Abstract
Background and Objective
Increasing interest has developed in the therapeutic potential of bone marrow-derived mesenchymal stem cells (MSCs) for the treatment of inflammatory bowel disease (IBD) and IBD-induced cancer. However, whether MSCs have the ability to suppress or promote tumor development remains controversial. The stromal cell-derived factor 1 (SDF-1)/C-X-C chemokine receptor type 4 (CXCR4) axis is well known to play a critical role in the homing of MSCs. In this study, we aimed to evaluate the role of CXCR4-overexpressing MSCs on the tumorigenesis of IBD.
Methods
MSCs were transduced with lentiviral vector carrying either CXCR4 or green fluorescent protein (GFP). Chemotaxis and invasion assays were used to detect CXCR4 expression. A mouse model of colitis-associated tumorigenesis was established using azoxymethane and dextran sulfate sodium (DSS). The mice were divided into three groups and then injected with phosphate buffer saline (PBS), MSC-GFP or MSC-CXCR4.
Results
Compared with the mice injected with MSC-GFP, the mice injected with MSC-CXCR4 showed relieved weight loss, longer colons, lower tumor numbers and decreased tumor load; expression of pro-inflammatory cytokines decreased, and signal transducer and activator of transcription 3 (STAT3) phosphorylation level in colon tissue was down-regulated.
Conclusion
CXCR4-overexpressing MSCs exhibited effective anti-tumor function, which may be associated with enhanced homing to inflamed intestinal tissues.
Keywords: Inflammatory bowel disease, tumorigenesis, mesenchymal stem cells, CXCR4, mice
Introduction
Inflammatory bowel disease (IBD), which includes ulcerative colitis and Crohn’s disease, represents chronic and idiopathic inflammatory disorder of the gastrointestinal tract [1, 2]. Patients with underlying IBD have been shown to be at risk for developing colorectal cancer (CRC), which is referred to as colitis-associated cancer (CAC) [3]. CAC is one of the most serious complications associated with IBD, and accounts for approximately 10–15% of all IBD patient deaths [4, 5]. In addition, it has been demonstrated that CAC patients exhibit poorer survival statistics compared with patients with sporadic CRC [6]. Recently, convincing data have demonstrated that the development from inflammation to cancer is a multifactorial and complicated process [7]. This, to some extent, results in limited treatment options for CAC patients.
As more novel treatments emerge from the drug-development pipeline and clinical research for both IBD and IBD-associated complications [8], growing interest has emerged in the use of cell therapy as a novel therapeutic approach. Mesenchymal stromal cells (MSCs) have become the primary candidate cell type studied due to their low immunogenicity and immunoregulatory properties [9, 10]. MSCs are a type of stem cells that can be derived from numerous different tissue sources, including bone marrow and adipose tissue [11]. They are currently used for the treatment of inflammatory diseases, including myocarditis, multiple sclerosis and IBD [12–14]. While questions remain surrounding the role of MSCs in tumorigenesis and tumor growth [15–17], the use of MSCs for the treatment of IBD has demonstrated promising results in both animal models and human clinical trials [14, 18, 19]. In addition, our previous work has indicated anti-tumor properties of MSCs in the tumorigenesis of IBD [20].
Nevertheless, an increasing number of studies have indicated that the homing of systemically administered MSCs is very low and transient [21–23], which could restrict the potential curative efficacy of this treatment. Thus, it is of importance to facilitate both the chemotaxis and retention of MSCs so as to expand the effectiveness of MSC-based cell therapy. Stromal cell-derived factor 1 (SDF-1) has been identified as one of the most crucial factors in the homing of stem cells to bone marrow and other injured tissues [24, 25]. C-X-C chemokine receptor type 4 (CXCR4) is the receptor for SDF-1, and is located on the cell surface of MSCs; however, its expression has been shown to be greatly reduced throughout the in vitro amplification of MSCs [26]. Emerging evidence has demonstrated that the SDF-1/CXCR4 axis may play a critical role in MSC homing and survival [27–30]. However, the effects of the SDF-1/CXCR4 axis in tumorigenesis of IBD have yet to be clearly investigated.
In this study, we established a mouse model of colitis-associated tumorigenesis in order to investigate the role of the SDF-1/CXCR4 axis in tumorigenesis of IBD as well as the potential mechanisms.
Methods
Mice
All-female C57BL/6 mice were obtained from the Laboratory Animal Center of Sun Yat-sen University, Guangzhou, China. Mice used in these studies were 7–8 weeks of age, with body weights between 18 and 20 g. These mice were maintained under specific pathogen-free (SPF) conditions at the Laboratory Animal Center of Sun Yat-sen University, Guangzhou, China. Mice were quarantined for 7 days prior to being used in an experiment and were provided with drinking water and fed a pellet-based diet. All animal experiments were carried out in accordance with the detailed rules of the Institutional Animal Care and Use Committee of Sun Yat-sen University. In addition, the experimental protocol used for this study was approved by the Ethical Committee of Sun Yat-sen University.
Isolation, culture and transduction of MSCs
MSCs were isolated from 3- to 4-week-old female C57BL/6 mice as described previously [31]. Briefly, mice were sacrificed by cervical dislocation. The bilateral femurs and tibias were then aseptically excised and stripped of connective tissue. Once the ends of the bones were trimmed, bone marrow was flushed from the marrow cavity using complete culture medium comprising Dulbecco’s modified Eagle’s medium (DMEM; Gibco, New York, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin (Gibco). The cell suspension was filtered through a 70-μm strainer (BD Biosciences; Franklin Lakes, NJ, USA) and then centrifuged at 600 × g for 3 min. Following removal of the supernatant, cells were re-suspended and plated in plastic tissue culture flasks (Corning; New York, NY, USA) at a density of 1 × 106 cells/cm2 in the complete culture medium described above. Medium was refreshed every 2 days to remove non-adherent cells. When the culture reached over 80% confluency, cells were digested using 0.25% trypsin for 2 min at room temperature and sub-cultured. For all experiments, cells were used at passage 3 or 4.
Stable CXCR4-overexpressing MSCs were generated by lentiviral transduction using a pWSLV-07-EF1α-Puro-GFPvector from ViewSolid Biotech (Beijing, China). The empty pWSLV-07-EF1α-Puro-GFPvector was used as a control. Both plasmids were verified by sequencing. The pWSLV-07-EF1α-Puro-GFP and pWSLV-07-EF1α-CXCR4-Puro-GFP plasmids were both co-transfected into 293 T cells with Lipofectamine 3000 (InvitrogenTM; Life Tech, Shanghai, China) using a packaging plasmid (psPAX2) and an enveloping plasmid (pMD2.G), respectively. The transfection and lentiviral infection processes were carried out as described previously by Yuan et al. [32].
Identification of MSCs
Cells were trypsinized, collected and washed with cold phosphate buffer saline (PBS). Cells were then incubated with phycoerythrin (PE)-conjugated anti-mouse CD105, allophycocyanin (APC)-conjugated anti-mouse CD11b, CD44, CD45 or fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD29, CD31 (BD Biosciences) in the dark at 4°C for 30 min. Following two washes with PBS (Gibco), cells were re-suspended and analysed using flow cytometry (BD FACSCanto™; BD Biosciences). A total of 10 000 viable events were collected and analysed.
