Abstract
Background
Few studies on anastomotic condition after rectal-cancer resection and its effect on anastomotic leakage (AL) are available up to now. This study aimed to investigate potential radiation-induced injury left on surgical margins of anterior resection after neoadjuvant chemoradiotherapy (nCRT) and its association with AL.
Methods
We retrospectively identified 161 consecutive patients who underwent anterior resection with nCRT, neoadjuvant chemotherapy without radiation (nCT) or no neoadjuvant therapy between 2014 and 2015. Tissue samples of resection margins were assessed using a specific histopathological score and microvessel density in submucosa. Propensity score matching was used to balance the baseline characteristics. Association between AL and histopathological features was analysed.
Results
AL occurred in 13 of 54 patients undergoing nCRT, 5 of 48 patients undergoing nCT and 7 of 59 patients without neoadjuvant therapy. Comparisons after matching showed median (range) histopathological scores as follows: 3 (0–8) vs 0 (0–3) vs 0 (0–2) for the proximal margin (P < 0.001); 4 (2–9) vs 0 (0–4) vs 0 (0–3) for the distal margin (P < 0.001). Correspondingly, mean (SD) microvessel densities were as follows: 21.7 (7.9) vs 27.2 (8.6) vs 27.3 (9.4) for the proximal margin (P = 0.003); 18.1 (9.3) vs 25.2 (12.9) vs 24.9 (7.4) for the distal margin (P < 0.001). Among patients undergoing nCRT, AL was associated with increased histopathological score (P = 0.003) and decreased microvessel density (P = 0.004) on the proximal margin.
Conclusions
Surgical margins of rectal-cancer resection are exposed to certain radiation-induced injury after nCRT. AL is associated with aggravated radiation damage on the proximal margin.
Keywords: Anastomotic leakage, rectal cancer, anterior resection, neoadjuvant chemoradiotherapy, radiation injury, histopathology
Introduction
Neoadjuvant chemoradiotherapy (nCRT) and total mesorectal excision are currently the standard treatments for locally advanced rectal cancer [1, 2]. New strategies have promoted the wide adoption of sphincter-saving procedures in the last decade [3, 4]. Despite advances in surgical techniques, an ever-present risk of anastomotic leakage (AL) remains after anterior resection for patients with rectal cancer, which is associated with increased morbidity and mortality [5, 6]. Major concerns have been reported on the effect of pre-operative radiation on AL with conflicting evidence [7–10]. A recent study with post hoc analysis of a randomized trial demonstrated the association between pre-operative long-course radiotherapy and AL after rectal-cancer resection [11]. Moreover, radiation proctitis was identified as a special predictor of AL in patients receiving nCRT, which suggests the potential effect of radiation-induced injury on anastomotic integrity.
Previous studies of radiation enteritis have shown the crucial role of bowel segments used for anastomosis, where irradiated bowels at both ends substantially contribute to the AL and mortality after surgery [12, 13]. Although great expectations have been placed on the improvement of radiotherapy technology to optimize the damage control in non-neoplastic tissues, little evidence has demonstrated the anastomotic condition after anterior resection following nCRT and its association with AL. Otherwise, some histopathological features of radiation-induced bowel injury have been described for rectal-cancer patients treated with radiotherapy [14, 15]. These studies assessed radiation damage possible in colorectal specimens.
When intact anastomoses are not accessible for post-operative examination, the surgical margins reflect the bowel segments united accordingly. This study aimed to investigate potential radiation-induced injury on surgical margins after nCRT for rectal cancer and to assess the effect of radiation damage on AL after anterior resection.
Patients and methods
Study population
We retrospectively studied a cohort of patients who underwent curative anterior resection combined with colorectal anastomosis for rectal cancer at the Sixth Affiliated Hospital of Sun Yat-sen University (Guangzhou, China) between 2014 and 2015. Patients were excluded for stage I rectal cancer, emergency operation or a history of inflammatory bowel disease (IBD). According to the pre-operative treatment, these consecutive patients included in the present study were divided into the nCRT group, the neoadjuvant chemotherapy (nCT) group and the control group without neoadjuvant therapy. All patients undergoing nCRT or nCT alone were recruited from a randomized trial of neoadjuvant fluorouracil, leucovorin and oxaliplatin (FOLFOX) chemotherapy with or without radiation compared with 5-fluorouracil (5-FU)-based chemoradiotherapy in the treatment of resectable rectal cancer (FOWARC study) [16]. Patients in the control group refused any pre-operative treatment or underwent surgery alone for a relatively early disease [17]. Clinical and treatment details were retrieved from the institutional database of colorectal cancer. The surgical specimens were obtained from the tissue bank of the hospital. Informed consents were signed for specimen collections and investigations. This study was approved by the Institutional Review Board of the Sixth Affiliated Hospital of Sun Yat-sen University.
