Summary
The inpatient burden of dysphagia has primarily been evaluated in patients with stroke. It is unclear whether dysphagia, irrespective of cause, is associated with worse clinical outcomes and higher costs compared to inpatients with similar demographic, hospital, and clinical characteristics without dysphagia. The aim of this study is to assess how a dysphagia diagnosis affects length of hospital stay (LOS), costs, discharge disposition, and in-hospital mortality among adult US inpatients. Annual and overall dysphagia prevalence, LOS, hospital charges, inpatient care costs, discharge disposition, and in-hospital mortality were measured using the AHRQ Healthcare Cost and Utilization Project (HCUP) National Inpatient Sample (2009–2013). Patients aged 45 years or older with ≤180 days of stay in hospital with and without dysphagia were included. Multivariable survey regression methods with propensity weighting were used to assess associations between dysphagia and different outcomes. Overall, 2.7 of 88 million (3.0%) adult US inpatients had a dysphagia diagnosis (50.2% male, 72.4% white, 74.6% age 65–90 years) and prevalence increased from 408,035 (2.5% of admissions) in 2009 to 656,655 (3.3%) in 2013. After inverse probability of treatment weighting adjustment, mean hospital LOS in patients with dysphagia was 8.8 days (95% CI 8.66–8.90) compared to 5.0 days (95% CI 4.97–5.05) in the non-dysphagia group (P < 0.001). Total inpatient costs were a mean $6,243 higher among those with dysphagia diagnoses ($19,244 vs. 13,001, P < 0.001). Patients with dysphagia were 33.2% more likely to be transferred to post-acute care facility (71.9% vs. 38.7%, P < 0.001) with an adjusted OR of 2.8 (95% CI 2.73–2.81, P < 0.001). Compared to non-cases, adult patients with dysphagia were 1.7 times more likely to die in the hospital (95% CI 1.67–1.74). Dysphagia affects 3.0% of all adult US inpatients (aged 45–90 years) and is associated with a significantly longer hospital length of stay, higher inpatient costs, a higher likelihood of discharge to post-acute care facility, and inpatient mortality when compared to those with similar patient, hospital size, and clinical characteristics without dysphagia. Dysphagia has a substantial health and cost burden on the US healthcare system.
Keywords: costs, dysphagia, hospitalization, inpatient, mortality
INTRODUCTION
Dysphagia or impaired swallowing is often classified anatomically as either oropharyngeal or esophageal. Prevalence in community-dwelling persons over age 50 years is estimated to be between 15% and 22%1-3, while 40% to 60% of assisted living facility and nursing home residents are reported to have feeding difficulties.3,4 A 2012 National Health Interview Survey estimated that 9.44 ± 0.33 million adults reported having a swallowing problem in the preceding 12 months with stroke being the most commonly reported etiology (11.2%) followed by other neurologic cause (7.2%) and head and neck cancer (4.9%).5 In fact, dysphagia affects 1 in 25 adults annually and has significant psychosocial burden with approximately 41% of patients reporting anxiety during mealtimes and 36% avoid eating with others because of their dysphagia.6 Despite health and psychosocial implications, only a third of those affected seek professional treatment.6
Consequences of untreated and unrecognized dysphagia can be profound including malnutrition, volume depletion, quality of life issues, and aspiration, which may ultimately be the pivotal factor that precipitates a decline in a patient's outcome.7,8 Given its high prevalence, surprisingly few studies have evaluated the economic and survival burden associated with dysphagia among inpatients, and those that have focus primarily on stroke patients. One study evaluating the impact of dysphagia among inpatients with stroke using 2005–2006 National Hospital Discharge Survey noted that a dysphagia diagnosis was associated with 40% increased length of hospital stay and patients undergoing rehabilitation had a 13-fold increased risk of mortality during their hospitalization.9 Another study evaluating post-stroke dysphagia at a single tertiary center found that it was an independent predictor of discharge disposition and institutionalization at 3 months, and that severe dysphagia requiring gastrostomy tube placement was associated with higher mortality.10 Similar results have been found in patients after anterior cervical discectomy (ACD) and patients with dementia who have dysphagia.11,12
These data suggest that dysphagia may have a significant economic and survival burden regardless of etiology. Nonetheless, the overall impact of dysphagia among US inpatients irrespective of primary admission diagnosis and comorbidities is unknown. Early recognition of dysphagia and intervention in the hospitalized patient should be prioritized if dysphagia is indeed associated with longer hospital length of stay and higher mortality. To address this void, this study aims to determine the clinical and economic burden associated with dysphagia among adult US inpatients using 2009–2013 hospital discharge data from the AHRQ Healthcare Cost and Utilization Project (HCUP) National Inpatient Sample.