Multi-potential differentiation of MSCs
In order to characterize MSCs in accordance with the International Society for Cellular Therapy statement [33], we utilized two different experimental procedures as described by previous studies [34, 35]. Multi-lineage potential of MSCs was assessed by incubating the cells under specific conditions in order to induce differentiation into adipocytes and osteoblasts. Briefly, adipocyte formation was assessed by staining of accumulated lipid vacuoles with Oil Red O (Sigma; Springdale, AR, USA). Osteoblast formation was assessed by measuring Alizarin red staining (Sigma). Control cells were fed only with MSCs complete culture medium.
Monitoring cell proliferation
MSC proliferation was analysed using real-time cell analysis (RTCA) with the xCELLigence systemE-Plate (ACEA Biosciences Inc.; San Diego, CA, USA). DMEM supplemented with 10% FBS was added to the E-Plate, and the background cell index (CI) values were recorded. The MSCs suspension (4000 cells/well) was then added to the medium and incubated. Cell proliferation was observed at 15-min intervals using the RTCA analyser. CI values were recorded, and cell proliferation was observed continuously for 120 h. Cell growth rate was calculated based on the slope of the line between two given time points [36].
Scratch assay
MSC-GFP or MSC-CXCR4 were seeded in six-well plates and cultured until they reached confluency. Monolayers were then gently scratched using a pipette tip to create a rectangular scratch. Culture medium and detached cells were then removed, and the monolayers were washed twice with PBS and maintained in DMEM containing 0.5% BSA for 24 h. Cells were stimulated by using 100 ng/mL SDF-1 over the next 24 h. Thereafter, cells that migrated into the wound line and along the wound line were imaged using a phase-contrast microscope (DMI4000B; Leica, Wetzlar, Germany). Wound closure was quantified as the percentage of the starting distance between the wound edges after 24 h (analysed by Image-Pro Plus 5.02; Media Cybernetics, Bethesda, MD, USA).
Cell-invasion assay
Invasion assays were carried out using Transwell plates (Corning) with 8-μm pore-sized membranes with coated Matrigel (BD Biosciences). For each Transwell plate, 8 × 104 MSC-GFP or MSC-CXCR4 cells were seeded on the upper chambers; the lower chambers were filled with culture medium supplemented with chemoattractant (SDF, 100 ng/mL). Following 24 h of incubation, cells that passed through the coated membrane to the lower surface were fixed with 4% paraformaldehyde and stained with hematoxylin. The cells were counted under a microscope (×100). Invading cells were quantified using ImageJ™ software (ImageJ 1.46r; Bethesda, MD, USA).
Animal-model induction and treatment
The model of colitis-associated tumorigenesis was induced by using azoxymethane (AOM; Sigma-Aldrich, St. Louis, MO, USA) and DSS (36–50 kDa; MP Biomedical; Santa Ana, CA, USA). Briefly, female mice were injected intraperitoneally with a single dose of AOM (10 mg/kg). Mice then received a course of 2% DSS in sterile drinking water for 1 week followed by a course of pure drinking water for 2 weeks. This was carried out for a total of three cycles. The AOM/DSS-treated mice were assessed daily to monitor general appearance, food uptake, body weight, stool consistency and rectal bleeding.
Twenty-four female C57BL/6 mice were randomized into four groups (six mice per group): two experimental groups (MSC-GFP group and MSC-CXCR4 group), one control group (PBS group) and one negative control group (NC group).
On Days 4, 14 and 24, mice in the experimental groups were injected with MSC-GFP or MSC-CXCR4 (1 × 106 cells in 0.3 mL of PBS) via the tail vein. Mice in the PBS group received 0.3 mL of PBS without MSCs. Mice in the NC group did not undergo the treatment of AOM or DSS or the treatment of MSC or PBS.
At the end of Week 12, all mice were sacrificed by cervical dislocation. Mouse colons were then isolated and slit longitudinally to count the number of tumors and measure the tumor size using a dissecting microscope. Segments of the distal colon were fixed in 10% neutral buffered formalin for subsequent paraffin embedding, or kept in RNA stabilization solution (RNA later; Ambion, Thermo Fisher Scientific, St Peters, MO, USA) as tissue samples for real-time polymerase chain reaction (RT-PCR) analysis.
Histopathological analysis
For each mouse, four sections of distal colon were histopathologically analysed. Colon samples were fixed in 10% neutral buffered formalin, embedded in paraffin and sliced into micrometer-thick sections prior to staining with hematoxylin and eosin (HE). The stained tissues were histologically evaluated in a double-blind fashion as previously reported, using a combined score for tissue injury (score, 0–3) and infiltration of inflammatory cells (score, 0–3) [37]. Briefly, for tissue injury, normal colonic mucosa, discrete lymphoepithelial lesions, surface mucosal erosion or focal ulceration and extensive mucosal damage and extension into deeper layers were scored as 0, 1, 2 and 3, respectively; in the case of infiltration of inflammatory cells, occasional presentation of inflammatory cells in the lamina propria, increasing number of inflammatory cells in the lamina propria, inflammatory cells extending into the submucosa and transmural extension of the infiltration were scored as 0, 1, 2 and 3, respectively. The histological score was defined as the sum of the two parameters above (ranging from 0 to 6).
RNA extraction and RT-PCR
Total RNA was extracted from cells or tissue samples using the TRIzol Reagent (Invitrogen; Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA concentration and purity were determined using a NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific; St. Peters, MO, USA). For each sample, 1000 ng of total RNA was then reverse-transcribed using the ReverTra Ace qPCR RT Kit (Toyobo Biochemicals; Kita-ku, Osaka, Japan) according to the manufacturer’s instructions. RT-PCR was carried out using the SYBR Green PCR Master Mix (Applied Biosystems; Foster City, CA, USA) on the Applied Biosystems 7500 Sequence Detection system utilizing the SYBR Green detection protocol, as described by the manufacturer. All PCR reactions were carried out in triplicate, and control reactions without cDNA templates were also carried out. The housekeeping gene, glyceraldehydephosphate dehydrogenase (GAPDH), was used as an endogenous control in these studies. The relative expression level of each gene was calculated and normalized using the 2–ΔΔCt method relative to GAPDH. All primer sequences are listed in Table 1.
Table 1.