All patients undergoing nCRT received intensity modulation radiotherapy (IMRT), with a total dose of 50 Gy in 25 fractions, five times weekly. The clinical target volume included the mesorectum and pelvic lymphatic area. The patients undergoing concurrent chemotherapy were treated with 5-FU infusion or the FOLFOX regimen. Patients in the nCT group received FOLFOX chemotherapy alone. The nCRT plan and chemotherapy regimens were the same as described previously [16]. Curative anterior resection was performed by senior surgeons specializing in colorectal surgery. For patients who received neoadjuvant therapy, surgery was performed 6–8 weeks after nCRT or 4 weeks after nCT. Mechanical bowel preparation was performed pre-operatively for all patients. Curative surgery was routinely performed with high ligation of the inferior mesenteric artery at the origin of the aorta. Generally, the rectum and the sigmoid colon within the pelvis were used for end-to-end anastomosis, whereas the descending colon was only used in patients with doubtful blood supply of the sigmoid colon. A diverting ileostomy was performed in most patients with nCRT, technical difficulties in dissection or very low anastomosis.
Outcome measures
AL was defined as a communication through the intestinal lumen due to defective integrity of the anastomosis, as well as a proximate pelvic abscess [18]. Clinical AL was defined based on symptoms/signs of abdominal sepsis or fistula formation, which required urgent operation or active intervention. Imaging examinations were requested for clinical concerns on AL or before reversal of the ileostomy. Radiologic AL was asymptomatic and required no active intervention.
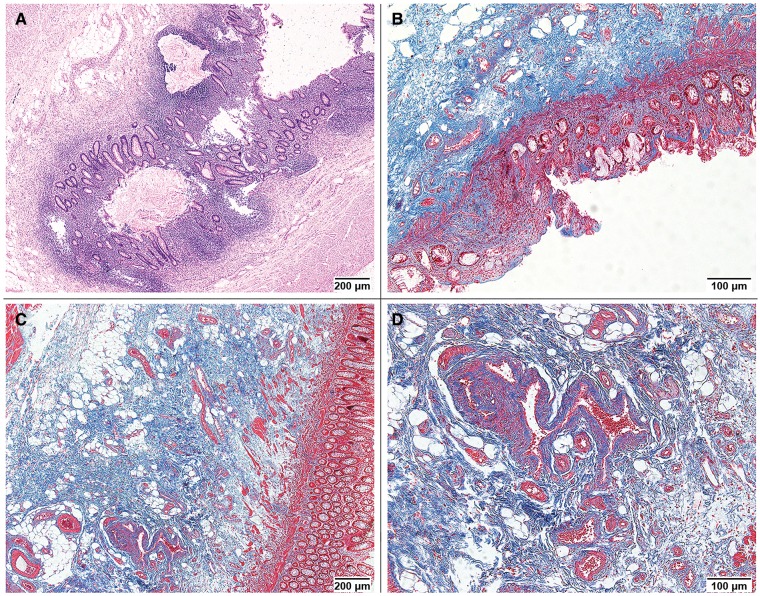
Transmural tissue samples were taken from both proximal and distal resection margins of each specimen. Formalin-fixed tissue samples were embedded in paraffin and cut into 4-μm sections. These sections were stained with Hematoxylin-Eosin and Masson’s trichrome for histopathological assessment. On the basis of published literature [14, 15, 19] and senior pathologists’ experience (X.F. and Y.H.), we identified nine morphologic features to evaluate the radiation-induced bowel injury, including mucosal erosions or ulcers, nuclear abnormalities of epithelial cells, crypt disarrangement, inflammatory infiltration in the lamina propria, mucosal edema, fibrosis of the lamina propria, fibrosis of submucosa, sclerosis of submucosal vessels and lymph congestion. A specific scoring system that consists of these morphologic features with intensity assessment was elaborated (Table 1). The total score of nine items ranges from 0 to 17. A high score indicates aggravated radiation damage. Using this semi-quantitative scoring system, we evaluated radiation-induced colitis, architectural distortions and the reparative disturbances of stromal and vascular cells (Figure 1).
Table 1.
Histopathological scoring system for radiation-induced bowel injury
| Item | Scorea |
|---|---|
| Mucosal erosions/ulcerations | 0: Absent |
| 1: Superficial erosions | |
| 2: Ulcerations | |
| Nuclear abnormalities of epithelial cells (Surface & Crypt) | 0: Absent |
| 1: Hyperchromatic, enlarged nuclei with irregular contours; atypical mitotic figures | |
| Crypt disarrangement | 0: Absent |
| 1: Crypt disarray | |
| 2: Crypt distortion with branching or shortening | |
| Mucosal inflammatory infiltrate | 0: Absent |
| 1: Mild increase in cellularity of the lamina propria | |
| 2: Moderate increase in lymphocytes and plasma cells; eosinophilic crypt abscess | |
| 3: Marked increase in lymphocytes and plasma cells with aggregation as ulcerative colitis | |
| Mucosal edema | 0: Absent |
| 1: Broadened mucosa with scattered cellularity | |
| Fibrosis of the lamina propria | 0: Absent |
| 1: Increase in hyalinized collagen fibers | |
| Fibrosis of submucosa | 0: Absent |
| 1: Mild increase in broadened and hyalinized collagen fibers | |
| 2: Moderate increase in dense collagen fibers; increased submucosal thickness | |
| 3: Marked fibrosis including muscularis | |
| Sclerosis of submucosal vessels | 0: Absent |
| 1: Fibromuscular thickening of the intima | |
| 2: Moderate thickening of the intima with luminal stenosis | |
| 3: Extreme sclerosis with marked stenosis or occlusion | |
| Lymph congestion | 0: Absent |
| 1: Dilated lymph vessels or cystic collections of lymph |
aThe total score of nine items ranges from 0 to 17. A higher score indicates aggravated radiation damage.
