Abstract
Community-acquired pneumonia in children is associated with significant morbidity and mortality; however, data are limited in predicting which children will have negative outcomes, including clinical deterioration, severe disease, or development of complications. The Pediatric Infectious Diseases Society/Infectious Diseases Society of America (PIDS/IDSA) pediatric pneumonia guideline includes criteria that were modified from adult criteria and define pneumonia severity to assist with resource allocation and site-of-care decision-making. However, the PIDS/IDSA criteria have not been formally developed or validated in children. Definitions for mild, moderate, and severe pneumonia also vary across the literature, further complicating the development of standardized severity criteria. This systematic review summarizes (1) the current state of the evidence for defining and predicting pneumonia severity in children as well as (2) emerging evidence focused on risk stratification of children with pneumonia.
Keywords: children, pneumonia, risk stratification, severity
Community-acquired pneumonia (CAP) is a leading cause of morbidity and mortality in children worldwide [1]. In the United States, CAP is one of the most frequent and costly reasons for childhood hospitalization [2, 3]. Despite its impact, significant variation exists in the diagnosis and management of children with pneumonia [4–6]. Although practice guidelines can assist with clinical decision making [1, 7, 8], no validated severity criteria exist for children with CAP. The objective of this systematic review is to summarize the current state of evidence for defining and predicting pneumonia severity in children.
PNEUMONIA SEVERITY SCORES
Numerous severity scores have been developed in adults with CAP (Table 1) [9–14]. They have been shown to reduce hospitalizations and administration of broad-spectrum antibiotics [15, 16], but they predict other outcomes with varied success.
Table 1.
Summary of Existing Pneumonia Severity Scoresa
| Severity Score | Components | Area Under the Receiver Operator Characteristic Curve |
|---|---|---|
| PSI [10] | Age, Nursing Home, Comorbidities, AMS, Tachypnea, Hypotension, Hypo- or Hyperthermia, Tachycardia, pH <7.35, BUN ≥30, Na <130, Glucose ≥250, Hematocrit <30%, Partial pressure of arterial oxygen <60 mmHg, Pleural Effusion | 30-day Mortality: 0.70–0.89 CAP Complications: 0.58–0.85 |
| CURB-65 [11] | Confusion, Urea ≥7 mmol/L, Tachypnea, Hypotension and Age ≥65 | 30-day Mortality: 0.73–0.87 CAP Complications: 0.60–0.78 |
| IDSA/ATS 2007 [14] | Minor Criteria: Tachypnea, PaO2/FiO2 ≤250, Multilobar Infiltrates, Confusion, BUN ≥20, WBC ≤4000, Platelets ≤100000, Hypothermia, Hypotension Major Criteria: Invasive Mechanical Ventilation, Need for Vasopressors |
30-day Mortality: 0.63–0.67 CAP Complications: 0.85–0.88 |
| SMART-COP [9] | Hypotension, Multilobar Infiltrates, Albumin <3.5 g/dL, Tachypnea, Tachycardia, Confusion, Hypoxemia and Arterial pH <7.35 | 30-day Mortality: Not assessed CAP Complications: 0.83–0.87 |
| SCAP [12] | Major Criteria: pH <7.30, Hypotension Minor Criteria: Confusion, Urea >30 mg/dL, Tachypnea, Multilobar Infiltrates, Hypoxemia, Age ≥80 |
30-day Mortality: Not assessed CAP Complications: 0.75–0.83 |
| Williams et al [18] (Pediatric) | AgeR,E, SexE, RaceE, ComorbiditiesR, Household smoke exposure, Season, Symptom Duration, Vomiting/feeding refusal, Hypo/HyperthermiaE, TachypneaR,E, TachycardiaR,E, HypotensionR,E, HypoxemiaR,E, AMSR, Chest IndrawingR, Asymmetric breath sounds, Wheezing, White blood cell countE, Infiltrate PatternR, Pleural EffusionR | Death or CAP Complications: 0.78–0.81 |
| Araya et al [19] (Pediatric) | Age, Comorbidities, Hypoxemia, Hypotension, Bacteremia, Multilobar/Complicated Pneumonia, Kidney/Liver Failure and Acute Respiratory Distress Syndrome | Death: 0.94 |
Abbreviations: AMS, altered mental status; CAP, community-acquired pneumonia; E, components of electronic health record-based variable model; IDSA/ATS, Infectious Diseases Society of America/Amercan Thoracic Society; PSI, pneumonia severity index; R, components of reduced variable model; SCAP, severe CAP.
aTable adapted from Pereira JM, Paiva JA, Rello J. Assessing severity of patients with community-acquired pneumonia. Semin Respir Crit Care Med. 2012;33:272. CAP complications defined as need for intensive care unit admission, need for mechanical ventilation, or need for vasopressor support.
The World Health Organization (WHO) defines “pneumonia” in children as presence of cough or difficulty breathing associated with fast breathing or chest indrawing in children 2–59 months of age, whereas “severe pneumonia” is defined as pneumonia plus inability to drink, persistent vomiting, convulsions, lethargy, stridor, or severe malnutrition [1]. These criteria were developed for use in countries with limited resources, and they are highly sensitive at the cost of specificity.
In the developed world, pediatric CAP guidelines include severity criteria intended to assist site-of-care decision making. The British Thoracic Society (BTS) [8] and the Pediatric Infectious Diseases Society/Infectious Diseases Society of America (PIDS/IDSA) [7] criteria were developed by author consensus (Table 2) and have not been formally derived and validated in children. A recent study found that >50% of children who met PIDS/IDSA severity criteria were safely discharged home from the emergency department (ED) [17].
Table 2.
Summary of BTS and PIDS/IDSA Criteria for Severe Pneumonia
| British Thoracic Society [8] | Pediatric Infectious Diseases Society/Infectious Diseases Society of America [7] |
|---|---|
| Temperature >38.5°C Respiratory rate • >70 in infants • >50 in older children Moderate/severe recession in infants Severe difficulty in breathing in children Not feeding in infants Nasal flaring Cyanosis Apnea Grunting Tachycardia Signs of dehydration Capillary refill ≥2 seconds |
Major Criteria: Invasive mechanical ventilation Fluid refractory shock Acute need for noninvasive positive pressure ventilation Hypoxemia requiring FiO2 at a higher concentration or flow feasible in general care area Minor Criteria: Tachypnea for age: • 0–2 months: respiratory rate >60 • 2–12 months: respiratory rate >50 • 1–5 years: respiratory rate >40 • >5 years: respiratory rate >20 Apnea Increased work of breathing PaO2/FiO2 <250 Multilobar infiltrates Pediatric Early Warning Score >6 Altered mental status Hypotension Pleural effusion Comorbid conditions Unexplained metabolic acidosis |
Abbreviations: BTS, British Thoracic Society; FiO2, fraction of inspired oxygen; IDSA, Infectious Diseases Society of America; PaO2, oxygen partial pressure; PIDS, Pediatric Infectious Diseases Society.
There are few severity scoring systems developed in children with CAP. A 2016, large-scale, multicenter, prospective cohort study developed a prediction model for severe outcomes (defined as death or need for mechanical ventilation or vasoactive medications) in children hospitalized with CAP [18]. Age extremes, vital signs, chest indrawing, and radiographic infiltrate pattern were the most important predictors. Given its derivation in hospitalized children, its applicability to the ED or clinic is currently unknown.
In a developing nation, Araya et al [19] developed a scoring system (based on age, comorbidities, hypoxemia, hypotension, bacteremia, multilobar/complicated pneumonia, kidney/liver failure, and acute respiratory distress syndrome) to predict mortality in children hospitalized with pneumonia. The application of this system in developed nations is limited.
METHODS
We conducted a systematic review using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology [20].