Primer sequences of real-time polymerase chain reaction
| Gene | Sequence (5'-3') | |
|---|---|---|
| SDF-1 | Forward primer | GAGAGCCACATCGCCAGAGC |
| Reverse primer | GGATCCACTTTAATTTCGGGTCAA | |
| CXCR4 | Forward primer | GACCGCCTTTACCCCGATAGC |
| Reverse primer | ACCCCCAAAAGGATGAAGGAGTC | |
| TNF-α | Forward primer | CCTCTCTCTAATCAGCCCTCTG |
| Reverse primer | GAGGACCTGGGAGTAGATGAG | |
| IL-1β | Forward primer | ACCTGCTGGTGTGTGACGTT |
| Reverse primer | TCGTTGCTTGGTTCTCCTTG | |
| IL-6 | Forward primer | GAGGATACCACTCCCAACAGACC |
| Reverse primer | AAGTGCATCATCGTTGTTCATACA | |
| IL-8 | Forward primer | AAATTTGGGGTGGAAAGGTT |
| Reverse primer | TCCTGATTTCTGCAGCTCTGT | |
| GAPDH | Forward primer | TCAATGAAGGGGTCGTTGAT |
| Reverse primer | CGTCCCGTAGACAAAATGGT |
SDF-1, stromal cell-derived factor 1; CXCR4, C-X-C chemokine receptor type 4; TNF-α, tumor necrosis factor α; IL-1β, interleukin 1β; IL-6, interleukin 6; IL-8, interleukin 8; GAPDH, glyceraldehydephosphate dehydrogenase.
Western blot analysis
Tissue samples or cells were lysed in radio-immunoprecipitation assay lysis buffer containing 150 mmol/L NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mmol/L Tris (pH8) and a protease inhibitors cocktail (Promega; Fitchburg, WI, USA). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. Membranes were blocked using 5% skimmed milk (BD Biosciences) in Tris-buffered saline with Tween 20 and then probed with specific primary antibodies against the following proteins: SDF-1 (1: 1000, ab9797; Abcam, Cambridge, MA, USA); GFP (1: 1000, ab290; Abcam); CXCR4 (1: 1000, #2024; Cell Signaling, Danvers, MA, USA); signal transducer and activator of transcription 3 (STAT3; 1: 2000, #4904; Cell Signaling, Danvers, MA, USA); p-STAT3 (1: 2000, #9145; Cell Signaling); GAPDH (1: 10000, #5632–1; Epitomics, Burlingame, CA, USA). Membranes were then subsequently incubated with horseradish peroxidase-coupled anti-rabbit or anti-mouse antibodies (both 1: 2500; Cell Signaling). Specific bands were visualized using ECL Blotting Detection Reagents (Amersham Biosciences; Little Chalfont, Buckinghamshire, UK).
Immunohistochemistry
Formalin-fixed paraffin-embedded tissues were cut serially into 4-μm sections on silanized glass slides for immunohistochemical staining. Briefly, slides were deparaffinized with dimethylbenzene and rehydrated through graded alcohols prior to retrieving the antigen by incubation in sodium citrate buffer. Endogenous peroxidase activity was blocked using hydrogen peroxide solution and the slides were then incubated with the corresponding primary antibody (anti-CXCR4, anti-p-STAT3; Cell Signaling) at 1:250 dilutions overnight at 4°C. After being washed three times with PBS, sections were incubated with the appropriate secondary antibodies at a 1:500 dilution for 30 min at 37°C. Finally, sections were visualized by incubating in 3,3’-diaminobenzidinewith 0.05% H2O2 for 3 min in order to induce acolorimetric reaction.
Statistics analysis
All values are expressed as mean ± standard error mean (SEM) and were analysed using the Student’s t-test with SPSS version 22 (IBM; New York, NY, USA). A P-value of <0.05 was considered statistically significant.
Results
Characteristics and genetic modification of MSCs
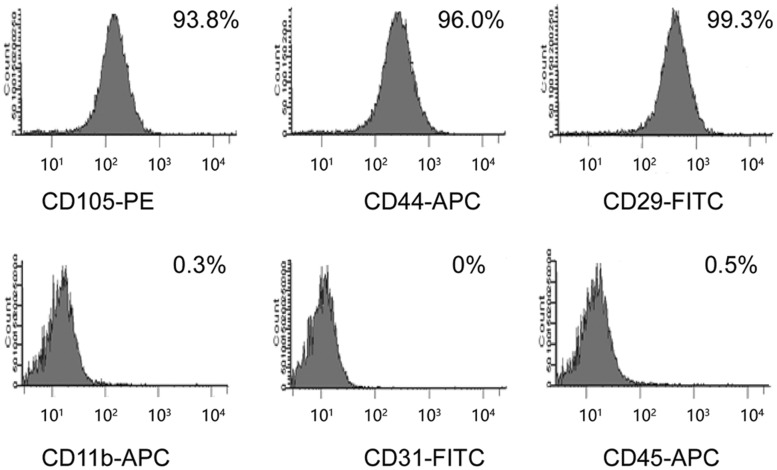
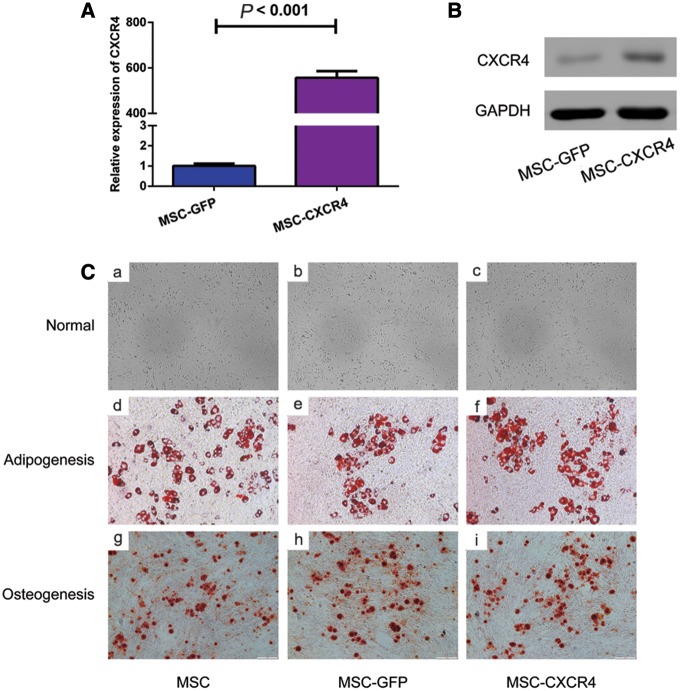
Cell surface characteristics of MSCs were analysed by using flow cytometry, and the results demonstrated that MSCs were positive for CD105, CD44 and CD29 expressions, and were negative for CD11b, CD31 and CD45 expressions (Figure 1). Following transfection of MSCs with the sense-strand lentiviral vectors, pWSLV-07-EF1α-Puro-GFP or pWSLV-07-EF1α-CXCR4-Puro-GFP, CXCR4 expression was examined at both the mRNA and protein levels. The results of RT-PCR and Western blot showed that CXCR4 expression was significantly higher in MSC-CXCR4 than in MSC-GFP in both gene level (Figure 2a) and protein level (Figure 2b). MSCs were expanded under normal culture conditions and exhibited a fusiform shape or uniform morphology following several passages. Genetically modified MSCs were then tested for their multi-lineage differentiation potential as well. In in vitro experiments, after being cultured in the appropriate inductive culture condition, MSCs presented either adipogenic or osteogenic differentiation. No changes were noted in this study between genetically modified MSCs and unmodified MSCs in their ability to differentiate into either adipolineage cells or osteocytes (Figure 2c).
