Abstract
Background
Chronic antibiotic-refractory pouchitis (CARP) is a complication of ileal pouch-anal anastomosis (IPAA), which poses a therapeutic challenge. Vedolizumab, a gut-selective monoclonal antibody to the α4β7 of integrin, has been used in such patients, but data on its efficacy are limited. Our aim was to assess the efficacy and safety of vedolizumab as induction therapy in CARP patients.
Methods
In this single-center, historic cohort, patients with CARP who received vedolizumab between January 2015 to June 2017 were identified and analysed. Patients were included if they had active pouchitis with a total of modified pouch disease activity index (mPDAI) score ≥5 or if unavailable clinician diagnosis of active pouchitis. Pre-treatment and at 3-month post-therapy pouchoscopy and clinical visits were used to calculate mPDAI.
Results
A total of 19 patients were included in the study. The mean age was 26.7 ± 12.8 years, with 10 (53%) males. Nine (47%) patients had been treated with anti-tumor necrosis factor (TNF) agents before colectomy and 10 (53%) had anti-TNFs after colectomy and IPAA. Six (32%) patients had improvement in the mPDAI symptom subscores (P = 0.031) and 14 (74%) had improvement in both endoscopic and total mPDAI scores with a median change of –2 units (both P = 0.031). Adverse events were noted only in two (11%) patients and four (21%) required surgery for CARP.
Conclusions
Our study suggests that vedolizumab has efficacy and can be safely used for CARP patients. Larger studies with a higher number of patients are required to confirm these findings.
Keywords: Vedolizumab, chronic antibiotic-refractory puchitits, modified pouch disease activity index
Introduction
Restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) is the treatment of choice for medically refractory ulcerative colitis (UC), colitis-associated dysplasia and familial adenomatous polyposis (FAP) [1]. Pouchitis is the most common complication in patients with IPAA, which is almost exclusive in UC patients with IPAA as compared to FAP counterparts, with a reported cumulative prevalence ranging from 23 to 46 % and an annual incidence up to 40% [2, 3]. Symptomatic patients typically present with an increase in stool frequency, urgency, incontinence and abdominal, rectal or pelvic pain. Diagnosis is made by a combination of clinical, endoscopic and histologic variables. Antibiotics are the mainstay of the treatment in such patients but, in certain patients, symptoms persist despite various antibiotic therapies. About 5–19% of these patients develop chronic relapsing or treatment refractory disease [4]. Chronic antibiotic-refractory pouchitis (CARP), described as the persistence of symptoms after 2 weeks of receiving a course of ciprofloxacin, metronidazole or rifaximin, alone or in combination for pouchitis, has emerged as a challenge for physicians treating pouchitis patients [5]. In cases of antibiotic resistance, therapeutic interventions may escalate to include steroids or biologics such as anti-tumor necrosis factor (TNF) agents. Surgical interventions are used in failed medical therapy and include pouch resection or redo.
Vedolizumab is a gut-specific monoclonal antibody, which acts on the α4β7 isomer of integrin and blocks gut lymphocyte trafficking [6]. It has been approved by the Food and Drug Administration for the induction and maintenance of remission in moderate to severe Crohn’s disease (CD) and UC. Recently, it has been used off-label for CARP and CD of the pouch, and has been shown to be beneficial with symptomatic and endoscopic improvement [7–9]. In a recent multicenter study from Germany, vedolizumab after 14 weeks of therapy was shown to cause significant improvement in Orseland score (OS) and pouch disease activity index (PDAI) in patients with CARP and chronic antibiotic-dependent pouchitis (CADP) [10]. In a small case-series of four patients, we also showed improvement in symptoms and endoscopic appearance of the pouch after 3 months of therapy with vedolizumab in patients who initially failed all other therapies including anti-TNF agents [11].
We herein aim to assess the efficacy and safety profile of vedolizumab in CARP, which might help physicians to guide therapy in such patients and, in the long term, possibly help to avoid or delay the need for pouch surgery.
