Abstract
Objective
Difficulties managing medications, particularly among older adults experiencing cognitive deficits, is an important contributing factor to medication nonadherence that may have significant negative financial and health outcomes. The current study examined the performance of healthy older adults’ (HOA) and individuals with amnestic mild cognitive impairment (aMCI) on the medication management abilities assessment’s (MMAA, a performance-based measure of medication management) original scoring criteria and derived error process measures, assessing medication overtaking and undertaking magnitude. Exploratory correlations between performances on the MMAA and self-reported confidence in medication management skills and cognitive abilities were also examined.
Method
A sample of 25 HOAs with aMCI and 25 age- and education-matched HOAs completed the MMAA, a self-reported medication management confidence rating and a battery of neuropsychological tests.
Results
HOAs performed significantly better on the MMAA score and committed significantly less process errors than individuals with aMCI. Despite these differences in MMAA performance, the HOA and aMCI groups rated similar high levels of confidence in their ability to manage a new medication routine. Notably, while the HOA group’s performance on all of the MMAA measures did not relate to cognitive measures, the aMCI group’s performance on the MMAA score was significantly related to memory and executive functioning and a new process error score for overtaking was related to processing speed.
Conclusions
Although these results present promising potential for the MMAA as a measure of medication management in a clinical setting, further studies need to examine the validity of the MMAA against real-world adherence measures.
Keywords: Everyday functioning, Mild cognitive impairment, Executive functioning, Learning and memory
Introduction
It is estimated that medication nonadherence is prevalent in 50% of the community dwelling older adult population and is as high as 75% in individuals with mild cognitive impairment (MCI; Murray, 2004; Pasina et al., 2014; Turner, Hochschild, Burnett, Zulfiqar, & Dyer, 2012). Significant negative outcomes have been associated with nonadherence, including annual health care costs of $100–$300 billion, emergency department admissions, and mortality (Benjamin, 2012; Fitzgerald et al., 2011; Pirmohamed et al., 2004; Pretorius, Gataric, Swedlund, & Miller, 2013). Contributing factors to medication nonadherence include side effects, attitudes towards medications and the ability to manage medications (Smith et al., 2017; Yap, Thirumoorthy, & Kwan, 2015). Medication nonadherence encompasses intentional and unintentional deviations from medical instructions (e.g., choosing not to and being unable to take medications). Medication management, specifically, refers to the abilities necessary to store, refill and remember when and how to take medications, as well as remember whether medications have already been taken (NIH Senior Health, n.d.). In this study, we compare medication management abilities of HOA and individuals with aMCI and examine neurocognitive correlates of medication management abilities.
In prior research, biomedical, naturalistic in-home techniques, and laboratory measures have been used to examine medication adherence and management. Although biomedical and in-home measures like blood testing, pill counting and medication event monitoring systems (MEMS; e.g., pill caps that record the date and time a pill bottle is opened) are often considered more accurate measurements of adherence, these measures also require significant resources (e.g., time and cost). In comparison, laboratory measures, such as self-report and performance-based measures, are designed as proxy measures of adherence. These proxy measures generally assess aspects of medication use, for example medication management, that are hypothesized to contribute to adherence.
Self-report measures are the most practical and widely used laboratory measures for assessing medication use because they are low-cost, flexible, and quick to administer (Stirratt et al., 2015). However, self-report measures also have significant disadvantages, including social desirability, inaccurate recall and reporting, and ceiling effects (Wilson, Carter, & Berg, 2009). Shi and colleagues (2010) conducted a meta-analysis of 11 studies that examined differences between self-reported adherence and MEMS measurements of adherence within the general public (age range of 22–62 years). Although the average self-reported adherence was 84% (i.e., ranging from 68.5% to 95.0%), the MEMS’ adherence rate was 74.9% (i.e., ranging from 53.4% to 92.9%) suggesting that self-reports may yield higher rates of adherence than MEMS within the general public. In an older adult sample (Mage = 77.1, SD = 8.6), self-reports of medication adherence may be further exaggerated as the mean self-reported medication adherence in one study was 95.8%, while the average pill count adherence was significantly lower at 74.0% (Grymonpre, Didur, Montgomery, & Sitar, 1998). In another study, 93% of OA self-reported having “excellent adherence”, but only 53% of the sample was adherent as measured by an electronic pill box (Hayes, Larimer, Adami, & Kaye, 2009). Hayes and colleagues (2009) further found that a subsample of cognitively compromised OAs were significantly less adherent to their medication routine compared to HOAs despite self-reporting similarly high levels of medication adherence. This suggests that inaccurate recall impacts the validity of self-report measures of adherence more in individuals with comprised cognition.
Unlike self-report measures, performance-based medication management assessments provide objective and quantifiable measures, which can be useful tools in clinical settings to identify difficulties in self-managing medication, thereby reducing risk of medication nonadherence (Elliott & Marriott, 2009; Wesson, Clemson, Brodaty, & Reppermund, 2016). The most common performance-based task used with OAs is the drug regimen unassisted grading scales (DRUGS, Edelberg, Shallenberger, & Wei, 1999; Wesson et al., 2016). This task measures a patient’s ability to manage their own medication routine by requiring participants to bring their own medications into the clinic (i.e., brown bag task) and demonstrate their ability to recall their own medication routine (i.e., dosage and times). The medication routine they recall is then compared against their medical records. The medication management abilities assessment (MMAA; Patterson et al., 2002), which mimics organizing a new medication routine, also assesses medication management. Performance on the MMAA has been found to correlate with the DRUGS in community dwelling OAs (Hutchison, Jones, West, & Wei, 2006). An advantage of the MMAA over other performance-based medication measures (e.g., DRUGS) is that it is less of a disruption to an individuals’ personal medication management (i.e., not a brown bag task). Another advantage is that the MMAA requires participants to manage a new medication routine with four novel medication and food rules reducing a familiarity bias. Notably, the number of medications the MMAA requires participants to manage is comparable to the average OA’s medication routine (i.e., 35.8% of OAs managing five medications; Qato, Wilder, Schumm, Gillet, & Alexander, 2016).