METHODS
This research received institutional review board approval from the Vanderbilt University Medical Center (IRB#610481). Informed consent was waived due to use of the de-identified HCUP database.
Data source
We used 2009–2013 hospital discharge data from the AHRQ-sponsored HCUP National Inpatient Sample (NIS) to assess prevalence of dysphagia in adult patients and to determine the effect of having a diagnosis of dysphagia on length of stay (LOS), in-hospital mortality, discharge disposition, total charges, and in-hospital costs. The NIS is a nationally representative 20% stratified sample of more than 94% of discharges from US hospitals (excluding rehabilitation and long-term, acute-care hospitals) with survey weights available to compute national estimates, representing more than 95% of the US population.13
Included in this analysis were patients aged 45 years or older with ≤180 days of stay in hospital. Age ≥ 45 years was selected because evidence suggests that dysphagia becomes more prevalent after this age14 and to permit balanced propensity weighting between patients with and without dysphagia. Dysphagia was defined using International Classification of Diseases (ICD)-9 discharge diagnosis codes (primary and/or secondary diagnosis) 787.20 to 787.29 and are referred to herein as ‘cases’. Patients without dysphagia ICD-9 codes formed the ‘non-case’ group. Variables with potential or expected relationship to the exposure and outcomes were extracted from NIS. These included patient characteristics (sex, race, age), type of admission to hospital (elective, nonelective), payer status (Medicare, Medicaid, private insurance, self-pay, no charge, other), comorbidities, hospital characteristics including hospital's census region (Northeast, Midwest, South, and West), and bed size (small, medium, and large). Elixhauser comorbidity index was used to quantify comorbidity, which was then stratified into zero, 1–2, or ≥3 total scores.15
Outcomes
Four outcomes were considered: (1) hospital length of stay, (2) total charges and inpatient care costs, (3) discharge disposition, and (4) in-hospital mortality. Cost of inpatient care per discharge was calculated based on the total charge of the hospitalization and the group average all-payer inpatient cost to charge ratio estimated by the HCUP. Hospital length of stay (LOS) equals the days between hospital admission and discharge. Post-hospitalization discharge disposition was categorized into home/routine care, acute care facilities (e.g. skilled nursing, home health), died in hospital, or other discharges (e.g. court, law enforcement, against medical advice, or destination unknown).
Statistical analysis
To account for the complex sampling design, analyses were conducted using survey methods in SAS 9.4 (SAS Institute, Cary, NC) and results reflect weighted estimates with standard errors computed using Taylor series estimation method. First, unweighted and weighted sample size and the percentage distribution of dysphagia for each of the five sampled calendar years (NIS 2009–2013) were determined. The distribution of basic characteristics including age, comorbidity, sex, race, hospital region, bed size, payer status, and type of admission were generated by dysphagia status using trend weights provided by HCUP and presented as weighted percentage (95% CI) and compared by Χ2 test. Standardized difference of proportions was calculated for each level of the covariate. Means with 95% confidence intervals are provided for continuous outcome variables using survey trend weights and are compared between cases and non-cases using t-test.
To assess the effect of dysphagia with multiple outcomes and with distribution of covariates similar to that of the sample without a dysphagia diagnosis, we conducted propensity score analysis using inverse probability treatment weighting (IPTW) method to bring a balance on the aforementioned covariates between groups. Propensity scores (pi) were generated with survey weights, design variables, all covariates, and interactions as predictors of dysphagia in the logistic regression model. According to this method, cases received a weight equal to 1/pi and the non-cases received a weight equal to 1/(1-pi) and extreme weights were trimmed at 99th percentile.16 Finally, we generated new weights by multiplying survey weights with IPTW to account for survey design and to achieve an unbiased effect estimate.17
Covariate balance between cases and non-cases were checked using absolute standardized difference of proportions in the two groups after applying new weights. An absolute standardized difference of <0.10 for a covariate was considered to have a relatively small imbalance between groups.18 Weighted (new IPTW) multivariate survey regression analyses were performed to assess the association between dysphagia with different outcomes and compared the difference in outcomes from 2009 to 2013 using year as an independent term in the regression procedures. Complete case analysis was performed and all statistical tests were two-sided with a significance level of P < 0.001.