Patients and methods
After obtaining approval from the Cleveland Clinic Institutional Review Board (IRB), we reviewed all pouchitis patients who were regularly followed in the Center for Ileal Pouch Disorders between January 2015 and June 2017. Using ICD-9 codes, patients who had received vedolizumab for CARP (300 mg at Weeks 0, 2, 6 and 10) were identified and analysed.
Inclusion and exclusion criteria
Patients were included if they had underlying inflammatory bowel disease (IBD); an ileal pouch; diagnosis of active pouchitis with a total modified pouch disease activity (mPDAI) score ≥5 or, if unavailable, clinician diagnosis of active pouchitis; vedolizumab for the treatment of CARP who received at least one infusion of vedolizumab; pre- and post-treatment pouchoscopy; follow-up at 3 months post therapy (endoscopic or clinical); and if they were older than 18 years of age. They were excluded if they had underlying FAP; CD of the pouch; diverting loop ostomy; or were younger than 18 years of age.
The diagnosis of CARP was made if the patient had symptoms of active pouchitis after receiving a 2-week course of ciprofloxacin, metronidazole or rifaximin for pouchitis [5].
Clinical variables
A retrospective chart review was performed by one investigator (A.S.) to extract relevant data and demographic information, including age, gender, body mass index (BMI), smoking history, family history of IBD, chronic medical issues, history of colon cancer and clinical symptoms including presence or absence of extra-intestinal manifestations (EIM). Pouch-related variables were also collected, including the indication of pouch surgery, duration of the disease, type of pouch, history of pouchitis and past or current immunosuppressants (azathioprine, 6-mercaptopurine [6-MP], methotrexate [MTX] and anti-TNF or anti-integrin agents).
In addition, we recorded information regarding vedolizumab-related adverse events, exacerbation of EIM, pouch failure (defined by a need for pouch revision or excision and permanent ileostomy) or death. The diagnosis of pouchitis was made on the basis of the triad of compatible symptoms, endoscopic and histologic findings. A pre- and 3-month post-vedolizumab mPDAI was also calculated, with a score ≥5 suggestive of the diagnosis of active pouchitis [12].
Outcome measurements
The primary outcomes were to assess the improvement or reduction in the mPDAI symptom (range: 0–6) and endoscopy (range: 0–6) subscores after 3 months of vedolizumab therapy for CARP. The secondary outcome was the adverse events related to vedolizumab.
Statistical analysis
Data are presented as a mean ± standard deviation, median (25th, 75th percentiles) or frequency (percent). A Sign test was used to assess whether the difference between post- and pre-vedolizumab mPDAI scores was significantly different from 0. Univariable analysis was conducted to assess factors associated with changes in mPDAI; Kruskal–Wallis tests were used for categorical data and Spearman’s correlation coefficients (ρ) were used for continuous data. In addition, quantile regression was used to assess the relationship between the variables and change in mPDAI adjusting for baseline mPDAI. All analyses were performed using SAS (version 9.4, The SAS Institute, Cary, NC) and a P < 0.05 was considered statistically significant.
Ethical considerations
Data were collected using electronic medical records without any direct contact with the patients for the purpose of the study. The need for informed consent was waived and the Cleveland Clinic IRB approved the study.
Results
A total of 19 patients who received vedolizumab for CARP were included in the final analysis. They were followed for treatment response and adverse events at 3 months post therapy.
Demographic and clinical variables
A summary of demographic and clinical characteristics of patients included in the study is shown in Table 1. The mean age was 26.7 ± 12.8 years, 10 (53%) were males, mean BMI was 27.2 ± 6.4 kg/m2, 14 (74%) of the patients never smoked, 4 (21%) had autoimmune diseases, 6 (32%) had family history of IBD, 9 (47.4%) had EIM and the mean age at the time of start of therapy was 44.7 ± 12.4 years. Current or past non-steroidal anti-inflammatory drugs (NSAIDs) use was found in 10 (53%) patients. Five (26%) had past Clostridium difficile pouchitis and 1 (5%) had past cytomegalovirus (CMV) infection of the pouch. Overall, 9 (47%) were treated with anti-TNF agents before colectomy and 10 (53%) had anti-TNFs after colectomy and IPAA (Table 2).
Table 1.