Several studies have been conducted comparing performance on the MMAA between healthy control participants and clinical populations (i.e., Parkinson’s disease, schizophrenia and mental illness). Individuals with severe mental illness and with Parkinson’s disease MCI were found to perform more poorly than control groups on the MMAA (Patterson et al., 2002; Pirogovsky et al., 2013, 2014; Wesson et al., 2016). In contrast, individuals with Parkinson’s disease without MCI did not differ from healthy adults (Pirogovsky et al., 2014), suggesting that the MMAA may be sensitive to changes in cognition.
Generally, both poorer medication adherence as measured by MEMS and pill counts and lower performance on the MMAA have been related to poorer global cognitive scores (Depp et al., 2008; Hayes et al., 2009; Jeste et al., 2003; Patterson et al., 2002). When specific cognitive factors are examined, the literature suggests that memory and executive functioning are most related to medication adherence in OAs with and without cognitive impairment (Insel, Morrow, Brewer, & Figueredo, 2006; Manning et al., 2012; Smith et al., 2017; Stoehr et al., 2008). Some research further suggests that executive functioning (i.e., a composite of tests assessing shifting and working memory) may relate to medication adherence over and above memory (Insel et al., 2006). Unlike medication adherence, there is limited research examining specific neurocognitive correlates of medication management. Pirogovsky and colleagues (2014) reported no significant relationships between performance on the MMAA and neuropsychological measures in patients with Parkinson’s disease MCI. However, in a sample of OAs with serious mental illness, worse MMAA performance was significantly related to poorer performance on working memory, executive functioning, verbal fluency and memory measures (Pratt, Mueser, Driscoll, Wolfe, & Bartels, 2006). Additionally, slower processing speed and cognitive flexibility have been reported to significantly relate to poorer medication management tasks in Parkinson’s disease and community dwelling OAs (Fellows, Dahmen, Cook, & Schmitter-Edgecombe, 2017; Manning et al., 2012). This limited research suggests that, similar to medication adherence, memory and executive functioning as well as processing speed may impact medication management. Changes in these cognitive domains are well documented in the aging population and in individuals with aMCI (Harada, Natelson Love, & Triebel, 2013; Kramer et al., 2006).
In addition to examining neurocognitive correlates affecting MMAA performance in healthy and individuals with aMCI, we also aimed to further understand processes which contribute to overall MMAA performance. Although a total score is often easier to calculate and convenient, clinical and healthy populations can sometimes perform similarly on total scores, but their process and errors can be relatively different (Kaplan, 1988). Therefore, the present study sought to expand the MMAA scoring system by developing process error scores that would allow for a more detailed understanding of the processes that may impact performance on the MMAA. More specifically, process measures were developed to examine the magnitude and direction (overtaking, undertaking) of errors.
In this study, HOA and individuals with aMCI were administered the MMAA, provided a self-report rating of confidence in their medication management abilities and were administered a battery of neuropsychological tests. To the authors’ best knowledge, MMAA performance has not been examined in aMCI samples. Since prior studies have provided preliminary evidence of the MMAA being sensitive to cognitive changes, examining aMCI participants’ MMAA performance could provide information about their medication management abilities. We hypothesized that individuals with aMCI will perform more poorly on the MMAA and have poorer process error scores compared to the HOAs. We also predicted that both the HOA and aMCI groups would rate similar high levels of medication management abilities and that, like past studies (Hutchison et al., 2006), HOA and aMCI participant’s confidence ratings of their medication management abilities would not significantly relate to their performance on the MMAA or error process scores. To examine cognitive correlates of medication management, exploratory associations between HOA and individuals with aMCI’s performance on the MMAA and on measures of memory, executive functioning (i.e., mental flexibility, verbal fluency, and working memory), and processing speed were examined.
Methods
Participants
The sample is composed of 25 Caucasian individuals diagnosed with aMCI and 25 age- and education-matched cognitively healthy Caucasian OAs (see Table 1). All participants were recruited through community advertisements or referrals from physicians and local community agencies in the Whitman and Spokane counties of Washington State. The data was collected between 2012 and 2017 as part of a larger study investigating the relationship between cognition and everyday functioning in community-dwelling older adults (N = 347) at Washington State University. An initial phone interview, comprising a medical interview and the Telephone Interview of Cognitive Status (Brandt & Folstein, 2003), was conducted first. These screeners served to identify individuals who would likely be unable to complete the assessment due to significant cognitive (i.e., dementia), motor or sensory deficits. Individuals who did not meet exclusionary criteria completed two testing sessions. All of the data reported in this study was collected in the first session, which required that participants complete a battery of standardized and experimental neuropsychological tests in a laboratory. This protocol was reviewed and approved by the Washington State University institutional review board, and all participants gave verbal consent and signed the institutional review board approved consent document.
Table 1.
Table of mean (standard deviation) raw data from the aMCI and HOA groups on the demographic and neuropsychological measures along with statistical comparisons between the groups and eta squared effect size estimates.
| Variables or test | aMCI (n = 25) | HOA (n = 25) | t | p | η2 | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Demographics | |||||||
| Age (years) | 70.80 | 7.14 | 70.68 | 7.16 | −.059 | .953 | <.000 |
| Education (years) | 16.40 | 2.91 | 16.40 | 2.29 | −.054 | .957 | <.000 |
| Gender (% female)a | 68 | 80 | – | .333 | |||
| TICS | 33.00 | 2.47 | 35.92 | 2.84 | 3.88 | <.001 | .239 |
| Neuropsychological correlates | |||||||
| TMT A (sec) | 36.16 | 9.64 | 29.04 | 6.67 | −3.04 | .004 | .161 |
| LF | 39.28 | 14.98 | 41.40 | 11.57 | .560 | .578 | .006 |
| TMT B/A (sec) | 2.51 | .608 | 2.26 | .60 | −1.48 | .146 | .043 |
| DSB | 8.16 | 2.03 | 8.56 | 1.91 | .716 | .478 | .011 |
| MAS LD | 8.36 | 2.23 | 11.56 | .65 | 6.88 | <.001 | .496 |
aχ2 (1, N = 50) = .936 p < .333
Notes: Unless otherwise indicated, mean scores are raw scores. aMCI = amnestic Mild Cognitive Impairment; TICS = Telephone Interview of Cognitive (Brandt & Folstein, 2003); TMT A = Trails A (Reitan, 1992); LF = Letter Fluency (Delis et al., 2001); TMT B/A = Trails B/Trails A (Reitan, 1992); DSB = Digit Span Backwards (Wechsler, 2008); MAS LD = Memory Assessment Scale long delay list recall (Williams, 1991).