RESULTS
Overall, 2.7 of 88 million (3.0%) adult US inpatients 45 years of age or older had a dysphagia diagnosis (50.2% male, 72.4% white, 74.6% age 65–90 years) (Table 1) and its prevalence increased from 408,035 (2.5% of admissions) in 2009 to 656,655 (3.3%) in 2013. Cases were older (75% vs. 57% aged 65–90 years), more likely to have Medicare insurance (76% vs. 61%), and higher comorbidity scores (72% vs. 53% had comorbidity index ≥3). The five most common Diagnosis Related Groups (DRG) among cases were (1) septicemia (7.9%; DRG 871), (2) intracranial hemorrhage or cerebral infarction (7.7%; DRG 64–65), (3) rehabilitation (6.4%; DRG 945), (4) respiratory infections (4.1%; DRG 177), and (5) esophagitis, gastroenteritis, and miscellaneous digestive disorders (3.6; DRG 392). All the demographic characteristics including age, comorbidity, sex, race, hospital region, hospital size, payer status, and type of hospital admission were significantly different between cases and non-cases (P < 0.001). After adjustment with IPTW, the imbalance between cases and non-cases reduced from a mean standardized difference of 0.127 to 0.008 with an average overall reduction in bias of 93.7% (Table 1).
Table 1.
Characteristics of adult inpatients in United States (HCUP-NIS 2009–2013)
| Absolute | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Unadjusted | IPTW adjusted | IPTW adjusted | standardized | ||
| dysphagia | no dysphagia | dysphagia | no dysphagia | difference | ||
| % (95%CI) | % (95%CI) | % (95%CI) | % (95%CI) | after IPTW | ||
| Total (weighted) | 2,656,196 (2.90%) | 88,054,454 (97.10%) | 2,630,428 (2.90%) | 88,068,657 (97.10%) | ||
| Age | 45–64 | 25.4 (25.01–25.72) | 43.0 (42.65–43.31) | 41.8 (41.32–42.23) | 42.5 (42.14–42.79) | 0.014 |
| 65–90 | 74.6 (74.28–74.99) | 57.0 (56.69–57.35) | 58.2 (57.77–58.68) | 57.5 (57.21–57.86) | 0.014 | |
| Sex | Female | 49.8 (49.57–50.01) | 53.4 (53.23–53.53) | 53.2 (52.95–53.40) | 53.3 (53.15–53.40) | 0.002 |
| Male | 50.2 (49.99–50.43) | 46.6 (46.47–46.77) | 46.8 (46.60–47.05) | 46.7 (46.60–46.85) | 0.002 | |
| Race | Caucasian | 72.4 (71.50–73.30) | 73.6 (72.69–74.52) | 74.0 (73.18–74.72) | 73.6 (72.77–74.37) | 0.009 |
| African American | 13.7 (13.13–14.33) | 13.3 (12.68–13.84) | 13.3 (12.75–13.78) | 13.3 (12.76–13.79) | 0.000 | |
| Hispanic | 7.7 (7.09–8.32) | 7.9 (7.34–8.52) | 7.7 (7.15–8.22) | 7.9 (7.40–8.45) | 0.009 | |
| Asian/Pacific Islander | 3.0 (2.73–3.35) | 1.9 (1.71–2.06) | 1.9 (1.72–2.01) | 1.9 (1.77–2.06) | 0.004 | |