Figure 1.
Morphologic features of radiation-induced bowel injury. (A) Crypt disarrangement and mucosal inflammatory infiltrate (Hematoxylin & Eosin staining, 40× field; histopathological score, 2 for both). (B) Mucosal erosions, fibrosis of the lamina propria and fibrosis of submucosa (Masson’s trichrome staining, 100× field; histopathological score, 1, 1 and 2, respectively). (C) Fibrosis of submucosa (Masson’s trichrome staining, 40× field; histopathological score, 2). (D) Sclerosis of submucosal vessels (Masson’s trichrome staining, 100× field; histopathological score, 2).
We also assessed the microvessel density in submucosal areas for each sample. Microvessel staining and counting followed the modified procedures as previously reported by Weidner et al. [20]. Briefly, microvessels were highlighted by staining endothelial cells for cluster of differentiation-31 (CD31), using the immunohistochemistry technique (Supplementary Figure 1). A brown-stained endothelial cell or cluster, clearly separate from adjacent elements, was considered as a single microvessel. The lumen was not necessary for microvessel definition. Microvessel counts were assessed in vascularized areas of submucosa, which contain the highest number of capillaries and venules per area. These neovascular areas were identified at low magnification (40× and 100×) and found most frequently adjacent to the muscularis mucosa. Individual microvessel counts were made on a 200× field (0.140 mm2 per field). Microvessel density was expressed as the mean number of microvessels identified on 10 representative 200× fields.
For histopathological scoring, all slides were examined simultaneously by two of the authors (Y.Z. and X.F.) using a multi-headed light microscope. Consensus was reached before any score was made. Microvessel counts were also made by two of the authors (Y.Z. and P.W.) simultaneously, both of whom had to agree on the identification of a single microvessel before any vessel was included in the count. A third pathologist (Y.H.) repeated the tissue scoring and microvessel counts with the same criteria independently and separately. Good consistency was shown between double verifications (Supplementary Table 1). All assessments were performed without knowledge of any patient’s outcome, treatment group and other pertinent information.
Statistical analysis
Comparisons between groups were performed using the Kruskal–Wallis H-test or Mann–Whitney U-test for quantitative variables and χ2 test or Fisher exact test for categorical variables. The Wilcoxon matched-pairs signed-ranks test was used for comparisons between the proximal and distal margins. Because the nature of retrospective cohort leads to certain selection bias, we conducted a propensity score-matching analysis to compensate for the differences in baseline characteristics. Comparisons between treatment groups were initially performed and a propensity score (the probability that a patient received nCRT or not) was calculated using a logistic regression with imbalanced variables. Patients were matched according to propensity scores to make an even distribution of potential confounding factors among treatment groups. For patients undergoing nCRT, we performed univariate analyses to identify factors associated with AL and to calculate odds ratio (OR) and 95% confidence interval (CI). A two-sided P ≤ 0.05 was considered statistically significant. All statistical analyses were carried out by using SPSS Statistics 22.0 (IBM Corp, Armonk, NY, USA).
Results
Demographic and clinical data
In the entire cohort of 161 patients, 54 patients were treated with nCRT, 48 with nCT alone and 59 without any neoadjuvant therapy (Table 2). The patients in the nCRT group were younger, had more diverting stomas and longer proximal resection margins than the nCT and control groups. Patients receiving neoadjuvant therapy had more advanced-stage cancer than that of the control group. A propensity score was calculated for each patient with identified variables that were not equally distributed among the three treatment groups. After matching on propensity scores, the three groups were nearly balanced except for the use of diverting stoma. Overall, the occurrence rate of AL was 15.5% (25/161) and the rate of clinical AL was 8.1% (13/161). Only one patient underwent salvage operation with colostomy 7 days after curative surgery; the other 12 patients with clinical AL received active drainage or transanal lavage within 30 days post-operatively. Noticeably, the total AL rate of the nCRT group was twice that of the nCT or control group, no matter before or after propensity score matching (Table 2).
Table 2.