Demographic and clinical characteristics
| Factor | Total (N = 19) |
|---|---|
| Age at IBD diagnosis, years | 26.7 ± 12.8 |
| Male gender | 10 (53) |
| Smoking | – |
| Past | 5 (26) |
| Never | 14 (74) |
| Family history of CRC | 3(16) |
| Family history of IBD | 6 (32) |
| Age at 1st visit after vedolizumab, years | 44.7±12.4 |
| IBD duration at first visit after vedolizumab, years | 13 [9, 25] |
| BMI at time of vedolizumab initiation, kg/m2 | 27.2 ± 6.4 |
| Autoimmune disease | 4 (21) |
| Prior surgical history | 5 (26) |
| Prior surgery type | |
| None | 14 (74) |
| Hernia repair | 2 (11) |
| Hernia repair + pouch redo | 1 (5) |
| Redo pouch | 1 (5) |
| Appendectomy | 1 (5) |
| Extra-intestinal manifestations | 9 (47) |
| Liver (primary sclerosing cholangitis) | 1 (5) |
| Joint | 6 (32) |
| Skin | 1 (5) |
| Eyes | 1 (5) |
| NSAIDs use | |
| Never | 9 (47) |
| Current | 3 (16) |
| Past | 7 (37) |
| Past cytomegalovirus infection | 1 (5) |
| Past Clostridium difficile of pouch | 5 (26) |
| Age at colectomy, years | 32.5 ± 11.7 |
| Pouch type | |
| J | 18 (95) |
| K/Barnett | 1 (5) |
Data presented as mean ± Standard deviation, median (P25, P75) or number (%).
IBD, inflammatory bowel disease; CRC, colorectal cancer; BMI, body mass index; NSAIDs, non-steroidal anti-inflammatory drugs.
Table 2.
Pre-surgery and pre-vedolizumab medications
| Factor | Total (N = 19) |
|---|---|
| Biologics prior to colectomy, n (%) | 9 (47) |
| Pre-colectomy infliximab | 6 (32) |
| Pre-colectomy adalimunab | 6 (32) |
| Pre-colectomy certolizumab | 1 (5) |
| Use of non-biologics for pouchitis, n (%) | 19 (100) |
| Antibiotics (more than one course) | 19 (100) |
| Mesalamine (>3 months, oral/topical) | 12 (63) |
| Systemic steroids (>3 months) | 18 (95) |
| Topical steroids (>3 months) | 10 (53) |
| Azathioprine/6-mercaptopurine (>3 months) | 3 (16) |
| Methotrexate (>3 months) | 3 (16) |
| Pre-vedolizumab biologics, n (%) | 10 (53) |
| Infliximab (>3 months) | 4 (21) |
| Adalimumab (>3 months) | 9 (47) |
Clinical efficacy and adverse effects of vedolizumab
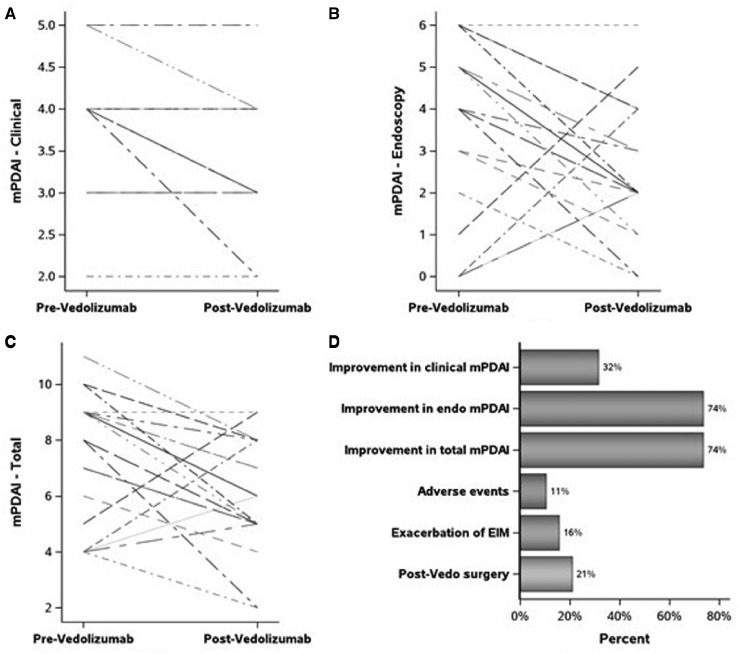
Six (32%) patients demonstrated improvement in the mPDAI symptom subscores (P = 0.031). On the other hand, 14 (74%) patients had improvement in both endoscopic and total mPDAI scores with a median change of –2 units (both P = 0.031) (Table 3). On univariate analysis, patients who did not receive adalimumab prior to colectomy had a higher decrease in endoscopic and total mPDAI (P = 0.020 and 0.038, respectively; Figure 1 and Table 4). Higher BMI was associated with a greater reduction in endoscopic and total mPDAI (ρ = –0.46 [P = 0.047] and –0.51 [P = 0.020], respectively). Older age at first visit post vedolizumab was also associated with a greater reduction in total mPDAI (ρ = –0.46, P = 0.048). Neither of these associations held after adjusting for baseline mPDAI score (P = 0.52, 0.16 and 0.20, respectively).