| Native American | 0.4 (0.36–0.52) | 0.6 (0.49–0.71) | 0.6 (0.51–0.73) | 0.6 (0.50–0.69) | 0.004 | |
| Other | 2.7 (2.42–2.96) | 2.7 (2.43–3.01) | 2.6 (2.36–2.87) | 2.7 (2.45–2.99) | 0.006 | |
| Comorbidity scores | 0 | 2.5 (2.45–2.60) | 8.6 (8.41–8.71) | 7.3 (7.16–7.52) | 8.4 (8.25–8.52) | 0.039 |
| 1–2 | 25.1 (24.72– 25.47) | 38.8 (38.54–39.04) | 38.3 (37.89–38.66) | 38.4 (38.16–38.61) | 0.002 | |
| ≥3 | 72.4 (71.96–72.81) | 52.7 (52.30–52.99) | 54.2 (52.95–53.87) | 53.2 (52.92–53.53) | 0.023 | |
| Region | Northeast | 17.0 (15.74–18.31) | 19.6 (18.34–20.90) | 19.6 (18.42–20.71) | 19.5 (18.57–20.52) | 0.001 |
| Midwest | 18.7 (17.44–20.04) | 19.9 (18.60–21.10) | 19.9 (18.83–20.96) | 19.8 (18.87–20.76) | 0.002 | |
| South | 43.7 (41.91–45.47) | 42.5 (40.92–44.03) | 42.6 (41.34–43.79) | 42.5 (41.43–43.59) | 0.001 | |
| West | 20.5 (19.12–21.96) | 18.1 (16.92–19.19) | 18.0 (17.04–18.90) | 18.1 (17.40–18.86) | 0.004 | |
| Bed Size | Small | 12.0 (11.18–12.83) | 13.3 (12.60–14.03) | 13.2 (12.52–13.89) | 13.3 (12.73–13.83) | 0.002 |
| Medium | 25.0 (23.64–26.27) | 25.3 (24.18–26.42) | 25.3 (23.35–26.32) | 25.3 (24.48–26.11) | 0.001 | |
| Large | 63.0 (61.51–64.57) | 61.4 (60.04–62.73) | 61.5 (60.30–62.63) | 61.4 (60.44–62.42) | 0.001 | |
| Payer | Medicare | 75.7 (75.20–76.17) | 60.8 (60.38–61.24) | 62.2 (61.67–62.66) | 61.3 (60.87–61.62) | 0.019 |
| Medicaid | 6.7 (6.50–6.97) | 8.3 (7.94–8.57) | 8.2 (8.00–8.47) | 8.2 (7.94–8.48) | 0.001 | |
| Private insurance | 13.4 (13.04–13.81) | 23.5 (23.10–23.92) | 22.5 (22.02–22.91) | 23.2 (22.86–23.57) | 0.018 | |
| Self-pay | 2.1 (1.92–2.19) | 4.1 (3.85–4.26) | 3.9 (3.65–4.04) | 4.0 (3.81–4.18) | 0.007 | |
| No charge | 0.2 (0.19–0.30) | 0.5 (0.40–0.56) | 0.5 (0.37–0.52) | 0.5 (0.40–0.55) | 0.003 | |
| Other | 1.9 (1.73–1.97) | 2.9 (2.74–3.04) | 2.8 (2.69–2.98) | 2.9 (2.73–2.99) | 0.001 | |
| Type of admission | Nonelective | 84.5 (83.92–85.05) | 76.8 (76.26–77.27) | 78.0 (77.36–78.56) | 77.0 (76.55–77.42) | 0.023 |
| Elective | 15.5 (14.95–16.08) | 23.2 (22.73–23.74) | 22.0 (21.44–22.64) | 23.0 (22.58–23.45) | 0.023 | |
All unadjusted comparisons are significantly different at P < 0.001.
IPTW, inverse probability treatment weighting.
Hospital length of stay
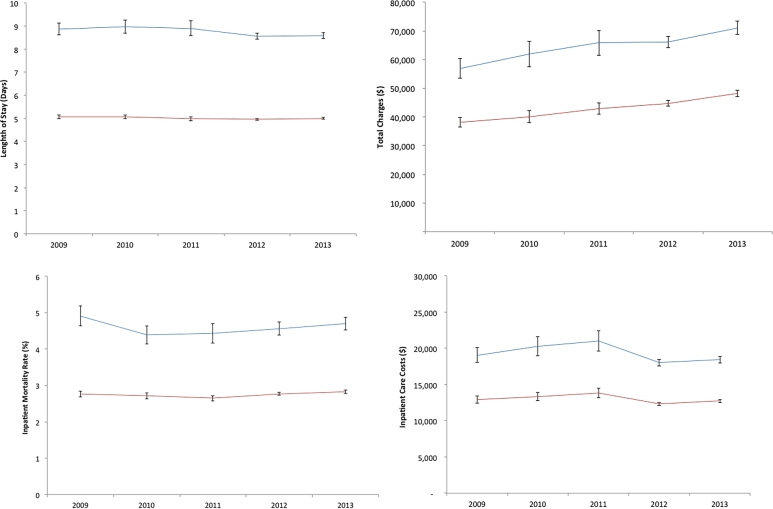
Hospital LOS per admission was stable over time in both groups between 2009 and 2013 (Fig. 1). Cases were hospitalized a mean 8.8 days (95% CI 8.66–8.90) compared with 5.0 days (95% CI 4.97–5.05) among non-cases (P < 0.001). Thus, cases had 3.8 additional hospital days or a 43% longer LOS than non-cases (Table 2).
Fig. 1.