Additional exclusionary criteria for this study included a history of brain surgery, head trauma with permanent brain lesion, psychoactive substance abuse within the past year, stroke, or a known medical, neurological or psychiatric cause of cognitive dysfunction. Two experienced neuropsychologists evaluated the neuropsychological testing data, the Telephone Interview of Cognitive Status, collateral medical information (e.g., laboratory or imaging results) and the information provided by the participant and their knowledgeable informant to assess whether a participant met clinical criteria for aMCI. Several of the neuropsychological measures used in diagnostic decision-making (i.e., Trail Making Test; Memory Assessment Scale long delay list recall) were used to explore possible neurocognitive correlates of the MMAA. The MMAA was not, however, used in diagnostic decision-making. Inclusion criteria for aMCI were consistent with the diagnostic criteria defined by Petersen and colleagues (Petersen & Morris, 2005; Petersen et al., 2001). Of the MCI participant pool (n = 50), 37 participants met criteria for aMCI, and 25 of the aMCI participants completed the MMAA. Reasons for missing data included non-test administration when testing was running long (n = 9), participant discontinuing the MMAA task prior to task completion (n = 1) due to task difficulty and tester’s discontinuing testing due to participants not completing the task within 15 min (n = 2). Participants diagnosed with both single-domain aMCI (n = 15) and multi-domain aMCI (n = 10) are represented in this sample. The HOA group consisted of a subsample (n = 25) of the 175 HOA, who best matched the aMCI group in age and education (±2 years). If more than one HOA matched an aMCI participant, the HOA with the closest age and education was selected. If multiple matches still existed, the HOA with the same gender as the aMCI participant was selected. Matching of participants was conducted prior to any analyses being conducted. No significant differences in age, education, and gender were found between the two groups (see Table 1).
Neuropsychological Tests
To investigate the cognitive processes of speed of processing, executive functioning, and memory that may be related to medication management abilities, participants completed several neuropsychological tests that measure these constructs. Although these measures are not pure representations of these constructs, they have consistently been associated with these cognitive constructs in the literature.
Speed of processing
The total number of seconds to complete the Trails A subtest of the Trail Making Test (TMT-A; Reitan, 1992) was used as a measure of speeded processing. Trails A require participants to scan a page with numbers and quickly connect the numbers in order.
Executive Functioning
Verbal fluency – The total number correct from the letter fluency subtest of the Delis–Kaplan Executive Function System (D-KEFS; Delis, Kaplan & Kramer, 2001) was used as a measure of executive functioning. The D-KEFS letter fluency test is a novel task that requires individuals to quickly retrieve and name words beginning with given letter.
Mental flexibility – Trails B requires participants to scan a page of numbers and letters and quickly switch between connecting the numbers and letters in order. The total number of seconds on Trails B (TMT-B) was divided by the TMT A subtest from the TMT (Arbuthnott & Frank, 2000; Reitan, 1992) and used as a measure of mental flexibility.
Working Memory – To measure working memory, the total of correct number sequences recalled backwards on the Digit Span Backwards condition of the Digit Span subtest from the Wechsler Adult Intelligence Scale (Wechsler, 2008) was used. This subtest requires participants to recall a sequence of numbers in reverse order.
Long delay memory
Participants were required to learn a list of 12 words across several trials and then recall the words after a delay of approximately 20 min. The total number of 12 words correctly recalled from the Memory Assessment Scales list learning subtest after the delay administration was used to assess episodic memory (Williams, 1991).
Confidence rating
Prior to the administration of the MMAA task, a measure of each individual’s confidence in completing the MMAA task was assessed. Specifically, after being read the instructions for the MMAA task (see description below), the tester asked the participant, “On this scale from 0 to 100, where 0 equals complete lack of confidence and 100 equals strong confidence, how confident are you that you will be able to look at some medication bottles and organize your own medication schedule?”.
Medication Management Abilities Assessment
The MMAA is a laboratory performance-based roleplay measurement of a participant’s ability to organize medication after an introduction to a fictitious four medication regimen. While playing the role of a doctor, the tester first introduces the task to the participant by presenting the medication bottles and reading the instructions, which include: two tablets of Parlenol twice a day with food, one tablet of BRB three times a day, two tablets of Cyclomeovan three times a day without food, and two tablets of Linophen four times per day. Following a 45–60 min delay where additional cognitive tests are administered, the participant is provided pill bottles, with instructions printed on the label, and asked to walk the tester through their day starting with the time the participant would wake up in the morning to the time they go to bed at night. The participants were required to state the time they would take each medication and to give the examiner the number of pills they would take at that time. The tester recorded the medication type, number of pills, time of day and food or milk consumption for all medications presented during the task (including additional attempts and pills). The tester placed the medications out of sight into a pill holder and was not permitted to provide information on which medications or the number of pills the participant has already “taken”. For this study, the MMAA was scored using the MMAA scoring method and a new method based on process error analysis.
MMAA Scoring
The MMAA scoring method uses a 33-point scoring system, where a value of 33 indicates a “perfect” score (Patterson et al., 2002). For each medication, the scoring criteria are divided into three categories: accurate frequency (i.e. number of attempts), quantity (i.e. number of pills) and adherence to food rules (i.e. with or without food). A point is awarded for every occurrence where the participant indicates attempting to take the medication, taking the correct number of pills and adhering to the correct food rules. Another point is awarded for achieving the correct frequency per medication.
Process Error Scores
Although the MMAA scoring method accurately captures the frequency of noncompliance to the medication plan, it does not capture mistake magnitude or direction. Therefore, additional variables were created to capture the magnitude and direction of mistakes committed.