Table 3.
Pre- and post-vedolizumab modified pouch disease activity index (mPDAI) score
| Factor | Total (N = 19) | P-value |
|---|---|---|
| mPDAI—clinical | 0.031 | |
| Pre vedolizumab | 4.0 [4.0, 4.0] | |
| Post vedolizumab | 4.0 [3.0, 4.0] | |
| Post – pre | 0.00 [–1.00, 0.00] | |
| Any improvement (reduction) | 6 (32) | |
| mPDAI—endoscopic | 0.031 | |
| Pre vedolizumab | 4.0 [2.0, 5.0] | |
| Post vedolizumab | 2.0 [2.0, 4.0] | |
| Post – pre | –2.0 [–2.0, 0.00] | |
| Any improvement (reduction) | 14 (74) | |
| mPDAI—total | 0.031 | |
| Pre vedolizumab | 8.0 [5.0, 9.0] | |
| Post vedolizumab | 6.0 [5.0, 8.0] | |
| Post – pre | –2.0 [–3.0, 0.00] | |
| Any improvement (reduction) | 14 (74) |
Data presented as median [P25, P75] or number (%).
Figure 1.
Vedolizumab outcomes. mPDAI, modified pouch disease activity index; EIM, extra-intestinal manifestations.
Table 4.
Univariate relationships between change in mPDAI scores and several categorical variables of interest
| Factor | No. | Post – pre clinical mPDAI |
Post – pre endoscopic mPDAI |
Post – pre total mPDAI |
|||
|---|---|---|---|---|---|---|---|
| Median | P-value | Median | P-value | Median | P-value | ||
| Gender | 0.76 | 0.097 | 0.21 | ||||
| Male | 10 | 0.00 [–1.00, 0.00] | –2.0 [–3.0, –2.0] | –2.0 [–3.0, –2.0] | |||
| Female | 9 | 0.00 [–1.00, 0.00] | –1.00 [–2.0, 2.0] | –2.0 [–2.0, 2.0] | |||
| Smoking | 0.73 | 0.39 | 0.39 | ||||
| Past | 5 | 0.00 [–1.00, 0.00] | –2.0 [–2.0, –2.0] | –2.0 [–3.0, –2.0] | |||
| Never | 14 | 0.00 [–1.00, 0.00] | –2.0 [–2.0, 2.0] | –2.0 [–3.0, 1.00] | |||
| Family history of IBD | 0.75 | 0.52 | 0.53 | ||||
| No | 13 | 0.00 [–1.00, 0.00] | –2.0 [–2.0, 0.00] | –2.0 [–3.0, 0.00] | |||
| Yes | 6 | 0.00 [–1.00, 0.00] | –2.0 [–3.0, –1.00] | –2.0 [–3.0, –2.0] | |||
| Prior surgical history | 0.50 | 0.23 | 0.23 | ||||
| No | 14 | 0.00 [–1.00, 0.00] | –2.0 [–3.0, 0.00] | –2.0 [–3.0, 0.00] | |||
| Yes | 5 | 0.00 [0.00, 0.00] | –1.00 [–2.0, –1.00] | –2.0 [–2.0, –1.00] | |||
| Extra-intestinal manifestation | 0.071 | 0.47 | 0.36 | ||||
