and utility values.
| mRS score | mRS 0 | mRS 1 | mRS 2 | mRS 3 | mRS 4 | mRS 5 | mRS 6 |
|---|---|---|---|---|---|---|---|
| mRS score at 7–10 days after stroke or discharge 10 | |||||||
| Solitaire® + IV t-PA (n = 98) | 16.33% | 19.39% | 9.18% | 11.22% | 14.29% | 27.55% | 2.04% |
| IV t-PA (n = 93) | 5.38% | 7.53% | 6.45% | 10.75% | 24.73% | 39.78% | 5.38% |
| mRS score at 90 days after stroke 10 | |||||||
| Solitaire® + IV t-PA (n = 98) | 17.35% | 25.51% | 17.35% | 12.24% | 15.31% | 3.06% | 9.18% |
| IV t-PA (n = 93) | 8.60% | 10.75% | 16.13% | 17.20% | 21.51% | 12.90% | 12.90% |
|
| |||||||
| Relative risk of dying23 | 1.00 | 1.00 | 1.12 | 1.66 | 1.92 | 2.57 | – |
|
| |||||||
| Base case values 24,25 | – | ||||||
| Time trade off | 0.936 | 0.817 | 0.681 | 0.558 | 0.265 | −0.054 | – |
| Sensitivity Analysis 35 | – | ||||||
| VAS | 0.90 | 0.68 | 0.47 | 0.20 | 0.07 | −0.02 | – |
| Double gamble | 0.90 | 0.78 | 0.48 | 0.26 | −0.04 | −0.72 | – |
|
| |||||||
| Recurrence Risk21 | |||||||
|
| |||||||
| From 90 days to 1 year after stroke | 4.91% | ||||||
| From 1 year after stroke | 2.01% | ||||||
|
| |||||||
| Patient age10 | 66 | ||||||
|
| |||||||
| Adverse events | Base case value11 (REVASCAT) | Sensitivity Analysis10 (SWIFT-PRIME) | |||||
|
| |||||||
| Mechanical thrombectomy + IV t-PA | |||||||
| Symptomatic intracranial haemorrhage | 1.94% | 0.00% | |||||
| Malignant cerebral oedema with craniectomy | 2.91% | 2.60% | |||||
| IV t-PA alone | |||||||
| Symptomatic intracranial haemorrhage | 1.94% | 3.09% | |||||
| Malignant cerebral oedema with craniectomy | 5.83% | 2.60% | |||||
IV t-PA: intravenous tissue plasminogen activator; mRS: modified Rankin scale; VAS: visual analogue scale.
Quality of life
The quality-adjusted life year (QALY) is a measure which reflects both quantity and quality of life lived, calculated by weighting the life years gained (LYG) by the utility value. Utilities were assigned to health states and are often expressed as a numerical scale with extreme values of 0 (death) and 1 (optimal health). Negative utilities would indicate that living in this state is considered worse than death. Utility values for each health state, described in Table 1, were based on patients in the Oxford Vascular Study.22,23
Costs
A Spanish National Health System (NHS) perspective was considered. Therefore, only direct medical costs were included, comprising acute and long-term stroke management, treatment (mechanical thrombectomy, IV t-PA and non-thrombolytic treatment) and adverse event management costs. Alternative scenarios also considered formal care costs (nursing/residential care costs). Costs were expressed in euros of year 2016. An annual discount rate of 3.0% was applied to costs and health outcomes.24 Unit costs were obtained from available published sources, Spanish healthcare cost databases and market prices and endorsed by an expert panel of neurologists and interventional neuroradiologists.25,27–30 Acute, long-term management and formal care cost were broken down by mRS score28,29 (Table 2). However, available costs were reported using various categories (minor [mRS 0–2] or major stroke [mRS 3–5] for acute management costs and by dependency level [Barthel index score] for long-term management costs). Thus, available costs were weighted by ±10%, as necessary, to allow to discriminate acute costs between health states. Long-term management costs by dependency level were translated into their corresponding mRS value according to a categorisation scheme.31 Treatment costs were obtained from unit costs and resource use based on current clinical practice provided by the expert panel (Table 3). Non-thrombolytic treatment was included in both thrombectomy + IV t-PA (€8,680.66) and IV t-PA total treatment costs (€1,654.48).
Table 2.