Outcomes comparing US inpatients with and without dysphagia (HCUP-NIS 2009–2013). Panels: (A) adjusted mean length of stay (95% confidence interval), (B) adjusted mean total charges (95% confidence interval), (C) adjusted mean in-hospital mortality (95% confidence interval), and (D) adjusted mean inpatient care costs (95% confidence interval). Blue = dysphagia, Red = no dysphagia.
Table 2.
Comparison of length of stay, total charges, and inpatient care costs between patients with and without dysphagia (HCUP-NIS 2009–2013)
| Unadjusted | Unadjusted | IPTW adjusted | IPTW adjusted | |
|---|---|---|---|---|
| dysphagia | no dysphagia | dysphagia | no dysphagia | |
| mean (95%CI) | mean (95%CI) | mean (95%CI) | mean (95%CI) | |
| Length of stay (days) | 9.11 (9.01–9.21) | 4.99 (4.97–5.01) | 8.78 (8.66–8.90) | 5.01 (4.97–5.05) |
| Total charges ($) | 68,540 (66,956–70,124) | 42,987 (42,269–43,705) | 65,181 (63,707–66,655) | 43,069 (42,350–43,788) |
| Inpatient care cost ($) | 19,907 (19,478–20,336) | 12,987 (12,790–13,184) | 19,244 (18,823–19,665) | 13,001 (12,804–13,198) |
IPTW, inverse probability treatment weighting.
Total charges & inpatient care cost
Total charges increased over the study period (Fig. 1). The adjusted mean total charges for cases was $65,181 (95% CI $63,707–66,655) compared to $43,069 in non-cases (95% CI $42,350–43,788; P < 0.001, Table 2). Inpatient care costs were relatively stable in both cases and non-cases (Fig. 1). Overall mean inpatient care costs were $19,244 (95% CI 18,823–19,665) and $13,001 (95% CI $12,804–13,198) in cases and non-cases (P < 0.001, Table 2). Thus, cases had 34% higher total charges and 33% higher inpatient care costs than non-cases. Even though both the total charges and inpatient care costs were significantly different between cases and non-cases (P < 0.001), there was no significant trend in the magnitude of the difference over the 5-year period.
Discharge disposition & mortality
Mortality rates were stable over the study period in both groups (Fig. 1), with the unadjusted in-hospital mortality rate more than double among cases than non-cases (5.6% vs. 2.7%, P < 0.001). After IPTW adjustment, patients with dysphagia were 1.7 times more likely to die in the hospital (95% CI 1.67–1.74) than non-cases. The disposition of surviving adult inpatients was also different. A higher proportion of cases were transferred to post-acute care facilities (Table 3). Specifically, 33.2% more cases were transferred to post-acute care facilities. Thus, compared to non-cases, patients with dysphagia were significantly more likely to be discharged to post-acute care facilities (adjusted OR 2.8, 95% CI 2.73–2.81) than to be discharged to routine care/home.
Table 3.
Association of dysphagia with discharge disposition among survivors (HCUP-NIS 2009–2013)
| Dysphagia | No dysphagia | Crude | IPTW adjusted | |
|---|---|---|---|---|
| % (95% CI)† | % (95% CI)† | OR (95%CI) | OR (95%CI) | |
| Routine care | 27.5 (27.12–27.85) | 60.3 (59.96–60.57) | Referent | Referent |
| HHC, transfers | 71.9 (71.57–72.29) | 38.7 (38.34–38.96) | 4.1 (4.02–4.14)* | 2.8 (2.73–2.81)* |
| Other | 0.6 (0.54–0.62) | 1.1 (1.05–1.12) | 1.2 (1.10–1.27)* | 1.0 (0.98–1.11) |
†Unadjusted.
*P < 0.001; IPTW, inverse probability treatment weighting.
DISCUSSION
This study is first to describe the significant economic and survival burden associated with a diagnosis of dysphagia among all inpatients. Compared to other inpatients with similar characteristics, those with dysphagia had 3.8 days longer length of stay, 33% higher inpatient care costs, were 2.8-times more likely to require post-acute care services, and had 1.7-fold higher odds of dying in the hospital. Thus, a diagnosis of dysphagia appears to be an indicator of worse outcomes and higher costs.