| No | 10 | –0.50 [–1.00, 0.00] | –2.0 [–2.0, –1.00] | –2.0 [–3.0, –2.0] | |||
| Yes | 9 | 0.00 [0.00, 0.00] | –2.0 [–2.0, 2.0] | –2.0 [–3.0, 2.0] | |||
| NSAIDs | 0.76 | 0.39 | 0.64 | ||||
| None | 9 | 0.00 [–1.00, 0.00] | –2.0 [–4.0, –1.00] | –2.0 [–4.0, –1.00] | |||
| Past or present | 10 | 0.00 [–1.00, 0.00] | –2.0 [–2.0, 0.00] | –2.0 [–3.0, 0.00] | |||
| Clostridium difficile of pouch | 0.73 | 0.56 | 0.57 | ||||
| Never | 14 | 0.00 [–1.00, 0.00] | –2.0 [–2.0, 0.00] | –2.0 [–3.0, 0.00] | |||
| Past | 5 | 0.00 [–1.00, 0.00] | –2.0 [–2.0, –2.0] | –2.0 [–3.0, –2.0] | |||
| Biologics prior to colectomy | 0.37 | 0.055 | 0.054 | ||||
| No | 10 | 0.00 [–1.00, 0.00] | –2.0 [–3.0, –2.0] | –2.0 [–3.0, –2.0] | |||
| Yes | 9 | 0.00 [0.00, 0.00] | 0.00 [–2.0, 2.0] | 0.00 [–2.0, 2.0] | |||
| Pre-colectomy infliximab | 0.33 | 0.12 | 0.079 | ||||
| No | 13 | 0.00 [–1.00, 0.00] | –2.0 [–2.0, –2.0] | –2.0 [–3.0, –2.0] | |||
| Yes | 6 | 0.00 [0.00, 0.00] | 1.00 [–2.0, 2.0] | 0.50 [–2.0, 2.0] | |||
| Pre-colectomy adalimunab | 0.99 | 0.020 | 0.038 | ||||
| No | 13 | 0.00 [–1.00, 0.00] | –2.0 [–3.0, –2.0] | –2.0 [–3.0, –2.0] | |||
| Yes | 6 | 0.00 [–1.00, 0.00] | 1.00 [–2.0, 4.0] | 0.50 [–2.0, 4.0] | |||
| Mesalamine (>3 months) | 0.76 | 0.25 | 0.43 | ||||
| No | 7 | 0.00 [–1.00, 0.00] | –1.00 [–2.0, 4.0] | –2.0 [–3.0, 4.0] | |||
| Yes | 12 | 0.00 [–1.00, 0.00] | –2.0 [–2.5, –1.00] | –2.0 [–3.0, –1.00] | |||
| Topical steroids (>3 months) | 0.76 | 0.39 | 0.64 | ||||
| No | 9 | 0.00 [–1.00, 0.00] | –1.00 [–2.0, 0.00] | –2.0 [–3.0, 0.00] | |||
| Yes | 10 | 0.00 [–1.00, 0.00] | –2.0 [–2.0, –2.0] | –2.0 [–3.0, –2.0] | |||
| Pre-vedolizumab biologics | 0.23 | 0.86 | 0.83 | ||||
| No | 9 | 0.00 [–1.00, 0.00] | –2.0 [–2.0, 0.00] | –2.0 [–3.0, 0.00] | |||
| Yes | 10 | 0.00 [0.00, 0.00] | –2.0 [–2.0, –1.00] | –2.0 [–3.0, –1.00] | |||
Data presented as Median [P25, P75]. P-values correspond to Kruskal–Wallis test.
mPDAI, modified pouch disease activity index; IBD, inflammatory bowel disease; NSAIDs, non-steroidal anti-inflammatory drugs.