Acute and long-term management costs.
| Acute and long-term management costs |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Acute cost
|
Annual long-term cost
|
|||||||||
| Health state | Acute care cost28 | Weight | Total acute care cost | Long-term care cost28 | Weight | Long-term care total cost | Nursing and residential cost29 | Weight | Nursing and residential total cost | Total |
| mRS 0 | €5,070.00 | 0.90a | €4,563.00 | €1,440.00 | 0.90a | €1,296.00 | €1,533.40 | 0.90a | €1,380.06 | €2,676.06 |
| mRS 1 | €5,070.00 | 1.00a | €5,070.00 | €1,440.00 | 1.00a | €1,440.00 | €1,533.40 | 1.00a | €1,533.40 | €2,973.40 |
| mRS 2 | €5,070.00 | 1.10a | €5,577.00 | €1,440.00 | 1.10a | €1,584.00 | €1,533.40 | 1.10a | €1,686.74 | €3,270.74 |
| mRS 3 | €6,951.00 | 0.90a | €6,255.90 | €24,984.00 | 0.90a | €22,485.60 | €9,488.60 | – | €9,488.60 | €31,974.20 |
| mRS 4 | €6,951.00 | 1.00a | €6,951.00 | €24,984.00 | 1.00a | €24,984.00 | €26,601.30 | – | €26,601.30 | €51,585.30 |
| mRS 5 | €6,951.00 | 1.10a | €7,646.10 | €24,984.00 | 1.10a | €27,482.40 | €37,701.40 | – | €37,701.40 | €65,183.80 |
| mRS 630 | €3,912.75 | 1.00a | €3,912.75 | |||||||
mRS: modified Rankin scale.
Weights informed by expert clinical opinion.
Table 3.
Treatment costs and adverse event management costs.
| TREATMENT COSTS | Units | Unit cost | Final cost | |
|---|---|---|---|---|
| Mechanical thrombectomy | ||||
| Materials (number of units needed for the intervention) | ||||
| Stent retriever (Solitaire) | 1.2 | €3,300.00 | €3,960.00 | |
| Guidewire (Avigo) | 1.0 | €312.00 | €312.00 | |
| Intracranial catheter (Navien)/Balloon guide catheter (Cello) | 1.1 | €630.00 | €693.00 | |
| Microcatheter (Rebar) | 1.1 | €656.24 | €721.86 | |
| Introducer | 1.0 | €200.00 | €200.00 | |
| Procedure packa | 1.0 | €30.00 | €30.00 | |
| Gloves | 2.0 | €0.50 | €1.00 | |
| Diagnosis catheter | 1.0 | €56.42 | €56.42 | |
| Contrast | 1.0 | €77.04 | €77.04 | |
| PTA balloon catheter | 0.2 | €564.20 | €112.84 | |
| Carotid stent | 0.1 | €1,129.00 | €112.90 | |
| Personnel (number of hours of personnel needed for the intervention) | ||||
| Anaesthetist | 2.0 | €35.00 | €70.00 | |
| Interventional neuroradiologist | 3.0 | €35.00 | €104.99 | |
| Neurologist | 2.0 | €35.00 | €70.00 | |
| Resident doctor | 2.0 | €12.15 | €24.29 | |
| Nurse | 6.0 | €21.29 | €127.73 | |
| Tests (number of tests required) | ||||
| Cranial computerised tomography scan | 1.0 | €82.04 | €82.04 | |
| Computerised tomography angiography | 1.0 | €270.07 | €270.07 | |
| Total |
€7,026.17 | |||
| IV t-PA thrombolysis |
||||
| Materials (number of units needed for the intervention) | ||||
| Alteplase (0.9 mg/kg; average patient weight, 75 kg) | €643.68 | |||
| Personnel (number of hours of personnel needed for the intervention) | ||||
| Neurologist | 1.0 | €35.00 | €35.00 | |
| Resident doctor | 3.0 | €12.15 | €36.44 | |
| Nurse | 4.0 | €21.29 | €85.15 | |
| Nursing assistant | 1.0 | €12.64 | €12.64 | |
| Tests (number of tests required) | ||||
| Cranial computerised tomography scan | 1.0 | €82.04 | €82.04 | |
| Computerised tomography angiography | 0.5 | €270.07 | €135.03 | |
| Total |
€1,029.98 | |||
| Non-thrombolytic treatment |
||||
| Personnel (number of hours of personnel needed for the intervention) | ||||
| Neurologist | 0.5 | €35.00 | €17.50 | |
| Resident doctor | 1.0 | €12.15 | €12.15 | |
| Nurse | 2.0 | €21.29 | €42.58 | |
| Interventional neuroradiologist | 0.5 | €35.00 | €17.50 | |
| Tests (number of test required) | ||||
| Cranial computerised tomography scan | 1.0 | €82.04 | €82.04 | |
| Blood test | 1.0 | €44.59 | €44.59 | |
| Transcranial duplex | 1.0 | €128.00 | €128.00 | |
| Electrocardiogram | 1.0 | €38.94 | €38.94 | |
| TREATMENT COSTS |
Units | Unit cost | Final cost | |
| Chest radiograph | 1.0 | €25.71 | €25.71 | |
| Computerised tomography angiography | 0.5a | €270.07 | €135.03 | |
| Perfusion computerised tomography | 0.33 | €243.88 | €80.48 | |
| Total |
|
|
|
€624.50 |
| ADVERSE EVENT MANAGEMENT COSTS |