Literature Search
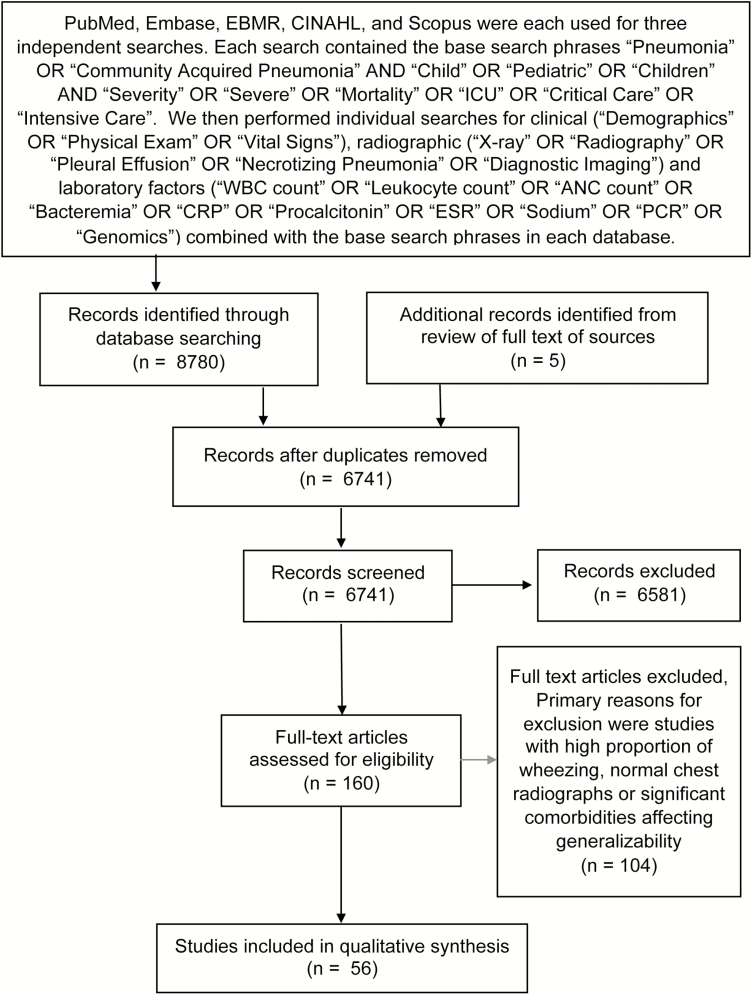
We searched PubMed, Embase, EBM Reviews, CINAHL, and SCOPUS in February 2018. We limited our search to English language articles in children over the past 20 years. We excluded studies in which a high percentage of subjects had wheezing or diarrhea, use of WHO-definitions of pneumonia without requiring focality on exam or chest x-ray confirmation, or if severe comorbidities (ie, human immunodeficiency virus [HIV] or malaria) affected generalizability. Our search strategy and results are listed in Figure 1. Included studies are listed in Table 3.
Figure 1.
Results from literature search.
Table 3.
Studies Included in Systematic Review
| Study, Year | Region, Setting | Study Design | Age | n | Limitations and Potential Biases |
|---|---|---|---|---|---|
| Florin et al [17], 2018 | Single center, US, ED | Retrospective | 3 months–18 years old | 518 | Single center, use of ICD coding may have introduced misclassification bias |
| Williams et al [18], 2016 | Multicenter, US, inpatient | Prospective | <18 years old | 2319 | Definition of mild, moderate, and severe pneumonia based on author opinion, limited to hospitalized patients |
| Araya et al [19], 2016 | Single center, Paraguay, inpatient | Retrospective | <15 years old | 860 | Single center, retrospective, exclusively inpatient, significant rates of comorbidities |
| Duke et al [22], 2001 | Single center, Papua New Guinea, inpatient | Prospective | 28 days–5 years old | 703 | High rates of malnourished children, lack of invasive interventions available, elevation of 1600 m, use of WHO severity criteria |
| Djelantik et al [23], 2003 | Single center, Indonesia, inpatient | Retrospective | <24 months | 4531 | Possible selection bias, high rates of malnourishment, lack of invasive interventions, use of WHO severity criteria |
| Nantanda et al [24], 2008 | Single Center, US, ICU and General Wards | Retrospective | 2–59 months | 157 | Retrospective, single center, developing nation with high rates of malnourishment limiting generalizability, used WHO-definitions but required either CXR confirmation or excluded children with wheezing with negative CXR |
| Reed et al [25], 2012 | Single center, South Africa, inpatient | Retrospective | <24 months | 4148 | Limited to young infants, high rates of malnourished children |
| Wolf et al [26], 2015 | Single center, US, inpatient | Post hoc analysis of prospective population-based study | <18 years old | 336 | Single-site analysis of a multicenter study introducing possible selection bias |
| Neuman et al [27], 2012 | Large-scale Multicenter, US, inpatient | Retrospective | <18 years old | 82 566 | Children may have been readmitted at nonincluded facility, admission decisions can show great variation between institutions |
| Mamtani et al [28], 2009 | Multicenter, 8 developing countries, inpatient | Post hoc analysis of previous RCT | 3–59 months | 889 | Developing countries, use of WHO severity criteria, treatment dose of amoxicillin 45 mg/kg per day, did not comment on percentage of children with wheezing (bronchiolitis, reactive airway disease) or HIV status |
| Tiewsoh et al [34], 2009 | Single center, India, inpatient | Prospective | 2–60 months | 200 | Use of WHO severity criteria, high rates of malnourishment and overcrowding, high proportion of children had wheezing, possible recall bias |
| Basnet et al [35], 2006 | Single center, Nepal, outpatient/ED | Retrospective | 2–60 months | 250 | Use of WHO severity criteria, developing country, 1300+m above sea level likely influencing degree of hypoxemia |
| Kuti et al [36], 2013 | Single center, Gambia, inpatient | Prospective | 2–59 months | 420 | Used WHO definitions for pneumonia diagnosis and severity, and did not require CXR confirmation (however, did exclude children with wheezing or cough for >2 weeks), single center and developing nation limiting generalizability |
| Demers et al [37], 2000 | Single center, Central African Republic, inpatient | Prospective | <5 years old | 395 | Developing nation with limited resources and high rates of malnourishment, possible observer bias as “alteration of general status” based on physician opinion and not validated scoring system, possible selection bias (significant proportion of patients absconded due to military uncertainty during the study, and not all patients had CXR performed) |
| Chisti et al [38], 2013 | Single center, Bangladesh, ICU | Retrospective | <5 years old | 140 | Developing nation, high rates of malnourishment, retrospective, limited to ICU |
| Hsu et al [41], 2015 | Multicenter, Taiwan, ICU | Retrospective | <18 years old | 12577 | Retrospective, dependent upon ICD-9 coding, only ICU setting limiting generalizability |
| Hirsch et al [42], 2016 | Multicenter, US, inpatient | Retrospective | Children | 12097 | Retrospective, used administrative database relying on ICD coding, all sites are tertiary care referral centers limiting generalizability |
| Champatiray et al [43], 2017 | Single center, India, inpatient | Prospective | 2 months– 5 years old | 141 | Single center, exclusively inpatient, use of WHO definitions, CXR were obtained on admission, but study does not mention whether cases were radiographically confirmed, study did not comment on rate of wheezing, history of present illness/social history subject to recall bias, much longer LOS (8–9 days) and much higher mortality rate (22%) than US studies |
| Muszynski et al [44], 2011 | Single Center, US, ICU | Retrospective | <18 years old | 23 | Retrospective, single center, small n, limited to ICU setting |
| Grafakou et al [48], 2004 | Single center, Greece, inpatient | Retrospective | 1–14 years old | 167 | Single center and only inpatient limiting generalizability, markers for severity were duration of fever and LOS |
| Kin et al [49], 2009 | Single center, Brazil, inpatient | Prospective | <5 years old | 113 | Single center, excluded bilateral pulmonary infiltrates that could present with more severe disease, severity criteria were WHO and BTS guidelines that are not validated, CXR interpreted by single radiologist |
| Patria et al [50], 2013 | Single center, Italy, ED | Retrospective | <14 years old | 335 | Single center, not all children with CAP during the study period had CXR performed introducing possible selection bias for more severe disease, CXR interpreted by single radiologist, higher than expected mean age (7.5 years old) |
| Mclain et al [51], 2014 | Multicenter, US, inpatient | Retrospective | 60 days–18 years old | 406 | Retrospective, CXR interpreted by single radiologist, potential selection bias as this was a sampling of a larger cohort |
| Ferrero et al [52], 2010 | Multicenter, Developing nations, inpatient | Prospective | 3–59 months | 2536 | Exclusively inpatient, use of WHO severity criteria, patients not vaccinated against pneumococcus significantly limiting generalizability, each CXR interpreted by 1 reviewer (possible interobserver bias) |
| Tapisiz et al [53], 2011 | Single center, Turkey, inpatient | Retrospective | <18 years old | 501 | Single center, retrospective, pre- pneumococcal and Haemophilus vaccination era in this region |
| Erlichman et al [56], 2017 | Multicenter, Jerusalem, inpatient | Retrospective | <18 years old | 144 | Retrospective, demographics limit generalizability |
| Langley et al [57], 2008 | Multicenter, Canada, inpatient | Retrospective | <18 years old | 251 | Retrospective, reliant upon chart review and ICD-coding, did not require specific WBC cutoff in pleural fluid to verify diagnosis of empyema |
| Goldbart et al [58], 2009 | Single center, Israel, inpatient | Retrospective | ≤8 years old | 112 | Retrospective, single center, and patients not vaccinated against pneumococcus significantly limiting generalizability, each CXR interpreted by 1 reviewer (possible interobserver bias) |
| Sawicki et al [61], 2008 | Single center, US, inpatient | Retrospective | Children | 80 | Single-center and retrospective design limit generalizability |