Figure 1.
Flow cytometry analysis of C57BL/6 bone marrow MSCs. Fluorescent immunostaining results show that cultivated cells express CD29, CD44 and CD105, but do not express CD11b, CD31 or CD45. MSCs, mesenchymal stem cells.
Figure 2.
Transfection efficiency in MSCs and the capability of multi-potential differentiation. The results of RT-PCR (A) and Western blot analysis (B) show that CXCR4 mRNA and protein expressions are higher in MSCs infected with pWSLV-07-EF1α-CXCR4-Puro-GFP than in MSCs infected with pWSLV-07-EF1α-Puro-GFP. The results of inductive experiment (C) indicate that MSC, MSC-GFP and MSC-CXCR4 present similar capability of multi-potential differentiation. Cells exhibit basic features as fibroblast-like morphology (a, b, c). MSC, MSC-GFP and MSC-CXCR4 are all able to differentiate into adipocytes (d, e, f) and osteocytes (g, h, i) (magnification, ×200). MSCs, mesenchymal stem cells; RT-PCR, real-time polymerase chain reaction; CXCR4, C-X-C chemokine receptor type 4; GFP, green fluorescent protein.
Effects of CXCR4 expression on cellular proliferation, chemotaxis and invasiveness toward SDF-1 of MSCs
In order to investigate whether CXCR4 altered the proliferation of MSCs, the RTCA assay was adopted to generate growth curves for MSC-GFP and MSC-CXCR4 in an effort to detect differences in the proliferation rates in vitro. No significant differences were observed in proliferation rates between the two cell types (Figure 3a), indicating that CXCR4 expression does not affect the proliferative capacity of MSCs. A scratch assay demonstrated that MSC-CXCR4 stimulated with SDF-1 migrated toward the free area to a much greater extent compared with MSC-GFP, which failed to close the scratch following SDF-1 stimulation. Similarly to the results of the scratch assay, an increased potential of MSC-CXCR4 to transmigrate through Matrigel-coated membranes was observed in the invasion assay (Figure 3b–e). These results indicated that CXCR4 overexpression enhanced the chemotaxis potential and invasiveness toward SDF-1 of MSCs.
Figure 3.
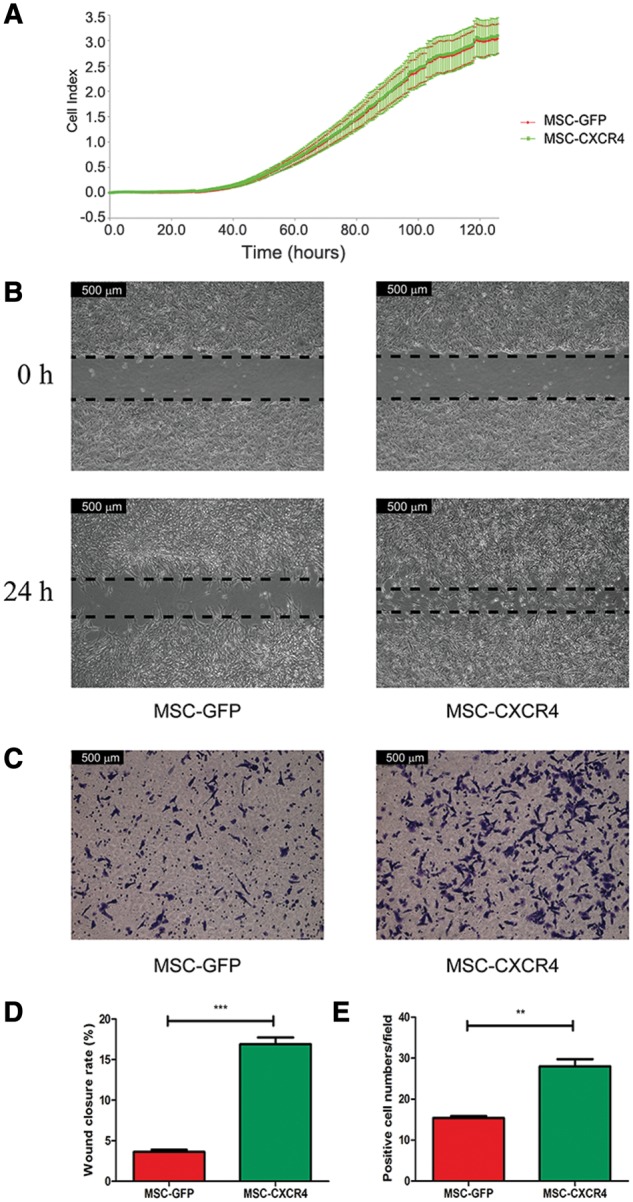
Regulation of CXCR4 expression affects the proliferation, chemotaxis and invasiveness toward SDF-1 of MSCs. (A) RTCA assay results show no differences in proliferation rates of MSC-GFP and MSC-CXCR4. The results of scratch assay (B) and invasion assay (C) indicate that CXCR4 overexpression enhanced the chemotaxis potential and invasiveness toward SDF-1 of MSCs (magnification, ×200). Quantitative results of the scratch assay (D) and the invasion assay (E) indicate that the wound-closure rate of MSC-CXCR4 is significantly higher than that of MSC-GFP and more cells pass through the membrane in the MSC-CXCR4 group than in the MSC-GFP group. MSCs, mesenchymal stem cells; CXCR4, C-X-C chemokine receptor type 4; SDF-1, stromal cell-derived factor 1; RTCA, real-time cell analysis; GFP, green fluorescent protein.
MSC-CXCR4 played a more effective role in attenuating AOM/DSS-induced colitis-associated tumorigenesis
In order to determine the role of MSC-GFP or MSC-CXCR4 on the colitis-associated tumorigenesis, C57BL/6 mice were administrated with AOM/DSS, followed by being injected with MSC-GFP or MSC-CXCR4. A scheme of the treatment protocol used for this study is depicted in Figure 4a.
Figure 4.