Units | % patients | Unit cost | Final cost |
| Symptomatic intracranial haemorrhage | ||||
| Specialist visits | ||||
| Neurologist | 10.0 | 100.0 | €138.22 | €1,382.23 |
| Anaesthesiology/Intensive Care | 5.0 | 50.0 | €57.04 | €142.60 |
| Neurosurgery | 1.0 | 25.0 | €151.99 | €38.00 |
| Haematology | 1.0 | 100.0 | €110.34 | €110.34 |
| Rehabilitation/Physiotherapy | 90.0 | 75.0 | €53.97 | €3,642.98 |
| Nutrition | 2.0 | 50.0 | €82.75 | €82.75 |
| Speech therapy | 4.0 | 25.0 | €65.52 | €65.52 |
| Tests | ||||
| Cranial CT scan | 2.0 | 100.0 | 153.00 | €306.01 |
| Hospital admissions | ||||
| Intensive Care Unit | 5.0 | 50.0 | €1,127.71 | €2,819.28 |
| Stroke Unit | 3.0 | 75.0 | €559.81 | €1,259.57 |
| Neurology Unit | 7.0 | 100.0 | €559.81 | €3,918.67 |
| Interventions | ||||
| Craniectomy | 1.0 | 5.0 | €20,190.42 | €1,009.52 |
| Haematoma removal surgery | 1.0 | 25.0 | €2,637.20 | €659.30 |
| Ventricular drain placement | 1.0 | 10.0 | €314.25 | €31.42 |
| Pharmaceutical treatment | ||||
| Intravenous labetalol (300 mg/day) | 3.0 | 50.0 | €0.03 | €11.75 |
| Intravenous urapidil (250 mg/day) | 3.0 | 50.0 | €0.05 | €20.09 |
| Total |
€15,500.02 | |||
| Malignant cerebral oedema requiring haemicraniectomy |
Total cost | |||
| ICD-9-CM 384.5 – cerebral oedema | €4,922.98 | |||
| Craniotomy (age >17 without major complications) | €11,048.15 | |||
| Total | €15,971.13 | |||
CT: computed tomography; IV t-PA: intravenous tissue plasminogen activator; PTA: percutaneous transluminal angioplasty.
It is estimated that half of the patients will not respond to IV t-PA and physicians will want to rule out a large arterial occlusion by performing a computerised tomography angiography.
Concerning adverse event management costs, symptomatic intracranial haemorrhage cost was obtained from resource use (€15,500). Malignant cerebral oedema requiring craniectomy was calculated considering the Diagnosis Related Group costs of cerebral oedema and craniectomy intervention cost (€15,971).31
Cost-effectiveness analysis
The model calculated total costs and health outcomes per treatment arm for a hypothetical cohort of 1,000 patients. A lifetime horizon was considered in the base case scenario of this analysis.
The cost-effectiveness of mechanical thrombectomy after IV t-PA versus IV t-PA alone was assessed by the incremental cost-utility ratio (ICUR), comparing both costs and QALYs generated by these alternatives
Alternatively, the results were expressed by net monetary benefit (NMB), which included the commonly used cost-effectiveness threshold in Spain of €30,000/QALY gained,32,33 which is the willingness to pay for the additional QALYs obtained with the more effective treatment
A positive NMB value would indicate that combined therapy of stent-retriever mechanical thrombectomy and IV t-PA would be an efficient treatment option for acute ischaemic stroke according to the cost-effectiveness threshold.
Alternative scenarios and sensitivity analysis (SA)
Alternative scenarios considered different time horizons than lifetime (base case scenario): 1, 2, 5, 20 and 25 years. These analyses were also conducted including formal care costs.
Deterministic and probabilistic SAs were conducted to assess the robustness of the results. One-way deterministic SA varied the value of one base case input at a time, especially those with the greatest uncertainty: number of Solitaire devices required (1–2), discount rates (0–5%), starting age (55–75), health states utilities34 (values obtained from visual analogue scale [VAS] and Double Gamble methods), recurrent stroke rates at 90 days (0–10%) and one year (0.5–5%), relative risk of dying (double). All costs varied by 10 and 25%. A probabilistic SA was also undertaken to assess the joint uncertainty in the model parameters (1,000 simulations). The value of each key parameter would vary within a specific probability distribution assigned to each parameter: mRS score at 90 days (Dirichlet), mortality relative risks (Lognormal), utilities (Beta) and costs (Gamma).