| Bender et al [62], 2008 | Single center, US, inpatient | Retrospective | <18 years old | 33 | Small n, single-center and retrospective design limit generalizability |
| Krenke et al [63], 2015 | Single center, Poland, inpatient | Retrospective | 1 months–18 years old | 32 | Small n, single-center and retrospective design limit generalizability |
| Donnelly et al [64], 1998 | Single center, US, inpatient | Retrospective | 6 months–16 years old | 17 | Very small n, single center, retrospective |
| Hacimustafaoglu et al [65], 2004 | Singe center, Turkey, inpatient | Prospective | 6 months–14 years old | 108 | Single center, each radiographic study interpreted by 1 reviewer (possible interobserver bias) |
| Hsiesh et al [66], 2011 | Single center, Taiwan, inpatient | Retrospective | <18 years old | 112 | Retrospective, single center, relatively few number of cases with BPF (18) |
| Hsiesh et al [67], 2015 | Multicenter, Taiwan, inpatient | Prospective | <18 years old | 94 | All cases limited to 1 region, images were not independently reviewed by 2 pediatric radiologists |
| Chen et al [70], 2017 | Single center, Taiwan, inpatient | Retrospective | 6 months–18 years old | 142 | Single center, retrospective, exclusively inpatient, not all patients during study had an ultrasound performed introducing possible selection bias, intrinsic limitation of ultrasound is that quality of images are operator-dependent (significant number of cases were excluded due to suboptimal images) |
| Lai et al [71], 2015 | Single center, Taiwan, inpatient | Retrospective | Children | 236 | Retrospective, single center, potential selection bias as children who had lung ultrasound were likely to have more severe pneumonia, ultrasound inherently is operator-dependent |
| Williams et al [72], 2015 | Multicenter, US, inpatient | Retrospective | <18 years old | 153 | Sampling from larger cohort that only included patients that had CRP and WBC performed thus introducing possible selection bias, retrospective |
| Don et al [74], 2009 | Single center, Italy, ED | Prospective | Children | 100 | Used hospital admission (institutional and provider differences can play a role) and alveolar infiltrate (vs interstitial) as markers for severity, single center |
| Prat et al [75], 2003 | Single center, Spain, ED | Prospective | 6 months–10 years old | 85 | Primarily studied PCT, ESR, and WBC’s ability to predict etiology of CAP; however, secondary analyses revealed no association between WBC and bacteremic patients, which may indicate a more severe disease course, limitations include a relatively small n at a single center limiting generalizability |
| Wu et al [76], 2015 | Single center, China, inpatient | Retrospective | Children | 865 | Use of WHO definition and severity criteria that are not specific, single center, no mention of exclusion criteria, no mention of how many patients had CXR and what the results of those potential imaging studies may have been, no mention of additional outcomes of cases (ie, mortality, ICU admission, invasive interventions) |
| Agnello et al [78], 2015 | Single center, Italy, inpatient | Retrospective | 1–14 years old | 119 | Single center, excluded patients who were hospitalized for more than 48 hours introducing selection bias against more severe cases, clinical markers for severity were hypoxemia (SpO2 <92%), dyspnea and tachycardia but not more severe markers or outcomes |
| Stockmann et al [79], 2017 | Multicenter, US, inpatient | Post hoc analysis of prospective study | <18 years old | 532 | Possible selection bias as only those with residual serum available for analysis were included (patients in the ICU were more likely to have residual serum), median time during admission PCT obtained was 1 day, thus limiting applicability to risk stratification on initial presentation |
| Yadav et al [80], 2015 | Single center, India, inpatient | Prospective | 2 months–5 years old | 50 | Single center, small n |
| Korppi et al [81], 2003 | Multicenter, Finland, Primary Care | Retrospective | ≤15 years old | 190 | Retrospective, serum samples run for PCT over 15 years after they were collected, pre-pneumococcal and Haemophilus vaccination era, performed in 1 region of Finland limiting generalizability |
| Singhi et al [82], 1992 | Single center, India, inpatient | Prospective | Children | 264 | Single-center study in a developing nation during the pre-routine vaccination era significantly limiting generalizability, LOS much longer than average LOSs in current studies in the developed world |
| Wrotek et al [83], 2013 | Single center, Poland, inpatient | Retrospective | <18 years old | 312 | Significant number of patients did not have sodium measured introducing possible selection bias, retrospective, single center, severity assessment based on clinical factors and inflammatory markers but did not evaluate for more severe outcomes, average hospitalization length (8–9 days), significantly longer than other current studies in the developed world |
| Don et al [84], 2008 | Single center, Italy, ED | Prospective | Children | 108 | Small percentage of patients did not have sodium samples introducing possible selection bias, single center, severity assessment based on clinical factors and inflammatory markers but did not evaluate for more severe outcomes |
| Wang et al [85], 2013 | Single center, Taiwan, inpatient | Retrospective | <18 years old | 84 | Single center, retrospective, pre- pneumococcal and Haemophilus vaccination era |
| Shah et al [86], 2011 | Multicenter, US, ED | Retrospective | ≤18 years old | 291 | Few number of bacteremic patients (6) limits statistical power of assessment of severity |
| Neuman et al [88], 2017 | Multicenter, US, ED | Retrospective | 3 months–18 years old | 2568 | Retrospective, wide variation between sites in rates of obtaining blood cultures, which introduces possible selection bias, primary objective of study was to evaluate rate of bacteremia in hospitalized children with CAP and determine susceptibility of pathogens to standard care, evaluation of +blood culture impact on severity came via rates of +cultures in complicated vs noncomplicated CAP and did not evaluate for other outcomes |
| Myers at al [90], 2013 | Multicenter, US, inpatient | Retrospective | 60 days–18 years old | 369 | Retrospective, inpatient study limiting generalizability, evaluated severity based on hypoxemia, LOS, ICU admission, and complicated pneumonia including respiratory failure but no mention of assessment on mortality |
| Banerjee et al [91], 2011 | Multicenter, North America, inpatient | Retrospective survey | Children | 37 | Retrospective survey of pediatric infectious disease physicians thus potential for reporting bias with potential preference to report more severe cases |
| Muñoz et al [93], 2011 | Single center, Spain, inpatient | Prospective | <18 years old | 206 | Single center, evaluated severity by LOS and ICU admission but not by other clinical factors or more severe outcomes |
| Shen et al [94], 2011 | Single center, Taiwan, inpatient | Retrospective | Children | 119 | Single center, retrospective, only pneumococcal and exclusively inpatient limiting generalizability, urinary antigen tests may have detected colonization and not acute infection in some cases |
| Pettigrew et al [95], 2016 | Single center, US, inpatient | Retrospective | 6 months to <18 years old | 363 | Single center, retrospective, exclusively inpatient, approximately 50% of children had asthma/reactive airway disease, ethnic distribution not representative of general population, 20% had received antibiotics before sputum collection, limited generalizability |
Abbreviations: BPF, bronchopleural fistulas; BTS, British Thoracic Society; CAP, community-acquired pneumonia; CRP, C-reactive protein; CXR, chest radiographs; ED, emergency department; ESR, erythrocyte sedimentation rate; HIV, human immunodeficiency virus; ICD, International Classification of Diseases; ICU, intensive care unit; LOS, length of stay; PCR, polymerase chain reaction; PCT, procalcitonin; RCT, randomized clinical trial; SpO2, blood oxygen saturation; US, United States; WBC, white blood cells; WHO, World Health Organization.