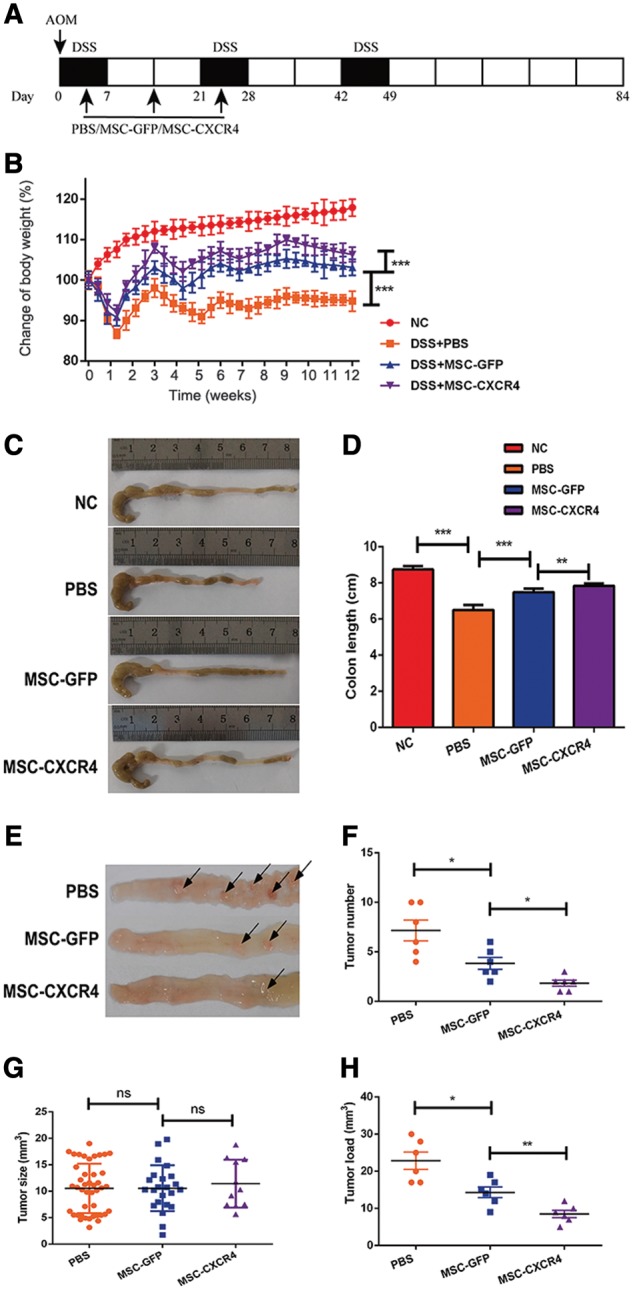
MSC-CXCR4 plays a more effective role in attenuating AOM/DSS-induced colitis-associated tumorigenesis. (A) Schematic overview of MSCs administration during colitis-associated tumorigenesis induced by AOM/DSS. PBS, MSC-GFP or MSC-CXCR4 was administered by injection via the tail vein on Days 4, 14 and 24. Mice were sacrificed on Day 84. (B) Changes in the body weight of mice show that the body-weight loss was significantly relieved in the MSCs-CXCR4 treated group. (C, D) DSS treatment shortened the length of the colon, and the following MSC treatment reduced the extent of colon shortening. The colons isolated from the mice are longer in the MSC-CXCR4 group than in the MSC-GFP group. (E–H) The tumor number is less and tumor load (measured as the sum of all tumor diameters in a given mouse) is smaller in the MSC-CXCR4 group than in the MSC-GFP group. However, the tumor size shows no significant difference among three groups. Arrows in (E) indicate tumors. Values are expressed as mean ± standard error mean (n = 6 mice per group). *P < 0.05, **P < 0.01, ***P < 0.001. MSCs, mesenchymal stem cells; CXCR4, C-X-C chemokine receptor type 4; AOM, azoxymethane; DSS, dextran sulfate sodium; GFP, green fluorescent protein; ns, no significant difference.
All AOM/DSS-treated mice developed clinical signs, including body-weight loss, bloody diarrhea and shortening of the colon. Mice that received no AOM/DSS in their drinking water did not exhibit any of these signs mentioned above, and gained weight over time. The body-weight loss was significantly relieved in the MSCs-treated group, and this effect was more prominent when CXCR4 was up-regulated in MSCs (P < 0.001) (Figure 4b). At the end of Week 12, all mice were sacrificed, and the entire colon of each mouse was placed on cellulose without tension in order to measure its length. The length of the colon was shorter in the AOM/DSS group than in the NC group. However, infusion of MSCs (especially MSC-CXCR4) reduced the extent of this colon shortening. Colons isolated from the mice in the MSC-CXCR4 group were longer than those isolated from mice in the MSC-GFP group (78.3 vs 74.9 mm, P = 0.005) (Figure 4c and d).
Tumors were observed between the mid-colon and the distal rectum in all mice treated with AOM/DSS, but not in any mice in the NC group. Mice in the PBS group developed numerous confluent tumor masses. The tumor multiplicity was reduced in mice treated with MSCs, and the number of tumors was lower in mice in the MSC-CXCR4 group than in mice in the MSC-GFP group (1.8 vs 3.8 per mouse, P = 0.014) (Figure 4e and f). Nevertheless, no difference was observed in tumor size among mice in the PBS, MSC-GFP and MSC-CXCR4 groups (Figure 4g). The tumor load (measured as the sum of all tumor diameters in a given mouse) was reduced after MSCs treatment. In addition, the tumor load was smaller in the MSC-CXCR4 group compared with that in the MSC-GFP group (the sum of all tumor diameters in a given mouse, 8.50 vs 14.33, P = 0.008) (Figure 4h).
The SDF-1/CXCR4 pathway plays a crucial part in the homing of MSCs to inflamed intestine in vivo
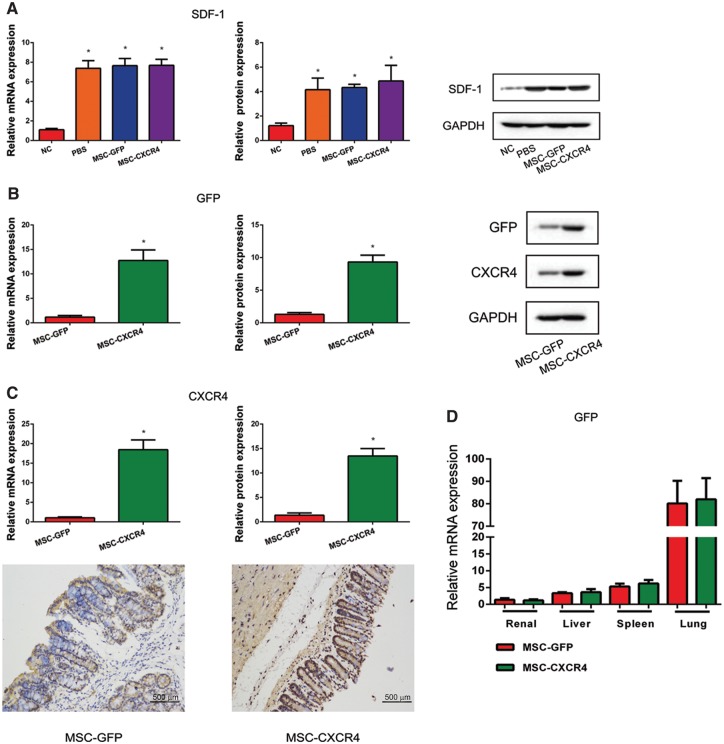
The SDF-1 expression in both mRNA and protein levels were up-regulated in inflamed intestines of mice treated with AOM/DSS (Figure 5a). In addition, the expressions of GFP and CXCR4 were significantly higher in mice in the MSC-CXCR4 group than in those in the MSC-GFP group. CXCR4 expression levels in the intestine were confirmed by immunohistochemistry (IHC) (Figure 5b and c). GFP expression in gene level was also detected in the intestine as well as the renal, liver, spleen and lung (Figure 5d). These results suggested that overexpression of CXCR4 improved the rate of homing to inflamed intestines of mice.