Results
In the base case scenario, mechanical thrombectomy after IV t-PA resulted in a more effective and less costly treatment than IV t-PA alone over a patient’s lifetime. Therefore, it is considered a dominant alternative. With the combined therapy, a total cost of €123,866 was estimated versus €168,224 with IV t-PA alone, resulting in a savings of €44,378. More LYG and QALYs were gained with the Solitaire device plus IV t-PA therapy (11.71 and 7.62, respectively) compared to the IV t-PA alone (10.54 and 5.11). Additionally, an NMB of €119,744 was obtained considering a willingness-to-pay threshold of €30,000/QALY gained. The results of the base case analysis are shown in Table 4.
Table 4.
Results of cost-effectiveness analysis (base case and alternative scenarios).
| Solitaire + IV t-PA | IV t-PA | Incremental value | ||
|---|---|---|---|---|
| Costs | ||||
| Treatment costs | €8,428.00 | €1,606.00 | €6,822.00 | |
| Adverse event costs | €743.83 | €1,195.46 | −€451.63 | |
| Acute management costs | €5,630.00 | €6,083.00 | −€453.00 | |
| Long-term management costs | €105,624.00 | €157,668.00 | −€52,044.00 | |
| Stroke recurrence costs | €3,441.00 | €1691 | €1,749.00 | |
| Total cost | €123,866 | €168,244 | −€44,378 | |
| Health results | ||||
| Total QALYs | 7.62 | 5.11 | 2.51 | |
| LYG (without discount) | 15.869 | 14.038 | 1.831 | |
| LYG (with discount) | 11.708 | 10.536 | 1.172 | |
| Incremental cost-utility ratio (ICUR) | Dominant | |||
| Net monetary benefit (NMB) (threshold 30.000€/QALY) gained |
€119,744 | |||
| Alternative scenarios (varying time horizon and formal costs inclusion) | ||||
| Scenarios |
Time horizon |
Incremental costs (€) |
Incremental QALYs |
ICUR (€/QALY) gained |
| Base case Formal costs not included Efficacy data from SWIFT PRIME |
1 year | 2,560 | 0.17 | 15,044 |
| 2 years | −1,928 | 0.33 | Dominant | |
| 5 years | −13,976 | 0.78 | Dominant | |
| 20 years | −43,261 | 2.24 | Dominant | |
| 25 years | −44,386 | 2.43 | Dominant | |
| Lifetime |
−44,378 |
2.51 |
Dominant |
|
| Alternative scenario Formal costs included Efficacy data from SWIFT PRIME | 1 year | −1,413 | 0.17 | Dominant |
| 2 years | −10,970 | 0.33 | Dominant | |
| 5 years | −36,736 | 0.78 | Dominant | |
| 20 years | −100,280 | 2.24 | Dominant | |
| 25 years | −102,807 | 2.43 | Dominant | |
| Lifetime | −102,834 | 2.51 | Dominant | |
IV t-PA: intravenous tissue plasminogen activator; LYG: life years gained; QALY: quality-adjusted life year.
Alternative scenarios showed that in time horizons of two years and beyond, regardless of including formal care costs, mechanical thrombectomy after IV t-PA was always a dominant treatment option versus IV t-PA alone. Considering a one-year time horizon, the combined therapy had a ICUR of €15,044/QALY gained versus IV t-PA if formal care costs were not included, and it was dominant if formal care costs were considered (Table 4).