CLINICAL FACTORS
Hypoxemia
The PIDS/IDSA guideline recommends that children who require a fraction of inspired oxygen (FiO2) of ≥0.50 to maintain an oxygen saturation of >92%, or those with an arterial oxygen partial pressure (PaO2)/FiO2 <250, should be admitted to the intensive care unit (ICU) or a unit with continuous cardiorespiratory monitoring [7]. Hypoxemia is also a component of the Pediatric Early Warning Score (PEWS), a validated general early warning score to predict need for ICU care in children [21]. Although the association with hypoxemia with severe outcomes is well recognized, exact thresholds for defining hypoxemia are variable (BTS <92%, PIDS/IDSA <90%) [7, 8].
Numerous studies in developing countries show a consistent association between hypoxemia and mortality in childhood pneumonia [19, 22–25]. In the developed world, supplemental oxygen requirement was the most significant determinant in predicting time to clinical stability in hospitalized children with CAP [26]. The PF ratio has also been shown to be the factor most strongly associated with severe outcomes [18].
Age
The PIDS/IDSA guideline states that infants and young children are at highest risk for severe disease [7]. In the developed world, 1-year-olds have increased odds of severe disease compared with 2-year-olds [18], and infants are more likely to have pneumonia-specific readmissions (adjusted odds ratio [aOR], 1.36; 95% confidence interval [CI], 1.14–1.61) [27]. In children hospitalized with pneumonia in the developing world, age <6 months was the strongest factor associated with enteral treatment failure (aOR, 5.15; 95% CI, 2.94–9.02) among children 3–59 months [28], age <4 months was associated with mortality (relative risk [RR], 3.5; 95% CI, 3.0–4.2) among children <24 months [23], and age <6 months was associated with mortality (OR, 2.2; 95% CI, 1.1–4.2) among children <15 years [19].
Tachypnea
Tachypnea is included in the WHO definition, the BTS and PIDS/IDSA severity criteria, and in PEWS [21]. In the developing world, WHO-defined tachypnea-for-age is associated with mortality [19]. In the developed world, studies found tachypnea-for-age was not associated with severe disease in infants but showed increasing association as children got older (aOR = 0.99–1.53, depending on age) [18].
The use of tachypnea as a severity sign has important limitations, including association with fever, dehydration, and acidosis [29]; physician impression of tachypnea has only fair interrater reliability (kappa 0.42) [30]; and age-based respiratory rate thresholds vary, including conflicting definitions from WHO [1], Pediatric Advanced Life Support [31], and Advanced Pediatric Life Support [32] (Table 4). Recent studies suggest thresholds could be broken down into smaller groupings, because respiratory rate shows significant variation based on age [33].
Table 4.
Respiratory Rate Cutoffs to Define Age-Specific Tachypnea
| Source | Respiratory Rate Thresholds Defining Tachypnea-for-Age |
|---|---|
| WHO [1] | 0–2 months: >60 |
| 2–12 months: >50 | |
| 1–5 years: >40 | |
| Older than 5 years: >20 | |
| PALS [31] | Infant: >53 |
| Toddler: >37 | |
| Preschooler: >28 | |
| School-aged child: >25 | |
| Adolescent: >20 | |
| APLS [32] | 0–3 months: >50 |
| 3–6 months: >45 | |
| 6–18 months: >40 | |
| 18–24 months: >35 | |
| 2–8 years: >30 | |
| 8–12 years: >25 Older than 12 years: >24 |
|
| Bonafide et al [33]a | 0–3 months: >62 |
| 3–6 months: >58 | |
| 6–9 months: >54 | |
| 9–12 months: >51 | |
| 12–18 months: >48 | |
| 18–24 months: >45 | |
| 2–3 years: >42 | |
| 3–4 years: >40 | |
| 4–6 years: >37 | |
| 6–8 years: >35 | |
| 8–12 years: >31 | |
| 12–15 years: >28 | |
| 15–18 years: >26 |
Abbreviations: APLS, Advanced Pediatric Life Support; PALS, Pediatric Advanced Life Support; WHO, World Health Organization.
a95th percentile-for-age cutoffs used for study by Bonafide et al [38].
Dyspnea
Examination findings associated with dyspnea include accessory muscle use, retractions, nasal flaring, and grunting. Williams et al [18] found that chest indrawing was associated with severe outcomes (aOR, 2.12; 95% CI, 1.62–2.78). Reed et al [25] found that chest indrawing was an independent predictor of mortality in HIV-negative children <24 months old hospitalized with lower respiratory tract infections (LRTIs) in South Africa (aOR, 4.6; 95% CI, 2.2–9.4). In children hospitalized with WHO-defined severe or very severe pneumonia in a developing nation, head bobbing was associated with mortality (RR, 8.3; 95% CI, 2.71–12.77) and mechanical ventilation (RR, 4.7; 95% CI, 1.50–6.36) [34]. Grunting is associated with hypoxemia [35, 36] and can suggest impending respiratory failure [7]. Retractions have fairly strong interrater reliability (kappa 0.62) among children with suspected CAP; however, nasal flaring, head bobbing, and grunting have only fair reliability (kappa 0.49, 0.25, and 0.33, respectively) [30].
Tachycardia
Tachycardia may be due to multiple factors including pain, anxiety/fear, fever, dehydration, and underlying disease processes. Tachycardia is included in PEWS [21] and as a severity marker in the BTS guideline [8]. The PIDS/IDSA guideline recommends admission to the ICU or continuous cardiopulmonary monitoring for sustained tachycardia [7].
Data specifically investigating tachycardia in pediatric pneumonia severity are limited. Although Williams et al [18] found that tachycardia was one of the factors associated with severe pneumonia (aOR = 1.59–2.90, depending on age), Reed et al [25] found that admission heart rate >170 was not associated with mortality (OR, 0.9; 95% CI, 0.3–3.2) in HIV-negative infants <24 months old.
Altered Mental Status
Altered mental status (AMS) in children with CAP is often multifactorial, and it can be due to hypercarbia, hypoxemia, severe dehydration, sepsis, or a combination. It is included in the WHO and PIDS/IDSA guidelines [1, 7]. Altered mental status was one of the factors most associated with severe outcomes in the Williams et al [18] study (aOR, 11.9; 95% CI, 6.41–22.23). Araya et al [19] found that a Glasgow Coma Score <13 was the factor most associated with mortality in children admitted with pneumonia (OR, 324; 95% CI, 131–805). In the ED, AMS was highly specific for hospital admission in children with pneumonia (LR+ 10.6) [17]. In children admitted with WHO-defined severe or very severe pneumonia, AMS was associated with mortality (RR, 5.44; 95% CI, 1.34–17.56) [34], and in children admitted with WHO-defined pneumonia in a developing nation, “alteration of general status” based on clinician impression was also associated with mortality (aOR, 3.23; 95% CI, 1.17–8.94) [37].