Adverse events were noted only in two (11%) patients. Three (16%) patients had an exacerbation of EIM, most commonly rheumatic manifestations. Four (21%) patients required surgery, among whom three had ileostomy and one had pouch-redo surgery (Figure 1). All four patients required surgery for CARP, one also had pouch-vaginal fistula and another one had associated anal abscess.
Discussion
CARP is a challenging entity to treat due to limited therapeutic options, has a great impact on patient quality of life and health, and is the most common cause of pouch failure. Given the dearth of therapeutic options, vedolizumab has been used off-label for the treatment of CARP. Our study showed that, at 3 months post vedolizumab therapy, there was an improvement in mPDAI score. Out of 19 patients included in the study, 6 (32%) had improvement in the clinical mPDAI score (P = 0.031); 14 (74%) had improvement in both the endoscopic and total mPDAI scores with a median change of –2 units (both P = 0.031). There were no significant adverse events or mortality and only four (21%) patients required surgical interventions after vedolizumab. Ten (53%) of these responders had failed anti-TNF therapy and 18 (95%) had failed systemic steroids before successful treatment with vedolizumab, which further provides evidence regarding the efficacy of vedolizumab in such patients.
The etiology of pouchitis is not fully understood but is felt to be multifactorial. Dysbiosis is believed to play a key role in the development of pouchitis [13], which makes antibiotics the first line of treatment [5]. Unfortunately, few patients develop severe inflammatory pouchitis, which is refractory to antibiotics. Some data suggest that a genetic predisposition to immune dysregulation contributes to this pathway. These patients have a significantly increased risk for pouch failure [14, 15]. Therefore, especially in this patient group, an aggressive therapeutic approach is warranted. Biologic agents have been used in the past with equivocal results. A meta-analysis by Herfarth et al. [4] showed infliximab (IFX) to be an anti-TNF agent with good clinical effectiveness (80% short-term and around 50% long-term response) in such patients. But, in this analysis, only 23% of the patients were treated with IFX monotherapy, 71% with a combination of IFX and azathioprine or 6-MP and 6% with IFX and MTX. Besides CARP, IFX was also used for CD or CD-like disease with fistulizing and stricturing disease of the pouch, which makes it difficult to draw any conclusion specific to CARP treatment. Data were limited to draw any conclusion regarding the efficacy of adalimunab, another anti-TNF medication [4]. Interestingly, 10 (53%) of our study subjects did not respond to anti-TNF agents, along with systemic steroids in 18 (95%) and immunomodulators in 6 (32%), but 14 (74%) of these patients had endoscopic and overall improvement in mPDAI with vedolizumab. Also, patients who did not use adalimumab prior to colectomy had a higher decrease in endoscopic and total mPDAI (both P < 0.05). A recent multicenter retrospective study of 20 patients who received three or four infusions of vedolizumab for CARP or CADP showed moderate to excellent improvement in clinical symptoms of 13/20 patients [10]. They validated these results by a reduction in the mean OS from 6.8 to 3.4 and the mean PDAI from 10 to 3 points, and a reduction in the need for anti-diarrheal and antibiotics. Only three patients had adverse effects (nausea, pruritus and bronchitis) but therapy was not discontinued in any of them. Compared to the study by Bar et al. [10], our study is from a single center with higher homogeneity of the patient population and implementation of the study protocol, with standardized reporting of endoscopy results. But, at the same time as being a single-center study, we cannot generalize the results to other patient populations. Also, we only used PDAI scores, compared to the OS, PDAI and physician assessment used by Bar et al. [10], to see the effect of vedolizumab in patients with CARP. Recently, in a small case-series of four patients, we showed improvement in symptoms and endoscopic findings after 3 months of vedolizumab in patients who initially failed to respond to antibiotics, mesalamine, steroids, immunomodulators, intravenous immunoglobulin therapy and anti-TNF agents. Fecal microbiota transplantation and temporary fecal diversion were also attempted in two of these patients [11]. Similarly, there are other case reports of a response with vedolizumab therapy, which initially failed other therapies [7, 8], further suggesting that vedolizumab can be effectively used in CARP patients after failure of other treatment modalities including anti-TNF agents.