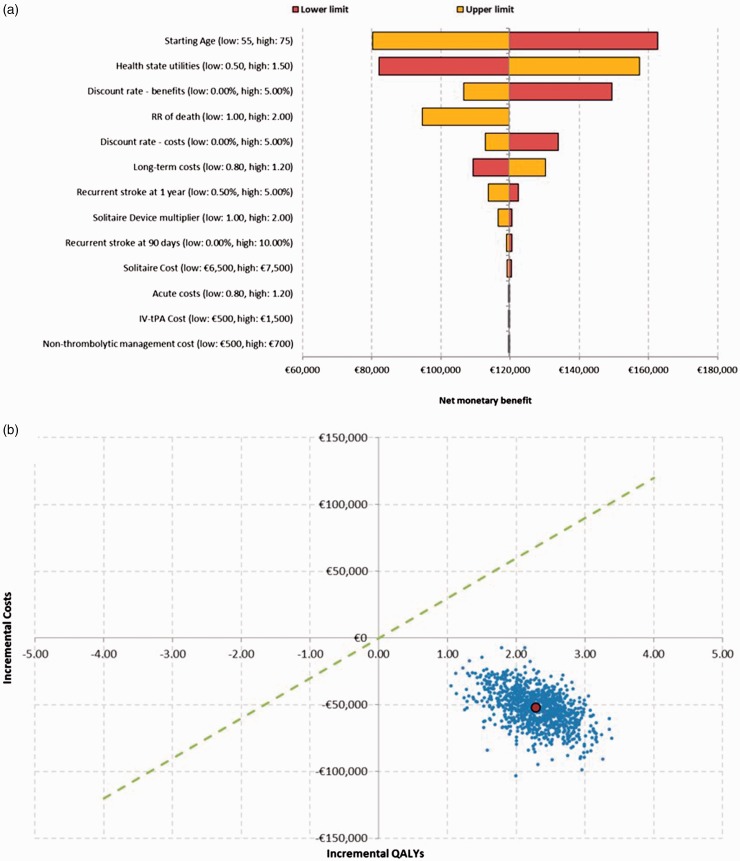
The robustness of the results was assessed with both deterministic and probabilistic SA. The deterministic analyses resulted in fewer costs and increased efficacy (more QALYs) for the combined therapy of the Solitaire device and IV t-PA versus IV t-PA alone. A positive value of NMB was always obtained in performing these analyses. The use of different utility values did not change the results (−€44,378 and 3.43 [double gamble] or 2.37 QALYs [EVA]). The starting age, utility values, discount rate and relative risk of dying were the key drivers of the analysis. Probabilistic analysis results also showed that mechanical thrombectomy plus IV t-PA was a dominant treatment alternative with lower associated costs and better health outcomes in 100% of the simulations performed. The results of both deterministic and probabilistic analyses are described in Figure 2.
Figure 2.
Results of deterministic (a) and probabilistic (b) sensitivity analyses.
Discussion
The management of patients with acute ischaemic stroke has improved greatly over the last two decades with better imaging techniques and new interventions, such as intravenous thrombolysis and/or endovascular treatment. The narrow time window available for an effective treatment with IV t-PA and the difficulty of breaking down the blood clots in patients with large vessel occlusions have encouraged the emergence of new interventional approaches to extend this treatment period and achieve better outcomes in artery recanalisation and functional independence.
Despite the failure of the first generation of mechanical embolectomy devices in demonstrating a significant improvement over intravenous thrombolysis,26,35,36 the following stent-retriever trials achieved better results in recanalisation rates and functional outcomes.7–11 Thus, endovascular treatment with stent retrievers after IV t-PA has become the gold standard of acute ischaemic stroke treatment in patients with large vessel occlusions.12
The results of this study showed that combined treatment of mechanical thrombectomy with a stent retriever device and IV t-PA for the treatment of acute ischaemic stroke in Spanish patients resulted in a dominant treatment alternative because of better health outcomes with a gain of 1.17 QALYs and lower costs with savings of €44,378 over IV t-PA alone during the patients’ lifetime. This treatment was associated with an NMB of €119,744, when a €30,000/QALY gained threshold was considered. When different time horizons were selected, stent-retriever mechanical thrombectomy after IV t-PA was always considered cost-effective and, over a two-year period, it was also a dominant therapy (less costly, more effective). The inclusion of long-term formal costs related to nursing and residential care resulted in a dominant therapy for all time horizons considered including the first year of analysis. Deterministic and probabilistic SA results showed robustness of the model inputs and revealed that the addition of endovascular treatment with stent retrievers to IV t-PA was a dominant alternative in 100% of the simulations.
A major strength of this analysis is that efficacy data were based on evidence from a randomised clinical trial data (SWIFT PRIME) which was stopped early due to proven efficacy. The only treatment alternatives considered in the study were Solitaire and IV t-PA. Inclusion of recurrent stroke in the model was an additional strength of the model because it assures that all clinical outcomes and related costs following a stroke were included. Additionally, the model related each mRS health status to patient outcomes of mortality, quality of life and resource use. Individual mRS scores, instead of combined dependence levels, better described the patients’ pathway.
However, this study was not exempt from some limitations. Efficacy data were taken from only one clinical trial contributing to meta-analyses. Because it was stopped early, it may have overestimated the effect of treatment. Although estimates are likely to be unaffected by the thrombectomy, they may lead to a reduction in difference between treatment and control over time in stroke survivors. The starting age of patients in SWIFT-PRIME was 66 years old, which seems to be lower than clinical practice. Thus, a reduction in benefits might occur in older patient populations, as shown in the deterministic SA, but a positive net benefit can still be achieved. Patients remain in the same mRS level unless they have a stroke recurrence, but mRS score could also be affected by age and comorbidities. However, these factors would vary equally in both treatment arms with no differences on incremental benefits. For cost estimation of acute and long-term management of stroke patients, costs per dependency levels by Barthel Index were converted into mRS, and therefore, they could not be completely representative of each mRS health state. These costs also needed to be weighted by ±10% to discriminate management costs between different mRS scores. However, despite the lack of available detailed cost data, these assumptions allowed the model to represent differences in management costs for the different disability degrees a patient could have. Additional costs related to patient transportation to centres where endovascular treatment is available were not included due to limited available information. However, these costs could apply in some situations. Utilities have been given by mRS score, but as a limitation, this scale has little bearing on pain and discomfort and anxiety/depression EQ-5D dimensions.