Temperature
Resolution of fever is a common sign of appropriate therapy and is often used to monitor clinical improvement; however, data are limited to suggest an association between height or duration of fever and pneumonia severity. The BTS guideline uses >38.5°C as a marker for severe pneumonia [8]. The PIDS/IDSA and WHO guidelines do not include fever in their severity criteria [1, 7]. Multiple studies in developing nations have found no association between degree of fever and pneumonia severity in children [22, 23, 25]. Hypothermia may be more indicative of severe disease, as Williams et al [18] found that temperature >39°C (aOR, 0.50; 95% CI, 0.39–0.65) was a protective factor against severe outcomes, whereas hypothermia (<35°C) was associated with severity (aOR, 2.0; 95% CI, 1.54–2.59).
Dehydration and Decreased Perfusion
In developing countries, clinical dehydration in malnourished children with radiographically confirmed pneumonia admitted to the ICU was associated with death (OR, 9.48; 95% CI, 2.42–37.19) [38]. Delayed capillary refill is one sign that dehydration has progressed to decreased perfusion. It is included in the BTS guideline (≥2 seconds) [8] and in PEWS [21]. Although widely used, the interrater reliability of capillary refill in suspected CAP is fair to poor (kappa 0.18) [30] and is highly variable in assessing dehydration (kappa 0.15–0.64) [39].
Hypotension
Age-specific hypotension is the defining factor of uncompensated septic shock [40]. Data examining hypotension specifically in pediatric pneumonia is limited and conflicting. Williams et al [18] found that systolic blood pressure <5th percentile-for-age was not associated with severe outcomes (aOR, 0.95–1.15). In developing nations, Chisti et al [38] found no association between hypotension and CAP severity in children; however, Araya et al [19] found a significant association between mean arterial pressure >2 standard deviation below mean-for-age and mortality in children hospitalized with pneumonia (OR, 48.7; 95% CI, 24.8–95.6).
Comorbidities
Araya et al [19] found that significant comorbidities (malnutrition, HIV, congenital heart disease [CHD], asthma, and Down syndrome) were associated with mortality (OR, 4.9–6.2). A retrospective study of children in the ICU with pneumonia found that cerebral palsy, epilepsy, and CHD were associated with mortality (OR, 1.49–2.37), whereas asthma was protective (OR, 0.17; 95% CI, 0.09–0.31) [41]. In a multicenter retrospective study comparing children hospitalized with aspiration pneumonia versus CAP, Hirsch et al [42] found that children with aspiration pneumonia had longer hospital length of stay (LOS) and were more likely to receive ICU care.
Duration of Symptoms/Time to Correct Antibiotic Therapy
Champatiray et al [43] found that in Indian children hospitalized with WHO-defined severe/very severe pneumonia, delayed presentation was associated with mortality, although the mortality rate in the study was high overall (22%). Muszynski et al [44] found that in children with pneumonia requiring invasive mechanical ventilation, time to correct antibiotic selection based on bacterial cultures was independently associated with duration of mechanical ventilation, hospital, and ICU LOS.
RADIOGRAPHIC FACTORS
Despite the widespread use of chest radiographs (CXR), challenges limit their accuracy and utility [45, 46], and clinical factors such as hydration status, degree of atelectasis, and time of presentation can influence interpretation [47]. International single-center studies suggest that various anatomic locations are associated with CAP severity in children (left lung [48], upper lobes [49], bilateral multifocal, and right hilum [50]). These studies evaluated severity based on LOS, duration of fever, dyspnea, tachypnea, and hydration status and did not consider more severe markers [51]. More recently, studies in developing and developed nations (including well powered prospective multicenter studies) have shown multilobar infiltrates are associated with severe outcomes including ICU admission, mechanical ventilation, vasoactive medications, and death [18, 19, 51].
In a recent multicenter cohort, pleural effusions of any size were associated with longer LOS (aOR, 2.6; 95% CI, 1.9–3.6) and duration of supplemental oxygen (aOR, 3.0; 95% CI, 1.4–6.5). Moderate or large effusions were associated with ICU admission (aOR, 3.2; 95% CI, 1.1–8.9) and mechanical ventilation (aOR, 14.8; 95% CI, 9.8–22.4) [51]. In a large multicenter study of children unvaccinated against Streptococcus pneumoniae hospitalized with WHO-defined severe pneumonia, Ferrero et al [52] found that pleural effusions were associated with pneumococcal bacteremia (OR, 3.1; 95% CI, 1.23–7.98). In children hospitalized with pneumonia, pleural effusions were the factors most associated with empiric parenteral ampicillin/sulbactam treatment failure (aOR, 5.74; 95% CI, 2.17–15.15) [53]. Complex loculated effusions contribute to treatment failure with conservative measures leading to increased LOS and need for surgical interventions [54, 55].
Empyema is also associated with severity including prolonged hospitalizations, bacteremia, and need for ICU admission, but not mortality [56, 57]. Goldbart et al [58] found that empyema was associated with LOS, ICU admission, and mechanical ventilation compared with children with nonpurulent effusions.
Necrotizing pneumonia is an increasingly recognized complication of CAP, defined by parenchymal liquefaction and necrosis, later replaced by air or fluid-filled cavities. Lung necrosis and abscesses are generally a result of bacterial pathogens, particularly S pneumoniae and Staphylococcus aureus, the latter of which is associated with a more severe disease course [59, 60]. Retrospective studies suggest that necrotizing pneumonia in children is associated with prolonged hospital stays, ICU admission, and surgical interventions, but not with mortality [56, 61–65]. Necrosis can extend through pleura leading to bronchopleural fistulas (BPF), which are associated with duration of fever, LOS, and mechanical ventilation when compared with culture-proven pneumococcal pneumonia without BPF [66, 67]. Pneumothoraces in children admitted with pneumonia are also associated with mortality (OR, 15; 95% CI, 2.9–76.6) [19].
Emerging evidence suggests that lung ultrasound is highly sensitive and specific for diagnosing pneumonia in children compared with CXR [68, 69]. Evidence of multifocal disease and fluid bronchograms on transthoracic ultrasound are associated with severity [70]. Lai et al [71] found that degree of impaired perfusion on ultrasound is associated with severity of necrosis and need for resection.
LABORATORY MARKERS
Complete Blood Count
Studies have consistently shown that leukocytosis alone is a poor predictor of pneumonia etiology and severity [7, 18, 19, 72–76]; however, Araya et al [19] found that leukopenia (<4000) was associated with mortality (OR, 6.5; 95% CI, 2.7–15.6). The PIDS/IDSA guideline recommends, with low-quality evidence, that a complete blood count should only be performed in severe CAP [7] to evaluate for severe complications such as hemolytic-uremic syndrome (HUS).
Inflammatory Markers
C-reactive protein (CRP) is an acute phase reactant that is associated with disease severity in bacterial infections in children [77]; however, studies have not shown substantial associations between CRP and CAP severity. A single-center cross-sectional study found that elevated CRP was not associated with hypoxemia, dyspnea, or tachycardia [78]. Another retrospective observational study of children admitted with CAP found that admission CRP was minimally associated with LOS and duration of fever (adjusted ratio of means 1.03 and 1.08, respectively) [72]. In children admitted with WHO-defined pneumonia, CRP was not associated with WHO severity criteria (OR, 1.01; 95% CI, 0.99–1.02) [76]. Reed et al [25] found that admission CRP was not associated with mortality in young children hospitalized with LRTIs.
Procalcitonin is a precursor of calcitonin that can increase in bacterial infections and inflammatory states. A multicenter, prospective study found increasing levels of procalcitonin were associated with ICU admission and empyema requiring drainage, and values <0.25 ng/mL were associated with decreased risk of ICU admission [79]. An Indian study found that elevation in admission procalcitonin in radiographically confirmed and WHO-defined severe or very severe pneumonia was associated with increased LOS and pneumonia complications [80]. In a prospective study of children diagnosed with CAP in the ED, Don et al [74] found that elevated procalcitonin was associated with hospitalization, whereas a cross-sectional study of hospitalized children found no association with markers for severity including tachycardia, hypoxemia, and dyspnea [78]. A primary care study found that procalcitonin was not associated with hospitalization in children with radiographically confirmed pneumonia [81]. These conflicting studies are limited by heterogenous populations, study settings, and varied outcomes.