Patient and treatment characteristics in three groups before and after propensity score matching
| Before matching |
After matching |
|||||||
|---|---|---|---|---|---|---|---|---|
| nCRT group | nCT group | Control group |
nCRT group | nCT group | Control group |
|||
| Variable | (n = 54) | (n = 48) | (n = 59) | P-value | (n = 48) | (n = 48) | (n = 48) | P-value |
| Age (years) | 50.5 (29–70) | 59 (22–77) | 59 (29–81) | 0.003 | 51.5 (30–70) | 59 (22–77) | 57 (29–81) | 0.064 |
| BMI (kg/m2) | 22.8 (17.5–27.6) | 22.9 (16.4–32.8) | 22.0 (14.2–32.8) | 0.641 | 22.7 (17.5–27.6) | 22.9 (16.4–32.8) | 22.3 (14.2–32.8) | 0.616 |
| Gender | 0.878 | 0.567 | ||||||
| Female | 19 (35.2) | 15 (31.3) | 21 (35.6) | 18 (37.5) | 15 (31.3) | 20 (41.7) | ||
| Male | 35 (64.8) | 33 (68.7) | 38 (64.4) | 30 (62.5) | 33 (68.7) | 28 (58.3) | ||
| Tumor stage | 0.002 | 0.264 | ||||||
| II | 4 (7.4) | 5 (10.4) | 18 (30.5) | 4 (8.3) | 5 (10.4) | 9 (18.8) | ||
| III | 50 (92.6) | 43 (89.6) | 41 (69.5) | 44 (91.7) | 43 (89.6) | 39 (81.2) | ||
| Tumor locationa | 0.436 | 0.419 | ||||||
| ≤7 cm | 27 (50.0) | 18 (37.5) | 25 (42.4) | 24 (50.0) | 18 (37.5) | 23 (47.9) | ||
| >7 cm | 27 (50.0) | 30 (62.5) | 34 (57.6) | 24 (50.0) | 30 (62.5) | 25 (52.1) | ||
| Chemotherapy | NA | NA | ||||||
| 5-FU | 23 (42.6) | 0 | NA | 22 (45.8) | 0 | NA | ||
| FOLFOX | 31 (57.4) | 48 (100) | NA | 26 (54.2) | 48 (100) | NA | ||
| Diverting stoma | < 0.001 | < 0.001 | ||||||
| Yes | 52 (96.3) | 20 (41.7) | 16 (27.1) | 46 (95.8) | 20 (41.7) | 16 (33.3) | ||
| No | 2 (3.7) | 28 (58.3) | 43 (72.9) | 2 (4.2) | 28 (58.3) | 32 (66.7) | ||
| Type of anastomosis | 0.819 | 0.929 | ||||||
| Sigmoid colon-rectum | 50 (92.6) | 43 (89.6) | 55 (93.2) | 45 (93.8) | 43 (89.6) | 44 (91.7) | ||
| Descending colon-rectum | 4 (7.4) | 5 (10.4) | 4 (6.8) | 3 (6.2) | 5 (10.4) | 4 (8.3) | ||
| Proximal margin (cm)b | 7.8 (3.0–15.5) | 6.4 (2.3–17.3) | 6.5 (2.3–18.0) | 0.030 | 7.0 (3.0–15.5) | 6.4 (2.3–17.3) | 6.5 (2.3–18.0) | 0.219 |
| Distal margin (cm)b | 2.0 (0.5–5.0) | 2.0 (0.5–6.5) | 2.0 (0.5–4.5) | 0.868 | 2.0 (0.5–5.0) | 2.0 (0.5–6.5) | 2.0 (0.5–4.5) | 0.855 |
| Anastomotic leakage | 0.071 | 0.017 | ||||||
| Clinical | 5 (9.3) | 2 (4.1) | 6 (10.2) | 5 (10.4) | 2 (4.1) | 4 (8.3) | ||
| Radiologic | 8 (14.8) | 3 (6.3) | 1 (1.7) | 8 (16.7) | 3 (6.3) | 0 | ||
| None | 41 (75.9) | 43 (89.6) | 52 (88.1) | 35 (72.9) | 43 (89.6) | 44 (91.7) | ||
Data are expressed as median (range) or number of patients (%).
nCRT, neoadjuvant chemoradiotherapy; nCT, neoadjuvant chemotherapy; BMI, body mass index; 5-FU, 5-fluorouracil; FOLFOX, fluorouracil, leucovorin and oxaliplatin; NA, not applicable.
aDistance from anal verge.
bLength measured in formalin-fixed specimens.
Histopathological assessment for surgical margins
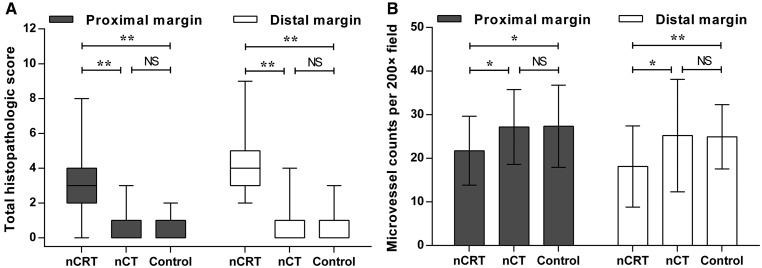
Histopathological features were compared among the three groups (Figure 2). Significant differences were found in architectural distortion, inflammation and extensive fibrosis (Supplementary Table 2). After propensity score matching, the median (range) of the total histopathological score was significantly higher in the nCRT group than in the nCT and control groups for both proximal margins [3 (0–8) vs 0 (0–3) and 0 (0–2), P < 0.001] and distal margins [4 (2–9) vs 0 (0–4) and 0 (0–3), P < 0.001]. Microvessel density corresponded with the difference in total histopathological score among the three groups. The average microvessel counts per 200× field were significantly lower in the nCRT group than in the other two groups for both proximal margins (21.7 ± 7.9 vs 27.2 ± 8.6 and 27.3 ± 9.4, P = 0.003) and distal margins (18.1 ± 9.3 vs 25.2 ± 12.9 and 24.9 ± 7.4, P < 0.001).