The model allowed the inclusion of only two adverse events. Symptomatic intracranial haemorrhage and malignant cerebral oedema requiring craniectomy were selected because they had the greatest impact on costs. The rest of the reported adverse events, such as vasospasm, were not included in the model, as they were not considered significant according to the expert panel. Adverse event rates for the base case scenario were selected from the REVASCAT Spanish trial, which assessed the efficacy of the inclusion of stent-retriever mechanical thrombectomy to standard medical management. Despite being obtained from a different study, these rates could be more representative of Spanish patients and management. These events contribute to short- and long-term costs, and their incidence was similar in both arms in SWIFT PRIME trial; therefore, they are unlikely to contribute to cost differences between treatments. Consequently, an SA with SWIFT PRIME rates was performed, and the results barely varied, in which combined treatment was still a dominant alternative for stroke management.
A cost-effectiveness study was published recently in the United Kingdom using the same model as in the present analysis.37 This study showed that stent retriever use after IV t-PA is a dominant treatment alternative versus IV t-PA alone, leading to a savings of £33,190 and increased health benefits of 2.31 QALYs gained and a 98.6% likelihood of cost-effectiveness according to a threshold of £20,000/QALY gained. Most of the clinical parameters included in this study were used in the present analysis, having different sources of adverse events rates (SWIFT PRIME study) and resource use and cost estimation. Analyses for the United States and Italy have also been performed with this model resulting in combined therapy as a cost-effective option.38,39
Additional cost-effectiveness analyses of mechanical thrombectomy for the treatment of acute ischaemic stroke and Health Technologies Assessment agencies reports have also been recently published for various NHSs.40–48 These studies included efficacy data from a single randomised clinical trial (SWIFT PRIME or MR CLEAN) or from meta-analyses of several endovascular treatment randomised clinical trials and considered standard of care or IV t-PA only as the study comparator. Most of them had a healthcare and/or social care perspective. Despite the different results obtained in each study, the results are consistent with the present analysis, and all concluded that mechanical thrombectomy associated with the standard treatment/IV t-PA was a cost-effective alternative versus the standard treatment or IV t-PA alone.
Conclusion
Mechanical thrombectomy using the Solitaire stent-retriever device in combination with intravenous thrombosis with IV t-PA resulted in a dominant alternative (less costly, more effective) versus IV t-PA alone for the treatment of acute ischaemic stroke in patients with confirmed occlusions in the proximal anterior intracranial circulation and an absence of large ischaemic core lesions. It was a cost-effective option in all assessed scenarios from the Spanish NHS perspective.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AD was the recipient of an unrestricted grant from Covidien (currently Medtronic) for conducting REVASCAT trial. MAdM, TS and PC declare that there is no conflict of interest in this work. AG has provided services to Medtronic Ibérica, S.A. as a consultant. FdA-N and MAC are developing their professional activity in PORIB, a consultancy firm, which specialises in economic evaluation of health technologies, which has received economic funding by Medtronic Ibérica, S.A. to conduct this project. MA is currently employed by Medtronic Ibérica, S.A. The authors hereby declare that this economic support has not interfered with the development of this project. The authors state that the sponsor did not participate or influence the analysis of the present study or the interpretations of its results.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially supported by Medtronic Inc.
Ethical approval
Not applicable.
Informed consent
No patients were recruited nor patient-level clinical data were used in this analysis.
Guarantor
Fernando de Andrés-Nogales.
Contributorship
FdA-N, MAC and MA researched literature and conceived the study. AD, MAdM, TS, AG and PC provided additional literature and validated the design and results of the model. FdA-N and MAC performed the analysis and wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
References
- 1.Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the Global Burden of Ischaemic and Hemorrhagic Stroke in 1990–2013: the GBD 2013 Study. Neuroepidemiology 2015; 45: 161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Instituto Nacional de Estadística. Defunciones según la causa de muerte. Año 2014. Madrid: Instituto Nacional de Estadística, http://www.ine.es/prensa/np963.pdf (2014, accessed 2 June 2016).