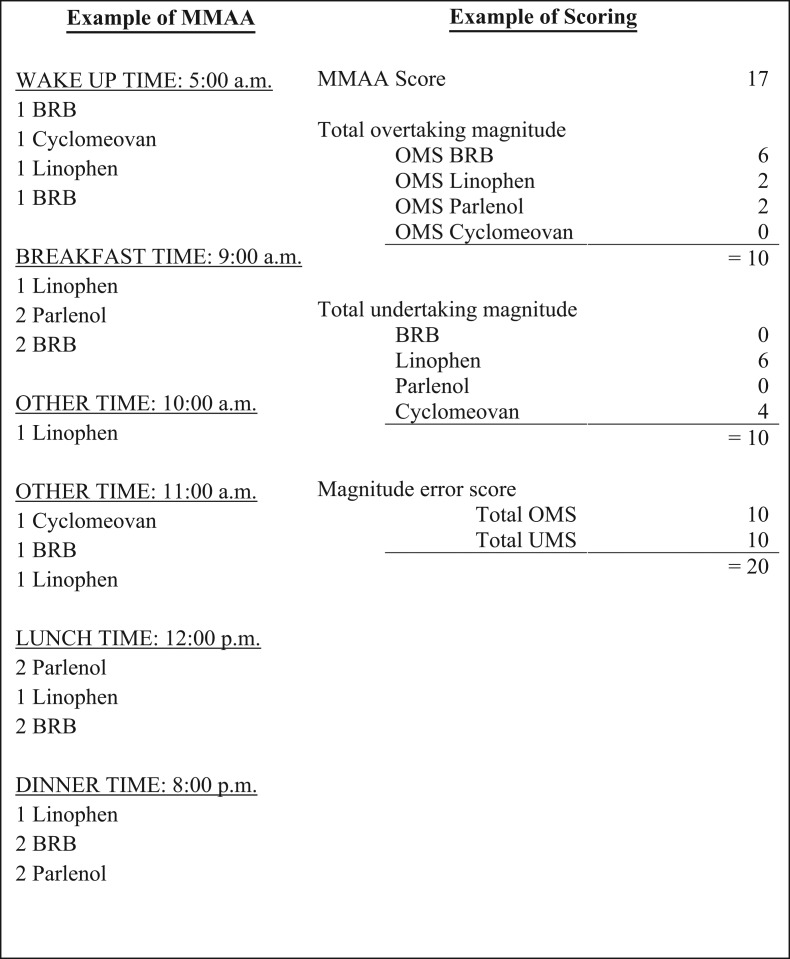
Total Overtaking Magnitude – The overtaking score represents the sum of the number of additional pills indicated taken within any attempt made and the pills indicated taken for any extra attempts made across all four medications. The BRB pills in Fig. 1 illustrate this scoring system. The participant indicated taking an extra pill at the first, second, fourth and fifth attempt, which is scored as a 4. The participant also indicated taking a fourth and fifth attempt, which adds two additional points, resulting in the participant having an overtaking magnitude subscore of 6 for BRB. The total overtaking magnitude score is then computed by summing the overtaking magnitude scores for each medication.
Fig. 1.
Example of MMAA and error scores.
Total Undertaking Magnitude – The undertaking score represents the sum of the number of missing pills within any attempt made and the missing pills from missing an attempt across all four medications. The Cyclomeovan pills in Fig. 1 illustrate this scoring system. The participant indicated taking one less Cyclomeovan pill for their first and second attempt, which is scored as a 2. The participant then did not indicate taking a third attempt, which adds two extra points for the two missing pills, resulting in the participant having an undertaking magnitude subscore of 4 for Cyclomeovan. The total undertaking magnitude score is then computed by summing the undertaking magnitude scores for each medication.
Magnitude Error Score – A magnitude error score was calculated by taking the absolute sum of the total overtaking and undertaking magnitude scores. The Linophen pills in Fig. 1 illustrates how the magnitude error score can increase when a participant indicates taking less pills and has more attempts than required.
Analyses
Prior to running analyses, an outlier analysis was conducted by running separate regressions between the MMAA variables and the HOA and aMCI groups in order to obtain studentized deleted residual, Cook’s and lever’s analyses. Both studentized deleted residual and Cook’s analyses suggested that one aMCI participant’s performance on the MMAA was abnormal. However, when the participant was removed from the sample a similar pattern of significance and effect size magnitude remained. Due to the small sample size, the similar pattern of data and the participant meeting aMCI criteria, the participant was not removed from the sample. Separate t-tests were conducted to evaluate for significant group differences on each neuropsychological test. The Shapiro–Wilks test was used to test data normality, and results of the analyses revealed that the confidence ratings and all of the MMAA measures were significantly non-normally distributed (ps < .05). Therefore, non-parametric statistics were used to analyze the data. Group differences on the MMAA score and process measures were analyzed using Mann–Whitney U test. Exploratory spearman’s rank correlations were conducted to examine the relationship between the confidence rating, the MMAA score and process measures. Also, the proportional reduction of error (i.e., r2) effect size was reported for all analyses.
Results
Neuropsychological Data
The mean and standardized deviation raw scores of HOAs and individuals with aMCI on the neuropsychological tests are shown in Table 1 along with statistical comparisons between the groups and eta squared effect size estimates. Separate t-tests were conducted to examine whether the mean scores for the HOA and aMCI groups differed across the neuropsychological measures. As seen in Table 1, t-tests revealed that the groups did not differ significantly on tests measuring executive functioning. However, the HOAs performed better than the aMCI group on measures assessing processing speed and memory.
Confidence Rating
A Mann–Whitney U test revealed that the aMCI and HOA groups did not differ in confidence for their ability to organize the MMAA medication schedule (see Table 2). More than half of the individuals with aMCI (52%) and HOAs (52%) reported having 100% confidence in their ability to look at the medication bottles and organize a medication schedule.
Table 2.