Costs
Total costs among inpatients with dysphagia were 42% to 44% higher per admission. Reasons for excess cost likely relate to longer length of stay, diagnostic testing, and additional treatment. This study was designed to provide an overview of costs and did not investigate more granular cost differences. Extrapolating these figures, dysphagia was responsible for between $4.3 to $7.1 billion in additional hospital costs annually, which is higher compared to inpatient cost of treating non-valvular atrial fibrillation ($2.93 billion)19 and comparable to inpatient cost of treating community-acquired pneumonia ($7.5 billion).20 Thus, patients with dysphagia incurred an additional $16.8 billion in inpatient costs between 2009 and 2013 compared to patients without the diagnosis. It is also important to note that reported costs relate only to inpatient care and underestimate the overall economic consequence of dysphagia, which would also include direct post-discharge costs (e.g. home health, skill-nursing facilities) and indirect costs to patient, family, and society.
Which patients are diagnosed?
Unique from non-cases, most common DRG’s associated with dysphagia were related to stroke and infection (i.e. septicemia, respiratory infections). To date, the largest literature focusing on the effect of dysphagia is in stroke patients. Post-stroke dysphagia is associated with longer hospital length of stay, greater utilization of post-acute care facilities upon discharge, and higher odds of mortality compared to stroke patients without dysphagia.10,21,22
As found herein, infectious etiologies like septicemia and respiratory infections (e.g. pneumonia) are commonly related to dysphagia. In one study of mechanically ventilated intensive care unit patients from 2009 to 2010 (excluded patients with stroke or neuromuscular disease), post-extubation dysphagia was independently associated with the composite outcome of pneumonia, reintubation, and death (OR 3.31, 95% CI of 1.89 to 5.90).23 Studies in elderly residents of long-term care facilities have also shown dysphagia to be the most important risk factor leading to pneumonia (OR 2.0, 95% CI 1.2–3.3).24,25 This study is first to quantify the effect of dysphagia in patients by comparing outcomes among patients possessing similar characteristics without dysphagia.
Is dysphagia a marker for disease severity?
Some argue that dysphagia is simply an indicator of overall disease severity. This was proposed in a study evaluating consequences of dysphagia using the National Hospital Discharge Survey (2005–2006), as patients with dysphagia had more overall diagnoses at the time of discharge (64.8% vs. 38.6%).9 Although certainly plausible, we found significantly higher hospital LOS and in-hospital mortality after adjusting for patient characteristics, comorbidity, and hospital-related factors. In fact, the absolute in-hospital mortality was 2.9% higher per year in patients with dysphagia, which translates into an additional 11,833 to 19,043 deaths per year among patients with dysphagia. A delay in recognizing dysphagia can also lead to significant morbidity due to risk of malnutrition and aspiration pneumonia, need for intubation, antibiotics, and potential need for enteral feeding using nasoenteral tubes or percutaneous gastrostomy tubes. This can lead to delay in hospital discharge, need for post-acute care facility and escalating hospital costs.
Implications
Findings from this study provide important insights into the burden of dysphagia. It behooves clinicians and hospitals to recognize and treat dysphagia early to reduce the risk of in-hospital mortality. Evidence exists that referrals for inpatient swallow studies have increased by 63% between 2007 and 2014 among 60–90+ year-old acute care geriatric-hospitalized patients.26 Promising early interventions are available. Novel interventions such as use of wi-fi-based wireless flexible endoscopic or the swallowing provocation test (SPT) and the simple SPT (S-SPT) for evaluation of swallowing among inpatients have been shown to detect silent aspiration and reduce risk of aspiration pneumonia and number of deaths during hospitalization.27-29 Early behavioral swallowing intervention including intensive speech therapy and dietary modification in inpatients with dysphagia and stroke has been associated with increased proportion of patients who returned to normal diet and recovered swallowing by 6 months.30 In another group with acute intracerebral hemorrhage, early intervention program for oral feeding with intensive oral care and early behavioral interventions was associated with lower incidence of chest infection (OR 0.48, 95% CI 0.26–0.88) and greater proportion of patients who could tolerate oral feeding (OR 3.13, 95% CI 1.59–6.15) compared to control group.31 Although current literature has primarily assessed impact of early intervention on dysphagia secondary to neurologic etiology, the high economic and survival burden in all adult US inpatients with dysphagia regardless of comorbidities suggest that earlier recognition and intervention should be considered for all inpatients with dysphagia.