Electrolytes
Several studies suggest that hyponatremia may be associated with CAP severity in children, although limitations prevent definitive conclusions. In a small-scale, single-center Indian study, hyponatremia was associated with LOS, complications, and mortality, although LOS and mortality rates were high overall [82]. More recently, 2 European studies (a retrospective study of children hospitalized with CAP [83] and a prospective ED-based study [84]) found that hyponatremia was associated with hospitalization, LOS, inflammatory markers, and degree of fever but not with respiratory rate, tachycardia, capillary refill, or defervescence.
Acidosis
Araya et al [19] found that HCO3− <15 was associated with mortality (OR, 26.7; 95% CI, 13–54), and Wang et al [85] found that metabolic acidosis was independently associated with mortality in children hospitalized with pneumonia (aOR, 8.5; 95% CI, 2.82–25.6).
Bacteremia
In the developed world, bacteremia in childhood pneumonia is uncommon, with rates <1% for outpatients, 2.5% for hospitalized children, and 13% in complicated pneumonia [7, 86–88]. Although blood cultures upon admission are recommended by the PIDS/IDSA guideline, the majority of organisms isolated from blood cultures in children with pneumonia were sensitive to guideline-recommended therapy (ie, penicillin) and rarely changed management [86, 88]. Bacteremia in childhood pneumonia has been associated with hypoxemia, LOS, and complications including effusions and empyema; however, data are limited for more severe measures [86, 89, 90].
Concern for Hemolytic-Uremic Syndrome
A 2011 survey of pediatric infectious disease physicians found that cases of S pneumoniae-associated HUS (diagnosed by microangiopathic hemolytic anemia, renal injury, and platelets <150000/mL) were associated with severe outcomes including ICU admission, invasive procedures/mechanical ventilation, and dialysis [91].
Molecular Diagnostics/Genomics
Molecular diagnostic tools are likely to improve severity classification in pediatric CAP. An association has been shown between pneumococcal load and pneumonia severity in adults [92]. In children, plasma pneumococcal load is associated with prolonged hospital stays (aOR, 3.53; 95% CI, 1.43–8.70) [93], and pleural pneumococcal load is associated with worsening necrosis including progression to BPF [67]. Urinary antigen tests have shown promise, and Shen et al [94] found that time to positivity and intensity of band reactions may correlate with pneumococcal CAP severity based on dyspnea, hypoxemia, bacteremia, LOS, and ICU admission. In addition, sputum microbiota profiles using 16S ribosomal ribonucleic acid may influence pneumonia severity, likely due to complex interactions between bacteria and a child’s immune response [95].
FUTURE DIRECTIONS
This systematic review highlights the current evidence regarding the factors that influence pneumonia severity in children. The current evidence suggests that hypoxemia, AMS, age <3–6 months, dyspnea, multilobar infiltrates, and moderate/large pleural effusions are the factors most predictive of pneumonia severity in children. The development and validation of pediatric pneumonia severity scoring systems for use in settings where site-of-care decisions are made have the potential to improve risk stratification.
Emerging technologies and molecular diagnostics will likely play an increasing role in severity assessment. Quantitative pneumococcal load and pneumococcal urinary antigen tests have shown promise in severity assessment in children [67, 93, 94], although pneumococcal pneumonia represents only one cause of CAP [96]. Gene expression profiling and other -omics approaches will provide new insights into CAP etiology and severity [97]. In nonpneumococcal cases, quantitative urinary metabolites have shown the potential to assess for mortality risk, although further research is still needed [98]. As the use of both rapid viral testing and polymerase chain reaction have become more common, viral coinfections have shown an association with CAP severity in children and may play a role in risk stratification [99, 100]. Assessing for impaired perfusion on lung ultrasound has shown promise in predicting pneumonia complications (necrosis) and may play a larger role in severity assessment going forward [71]. The development of a validated pneumonia severity scoring system across outpatient, ED, and inpatient settings, in combination with emerging technologies, will improve risk stratification and resource allocation.
Notes
Acknowledgments. We sincerely thank Elaine Grigg Dean for assistance and guidance with our literature search.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. Revised WHO Classification and Treatment of Childhood Pneumonia at Health Facilities: Evidence Summaries. Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- 2. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization. N Engl J Med 2015; 373:2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pfuntner A, Wier L, Stocks C.. Most Frequent Conditions in U.S. Hospitals, 2011. HCUP Statistical Brief #162. Rockville, MD: Agency for Healthcare Research and Quality, 2013. [PubMed] [Google Scholar]
- 4. Florin TA, French B, Zorc JJ, et al. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics 2013; 132:237–44. [DOI] [PubMed] [Google Scholar]
- 5. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J 2012; 31:1036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Handy LK, Bryan M, Gerber JS, et al. Variability in antibiotic prescribing for community-acquired pneumonia. Pediatrics 2017; 139:pii: 20162331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011; 53:e25–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 2011; 66(Suppl 2):ii1–23. [DOI] [PubMed] [Google Scholar]
- 9. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008; 47:375–84. [DOI] [PubMed] [Google Scholar]
- 10. Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336:243–50. [DOI] [PubMed] [Google Scholar]
- 11. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. España PP, Capelastegui A, Gorordo I, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med 2006; 174:1249–56. [DOI] [PubMed] [Google Scholar]
- 13. Pereira JM, Paiva JA, Rello J. Assessing severity of patients with community-acquired pneumonia. Semin Respir Crit Care Med 2012; 33:272–83. [DOI] [PubMed] [Google Scholar]
- 14. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jo S, Kim K, Jung K, et al. The effects of incorporating a pneumonia severity index into the admission protocol for community-acquired pneumonia. J Emerg Med 2012; 42:133–8. [DOI] [PubMed] [Google Scholar]
- 16. Chalmers JD, Singanayagam A, Akram AR, et al. Safety and efficacy of CURB65-guided antibiotic therapy in community-acquired pneumonia. J Antimicrob Chemother 2011; 66:416–23. [DOI] [PubMed] [Google Scholar]
- 17. Florin TA, Brokamp C, Mantyla R, et al. Validation of the PIDS/IDSA severity criteria in children with community-acquired pneumonia. Clin Infect Dis 2018. doi: 10.1093/cid/ciy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams DJ, Zhu Y, Grijalva CG, et al. Predicting severe pneumonia outcomes in children. Pediatrics 2016; 138:e20161019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Araya S, Lovera D, Zarate C, et al. Application of a prognostic scale to estimate the mortality of children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J 2016; 35:369–73. [DOI] [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parshuram CS, Hutchison J, Middaugh K. Development and initial validation of the Bedside Paediatric Early Warning System score. Crit Care 2009; 13:R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duke T, Mgone J, Frank D. Hypoxaemia in children with severe pneumonia in Papua New Guinea. Int J Tuberc Lung Dis 2001; 5:511–9. [PubMed] [Google Scholar]