Figure 5.
The role of the SDF-1/CXCR4 pathway in the homing of MSCs to inflamed intestine in vivo. (A) The results of RT-PCR and Western blot show that SDF-1 mRNA and protein levels were up-regulated in the intestinal tissue of mice after the treatment of AOM/DSS. (B, C) Homing of MSCs to inflamed intestine was measured by RT-PCR, Western blot and immunohistochemistry, and the results indicate that levels of GFP and CXCR4 were both up-regulated in the MSC-CXCR4 group compared with the MSC-GFP group. Recipients treated with MSC-GFP were used as control. *P < 0.05 vs control. (D) RT-PCR results show that GFP expression was detected in the intestine as well as the renal, liver, spleen and lung tissues. SDF-1, stromal cell-derived factor 1; CXCR4, C-X-C chemokine receptor type 4; MSCs, mesenchymal stem cells; RT-PCR, real-time polymerase chain reaction; GFP, green fluorescent protein.
MSC-CXCR4 reduced histological damage of colon tissue more effectively
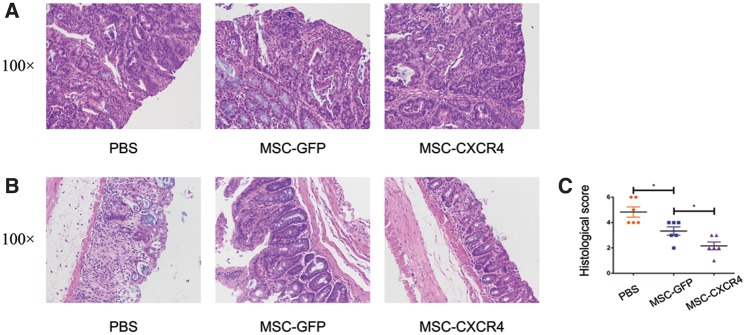
In an effort to further substantiate the role of MSC-CXCR4 in AOM/DSS-induced colitis-associated tumorigenesis, the colon tissue of mice was stained with HE, and histological scores were evaluated. All tumors isolated from both the PBS and MSCs-treated groups were confirmed to be adenomas with high-grade dysplasia (Figure 6a). As expected, histological scores of colon tissues from MSCs-treated mice were significantly decreased compared with those of colon tissues from PBS-treated mice. In addition, the infusion of MSCs-CXCR4 exhibited enhanced therapeutic effects compared with the administration of MSC-GFP (histological scores, 1.5 vs 3.3, P = 0.020) (Figure 6b and c).
Figure 6.
MSC-CXCR4 reduced histological damage of the colon tissue more effectively. (A) Representative HE staining of tumor tissues from PBS-, MSC-GFP- and MSC-CXCR4-treated mice. All tumors are characterized as adenomas with high-grade dysplasia. Representative HE staining (B) and histological score (C) of inflammatory tissue from the mice treated with PBS, MSC-GFP and MSC-CXCR4 show that histological scores of colon tissues from MSCs-treated mice were significantly decreased compared with those of colon tissues from PBS-treated mice. The infusion of MSCs-CXCR4 exhibited enhanced therapeutic effects compared with the administration of MSC-GFP. Values are expressed as the mean ± standard error mean (n = 4 mice per group). *P < 0.05. MSCs, mesenchymal stem cells; CXCR4, C-X-C chemokine receptor type 4; HE, hematoxylin and eosin; GFP, green fluorescent protein.
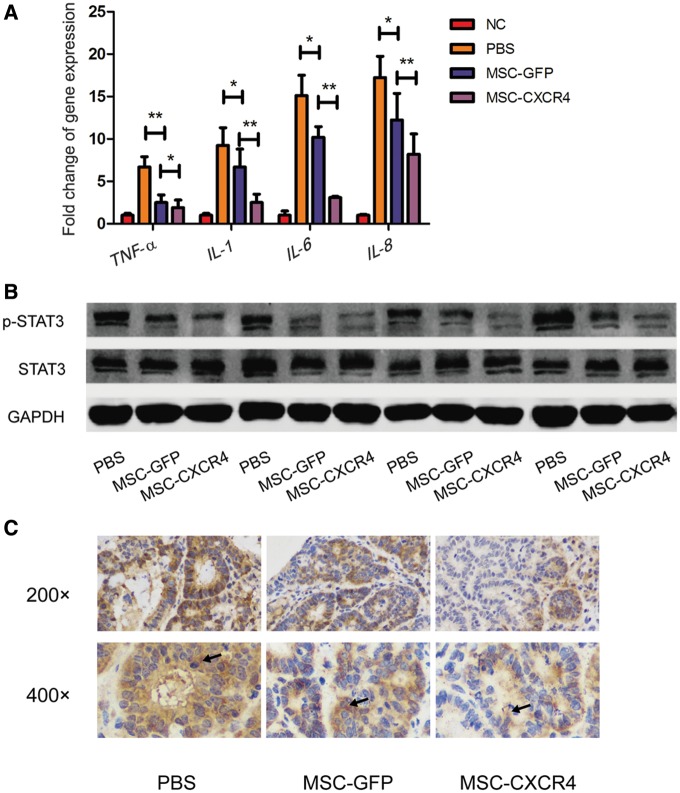
MSC-CXCR4 more effectively reduced pro-inflammatory cytokines and STAT3 phosphorylation in colon tissues
We also analysed the expression of numerous pro-inflammatory cytokines in gene level, including tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), interleukin 6 (IL-6) and interleukin 8 (IL-8) in colon tissues. As shown in Figure 7a, mRNA levels of these genes were down-regulated in colon tissues isolated from MSCs-treated mice. In addition, the expression levels of TNF-α, IL-1β, IL-6 and IL-8 were more dramatically reduced in mice from the MSC-CXCR4 group than in those from the MSC-GFP group. Furthermore, the results of Western blot showed that p-STAT3 expression in colon tissues was decreased after MSC treatment. CXCR4 overexpression further down-regulated the phosphorylation level of STAT3, which was confirmed by IHC (Figure 7b and c).
Figure 7.
MSC-CXCR4 more effectively reduced pro-inflammatory cytokines and STAT3 phosphorylation in colon tissue. (A) Real-time quantitative polymerase chain reaction results show mRNA expressions of inflammatory cytokines were down-regulated in colon tissues isolated from MSCs-treated mice, which could be more obviously observed in mice from the MSC-CXCR4 group than in mice from the MSC-GFP group. Values are expressed as the mean ± standard error mean (n = 4 mice per group). *P < 0.05, **P < 0.01. Both Western blot (B) and immunohistochemistry (C) results show that the level of STAT3 phosphorylation in colon tissues decreased after the treatment of MSC and the overexpression of CXCR4 further down-regulated the level of STAT3 phosphorylation. Arrows indicate positively stained areas. MSCs, mesenchymal stem cells; CXCR4, C-X-C chemokine receptor type 4; TNF-α, tumor necrosis factor α; IL-1β, interleukin 1β; IL-6, interleukin 6; IL-8, interleukin 8; GFP, green fluorescent protein; STAT3, signal transducer and activator of transcription 3.