Figure 2.
Total histopathological score (A) and microvessel density (B) for resection margins in three groups after propensity score matching. Score (range) for proximal margin: nCRT, 3 (0–8); nCT, 0 (0–3); control, 0 (0–2). Score for the distal margin: nCRT, 4 (2–9); nCT, 0 (0–4); control, 0 (0–3). Counts for the proximal margin: nCRT, 21.7 ± 7.9, nCT, 27.2 ± 8.6; control, 27.3 ± 9.4. Counts for the distal margin: nCRT, 18.1 ± 9.3; nCT, 25.2 ± 12.9; control, 24.9 ± 7.4. *P < 0.01, **P < 0.001. NS, not significant; nCRT, neoadjuvant chemoradiotherapy; nCT, neoadjuvant chemotherapy.
For patients undergoing nCRT, total histopathological score (P < 0.001) and microvessel density (P = 0.027) were significantly different between the proximal and distal margins; however, the differences were not significant for patients in the nCT and control groups (all P > 0.05). Besides, neither chemotherapy regimen of nCRT (Table 3) nor length of resection margin (Table 4) was associated with the histopathological features of both surgical margins.
Table 3.
Total histopathological score and microvessel density for resection margins in nCRT group according to the chemotherapy regimen
| 5-FU | FOLFOX | ||
|---|---|---|---|
| Variable | (n = 23) | (n = 31) | P-value |
| Total histopathologic score | |||
| Proximal margin | 3 (0–8) | 3 (1–7) | 0.872 |
| Distal margin | 4 (2–8) | 4 (2–9) | 0.761 |
| Microvessel densitya | |||
| Proximal margin | 21.4 ± 8.7 | 21.6 ± 7.2 | 0.827 |
| Distal margin | 16.3 ± 10.3 | 19.2 ± 8.9 | 0.137 |
Data are expressed as median (range) or mean ± standard deviation.
nCRT, neoadjuvant chemoradiotherapy; 5-FU, 5-fluorouracil; FOLFOX, fluorouracil, leucovorin and oxaliplatin.
aMicrovessel counts per 200× field.
Table 4.
Correlation between the histopathological features and length of resection margins
| nCRT group (n = 54) |
nCT group (n = 48) |
Control group (n = 59) |
||||
|---|---|---|---|---|---|---|
| Variable | Coefficienta | P-value | Coefficienta | P-value | Coefficienta | P-value |
| Total histopathological score and length of margin | ||||||
| Proximal | −0.195 | 0.158 | 0.119 | 0.420 | −0.168 | 0.203 |
| Distal | 0.252 | 0.066 | −0.151 | 0.307 | −0.002 | 0.986 |
| Microvessel density and length of margin | ||||||
| Proximal | 0.025 | 0.860 | 0.190 | 0.195 | −0.112 | 0.398 |
| Distal | −0.038 | 0.786 | −0.084 | 0.569 | 0.056 | 0.674 |
nCRT, neoadjuvant chemoradiotherapy; nCT, neoadjuvant chemotherapy.
aSpearman rank correlation coefficient.
Radiation-induced injury and AL
Among patients undergoing nCRT, significantly higher total histopathological score (P = 0.009) and lower microvessel density (P = 0.006) of the proximal margin were presented in patients with AL than in those without AL (Table 5), whereas those of the distal margin showed no significant difference between the two subgroups of patients. Among the patients in the nCT and control groups, neither total histopathological score nor microvessel density was significantly different between the patients with and without AL. Furthermore, univariate analysis for patients undergoing nCRT found that the occurrence of AL was only associated with increased total histopathological score (OR, 13.90; 95% CI, 1.65–116.96; P = 0.003) and decreased microvessel density (OR, 8.59; 95% CI, 1.68–43.95; P = 0.004) of the proximal margin (Table 6).
Table 5.
Total histopathological score and microvessel density for resection margins in three groups according to the presence of anastomotic leakage
| nCRT group |
nCT group |
Control group |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| AL (+) | AL (−) | AL (+) | AL (−) | AL (+) | AL (−) | ||||
| Variable | (n = 13) | (n = 41) | P-value | (n = 5) | (n = 43) | P-value | (n = 7) | (n = 52) | P-value |
| Total histopathological score | |||||||||
| Proximal margin | 4 (2–7) | 2 (0–8) | 0.009 | 0 (0–3) | 0 (0–2) | 0.670 | 0 (0–2) | 0 (0–2) | 0.400 |
| Distal margin | 4 (3–7) | 4 (2–9) | 0.183 | 0 (0–4) | 0 (0–2) | 0.974 | 1 (0–1) | 0 (0–3) | 0.362 |
| Microvessel densitya | |||||||||
| Proximal margin | 16.6 ± 4.7 | 23.2 ± 7.8 | 0.006 | 21.9 ± 5.6 | 26.8 ± 9.3 | 0.232 | 27.2 ± 11.5 | 27.1 ± 9.1 | 0.739 |
| Distal margin | 16.2 ± 9.8 | 18.6 ± 9.5 | 0.312 | 25.0 ± 6.3 | 25.2 ± 13.5 | 0.844 | 23.4 ± 4.3 | 25.1 ± 8.1 | 0.863 |
Data are expressed as median (range) or mean ± standard deviation.
nCRT, neoadjuvant chemoradiotherapy; nCT, neoadjuvant chemotherapy; AL, anastomotic leakage.
aMicrovessel counts per 200× field.