- 23. Djelantik IG, Gessner BD, Sutanto A, et al. Case fatality proportions and predictive factors for mortality among children hospitalized with severe pneumonia in a rural developing country setting. J Trop Pediatr 2003; 49:327–32. [DOI] [PubMed] [Google Scholar]
- 24. Nantanda R, Hildenwall H, Peterson S, et al. Bacterial aetiology and outcome in children with severe pneumonia in Uganda. Ann Trop Paediatr 2008; 28:253–60. [DOI] [PubMed] [Google Scholar]
- 25. Reed C, Madhi SA, Klugman KP, et al. Development of the Respiratory Index of Severity in Children (RISC) score among young children with respiratory infections in South Africa. PLoS One 2012; 7:e27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolf RB, Edwards K, Grijalva CG, et al. Time to clinical stability among children hospitalized with pneumonia. J Hosp Med 2015; 10:380–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neuman MI, Hall M, Gay JC, et al. Readmissions among children previously hospitalized with pneumonia. Pediatrics 2014; 134:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mamtani M, Patel A, Hibberd PL, et al. A clinical tool to predict failed response to therapy in children with severe pneumonia. Pediatr Pulmonol 2009; 44:379–86. [DOI] [PubMed] [Google Scholar]
- 29. Smyth A, Carty H, Hart CA. Clinical predictors of hypoxaemia in children with pneumonia. Ann Trop Paediatr 1998; 18:31–40. [DOI] [PubMed] [Google Scholar]
- 30. Florin TA, Ambroggio L, Brokamp C, et al. Reliability of examination findings in suspected community-acquired pneumonia. Pediatrics 2017; 140:pii: e20170310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. American Heart Association. In: Chameides L, Samson RA, Schexnayder SM, Hazinski MF, eds. PediatricAdvanced Life Support Provider Manual. Dallas, TX: American Heart Association, 2016. [Google Scholar]
- 32. Advanced Life Support Group. In: Samuels M, Wieteska S, eds. Advanced Paediatric Life Support: A Practical Approach to Emergencies. 6th ed. Chichester: John Wiley and Sons, 2016. [Google Scholar]
- 33. Bonafide CP, Brady PW, Keren R, et al. Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics 2013; 131:e1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tiewsoh K, Lodha R, Pandey RM, et al. Factors determining the outcome of children hospitalized with severe pneumonia. BMC Pediatr 2009; 9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Basnet S, Adhikari RK, Gurung CK. Hypoxemia in children with pneumonia and its clinical predictors. Indian J Pediatr 2006; 73:777–81. [DOI] [PubMed] [Google Scholar]
- 36. Kuti BP, Adegoke SA, Ebruke BE, et al. Determinants of oxygen therapy in childhood pneumonia in a resource-constrained region. ISRN Pediatr 2013; 2013:435976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Demers AM, Morency P, Mberyo-Yaah F, et al. Risk factors for mortality among children hospitalized because of acute respiratory infections in Bangui, Central African Republic. Pediatr Infect Dis J 2000; 19:424–32. [DOI] [PubMed] [Google Scholar]
- 38. Chisti MJ, Salam MA, Ashraf H, et al. Clinical risk factors of death from pneumonia in children with severe acute malnutrition in an urban critical care ward of Bangladesh. PLoS One 2013; 8:e73728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fleming S, Gill P, Jones C, et al. Validity and reliability of measurement of capillary refill time in children: a systematic review. Arch Dis Child 2015; 100:239–49. [DOI] [PubMed] [Google Scholar]
- 40. Goldstein B, Giroir B, Randolph A, et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8. [DOI] [PubMed] [Google Scholar]
- 41. Hsu CL, Lee YS, Chen CJ, et al. A population-based analysis of children with pneumonia among intensive care units in Taiwan. J Microbiol Immunol Infect 2015; 48:153–9. [DOI] [PubMed] [Google Scholar]
- 42. Hirsch AW, Monuteaux MC, Fruchtman G, et al. Characteristics of children hospitalized with aspiration pneumonia. Hosp Pediatr 2016; 6:659–66. [DOI] [PubMed] [Google Scholar]
- 43. Champatiray J, Satapathy J, Kashyap B, Mondal D. Clinico-aetiological study of severe and very severe pneumonia in two months to five years children in a Tertiary Health Care Centre in Odisha, India. J Clin Diagn Res 2017; 11:SC06–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muszynski JA, Knatz NL, Sargel CL, et al. Timing of correct parenteral antibiotic initiation and outcomes from severe bacterial community-acquired pneumonia in children. Pediatr Infect Dis J 2011; 30:295–301. [DOI] [PubMed] [Google Scholar]
- 45. Neuman MI, Lee EY, Bixby S, et al. Variability in the interpretation of chest radiographs for the diagnosis of pneumonia in children. J Hosp Med 2012; 7:294–8. [DOI] [PubMed] [Google Scholar]
- 46. Williams GJ, Macaskill P, Kerr M, et al. Variability and accuracy in interpretation of consolidation on chest radiography for diagnosing pneumonia in children under 5 years of age. Pediatr Pulmonol 2013; 48:1195–200. [DOI] [PubMed] [Google Scholar]
- 47. Gereige RS, Laufer PM. Pneumonia. Pediatr Rev 2013; 34:438–56. [DOI] [PubMed] [Google Scholar]
- 48. Grafakou O, Moustaki M, Tsolia M, et al. Can chest X-ray predict pneumonia severity?Pediatr Pulmonol 2004; 38:465–9. [DOI] [PubMed] [Google Scholar]
- 49. Kin Key N, Araújo-Neto CA, Nascimento-Carvalho CM. Severity of childhood community-acquired pneumonia and chest radiographic findings. Pediatr Pulmonol 2009; 44:249–52. [DOI] [PubMed] [Google Scholar]
- 50. Patria MF, Longhi B, Lelii M, et al. Association between radiological findings and severity of community-acquired pneumonia in children. Ital J Pediatr 2013; 39:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McClain L, Hall M, Shah SS, et al. Admission chest radiographs predict illness severity for children hospitalized with pneumonia. J Hosp Med 2014; 9:559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ferrero F, Nascimento-Carvalho CM, Cardoso MR, et al. Radiographic findings among children hospitalized with severe community-acquired pneumonia. Pediatr Pulmonol 2010; 45:1009–13. [DOI] [PubMed] [Google Scholar]
- 53. Tapısız A, Özdemir H, Çiftçi E, et al. Ampicillin/sulbactam for children hospitalized with community-acquired pneumonia. J Infect Chemother 2011; 17:504–9. [DOI] [PubMed] [Google Scholar]
- 54. Margenthaler JA, Weber TR, Keller MS. Predictors of surgical outcome for complicated pneumonia in children: impact of bacterial virulence. World J Surg 2004; 28:87–91. [DOI] [PubMed] [Google Scholar]
- 55. Ozcelik C, Inci I, Nizam O, Onat S. Intrapleural fibrinolytic treatment of multiloculated postpneumonic pediatric empyemas. Ann Thorac Surg 2003; 76:1849–53. [DOI] [PubMed] [Google Scholar]
- 56. Erlichman I, Breuer O, Shoseyov D, et al. Complicated community acquired pneumonia in childhood: different types, clinical course, and outcome. Pediatr Pulmonol 2017; 52:247–54. [DOI] [PubMed] [Google Scholar]
- 57. Langley JM, Kellner JD, Solomon N, et al. Empyema associated with community-acquired pneumonia: a Pediatric Investigator’s Collaborative Network on Infections in Canada (PICNIC) study. BMC Infect Dis 2008; 8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Goldbart AD, Leibovitz E, Porat N, et al. Complicated community acquired pneumonia in children prior to the introduction of the pneumococcal conjugated vaccine. Scand J Infect Dis 2009; 41:182–7. [DOI] [PubMed] [Google Scholar]
- 59. Spencer DA, Thomas MF. Necrotising pneumonia in children. Paediatr Respir Rev 2014; 15:240–5. [DOI] [PubMed] [Google Scholar]
- 60. Taffarel P, Bonetto G, Penazzi M, et al. Severe Staphylococcus aureus infection in three pediatric intensive care units: analysis of cases of necrotizing pneumonia. Arch Argent Pediatr 2014; 112:163–8. [DOI] [PubMed] [Google Scholar]
- 61. Sawicki GS, Lu FL, Valim C, et al. Necrotising pneumonia is an increasingly detected complication of pneumonia in children. Eur Respir J 2008; 31:1285–91. [DOI] [PubMed] [Google Scholar]