Mean (standard deviation), range, and median (standard error) raw data from the aMCI and HOA groups on the MMAA measures, along with statistical comparisons between the groups and eta squared effect size estimates
| Mean (SD) | Range | Median (SE) | U | p | η2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| aMCI | HOA | aMCI | HOA | aMCI | HOA | ||||
| Confidence | 89.56 (17.41) | 93.00 (8.42) | 30–100 | 75–100 | 100 (3.5) | 100 (1.68) | 304.0 | .857 | .0006 |
| MMAA score | 27.64 (6.30) | 31.60 (2.02) | 7–33 | 25–33 | 30.0 (1.26) | 32.0 (.40) | 130.5 | <.001 | .258 |
| Process Error Scores | |||||||||
| Total overtaking magnitude | 1.64 (1.60) | .44 (.87) | 0–5 | 0–3 | 2.0 (.32) | 0.0 (.17) | 179.0 | .004 | .169 |
| Total undertaking magnitude | −2.64 (3.42) | −.84 (1.24) | −13–0 | −4–0 | −1.0 (.68) | 0.0 (.30) | 204.0 | .026 | .099 |
| Magnitude error score | 4.28 (3.49) | 1.28 (1.40) | 0–14 | 0–4 | 3.0 (.70) | 1.0 (.28) | 118.5 | <.001 | .291 |
| Pill Magnitude Error Scores | |||||||||
| Cyclomeovan | 1.24 (1.39) | .36 (.76) | 0–5 | 0–2 | 1.0 (.29) | 0.0 (.15) | 187.0 | .006 | .153 |
| Parlenol | 1.08 (1.35) | .16 (.55) | 0–4 | 0–2 | 0.0 (.27) | 0.0 (.11) | 197.0 | .003 | .171 |
| BRB | .48 (.65) | .12 (.33) | 0–2 | 0–1 | 0.0 (.13) | 0.0 (.07) | 220.0 | .022 | .106 |
| Linophen | 1.48 (1.90) | .64 (.95) | 0–6 | 0–3 | 0.0 (.40) | 0.0 (.19) | 246.0 | .147 | .042 |
Note: For all error scores, higher numbers indicate more errors.
Medication Management Abilities Assessment
Table 2 displays the means, standard deviation, range, medians, and standard errors for each MMAA score by group. Individuals with aMCI performed more poorly than HOAs on the MMAA score and process error measures. More specifically, the aMCI group showed significantly poorer performance on measures of total overtaking error (η2 = .169), total undertaking error (η2 = .099), the magnitude error score (η2 = .291) and the MMAA score (η2 = .258).
Exploratory Analyses
Based on the finding that both overtaking and undertaking errors were contributing to the magnitude error score and ultimately the MMAA score, the authors were interested in whether the aMCI group had more difficulty with pills that were more complex (i.e., in frequency, number of pills, and food rules). Magnitude error scores were computed for each individual pill type, and group differences on individual pill magnitude error scores were analyzed using Mann–Whitney U test. Compared to the HOA group, the aMCI group performed significantly worse on the Cyclomeovan, Parlenol and BRB magnitude error scores (Table 2). No significant difference was found for the Linophen magnitude error score.
Exploratory Spearman correlations were conducted to examine the relationship between performance on the MMAA score and process error measures and the standardized neuropsychological tests (see Table 3). For the HOA group, a moderate negative correlation was found between confidence ratings and MMAA score (rs = –.413), such that better performance on the MMAA was associated with lower confidence ratings. In the aMCI group, moderate to large correlations emerged between performance on a speeded processing measure (i.e., TMT-A) and total overtaking magnitude (rs = .513) and with the magnitude error score (rs = .476). Measures of mental flexibility (rs = –.447) and memory (rs = .450) were both moderately correlated with MMAA score.
Table 3.
Spearman correlations for HOA and aMCI groups between MMAA measures and standardized neuropsychological tests
| Confidence | Total overtaking magnitude | Total undertaking magnitude | Magnitude error score | MMAA score | |
|---|---|---|---|---|---|
| HOA | |||||
| Confidence | −.021 | –.391 | .341 | −.413* | |
| TMT A (sec) | .183 | .092 | –.186 | .197 | −.279 |
| LF | .141 | −.224 | −.214 | .005 | −.135 |
| TMT B/A (sec) | −.199 | −.065 | .095 | −.117 | .155 |
| DSB | .058 | −.072 | .072 | −.135 | .043 |
| MAS LD | –.169 | –.199 | .355 | –.396 | .367 |
| aMCI | |||||
| Confidence | .033 | .142 | −.051 | .147 | |
| TMT A (sec) | .187 | .513* | .044 | .476* | –.122 |
| LF | .028 | –.133 | .069 | −.041 | .065 |
| TMT B/A (sec) | −.392 | .288 | −.317 | .344 | −.447* |
| DSB | .392 | −.164 | −.098 | .022 | −.033 |
| MAS LD | −.192 | −.018 | .299 | −.270 | .450* |
Note: TMT A = Trails A; LF = Letter Fluency; TMT B/A = Trails B/Trails A; DSB = Digit Span Backwards; MAS LA = Memory Assessment Scale learning acquisition.
aHigher scores on measure is associated with poorer performance.
*p < .05.
Discussion
Medication nonadherence can lead to significant adverse medical and financial consequences in the aging and MCI population (Kelly et al., 2016; Zhang et al., 2009). Medication management assessments for the laboratory or clinic could serve as objective and quantifiable tools for identifying individuals who are likely to experience difficulties with self-managing medications at home. We compared the performances of HOA and individuals with aMCI on the MMAA task and self-reported confidence to complete the MMAA. We also developed process error measures to better characterize MMAA performance and examined cognitive correlates with these measures.
Individuals with aMCI performed significantly worse than matched HOAs on the MMAA score and process error scores, suggesting that individuals with aMCI are more likely to have difficulty managing their medication regime. These findings are consistent with prior research, which has found that individuals with cognitive impairment (i.e., Parkinson’s disease MCI) perform worse on the MMAA compared to cognitively normal controls (Pirogovsky et al., 2014). Individuals with aMCI further engaged in more overtaking and undertaking errors compared to the HOA group when completing the MMAA. These findings mirror the medication adherence results of Hayes and colleagues (2009), which showed that cognitively compromised OAs were significantly less adherent to their medication routine compared to HOAs. To the author’s best knowledge, health outcomes related to medication management mistakes has not been explored in older adults with cognitive impairment. However as previously mentioned, under and over taking medications are related to serious health outcomes. Specifically, in a sample of community dwelling OAs, Budnitz, Lovegrove, Shehab and Richards (2011) reported that approximately 60% of hospitalizations were due to accidental overdose. Moreover, Wauters and colleagues (2016) reported that after controlling for the number of medications and misusing medications, risk for mortality and hospitalizations increased by 39% and 26%, respectively, for every medication an older adult underused.