- 3.Alvarez-Sabín J, Quintana M, Masjuan J, et al. Economic impact of patients admitted to stroke units in Spain. Eur J Health Econ 2017; 18: 449–458. [DOI] [PubMed]
- 4.de Andrés-Nogales F, Vivancos Mora J, Barriga Hernández FJ, et al. Use of healthcare resources and costs of acute cardioembolic stroke management in the Region of Madrid: The CODICE Study. Neurologia 2015; 30: 536–544. [DOI] [PubMed] [Google Scholar]
- 5.European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008; 25: 457–507. [DOI] [PubMed] [Google Scholar]
- 6.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischaemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 7.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischaemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 8.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischaemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 9.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischaemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 10.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 11.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischaemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 12.Fiehler J, Cognard C, Gallitelli M, et al. European Recommendations on Organisation of Interventional Care in Acute Stroke (EROICAS). Int J Stroke 2016; 11: 701–716. [DOI] [PubMed] [Google Scholar]
- 13.Touma L, Filion KB, Sterling LH, et al. Stent retrievers for the treatment of acute Ischaemic Stroke: a systematic review and meta-analysis of randomized clinical trials. JAMA Neurol 2016; 73: 275–281. [DOI] [PubMed] [Google Scholar]
- 14.Campbell BC, Hill MD, Rubiera M, et al. Safety and efficacy of solitaire stent thrombectomy: individual patient data meta-analysis of randomized trials. Stroke 2016; 47: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsivgoulis G, Safouris A, Katsanos AH, et al. Mechanical thrombectomy for emergent large vessel occlusion: a critical appraisal of recent randomized controlled clinical trials. Brain Behav 2016; 6: e00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boehringer Ingelheim. Alteplase for treating acute ischaemic stroke – single Technology Appraisal; manufacturer’s submission, 2012.
- 17.Naimark DM, Bott M, Krahn M. The half-cycle correction explained: two alternative pedagogical approaches. Med Decis Making 2008; 28: 706–712. [DOI] [PubMed] [Google Scholar]
- 18.Dávalos A, Cobo E, Molina CA, et al. REVASCAT trial investigators. Safety and efficacy of thrombectomy in acute ischaemic stroke (REVASCAT): 1-year follow-up of a randomised open-label trial. Lancet Neurol 2017; 16: 369–376. [DOI] [PubMed]
- 19.Mohan KM, Wolfe CD, Rudd AG, et al. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke 2011; 42: 1489–1494. [DOI] [PubMed] [Google Scholar]
- 20.Instituto Nacional de Estadística. 2013 Mortality tables of Spanish population. National results. In: INEbase. Madrid: Instituto Nacional de Estadística, http://www.ine.es (2013, accessed 25 February 2015).
- 21.Slot KB, Berge E, Sandercock P, et al. Causes of death by level of dependency at 6 months after ischaemic stroke in 3 large cohorts. Stroke 2009; 40: 1585–1589. [DOI] [PubMed] [Google Scholar]
- 22.Luengo-Fernandez R, Paul NL, Gray AM, et al. Population-based study of disability and institutionalization after transient ischaemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke 2013; 44: 2854–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivero-Arias O, Ouellet M, Gray A, et al. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Making 2010; 30: 341–354. [DOI] [PubMed] [Google Scholar]
- 24.López Bastida J, Oliva J, Antoñanzas F, et al. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias. Gac Sanit 2010; 24: 154–170. [DOI] [PubMed] [Google Scholar]
- 25.Rubio-Terrés C, Graefenhain de Codes R, Rubio-Rodríguez D, et al. Cost-effectiveness analysis of rivaroxaban versus acenocoumarol in the prevention of stroke in patients with non-valvular atrial fibrillation in Spain. JHEOR 2016; 4: 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischaemic stroke. N Engl J Med 2013; 368: 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mar J, Arrospide A, Begiristain JM, et al. The impact of acquired brain damage in terms of epidemiology, economics and loss in quality of life. BMC Neurol 2011; 11: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piñol C, Roze S, Valentine W, et al. Coste-efectividad de la adición de acarbosa al tratamiento de pacientes con diabetes tipo 2 en España. Gac Sanit 2007; 21: 97–104. [DOI] [PubMed] [Google Scholar]
- 29.Oblikue Consulting. eSalud Health Cost database [Internet]. Barcelona: Oblikue Consulting, http://www.oblikue.com/bddcostes/ (2016, accessed 25 February 2016).
- 30.Consejo General de Colegios Oficiales de Farmacéuticos. Base de datos del Conocimiento Sanitario – Bot Plus 2.0 [Internet]. Madrid: Consejo General de Colegios Oficiales de Farmacéuticos, https://botplusweb.portalfarma.com/ (2016, accessed 25 February 2016).