Discussion
The present study investigated the role of CXCR4-overexpressing MSCs on the tumorigenesis of IBD in vitro and in vivo, and the possible inflammatory cytokines involved in the process. Our results indicated that overexpression of CXCR enhanced the chemotaxis potential and invasiveness toward SDF-1 of MSCs. Results of the in vivo CAC model suggested that mice injected with MSC-CXCR4, compared with MSC-GFP, more remarkably showed relieved weight loss, longer colons, lower tumor numbers and decreased tumor load with declined level of pro-inflammatory cytokines and STAT3 phosphorylation in colon tissues. Our findings indicated that CXCR4-overexpressing MSCs exhibited effective anti-tumor function, which may be related to enhanced homing to inflamed intestinal tissues.
As is well known, MSCs are distinguished by their low immunogenicity and enhanced immunoregulation abilities, and can be isolated from the connective tissue of the majority of organs, including bone marrow and adipose tissue [11]. These advantageous properties have enabled MSCs to gain popular usage in both basic research and clinical trials [38, 39]. Recent studies have suggested that MSCs selectively proliferate to form tumors, contributing to the formation of tumor-associated stroma [40, 41]. However, the role of MSCs in tumorigenesis remains a controversial issue within the field. For example, Cuiffo et al. [17] demonstrated that MSCs have the ability to cause aberrant expression of microRNAs, including microRNA-199a and reduce FOXP2 expression, resulting in poor survival of breast cancer patients. Instead, Shahrokhi et al. [42] demonstrated that concomitant genetic modification of MSCs with TNF-α and CD40 ligand could contribute to the optimization of the anti-tumor immunity response in the presence of dendritic cells, resulting in an enhanced lifespan in mice. It should be noted that the tumor models used in these studies were generated through the injection of cancer cells into immunodeficient nude mice. In this regard, these models may be inappropriate, or at least defective, as tumors could also be induced by chronic inflammation—a remarkable characteristic of IBD-associated cancer [43]. In our study, we conducted an AOM/DSS-induced CAC model, which could better simulate the procedures involved in the progress of IBD-associated cancer.
IBD are multifactorial chronic relapsing diseases that are characterized by an abnormal, systemic dysregulation of the mucosal immune response [1, 2]. Carcinogenesis that is associated with IBD is thought to follow a sequence, which is distinct from that observed in the case of sporadic cancers. Prior work has shown that inflammation invokes a cascade within the abnormal epithelial proliferative zone, progressing through dysplasia, adenoma and finally carcinoma [44]. Given the crucial role that persistent inflammation plays in the process of CAC and the immune-regulatory properties of MSCs, we hypothesized that MSCs could potentially ameliorate carcinogenesis in IBD through the regulation of the pathways closely tied to inflammation and immunity. Towards this end, a mouse model of colitis-associated tumorigenesis was induced by AOM/DSS. In line with our previous work [18], we observed tumors in all mice that were treated with AOM/DSS in the present study, and these tumors were verified to be adenomas with high-grade dysplasia. We also found that MSCs contributed to the attenuation of AOM/DSS-induced colitis that is associated with tumorigenesis, with reduced histological damage of the colon tissue.
The in vivo administration of MSCs is conditioned by their ability to localize to and be retained within the appropriate tissue. Many studies have demonstrated the ability of MSCs to migrate to various sites of tissue injury and inflammation [45–47]. However, others within the field hold the opposite view. For example, it has been indicated that intraperitoneal injections failed to allow MSCs migration to the inflamed colon, and thus failed to attenuate colitis [48]. According to another study [49], the homing process relies on three different stages: chemotaxis/traffic, rolling and trans-endothelial migration, and finally integration into the parenchyma. MSCs express a broad range of chemokine receptors that are known to be involved in the first stage of homing, including CXCR4 [49, 50]. To investigate the relationship between MSC migration and its effect on tumorigenesis, we utilized a lentiviral system in order to stably overexpress functional CXCR4 in MSCs isolated from C57BL/6 mice. We then examined the chemotaxis and invasiveness properties toward SDF-1 of MSCs in vitro, in addition to its effects on tumorigenesis in vivo. Our results demonstrated that CXCR4 overexpression did not affect the ability of MSCs to differentiate into adipolineage cells or osteocytes. In addition, our results showed that CXCR4 overexpression did not affect the proliferative capacity of MSCs, but rather enhanced their potential of chemotaxis and invasiveness toward SDF-1. In accordance with the results of the in vitro experiment, we observed increased levels of GFP and CXCR4 in colon tissue isolated from mice obtained from the MSC-CXCR4 group in comparison to colon tissue isolated from mice of the MSC-GFP group. These results indicated that a greater number of MSCs were recruited to the inflamed intestinal tissue with CXCR4 up-regulation. In addition, MSC-CXCR4 was found to play a more effective role in the attenuation of AOM/DSS-induced colitis-associated tumorigenesis. This was demonstrated by a prolonged colon length, a reduced tumor load and an alleviated histological damage of the colon tissue. Taken together, these results suggested that overexpression of CXCR4 enabled the homing of MSCs to inflamed intestinal tissues more effectively, and allowed a more pronounced anti-tumor function.
Interestingly, we observed a significant decrease in the average number of tumors per mouse in the MSCs-treated group compared with that in the PBS group. However, we observed no differences in tumor size between the PBS, MSC-GFP and MSC-CXCR4 groups. Similar results were showed in the study by Nasuno et al. [51]. Given that differences in the average number of tumors per mouse typically provides evidence of factors involved in tumor initiation, and differences in the average tumor size provide evidence of factors that influence tumor progression [52], we hypothesized that MSCs could function to partially protect against AOM/DSS-induced tumor initiation, rather than repress tumor progression. In addition, in both the MSC-GFP group and the MSC-CXCR4 group, GFP expression was detected in the renal, liver, spleen as well as the lung of mice. This indicated that MSCs redistributed to not only the intestine, but also the renal, liver, spleen and lung. There were no differences observed in GFP levels between these two groups, suggesting that there may be other factors that could affect MSCs homing, which has also been suggested by other studies [53–56].