Table 6.
Univariate analysis of factors associated with anastomotic leakage in patients undergoing nCRT
| Variable | Category | No. of AL/total patients (%) | OR | 95% CI | P-value |
|---|---|---|---|---|---|
| Age (years) | ≤50 | 6/27 (22.2) | 1 | ||
| >50 | 7/27 (25.9) | 1.23 | 0.35–4.28 | 0.750 | |
| BMI (kg/m2) | ≤22.5 | 4/25 (16.0) | 1 | ||
| >22.5 | 9/29 (31.0) | 2.36 | 0.63–8.91 | 0.198 | |
| Gender | Female | 4/19 (21.1) | 1 | ||
| Male | 9/35 (25.7) | 1.30 | 0.34–4.95 | 0.961 | |
| Tumor stage | II | 1/4 (25.0) | 1 | ||
| III | 12/50 (24.0) | 0.95 | 0.09–9.98 | 1.000 | |
| Tumor location (cm)a | >7 | 7/27 (25.9) | 1 | ||
| ≤7 | 6/27 (22.2) | 0.82 | 0.23–2.85 | 0.750 | |
| Chemotherapy | 5-FU | 7/23 (30.4) | 1 | ||
| FOLFOX | 6/31 (19.4) | 0.55 | 0.16–1.93 | 0.346 | |
| Diverting stoma | Yes | 13/52 (25.0) | |||
| No | 0/2 (0) | NA | |||
| Type of anastomosis | Sigmoid colon-rectum | 13/50 (26.0) | |||
| Descending colon-rectum | 0/4 (0) | NA | |||
| Length of margin (cm)b | |||||
| Proximal margin | >7 | 5/28 (17.9) | 1 | ||
| ≤7 | 8/26 (30.8) | 2.04 | 0.57–7.33 | 0.267 | |
| Distal margin | >2 | 6/23 (26.1) | 1 | ||
| ≤2 | 7/31 (22.6) | 0.83 | 0.24–2.90 | 0.766 | |
| Total histopathological score | |||||
| Proximal margin | <3 | 1/23 (4.3) | 1 | ||
| ≥3 | 12/31 (38.7) | 13.90 | 1.65–116.96 | 0.003 | |
| Distal margin | <4 | 4/24 (16.7) | 1 | ||
| ≥4 | 9/30 (30.0) | 2.14 | 0.57–8.08 | 0.255 | |
| Microvessel densityc | |||||
| Proximal margin | >22 | 2/27 (7.4) | 1 | ||
| ≤22 | 11/27 (40.7) | 8.59 | 1.68–43.95 | 0.004 | |
| Distal margin | >16 | 5/27 (18.5) | 1 | ||
| ≤16 | 8/27 (29.6) | 1.85 | 0.52–6.63 | 0.340 |
nCRT, neoadjuvant chemoradiotherapy; AL, anastomotic leakage; OR, odds ratio; CI, confidence interval; BMI, body mass index; 5-FU, 5-fluorouracil; FOLFOX, fluorouracil, leucovorin and oxaliplatin; NA, not applicable.
aDistance from anal verge.
bLength measured in formalin-fixed specimens.
cMicrovessel counts per 200× field.
Discussion
Radiotherapy is the cornerstone of neoadjuvant therapy for rectal cancer, but there remain concerns about the effect of pre-operative radiation on anastomotic integrity after curative surgery [9–11, 21]. The evidence of surgical pathology is necessary to clarify this critical issue. The present study avoided the distraction of pre-operative chemotherapy [22, 23] and the propensity score-matched cohort demonstrated the adverse effect of pre-operative radiotherapy on AL. We identified the radiation-induced injury on resection margins by distinct histopathological features. This radiation damage was shown to be associated with the occurrence of AL in patients undergoing nCRT.
To assess the radiation-induced bowel injury, we referred to a series of morphologic features that cover the mucosal and submucosal areas. The specimens encompass the whole thickness of both resection margins to accommodate thorough evaluations. Previous studies have described the post-radiation intestinal changes in detail, but found no clinical implication of the distinct features [14, 15]. In this study, we observed significant tissue responses in architectural distortion, inflammation and extensive fibrosis after radiotherapy, which have been shown to attenuate the bowel anastomosis in animal experiments [24, 25]. Moreover, the original scoring system provides an objective evaluation of the histopathological features with intensity assessment and may be used to identify the radiation damage as a whole. Similar strategies have been widely used in the diagnosis of IBDs [26, 27]. Submucosal vasculature determines the tissue perfusion that has been confirmed to influence anastomotic dehiscence [28–30]. The decreased microvessel density on resection margins after nCRT indicates an impairment of blood supply in the anastomosis.