Limitations
HCUP data derive from administrative coding, which has inherent limitations. 2.7 million (3.0%) inpatients 45 years of age or older with a dysphagia diagnosis between 2009 and 2013 is likely an underestimate since many patients have subclinical dysphagia and/or a dysphagia diagnosis might not have been included among ICD-9 diagnoses at time of discharge. Furthermore, we used the validated Elixhauser comorbidity index to control for the comorbidities between the dysphagia and non-dysphagia group, which primarily relies on ICD diagnosis codes found in administrative data. This again relies on accuracy of ICD diagnosis codes entered into the hospital system, which can be subject of variability. However, in a systematic review analyzing comorbidity indices for evaluation of administrative data, Elixhauser comorbidity index was noted to have the best predictive characteristics especially for mortality.32-34 Generalizability of findings is also a concern since the analysis was restricted to inpatients aged 45 years or older. Adults 18–44 years of age were excluded because age and comorbidity distribution among those with and without dysphagia were not adequately balanced for IPTW. Recognizing this limitation, analyses were repeated for all adults (18+ years of age), which yielded similar hospital length of stay, inpatient costs, likelihood of discharge to post-acute care and inpatient mortality were observed. Finally, the most common ICD-9 code for dysphagia was 787.20 (dysphagia, unspecified). An ‘unspecified’ code precludes identifying the pathophysiological site of dysfunction, making it difficult to determine if majority of these patients had oropharyngeal or esophageal dysphagia. Nonetheless, given the profound health and cost implications of dysphagia among hospitalized patients, early identification and treatment of dysphagia should be encouraged. Further prospective longitudinal studies are needed to assess and evaluate for potential causes of higher mortality in this group of patients and develop cost-effective screening and treatment strategies.
CONCLUSION
Dysphagia affects 3.0% of all adult US inpatients 45 years of age or older and is associated with a significantly longer hospital length of stay, higher inpatient costs, a higher likelihood of discharge to post-acute care facility, and higher odds of inpatient mortality when compared to inpatients of similar ages and comorbidities.
Notes
Funding: National Foundation of Swallowing Disorders; Salary support for D.O.F. provided by grants K23DC013559 and L30DC012687 and for M.R.C. by grant R01DC014583 from the National Institute for Deafness and Communication Disorders of the National Institute of Health.
Disclosures and Conflicts of Interest: None by any author.
Specific author contributions: Interpretation of data: Dhyanesh Patel; Drafting of the manuscript: Dhyanesh Patel; Data management: Shanthi Krishnaswami; Analysis and interpretation of data: Shanthi Krishnaswami, David Francis; Critical revision of manuscript: Shanthi Krishnaswami; Study concept and design: Ed Steger, Ellen Conover, Michael Vaezi, Michelle Ciucci; Study concept: David Francis; Design: David Francis; Acquisition of data: David Francis; Obtained funding: David Francis; Critical revision of the manuscript: David Francis; Study supervision: David Francis.
References
- 1. Cook I J, Kahrilas P J. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology 1999; 116: 455–78. [DOI] [PubMed] [Google Scholar]
- 2. Lindgren S, Janzon L. Prevalence of swallowing complaints and clinical findings among 50-79-year-old men and women in an urban population. Dysphagia 1991; 6: 187–92. [DOI] [PubMed] [Google Scholar]
- 3. Barczi S R, Sullivan P A, Robbins J. How should dysphagia care of older adults differ? Establishing optimal practice patterns. Semin Speech Lang 2000; 21: 347–61. [DOI] [PubMed] [Google Scholar]
- 4. Siebens H, Trupe E, Siebens A et al. Correlates and consequences of eating dependency in institutionalized elderly. J Am Geriatr Soc 1986; 34: 192–8. [DOI] [PubMed] [Google Scholar]
- 5. Bhattacharyya N. The prevalence of dysphagia among adults in the United States. Otolaryngol Head Neck Surg 2014; 151: 765–9. [DOI] [PubMed] [Google Scholar]
- 6. Ekberg O, Hamdy S, Woisard V et al. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia 2002; 17: 139–46. [DOI] [PubMed] [Google Scholar]
- 7. Ekberg O, Feinberg M. Clinical and demographic data in 75 patients with near-fatal choking episodes. Dysphagia 1992; 7: 205–8. [DOI] [PubMed] [Google Scholar]
- 8. Gelperin A. Sudden death in an elderly population from aspiration of food. J Am Geriatr Soc 1974; 22: 135–6. [DOI] [PubMed] [Google Scholar]