When magnitude error scores were analyzed by pill type, the aMCI group performed significantly worse on the Cyclomeovan, Parlenol and BRB pills, but not the Linophen pill, when compared to HOAs. These results partially suggest that, compared to HOA, individuals with aMCI may struggle more with correctly managing medications with more rules. Specifically, two of these medications require the participant to adhere to food rules. Cyclomeovan needs to be taken an hour before a meal or two hours after a meal, but Parlenol needs to be taken with food. Parlenol also requires two doses, which differs from the Cyclomeovan and BRB, which require three doses. Interestingly, both aMCI and HOA individuals had numerically the most difficulty with the Linophen pill. Given that Linophen is presented after the more complex medications (i.e., Cyclomeovan and Parlenol) and only differs from the other medications in dosing frequency (i.e., take four times rather than three times per day), both the aMCI and HOA groups may not have allocated as much attentional resources to this difference and forget to follow the rule accurately. Alternatively, given that there are a higher number of Linophen doses to manage, this may also reflect that there was more room for error.
Although the findings reviewed thus far suggest that individuals with aMCI may be at greater risk for overtaking and undertaking medications or that complexity of medication plans may lead to more mistakes, it is unclear whether these errors reflect actual problems aMCI and HOA individuals experience when managing medications in the real-world environment. Additionally, unlike the home environment, this task is void of important compensatory strategies (e.g., pill box, routine) used to support independent living. It is therefore unclear whether these errors would persist if participants were allowed to use compensatory strategies or were engaging in their own medication routine.
As expected, the aMCI and HOA samples reported similar confidence ratings, suggesting that despite their different cognitive profiles, the aMCI sample still reported having similar confidence levels as the HOA participants in their ability to manage medications. Confidence ratings did not significantly relate to any of the cognitive or MMAA variables in the aMCI group, which is consistent with prior research (Hutchison et al., 2006). For the HOA, a significant association was found between MMAA and confidence ratings. However, this association revealed that as confidence ratings increased, performance on the MMAA score decreased, suggesting inaccuracies in self-report of medication management abilities. Other studies have also found little relationship between self-reported medication adherence and adherence as measured by pill counts or MEMS caps for both HOA and cognitively impaired populations (Grymonpre et al., 1998; Hayes et al., 2009). These findings when combined with other studies suggest that both HOA and cognitively impaired individuals may not provide accurate reports of their medication management abilities and that performance-based measures may provide a better alternative due to its objective properties. However, given that performance-based measures are administered outside the person’s everyday environment where routine and strategies can help compensate for declining cognitive capacity, it will be important for future work to replicate these findings and include actual medication adherence as an outcome measure.
Exploratory examination of the relationships between cognitive predictors and the process errors provided information about the cognitive domains contributing to performance on the MMAA in the aMCI group. The HOA group’s performance on the MMAA did not correlate with the cognitive measures. For the aMCI group, better performance on measures of mental flexibility and long delay memory were both significantly related to better performance on the MMAA score. These results suggest that those who performed worse on the MMAA may have experienced more difficulty switching between and remembering information necessary for accurate task performance. These findings are consistent with literature that has examined cognitive domains related to medication adherence, suggesting that similar cognitive domains (i.e., executive functioning and memory) are used in both medication adherence and medication management (Insel et al., 2006; Manning et al., 2012; Smith et al., 2017; Stoehr et al., 2008). The data further revealed that processing speed (i.e., TMT-A) was related to the total overtaking magnitude and the magnitude error scores and did not significantly relate to the MMAA score. Of all the process error scores, the overtaking magnitude score is the most unique from the MMAA score. This finding suggests that the overtaking magnitude score captured additional information about the aMCI group’s performance, which the MMAA score did not. Future research will be needed to determine whether the MMAA score and process error scores are capturing different aspects of performance that might dissociate based on disease groups or domains of cognitive deficits. Overall, our findings offer preliminary evidence supporting the MMAA as a method for identifying individuals, who may struggle with managing a novel medication routine.
The primary limitation of this study is that the relationships between medication management and adherence for personal medications, and performance on medication management laboratory measures is currently unknown. In addition, the confidence rating question was designed to mimic the requirements of the MMAA task and not medication adherence and lacks validity data. It therefore remains unclear whether these results would reflect an individual’s actual adherence and management in the real-world environment. To develop more valid measures of medication management, future studies should focus on examining relationships between actual everyday medication adherence and performance on proxy laboratory tasks. Additional limitations to the study include the small sample size, the generally high level of education and the race of the HOA and aMCI participants (i.e., 100% Caucasian sample). The small sample size may have limited our ability to detect generalizable relationships between performance on the MMAA and neuropsychological tests. Furthermore, the small sample size limited the types of statistical analyses that could be conducted and correlations can only be considered exploratory. The mixed sample of single and multi-domain aMCI also may have affected the power and generalizability of the findings. Additionally, the number of medications participants were managing would have served as a useful covariate in this study’s analyses. Together, future studies should examine more than one neuropsychological test per cognitive domain, control for the number of medications participants are managing, and examine potential differences between individuals who do and do not manage their own medications. Finally, as mentioned in the methods, 12 MMAAs were not administered or discontinued. Notably, 8 of these 12 participants met criteria for multi-domain aMCI and the average age of these participants was 77.92 (SD = 5.77), suggesting that the MMAA may be too difficult for individuals with more advanced MCI.