When the coverage of the distal resection margin by radiation volumes is predictable, it is surprising to observe some radiation-induced injury left on the proximal margin after nCRT. Although the aggravated damage on the distal margin may be due to the short distance to the tumor, we found no association between the length of the resection margin and radiation-induced injury. According to the guideline of IMRT contouring for rectal cancer, the superior margin of the clinical target volumes reaches the common iliac vessel bifurcation, with approximate boney landmark of the sacral promontory [31]. In the present cohort, the sigmoid colon was used for anastomosis in most cases. This suggests that the proximal margins within the pelvis may be subject to certain radiation by nCRT, though damage distributions need to be detailed in future studies.
In this study, both clinical and radiologic leaks were included for analysis, owing to their indications of unhealthy healing of the anastomosis. We applied propensity score matching to make the treatment groups comparable on the baseline, where more diversions did not compensate for the increased risk of AL caused by nCRT. Radiation-induced injury left on surgical margins is a possible explanation for adverse effects of pre-operative radiotherapy on anastomotic integrity. Among patients undergoing nCRT, we revealed the association between AL and aggravated radiation damage on the proximal margin, while the distal margin was injured indiscriminately. This sends a message that the occurrence of AL after nCRT could be partly due to the inadequate resections that retain both ends of the injured bowel for an unhealthy anastomosis.
The region of radiation damage is far more extensive than presented on gross inspection. Therefore, awareness of radiation damage should be raised for anterior resection after nCRT. Several animal studies have revealed that the anastomotic healing is impaired by radiation to both limbs of the bowel anastomosis [32–34]; however, the strength is not affected providing only one-sided limb is irradiated [35, 36]. Clinical evidence also shows that a safe anastomosis in the context of radiation enteritis should be constructed with non-irradiated bowel at least at one end [12, 13]. Therefore, a proximally extended resection out of the pelvis may be a feasible approach to constructing an anastomosis with at least one end of non-irradiated bowel after nCRT for rectal cancer.
The present study has several limitations. First, the outcomes were based on a limited cohort from a single center. Subgroup analysis with small numbers made it difficult to confirm the independent effect of radiation damage on AL. Besides, the retrospective design incurred certain bias, although we tried to minimize this by using propensity score-matching analysis. Further investigations with a large prospective cohort are certainly needed. Second, despite the assessment of radiation-induced bowel injury coming from solid histopathological observations, the original scoring system requires more evaluation in future studies. Finally, the status of the resection margins is an approximation of the intact anastomosis, of which the healing is a dynamic and multifactorial process.
Conclusions
The surgical margins of rectal-cancer resection are exposed to some degree of radiation-induced injury after nCRT. The aggravated radiation damage on the proximal margin is associated with AL, where both proximal and distal margins are generally involved in serious damage. A proximally extended resection may benefit particular patients undergoing nCRT in terms of a healthy end for the anastomosis.
Funding
This work was supported by Sun Yat-sen University Clinical Research 5010 Program (grant numbers 2017008) and National Natural Science Foundation of China (grant numbers 81573078).
Supplementary Material
Acknowledgements
The authors thank Dr Liudan Tu and Dr Lekun Fang for valuable advice on the article.
Conflict of interest: All authors declare no conflicts of interest.
References
- 1.National Comprehensive Cancer Network. NCCN Guidelines, Version 2. Rectal Cancer. Fort Washington, PA: National Comprehensive Cancer Network, 2017. [Google Scholar]
- 2. Ceelen WP, Van Nieuwenhove Y, Fierens K. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev 2009;D6041. [DOI] [PubMed] [Google Scholar]
- 3. Marwan K, Staples MP, Thursfield V et al. . The rate of abdominoperineal resections for rectal cancer in the state of Victoria, Australia: a population-based study. Dis Colon Rectum 2010;53:1645–51. [DOI] [PubMed] [Google Scholar]
- 4. Ferrari L, Fichera A. Neoadjuvant chemoradiation therapy and pathological complete response in rectal cancer. Gastroenterol Rep (Oxf) 2015;3:277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mirnezami A, Mirnezami R, Chandrakumaran K et al. . Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 2011;253:890–9. [DOI] [PubMed] [Google Scholar]
- 6. Espin E, Ciga MA, Pera M et al. . Oncological outcome following anastomotic leak in rectal surgery. Br J Surg 2015;102:416–22. [DOI] [PubMed] [Google Scholar]
- 7. Eriksen MT, Wibe A, Norstein J et al. . Anastomotic leakage following routine mesorectal excision for rectal cancer in a national cohort of patients. Colorectal Dis 2005;7:51–7. [DOI] [PubMed] [Google Scholar]
- 8. Jestin P, Pahlman L, Gunnarsson U. Risk factors for anastomotic leakage after rectal cancer surgery: a case-control study. Colorectal Dis 2008;10:715–21. [DOI] [PubMed] [Google Scholar]