What does our study add to the literature? Besides a multicenter study from Germany, few case reports and a small case-series regarding the efficacy of vedolizumab in CARP, there is a paucity of data regarding vedolizumab use in CARP. This is the first single-center study regarding the efficacy and safety of vedolizumab in CARP, demonstrating improvement in mPDAI after 3 months of therapy. For patients who failed steroids and other biologic therapies such as anti-TNFs for CARP, vedolizumab can be considered as the next therapeutic option. This study also highlighted disconnect between the endoscopic and clinical improvement, evidenced by the difference in clinical and endoscopic subscores, which has been well demonstrated by the previous studies [3, 16]. Therefore, relying only on clinical symptoms is not the best way to monitor response in these patients and should be confirmed by pouchoscopy with biopsy [3]. Besides vedolizumab, various other therapies have been tried for CARP including granulocyte and monocyte aphresis [17], green tea polyphenol epigallocatechin gallate (EGCG) [18] and Alicaforsen, an intercellular adhesion molecule-1 (ICAM-1) antisense oligonucleotide [19, 20], but data are limited regarding their long-term efficacy and safety. In our study, only two (11%) patients had adverse events; exacerbation of EIM was seen in three (16%), with exacerbation of rheumatic manifestation being the most common. This is in conjunction with the findings of Gemini studies, where arthralgia was one of the most common adverse events affecting patients who received vedolizumab [6, 21, 22]. Only four (21%) patients required surgery after 3 months of vedolizumab, three (16%) had ileostomy and one (5%) had pouch-revision surgery. The pouch-failure rate after initial IPAA creation at our institute is ∼2%, and ∼3% of the patients with chronic pouchitis require pouch-redo surgery [23, 24].
This study has some limitations. Our findings were based on a cohort of only 19 patients, which may limit the value of the findings. However, the use of vedolizumab for CARP so far is relatively uncommon in ileal pouches. Our study population was being followed up at a tertiary referral IBD center; this might have introduced a referral bias. We only record the pouch-related adverse events at our institution and could have missed interim events at the other hospitals. As this was a retrospective study, we were unable to record information regarding dietary risk factors. We used the recent endoscopic examination before and after the treatment to calculate mPDAI score—this might have resulted in bias, given the fluctuating nature of the disease course, with changes in the disease severity between endoscopic examinations. However, the time delay between endoscopic examinations and the evaluation status for CARP was short, thus minimizing the effect of this measurement bias.
In conclusion, our study suggests that vedolizumab has significant efficacy and can be safely used for CARP patients who have failed other treatment modalities including anti-TNF agents. Randomized–controlled trials with a greater number of patients are required to confirm these findings.
Funding
None.
Acknowledgements
Study concept and design: B.S., A.S., J.P.; acquisition of data: A.S.; analysis and interpretation of data: B.S., A.S., R.L.; drafting of the manuscript and critical revision of the manuscript for important intellectual content: all authors; statistical analysis: L.R., A.S., B.S.; administrative, technical or material support: J.P., B.S.; study supervision: B.S. All authors read and approved the final manuscript.
Conflict of interest statement: none declared.