- 9. Altman K W, Yu G P, Schaefer S D. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg 2010; 136: 784–9. [DOI] [PubMed] [Google Scholar]
- 10. Arnold M, Liesirova K, Broeg-Morvay A et al. Dysphagia in acute stroke: incidence, burden and impact on clinical outcome. PLoS One 2016;11:e0148424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Starmer H M, Riley L H 3rd, Hillel A T et al. Dysphagia, short-term outcomes, and cost of care after anterior cervical disc surgery. Dysphagia 2014; 29: 68–77. [DOI] [PubMed] [Google Scholar]
- 12. Paranji S, Paranji N, Wright S et al. A nationwide study of the impact of dysphagia on hospital outcomes among patients with dementia. Am J Alzheimers Dis Other Demen 2017; 32: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Databases H. Healthcare Cost and Utilization Project (HCUP). www.hcup-us.ahrq.gov/nisoverview.jsp:Agency for Heatlhcare Research and Quality, Rockville, MD, 2009–13. [PubMed] [Google Scholar]
- 14. Roden D F, Altman K W. Causes of dysphagia among different age groups: a systematic review of the literature. Otolaryngol Clin North Am 2013; 46: 965–87. [DOI] [PubMed] [Google Scholar]
- 15. Elixhauser A, Steiner C, Harris D R et al. Comorbidity measures for use with administrative data. Medical care 1998; 36: 8–27. [DOI] [PubMed] [Google Scholar]
- 16. Harder V S, Stuart E A, Anthony J C. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods 2010; 15: 234–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dugoff E H, Schuler M, Stuart E A. Generalizing observational study results: applying propensity score methods to complex surveys. Health Serv Res 2014; 49: 284–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Normand S T, Landrum M B, Guadagnoli E et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol 2001; 54: 387–98. [DOI] [PubMed] [Google Scholar]
- 19. Coyne K S, Paramore C, Grandy S et al. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health 2006; 9: 348–56. [DOI] [PubMed] [Google Scholar]
- 20. Niederman M S, McCombs J S, Unger A N et al. The cost of treating community-acquired pneumonia. Clin Ther 1998; 20: 820–37. [DOI] [PubMed] [Google Scholar]
- 21. Paciaroni M, Mazzotta G, Corea F et al. Dysphagia following Stroke. Eur Neurol 2004; 51: 162–7. [DOI] [PubMed] [Google Scholar]
- 22. Smithard D G, Smeeton N C, Wolfe C D. Long-term outcome after stroke: does dysphagia matter? Age Ageing 2007; 36: 90–4. [DOI] [PubMed] [Google Scholar]
- 23. Macht M, Wimbish T, Clark B J et al. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit Care 2011; 15: R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loeb M, McGeer A, McArthur M et al. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med 1999; 159: 2058–64. [DOI] [PubMed] [Google Scholar]
- 25. Marik P E, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest 2003; 124: 328–36. [DOI] [PubMed] [Google Scholar]
- 26. Leder S B, Suiter D M, Agogo G O et al. An epidemiologic study on ageing and dysphagia in the acute care geriatric-hospitalized population: a replication and continuation study. Dysphagia 2016; 31: 619–25. [DOI] [PubMed] [Google Scholar]
- 27. Sakakura K, Tazawa M, Otani N et al. Impact of a multidisciplinary round visit for the management of dysphagia utilizing a wi-fi-based wireless flexible endoscopic evaluation of swallowing. Ann Otol Rhinol Laryngol 2017; 126: 47–53. [DOI] [PubMed] [Google Scholar]
- 28. Teramoto S, Matsuse T, Fukuchi Y et al. Simple two-step swallowing provocation test for elderly patients with aspiration pneumonia. Lancet 1999; 353: 1243. [DOI] [PubMed] [Google Scholar]
- 29. Teramoto S, Yamamoto H, Yamaguchi Y et al. A novel diagnostic test for the risk of aspiration pneumonia in the elderly. Chest 2004; 125: 801–2. [DOI] [PubMed] [Google Scholar]
- 30. Carnaby G, Hankey G J, Pizzi J. Behavioural intervention for dysphagia in acute stroke: a randomised controlled trial. Lancet Neurol 2006; 5: 31–7. [DOI] [PubMed] [Google Scholar]
- 31. Takahata H, Tsutsumi K, Baba H et al. Early intervention to promote oral feeding in patients with intracerebral hemorrhage: a retrospective cohort study. BMC Neurol 2011; 11: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yurkovich M, Avina-Zubieta J A, Thomas J et al. A systematic review identifies valid comorbidity indices derived from administrative health data. J Clin Epidemiol 2015; 68: 3–14. [DOI] [PubMed] [Google Scholar]
- 33. Sharabiani M T, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care 2012; 50: 1109–18. [DOI] [PubMed] [Google Scholar]
- 34. Chu Y T, Ng Y Y, Wu S C. Comparison of different comorbidity measures for use with administrative data in predicting short- and long-term mortality. BMC Health Serv Res 2010; 10: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]