- 9. Chang JS, Keum KC, Kim NK et al. . Preoperative chemoradiotherapy effects on anastomotic leakage after rectal cancer resection: a propensity score matching analysis. Ann Surg 2014;259:516–21. [DOI] [PubMed] [Google Scholar]
- 10. Park JS, Choi GS, Kim SH et al. . Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg 2013;257:665–71. [DOI] [PubMed] [Google Scholar]
- 11. Qin Q, Ma T, Deng Y et al. . Impact of preoperative radiotherapy on anastomotic leakage and stenosis after rectal cancer resection: post hoc analysis of a randomized controlled trial. Dis Colon Rectum 2016;59:934–42. [DOI] [PubMed] [Google Scholar]
- 12. Galland RB, Spencer J. Surgical management of radiation enteritis. Surgery 1986;99:133–9. [PubMed] [Google Scholar]
- 13. Onodera H, Nagayama S, Mori A et al. . Reappraisal of surgical treatment for radiation enteritis. World J Surg 2005;29:459–63. [DOI] [PubMed] [Google Scholar]
- 14. Leupin N, Curschmann J, Kranzbuhler H et al. . Acute radiation colitis in patients treated with short-term preoperative radiotherapy for rectal cancer. Am J Surg Pathol 2002;26:498–504. [DOI] [PubMed] [Google Scholar]
- 15. Starzewski JJ, Pajak JT, Pawełczyk I et al. . The radiation-induced changes in rectal mucosa: hyperfractionated vs. hypofractionated preoperative radiation for rectal cancer. Int J Radiat Oncol Biol Phys 2006;64:717–24. [DOI] [PubMed] [Google Scholar]
- 16. Deng Y, Chi P, Lan P et al. . Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol 2016;34:3300–7. [DOI] [PubMed] [Google Scholar]
- 17. Glynne-Jones R, Wyrwicz L, Tiret E et al. . Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:v22–40. [DOI] [PubMed] [Google Scholar]
- 18. Rahbari NN, Weitz J, Hohenberger W et al. . Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the international study group of rectal cancer. Surgery 2010;147:339–51. [DOI] [PubMed] [Google Scholar]
- 19. Langberg CW, Waldron JA, Baker ML et al. . Significance of overall treatment time for the development of radiation-induced intestinal complications. an experimental study in the rat. Cancer 1994;73:2663–8. [DOI] [PubMed] [Google Scholar]
- 20. Weidner N, Semple JP, Welch WR et al. . Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991;324:1–8. [DOI] [PubMed] [Google Scholar]
- 21. Qin C, Ren X, Xu K et al. . Does preoperative radio(chemo)therapy increase anastomotic leakage in rectal cancer surgery? A meta-analysis of randomized controlled trials. Gastroenterol Res Pract 2014;2014:1–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bozdag AD, Peker Y, Derici H et al. . The effect of preoperative 5-fluorouracil on colonic healing: an experimental study. Hepatogastroenterology 2001;48:1631–4. [PubMed] [Google Scholar]
- 23. Kuzu MA, Koksoy C, Kale T et al. . Experimental study of the effect of preoperative 5-fluorouracil on the integrity of colonic anastomoses. Br J Surg 1998;85:236–9. [DOI] [PubMed] [Google Scholar]
- 24. Dominguez JM, Jakate SM, Speziale NJ et al. . Intestinal anastomotic healing at varying times after irradiation. J Surg Res 1996;61:293–9. [DOI] [PubMed] [Google Scholar]
- 25. Kuzu MA, Kuzu I, Koksoy C et al. . Histological evaluation of colonic anastomotic healing in the rat following preoperative 5-fluorouracil, fractionated irradiation, and combined treatment. Int J Colorectal Dis 1998;13:235–40. [DOI] [PubMed] [Google Scholar]
- 26. Geboes K, Riddell R, Ost A et al. . A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000;47:404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magro F, Langner C, Driessen A et al. . European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 2013;7:827–51. [DOI] [PubMed] [Google Scholar]
- 28. Millan M, Garcia-Granero E, Flor B et al. . Early prediction of anastomotic leak in colorectal cancer surgery by intramucosal pH. Dis Colon Rectum 2006;49:595–601. [DOI] [PubMed] [Google Scholar]
- 29. Vignali A, Gianotti L, Braga M et al. . Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum 2000;43:76–82. [DOI] [PubMed] [Google Scholar]
- 30. Fawcett A, Shembekar M, Church JS et al. . Smoking, hypertension, and colonic anastomotic healing; a combined clinical and histopathological study. Gut 1996;38:714–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Myerson RJ, Garofalo MC, El NI et al. . Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys 2009;74:824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saclarides TJ, Rohrer DA, Bhattacharyya AK et al. . Effect of intraoperative radiation on the tensile strength of small bowel anastomoses. Dis Colon Rectum 1992;35:151–7. [DOI] [PubMed] [Google Scholar]
- 33. Jahnson S, Graf W, Rikner G et al. . Anastomotic breaking strength and healing of anastomoses in rat intestine with and without chronic radiation damage. Eur J Surg 1995;161:425–30. [PubMed] [Google Scholar]
- 34. Kuzu MA, Koksoy C, Akyol FH et al. . Effects of preoperative fractionated irradiation on left colonic anastomoses in the rat. Dis Colon Rectum 1998;41:370–6. [DOI] [PubMed] [Google Scholar]
- 35. Biert J, Hoogenhout J, Wobbes T et al. . High-dose preoperative irradiation without detrimental effect on early repair of anastomoses in the colon of the rat. Radiat Res 1997;147:362–8. [PubMed] [Google Scholar]
- 36. Ceelen W, El MM, Cardon A et al. . Influence of preoperative high-dose radiotherapy on postoperative outcome and colonic anastomotic healing: experimental study in the rat. Dis Colon Rectum 2001;44:717–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.