References
- 1. Fazio VW, Ziv Y, Church JM. et al. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg 1995;222:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Penna C, Dozois R, Tremaine W. et al. Pouchitis after ileal pouch-anal anastomosis for ulcerative colitis occurs with increased frequency in patients with associated primary sclerosing cholangitis. Gut 1996;38:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sandborn WJ. Pouchitis following ileal pouch-anal anastomosis: definition, pathogenesis, and treatment. Gastroenterology 1994;107:1856–60. [DOI] [PubMed] [Google Scholar]
- 4. Herfarth HH, Long MD, Isaacs KL.. Use of biologics in pouchitis: a systematic review. J Clin Gastroenterol 2015;49:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shen B. Acute and chronic pouchitis--pathogenesis, diagnosis and treatment. Nat Rev Gastroenterol Hepatol 2012;9:323–33. [DOI] [PubMed] [Google Scholar]
- 6. Feagan BG, Rutgeerts P, Sands BE. et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 7. Mir F, Yousef MH, Partyka EK. et al. Successful treatment of chronic refractory pouchitis with vedolizumab. Int J Colorectal Dis 2017;32:1517–8. [DOI] [PubMed] [Google Scholar]
- 8. Schmid M, Frick JS, Malek N. et al. Successful treatment of pouchitis with vedolizumab, but not fecal microbiota transfer (FMT), after proctocolectomy in ulcerative colitis. Int J Colorectal Dis 2017;32:597–8. [DOI] [PubMed] [Google Scholar]
- 9. Khan F, Gao XH, Singh A. et al. Vedolizumab in the treatment of Crohn’s disease of the pouch. Gastroenterol Rep (Oxf) 2018;6:184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bar F, Kuhbacher T, Dietrich NA. et al. Vedolizumab in the treatment of chronic, antibiotic-dependent or refractory pouchitis. Aliment Pharmacol Ther 2018;47:581–7. [DOI] [PubMed] [Google Scholar]
- 11. Philpott J, Ashburn J, Shen B.. Efficacy of vedolizumab in patients with antibiotic and anti-tumor necrosis alpha refractory pouchitis. Inflamm Bowel Dis 2017;23:E5–6. [DOI] [PubMed] [Google Scholar]
- 12. Shen B, Achkar JP, Connor JT. et al. Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Dis Colon Rectum 2003;46:748–53. [DOI] [PubMed] [Google Scholar]
- 13. Coffey JC, Rowan F, Burke J. et al. Pathogenesis of and unifying hypothesis for idiopathic pouchitis. Am J Gastroenterol 2009;104:1013–23. [DOI] [PubMed] [Google Scholar]
- 14. Fazio VW, Kiran RP, Remzi FH. et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg 2013;257:679–85. [DOI] [PubMed] [Google Scholar]
- 15. Shen B, Lashner B.. Can we immunogenotypically and immunophenotypically profile patients who are at risk for pouchitis? Am J Gastroenterol 2004;99:442–4. [DOI] [PubMed] [Google Scholar]
- 16. Shen B, Achkar JP, Lashner BA. et al. Endoscopic and histologic evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterology 2001;121:261–7. [DOI] [PubMed] [Google Scholar]
- 17. Yamamoto T, Tanaka T, Yokoyama T. et al. Efficacy of granulocyte and monocyte apheresis for antibiotic-refractory pouchitis after proctocolectomy for ulcerative colitis: an open-label, prospective, multicentre study. Therap Adv Gastroenterol 2017;10:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehta M, Ahmed S, Dryden G.. Refractory pouchitis improves after administration of the green tea polyphenol EGCG: a retrospective review. Int J Colorectal Dis 2018;33:83–6. [DOI] [PubMed] [Google Scholar]
- 19. Greuter T, Biedermann L, Rogler G. et al. Alicaforsen, an antisense inhibitor of ICAM-1, as treatment for chronic refractory pouchitis after proctocolectomy: a case series. United European Gastroenterol J 2016;4:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miner P, Wedel M, Bane B. et al. An enema formulation of alicaforsen, an antisense inhibitor of intercellular adhesion molecule-1, in the treatment of chronic, unremitting pouchitis. Aliment Pharmacol Ther 2004;19:281–6. [DOI] [PubMed] [Google Scholar]
- 21. Baumgart DC, Bokemeyer B, Drabik A. et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice--a nationwide consecutive German cohort study. Aliment Pharmacol Ther 2016;43:1090–102. [DOI] [PubMed] [Google Scholar]
- 22. Sandborn WJ, Feagan BG, Rutgeerts P. et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 23. Remzi FH, Aytac E, Ashburn J. et al. Transabdominal redo ileal pouch surgery for failed restorative proctocolectomy: lessons learned over 500 patients. Ann Surg 2015;262:675–82. [DOI] [PubMed] [Google Scholar]
- 24. Lavryk OA, Stocchi L, Ashburn JH. et al. Case-matched comparison of long-term functional and quality of life outcomes following laparoscopic versus open ileal pouch-anal anastomosis. World J Surg 2018;42:3746–54. [DOI] [PubMed] [Google Scholar]