In summary, using a performance-based task, the study findings provide evidence showing that aMCI participants were less accurate in their medication management abilities than HOA participants. The aMCI group performed significantly worse on the MMAA score and the process measures compared to the HOA group. The aMCI also appeared to struggle more than HOAs with pills that required management of more rules. Similar to reported findings from research analyzing cognition and medication adherence, the aMCI group’s performance on the MMAA score related to memory and executive functioning abilities, suggesting similar neurocognitive correlates affect both medication adherence and MMAA performance. Unlike the MMAA score, the overtaking error score significantly related to processing speed, suggesting that this process score captured additional information about the aMCI group’s performance, which the MMAA score did not. Moreover, the aMCI group’s confidence in their medication management abilities did not differ from the HOA groups and was not related to actual performance on a medication management task, suggesting that the aMCI group had poor insight into their medication management abilities. Currently, additional research is needed to determine whether the MMAA and other performance-based tasks are good clinical tools for predicting medication management and adherence functioning. Future investigation should focus on whether medication management tasks reliably predict medication adherence in everyday life.
Acknowledgements
We thank Brooke Robinson her assistance in data entry. We also thank members of the WSU Cognitive Aging and Dementia laboratory for their help in collecting and scoring the data.
Funding
This work was supported by grants from the National Institute of Biomedical Imaging and Bioengineering [R01 EB009675 to M.S-E.] and U.S. Department of Education: Graduate Assistance in Areas of National Need [P200A150115 to M.S-E. and C.S.].
Conflict of interest
No conflicts of interest exist.
References
- Arbuthnott K., & Frank J. (2000). Trail Making Test, Part B as a measure of executive control: Validation using a set-switching paradigm. Journal of Clinical and Experimental Neuropsychology, 22, 518–528. 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- Benjamin R. M. (2012). Medication adherence: Helping patients take their medicines as directed. Public Health Reports, 127, 2–3. 10.1177/003335491212700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J., & Folstein M. (2003). Telephone interview for cognitive status. Lutz, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Budnitz D. S., Lovegrove M. C., Shehab N., & Richards C. L. (2011). Emergency hospitalizations for adverse drug events in older Americans. New England Journal of Medicine, 365, 2002–2012. 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- Delis D. C., Kaplan E., & Kramer J. H. (2001). Delis-Kaplan executive function system: Examiner’s manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Depp C. A., Cain A., Palmer B., Moore D., Eyler L., Lebowitz B., et al. (2008). Assessment of medication management ability in middle-aged and older adults with bipolar disorder. Journal of Clinical Psychopharmacology, 28, 225–229. 10.1097/JCP.0b013e318166dfed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelberg H. K., Shallenberger E., & Wei J. Y. (1999). Medication management capacity in highly functioning community-living older adults: Detection of early deficits. Journal of the American Geriatrics Society, 47, 592–596. [DOI] [PubMed] [Google Scholar]
- Elliott R. A., & Marriott J. L. (2009). Standardised assessment of patients’ capacity to manage medications: A systematic review of published instruments. BMC Geriatrics, 9, 27 10.1186/1471-2318-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows R. P., Dahmen J., Cook D., & Schmitter-Edgecombe M. (2017). Multicomponent analysis of a digital Trail Making Test. The Clinical Neuropsychologist, 31, 154–167. 10.1080/13854046.2016.1238510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald A. A., Powers J. D., Ho P. M., Maddox T. M., Peterson P. N., Allen L. A., et al. (2011). Impact of medication nonadherence on hospitalizations and mortality in heart failure. Journal of Cardiac Failure, 17, 664–669. 10.1016/j.cardfail.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Grymonpre R. E., Didur C. D., Montgomery P. R., & Sitar D. S. (1998). Pill count, self-report, and pharmacy claims data to measure medication adherence in the elderly. The Annals of Pharmacotherapy, 32, 749–754. 10.1345/aph.17423. [DOI] [PubMed] [Google Scholar]
- Harada C. N., Natelson Love M. C., & Triebel K. L. (2013). Normal cognitive aging. Clinics in Geriatric Medicine, 29, 737–752. 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes T. L., Larimer N., Adami A., & Kaye J. A. (2009). Medication adherence in healthy elders: Small cognitive changes make a big difference. Journal of Aging and Health, 21, 567–580. 10.1177/0898264309332836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison L. C., Jones S. K., West D. S., & Wei J. Y. (2006). Assessment of medication management by community-living elderly persons with two standardized assessment tools: A cross-sectional study. The American Journal of Geriatric Pharmacotherapy, 4, 144–153. 10.1016/j.amjopharm.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Insel K., Morrow D., Brewer B., & Figueredo A. (2006). Executive function, working memory, and medication adherence among older adults. The Journal of Gerontology, 61B, P102–P107. [DOI] [PubMed] [Google Scholar]
- Jeste S. D., Patterson T. L., Palmer B. W., Dolder C. R., Goldman S., & Jeste D. V. (2003). Cognitive predictors of medication adherence among middle-aged and older outpatients with schizophrenia. Schizophrenia Research, 63, 49–58. 10.1016/S0920-9964(02)00314-6. [DOI] [PubMed] [Google Scholar]
- Kaplan E. (1988). The process approach to neuropsychological assessment. Aphasiology, 2, 309–311. 10.1080/02687038808248930. [DOI] [PubMed] [Google Scholar]
- Kelly K., Grau-Sepulveda M. V., Goldstein B. A., Spratt S. E., Wolfley A., Hatfield V., et al. (2016). The agreement of patient-reported versus observed medication adherence in type 2 diabetes mellitus (T2DM). BMJ, 4, e000182 10.1136/bmjdrc-2015-000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. H., Nelson A., Johnson J. K., Yaffe K., Glenn S., Rosen H. J., et al. (2006). Multiple cognitive deficits in amnestic mild cognitive impairment. Dementia and Geriatric Cognitive Disorders, 22, 306–311. 10.1159/000095303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning K. J., Clarke C., Lorry A., Weintraub D., Wilkinson J. R., Duda J. E., et al. (2012). Medication management and neuropsychological performance in Parkinson’s disease. The Clinical Neuropsychologist, 26, 45–58. 10.1080/13854046.2011.639312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. (2004). A conceptual framework to study medication adherence in older adults. The American Journal of Geriatric Pharmacotherapy, 2, 36–43. 10.1016/S1543-5946(04)90005-0. [DOI] [PubMed] [Google Scholar]
- NIH Senior Health. (n.d.) Retrieved from https://nihseniorhealth.gov/takingmedicines/managingyourmedicines/01.html.