- 62. Bender JM, Ampofo K, Korgenski K, et al. Pneumococcal necrotizing pneumonia in Utah: does serotype matter?Clin Infect Dis 2008; 46:1346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Krenke K, Sanocki M, Urbankowska E, et al. Necrotizing pneumonia and its complications in children. Adv Exp Med Biol 2015; 857:9–17. [DOI] [PubMed] [Google Scholar]
- 64. Donnelly LF, Klosterman LA. Cavitary necrosis complicating pneumonia in children: sequential findings on chest radiography. AJR Am J Roentgenol 1998; 171:253–6 [DOI] [PubMed] [Google Scholar]
- 65. Hacimustafaoglu M, Celebi S, Sarimehmet H, et al. Necrotizing pneumonia in children. Acta Paediatr 2004; 93:1172–7. [DOI] [PubMed] [Google Scholar]
- 66. Hsieh YC, Wang CW, Lai SH, et al. Necrotizing pneumococcal pneumonia with bronchopleural fistula among children in Taiwan. Pediatr Infect Dis J 2011; 30:740–4. [DOI] [PubMed] [Google Scholar]
- 67. Hsieh YC, Chi H, Chang KY, et al. Increase in fitness of Streptococcus pneumoniae is associated with the severity of necrotizing pneumonia. Pediatr Infect Dis J 2015; 34:499–505. [DOI] [PubMed] [Google Scholar]
- 68. Guerra M, Crichiutti G, Pecile P, et al. Ultrasound detection of pneumonia in febrile children with respiratory distress: a prospective study. Eur J Pediatr 2016; 175:163–70. [DOI] [PubMed] [Google Scholar]
- 69. Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics 2015; 135:714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen IC, Lin MY, Liu YC, et al. The role of transthoracic ultrasonography in predicting the outcome of community-acquired pneumonia in hospitalized children. PLoS One 2017; 12:e0173343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lai SH, Wong KS, Liao SL. Value of lung ultrasonography in the diagnosis and outcome prediction of pediatric community-acquired pneumonia with necrotizing change. PLoS One 2015; 10:e0130082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Williams DJ, Hall M, Auger KA, et al. Association of white blood cell count and c-reactive protein with outcomes in children hospitalized for community-acquired pneumonia. Pediatr Infect Dis J 2015; 34:792–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Korppi M, Heiskanen-Kosma T, Leinonen M. White blood cells, C-reactive protein and erythrocyte sedimentation rate in pneumococcal pneumonia in children. Eur Respir J 1997; 10:1125–9. [DOI] [PubMed] [Google Scholar]
- 74. Don M, Valent F, Korppi M, Canciani M. Differentiation of bacterial and viral community-acquired pneumonia in children. Pediatr Int 2009; 51:91–6. [DOI] [PubMed] [Google Scholar]
- 75. Prat C, Domínguez J, Rodrigo C, et al. Procalcitonin, C-reactive protein and leukocyte count in children with lower respiratory tract infection. Pediatr Infect Dis J 2003; 22:963–8. [DOI] [PubMed] [Google Scholar]
- 76. Wu J, Jin YU, Li H, et al. Evaluation and significance of C-reactive protein in the clinical diagnosis of severe pneumonia. Exp Ther Med 2015; 10:175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rey C, Los Arcos M, Concha A, et al. Procalcitonin and C-reactive protein as markers of systemic inflammatory response syndrome severity in critically ill children. Intensive Care Med 2007; 33:477–84. [DOI] [PubMed] [Google Scholar]
- 78. Agnello L, Bellia C, Di Gangi M, et al. Utility of serum procalcitonin and C-reactive protein in severity assessment of community-acquired pneumonia in children. Clin Biochem 2016; 49:47–50. [DOI] [PubMed] [Google Scholar]
- 79. Stockmann C, Ampofo K, Killpack J, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatric Infect Dis Soc 2018; 7:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yadav KK, Awasthi S, Takia L, et al. Procalcitonin and C-reactive protein in WHO defined severe and very severe community acquired pneumonia: a hospital based cross-sectional study. Clin Epidemiol Glob Health 2015; 3:S3–9. [Google Scholar]
- 81. Korppi M, Remes S, Heiskanen-Kosma T. Serum procalcitonin concentrations in bacterial pneumonia in children: a negative result in primary healthcare settings. Pediatr Pulmonol 2003; 35:56–61. [DOI] [PubMed] [Google Scholar]
- 82. Singhi S, Dhawan A. Frequency and significance of electrolyte abnormalities in pneumonia. Indian Pediatr 1992; 29:735–40. [PubMed] [Google Scholar]
- 83. Wrotek A, Jackowska T. Hyponatremia in children hospitalized due to pneumonia. Adv Exp Med Biol 2013; 788:103–8. [DOI] [PubMed] [Google Scholar]
- 84. Don M, Valerio G, Korppi M, Canciani M. Hyponatremia in pediatric community-acquired pneumonia. Pediatr Nephrol 2008; 23:2247–53. [DOI] [PubMed] [Google Scholar]
- 85. Wang LJ, Mu SC, Lin CH, et al. Fatal community-acquired pneumonia: 18 years in a medical center. Pediatr Neonatol 2013; 54:22–7. [DOI] [PubMed] [Google Scholar]
- 86. Shah SS, Dugan MH, Bell LM, et al. Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J 2011; 30:475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. St Peter SD, Tsao K, Spilde TL, et al. Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: a prospective, randomized trial. J Pediatr Surg 2009; 44:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Neuman MI, Hall M, Lipsett SC, et al. Utility of blood culture among children hospitalized with community-acquired pneumonia. Pediatrics 2017; 140:pii: e20171013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Iroh Tam PY, Bernstein E, Ma X, Ferrieri P. Blood culture in evaluation of pediatric community-acquired pneumonia: a systematic review and meta-analysis. Hosp Pediatr 2015; 5:324–36. [DOI] [PubMed] [Google Scholar]
- 90. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J 2013; 32:736–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Banerjee R, Hersh AL, Newland J, et al. Streptococcus pneumoniae-associated hemolytic uremic syndrome among children in North America. Pediatr Infect Dis J 2011; 30:736–9. [DOI] [PubMed] [Google Scholar]
- 92. Rello J, Lisboa T, Lujan M, et al. Severity of pneumococcal pneumonia associated with genomic bacterial load. Chest 2009; 136:832–40. [DOI] [PubMed] [Google Scholar]
- 93. Muñoz-Almagro C, Gala S, Selva L, et al. DNA bacterial load in children and adolescents with pneumococcal pneumonia and empyema. Eur J Clin Microbiol Infect Dis 2011; 30:327–35. [DOI] [PubMed] [Google Scholar]
- 94. Shen CF, Wang SM, Liu CC. A new urinary antigen test score correlates with severity of pneumococcal pneumonia in children. J Formos Med Assoc 2011; 110:613–8. [DOI] [PubMed] [Google Scholar]
- 95. Pettigrew MM, Gent JF, Kong Y, et al. Association of sputum microbiota profiles with severity of community-acquired pneumonia in children. BMC Infect Dis 2016; 16:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. West DM, McCauley LM, Sorensen JS, et al. Pneumococcal urinary antigen test use in diagnosis and treatment of pneumonia in seven Utah hospitals. ERJ Open Res 2016; 2. doi: 10.1183/23120541.00011-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mejias A, Dimo B, Suarez NM, et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med 2013; 10:e1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ambroggio L, Florin TA, Shah SS, et al. Emerging biomarkers of illness severity: urinary metabolites associated with sepsis and necrotizing methicillin-resistant Staphylococcus aureus pneumonia. Pharmacotherapy 2017; 37:1033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Williams DJ, Hall M, Brogan TV, et al. Influenza coinfection and outcomes in children with complicated pneumonia. Arch Pediatr Adolesc Med 2011; 165:506–12. [DOI] [PubMed] [Google Scholar]
- 100. Dawood FS, Chaves SS, Perez A, et al. Complications and associated bacterial coinfections among children hospitalized with seasonal or pandemic influenza, United States, 2003–2010. J Infect Dis 2014; 209:686–94. [DOI] [PubMed] [Google Scholar]