- 31.Kwon S, Hartzema AG, Duncan PW, et al. Disability measures in stroke: relationship among the Barthel Index, the functional independence measure, and the modified Rankin scale. Stroke 2004; 35: 918–923. [DOI] [PubMed] [Google Scholar]
- 32.Sacristán JA, Oliva J, Del Llano J, et al. What is an efficient health technology in Spain? Gac Sanit 2002; 16: 334–343. [DOI] [PubMed] [Google Scholar]
- 33.De Cock E, Miravitlles M, González-Juanatey JR, et al. Valor umbral del coste por año de vida ganado para recomendar la adopción de tecnologías sanitarias en España: evidencias procedentes de una revisión de la literatura. Pharmacoecon Span Res Artic 2007; 4: 97–107. [Google Scholar]
- 34.Parody E, Pedraza S, García-Gil MM, et al. Cost–utility analysis of magnetic resonance imaging management of patients with Acute Ischaemic Stroke in a Spanish hospital. Neurol Ther 2015; 4: 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischaemic stroke. N Engl J Med 2013; 368: 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013; 368: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lobotesis K, Veltkamp R, Carpenter IH, et al. Cost-effectiveness of stent-retriever thrombectomy in combination with IV t-PA compared with IV t-PA alone for acute ischaemic stroke in the UK. J Med Econ 2016; 19: 785–794. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhry SA, Ishfaq MF, Sivagnanam K, et al. Cost effectiveness of stent-retriever thrombectomy compared with intravenous thrombolytic therapy alone in acute ischaemic stroke patients. Stroke 2016; 47: AWMP7. [Google Scholar]
- 39.Pizzo E, Morris S, Causin F. Cost-utility analysis of mechanical thrombectomy in acute ischaemic stroke in Italy. Cerebrovasc Dis 2016; 41: 1–2. [Google Scholar]
- 40.Ganesalingam J, Pizzo E, Morris S, et al. Cost-utility analysis of mechanical thrombectomy using stent retrievers in Acute Ischaemic Stroke. Stroke 2015; 46: 2591–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aronsson M, Persson J, Blomstrand C, et al. Cost-effectiveness of endovascular thrombectomy in patients with acute ischaemic stroke. Neurology 2016; 86: 1053–1059. [DOI] [PubMed] [Google Scholar]
- 42.Leppert MH, Campbell JD, Simpson JR, et al. Cost-effectiveness of intra-arterial treatment as an adjunct to intravenous tissue-type plasminogen activator for acute ischaemic stroke. Stroke 2015; 46: 1870–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kunz WG, Hunink MG, Sommer WH, et al. Cost-effectiveness of endovascular stroke therapy: a patient subgroup analysis from a US healthcare perspective. Stroke 2016; 47: 2797–2804. [DOI] [PubMed] [Google Scholar]
- 44.Shireman TI, Wang K, Saver JL, et al. SWIFT-PRIME investigators. Cost-effectiveness of solitaire stent retriever thrombectomy for Acute Ischaemic Stroke: results from the SWIFT-PRIME Trial (Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischaemic Stroke). Stroke 2017; 48: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Health Quality Ontario. Mechanical thrombectomy in patients with acute ischaemic stroke: a health technology assessment. Ont Health Technol Assess Ser 2016; 16: 1–79. http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ontario-health-technology-assessment-series/hta-mechanical-thrombectomy (accessed 7 April 2017). [PMC free article] [PubMed] [Google Scholar]
- 46.Frønsdal KB, Skår Å, Stoinska-Schneider A, et al. Mekanisk trombektomi ved akutt hjerneinfarkt [Mechanical thrombectomy for acute ischaemic stroke]. Rapport 2016. Oslo: Folkehelseinstituttet, https://www.fhi.no/globalassets/kss/filer/filer/publikasjoner/rapporter/20162/rapport_2016_mekanisk_trombektomi.pdf (2016, accessed 6 April 2017).
- 47.Stoinska-Schneider A, Robberstad B and Fure B. Mekanisk trombektomi ved akutthjerneinfarkt del 2. Helseøkonomisk evaluering [Mechanical thrombectomy for acute ischaemic stroke, part 2. Health economic evaluation]. Rapport 2016. Oslo: Folkehelseinstituttet, https://www.fhi.no/globalassets/dokumenterfiler/rapporter/trombektomi-helseokonomi-rapport-2016.pdf (2016, accessed 6 April 2017).
- 48.Cost-effectiveness analysis of thrombectomy for treatment of acute severe ischaemic stroke. Stockholm: Dental and Pharmaceutical Benefits Agency, http://www.tlv.se/Upload/English/Assessment_trombektomi.pdf (2016, accessed 6 April 2017).