- Pasina L., Brucato A. L., Falcone C., Cucchi E., Bresciani A., Sottocorno M., et al. (2014). Medication non-adherence among elderly patients newly discharged and receiving polypharmacy. Drugs & Aging, 31, 283–289. 10.1007/s40266-014-0163-7. [DOI] [PubMed] [Google Scholar]
- Patterson T. L., Lacro J., McKibbin C. L., Moscona S., Hughs T., & Jeste D. V. (2002). Medication management ability assessment: Results from a performance-based measure in older outpatients with schizophrenia. Journal of Clinical Psychopharmacology, 22, 11–19. 10.1097/00004714-200202000-00003. [DOI] [PubMed] [Google Scholar]
- Petersen R. C., Doody R., Kurz A., Mohs R. C., Morris J. C., Rabins P. V., et al. (2001). Current concepts in mild cognitive impairment. Archives of Neurology, 58, 1985–1992. 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen R. C., & Morris J. C. (2005). Mild cognitive impairment as a clinical entity and treatment target. Archives of Neurology, 62, 1160 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M., James S., Meakin S., Green C., Scott A. K., Walley T. J., et al. (2004). Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18 820 patients. BMJ, 329, 15–19. 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirogovsky E., Martinez-Hannon M., Schiehser D. M., Lessig S. L., Song D. D., Litvan I., et al. (2013). Predictors of performance-based measures of instrumental activities of daily living in nondemented patients with Parkinson’s disease. Journal of Clinical and Experimental Neuropsychology, 35, 926–933. 10.1080/13803395.2013.838940. [DOI] [PubMed] [Google Scholar]
- Pirogovsky E., Schiehser D. M., Obtera K. M., Burke M. M., Lessig S. L., Song D. D., et al. (2014). Instrumental activities of daily living are impaired in Parkinson’s disease patients with mild cognitive impairment. Neuropsychology, 28, 229–237. 10.1037/neu0000045. [DOI] [PubMed] [Google Scholar]
- Pratt S. I., Mueser K. T., Driscoll M., Wolfe R., & Bartels S. J. (2006). Medication nonadherence in older people with serious mental illness: Prevalence and correlates. Psychiatric Rehabilitation Journal, 29, 299–310. [DOI] [PubMed] [Google Scholar]
- Pretorius R. W., Gataric G., Swedlund S. K., & Miller J. R. (2013). Reducing the risk of adverse drug events in older adults. American Family Physician, 87, 331–336. [PubMed] [Google Scholar]
- Qato D. M., Wilder J., Schumm L. P., Gillet V., & Alexander G. C. (2016). Changes in prescription and over-the-counter medication and dietary supplement use among older sdults in the United States, 2005 vs 2011. JAMA Internal Medicine, 176, 473–482. 10.1001/jamainternmed.2015.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. (1992). Trail Making Test: Manual for administration and scoring. Tucson, AZ: Reitan Neuropsychology Laboratory. [Google Scholar]
- Shi L., Liu J., Fonseca V., Walker P., Kalsekar A., & Pawaskar M. (2010). Correlation between adherence rates measured by MEMS and self-reported questionnaires: A meta-analysis. Health and Quality of Life Outcomes, 8, 99 10.1186/1477-7525-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D., Lovell J., Weller C., Kennedy B., Winbolt M., Young C., et al. (2017). A systematic review of medication non-adherence in persons with dementia or cognitive impairment. PLoS One, 12, e0170651 10.1371/journal.pone.0170651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirratt M. J., Dunbar-Jacob J., Crane H. M., Simoni J. M., Czajkowski S., Hilliard M. E., et al. (2015). Self-report measures of medication adherence behavior: Recommendations on optimal use. Translational Behavioral Medicine, 5, 470–482. 10.1007/s13142-015-0315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoehr G., Lu S.-Y., Lavery L., Bilt J. V., Saxton J. A., Chang C.-C. H., et al. (2008). Factors associated with adherence to medication regimens in older primary care patients: The steel valley seniors survey. The American Journal of Geriatric Pharmacotherapy, 6, 255–263. 10.1016/j.amjopharm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A., Hochschild A., Burnett J., Zulfiqar A., & Dyer C. B. (2012). High prevalence of medication non-adherence in a sample of community-dwelling older adults with adult protective services-validated self-neglect. Drugs & Aging, 29, 741–749. 10.1007/s40266-012-0007-2. [DOI] [PubMed] [Google Scholar]
- Wauters M., Elseviers M., Vaes B., Degryse J., Dalleur O., Vander Stichele R., et al. (2016). Too many, too few, or too unsafe? Impact of inappropriate prescribing on mortality, and hospitalization in a cohort of community-dwelling oldest old: Too many, too few, or too unsafe? British Journal of Clinical Pharmacology, 82, 1382–1392. 10.1111/bcp.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (2008). Wechsler Adult Intelligence Scale (4th ed.). San Antonio, TX: Pearson. [Google Scholar]
- Wesson J., Clemson L., Brodaty H., & Reppermund S. (2016). Estimating functional cognition in older adults using observational assessments of task performance in complex everyday activities: A systematic review and evaluation of measurement properties. Neuroscience & Biobehavioral Reviews, 68, 335–360. 10.1016/j.neubiorev.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Williams J. M. (1991). Memory Assessment Scales. Odessa, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Wilson I. B., Carter A. E., & Berg K. M. (2009). Improving the self-report of HIV antiretroviral medication adherence: Is the glass half full or half empty? Current HIV/AIDS Reports, 6, 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap A. F., Thirumoorthy T., & Kwan Y. H. (2015). Medication adherence in the elderly. Journal of Clinical Gerontology and Geriatrics, 7, 64–67. 10.1016/j.jcgg.2015.05.001. [DOI] [Google Scholar]
- Zhang M., Holman C. D., Price S. D., Sanfilippo F. M., Preen D. B., & Bulsara M. K. (2009). Comorbidity and repeat admission to hospital for adverse drug reactions in older adults: Retrospective cohort study. BMJ, 338, 155–158. 10.1136/bmj.a2752. [DOI] [PMC free article] [PubMed] [Google Scholar]