Prior work has demonstrated that inflammatory mediators play an important role in the development of CAC [57, 58]. The pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6 and IL-8, are thought to promote tumor cell proliferation, and thereby promote carcinogenesis in CAC [59–61]. IL-6 is thought to be one of the most critical pro-inflammatory cytokines, and is produced primarily by myeloid cells [62]. This cytokine has been identified as a key promoter of carcinogenesis. STAT3 is known to function in the downstream of IL-6, with its role well documented in recent studies on the development of IBD-associated cancer [63].The IL-6/STAT3 signaling cascade has been shown to function as an important promoter of tumorigenesis [64, 65]. In addition, both IL-6 and TNF-α are known to be pro-inflammatory cytokines that are secreted by Th17, one of sunsets of T-cell dysregulation in IBD and IBD-associated cancer [66–68]. Importantly, MSCs have been demonstrated to exhibit immuno-depression and have been shown to have the ability to suppress T-cell proliferation and activation [69, 70]. In the present study, we observed decreased levels of pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6 and IL-8, as well as p-STAT3 in the MSCs-treated group compared with the PBS group. A more obvious reduction in expression levels of the indicators described above was detected in the mice from the MSC-CXCR4 group compared with those from the MSC-GFP group. Collectively, these results indicated that CXCR4-overexpressing MSCs could exert a more pronounced anti-tumor function. This could be at least partially due to the fact that CXCR4-overexpressing MSCs can more effectively ameliorate the dysregulation of T-cell sunset as well as pro-inflammatory cytokine levels and the IL-6/STAT3 signaling cascade.
One of the limitations of the current study is a failure to study the effect of MSCs-GFP/MSCs-CXCR4 on long-term survival in our CAC model. In our next study, we will examine whether the overexpression of CXCR4 can also enhance the long-term survival of mice with IBD-associated CRC and determine the molecular mechanisms involved in it. We will also try to investigate the factors that might impact the relationship between MSCs and the immune systems.
In summary, CXCR4-overexpressing MSCs could ameliorate CAC more effectively than MSCs in a mouse model, which could be associated with enhanced homing to inflamed intestinal tissues. This could contribute to the increased efficacy of MSCs-based therapy in CAC treatment, and could accelerate the process of translation of experimental evidence from basic research to daily clinical practice.
Funding
This work is supported by grants from the National Key Clinical Discipline, National Natural Science Foundation of China (No. 81300367 and No. 81400604), Guangdong Natural Science Foundation (No. 2015A030313108) and Science and Technology Planning Project of Guangdong Province (No. 2015B020229001).
Conflict of interest statement: none declared.
References
- 1. Torres J, Mehandru S, Colombel JF. et al. Crohn’s disease. Lancet 2017;389:1741–55. [DOI] [PubMed] [Google Scholar]
- 2. Ungaro R, Mehandru S, Allen PB. et al. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adami HO, Bretthauer M, Emilsson L. et al. The continuing uncertainty about cancer risk in inflammatory bowel disease. Gut 2016;65:889–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther 2003;18:1–5. [DOI] [PubMed] [Google Scholar]
- 5. Dugum M, Lin J, Lopez R. et al. Recurrence and survival rates of inflammatory bowel disease-associated colorectal cancer following postoperative chemotherapy: a comparative study. Gastroenterol Rep 2017;5:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peyrin-Biroulet L, Lepage C, Jooste V. et al. Colorectal cancer in inflammatory bowel diseases: a population-based study (1976-2008). Inflamm Bowel Dis 2012;18:2247–51. [DOI] [PubMed] [Google Scholar]
- 7. Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 8. Subramanian CR, Triadafilopoulos G.. Care of inflammatory bowel disease patients in remission. Gastroenterol Rep (Oxf) 2016;4:261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao L, Zhang Y, Hu B. et al. Phase II multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord-derived mesenchymal stromal cells in the prophylaxis of chronic graft-versus-host disease after HLA-haploidentical stem-cell transplantation. J Clin Oncol 2016;34:2843–50. [DOI] [PubMed] [Google Scholar]
- 10. Lazarus HM, Pavletic SZ.. Umbilical cord blood-derived mesenchymal stromal cells for reducing chronic graft-versus-host disease after haploidentical transplantation: just another labor-intensive strategy, or showing the way? J Clin Oncol 2016;34:2812–3. [DOI] [PubMed] [Google Scholar]
- 11. Mosna F, Sensebe L, Krampera M.. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev 2010;19:1449–70. [DOI] [PubMed] [Google Scholar]
- 12. Miteva K, Pappritz K, El-Shafeey M. et al. Mesenchymal stromal cells modulate monocytes trafficking in coxsackievirus B3-induced myocarditis. Stem Cells Transl Med 2017;6:1249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Connick P, Kolappan M, Crawley C. et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol 2012;11:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forbes GM, Sturm MJ, Leong RW. et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol 2014;12:64–71. [DOI] [PubMed] [Google Scholar]
- 15. Yang H, Zheng Y, Zhang Y. et al. Mesenchymal stem cells derived from multiple myeloma patients protect against chemotherapy through autophagy-dependent activation of NF-kappaB signaling. Leuk Res 2017;60:82–8. [DOI] [PubMed] [Google Scholar]
- 16. Ho IA, Toh HC, Ng WH. et al. Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells 2013;31:146–55. [DOI] [PubMed] [Google Scholar]
- 17. Cuiffo BG, Campagne A, Bell GW. et al. MSC-regulated microRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell Stem Cell 2014;15:762–74. [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez-Rey E, Delgado M.. Therapeutic application of mesenchymal stromal cells in murine models of inflammatory bowel disease. Methods Mol Biol 2014;1213:331–9. [DOI] [PubMed] [Google Scholar]
- 19. Robinson AM, Sakkal S, Park A. et al. Mesenchymal stem cells and conditioned medium avert enteric neuropathy and colon dysfunction in guinea pig TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol 2014;307:G1115–29. [DOI] [PubMed] [Google Scholar]
- 20. Chen Z, He X, He X. et al. Bone marrow mesenchymal stem cells ameliorate colitis-associated tumorigenesis in mice. Biochem Biophys Res Commun 2014;450:1402–8. [DOI] [PubMed] [Google Scholar]
- 21. Riella LV, Chandraker A.. Stem cell therapy in kidney transplantation. JAMA 2012;308:130. [DOI] [PubMed] [Google Scholar]
- 22. Mylotte LA, Duffy AM, Murphy M. et al. Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells 2008;26:1325–36. [DOI] [PubMed] [Google Scholar]
- 23. Wang M, Yang G, Jiang X. et al. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) regulates the expression of B-cell lymphoma/leukemia-2 (Bcl-2) and promotes the survival of mesenchymal stem cells (MSCs) via PGC-1alpha/ERRalpha interaction in the absence of serum, hypoxia, and high glucose conditions. Med Sci Monit 2017;23:3451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Won YW, Patel AN, Bull DA.. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials 2014;35:5627–35. [DOI] [PubMed] [Google Scholar]
- 25. Lau TT, Wang DA.. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther 2011;11:189–97. [DOI] [PubMed] [Google Scholar]
- 26. Kyriakou C, Rabin N, Pizzey A. et al. Factors that influence short-term homing of human bone marrow-derived mesenchymal stem cells in a xenogeneic animal model. Haematologica 2008;93:1457–65. [DOI] [PubMed] [Google Scholar]
- 27. Naderi-Meshkin H, Matin MM, Heirani-Tabasi A. et al. Injectable hydrogel delivery plus preconditioning of mesenchymal stem cells: exploitation of SDF-1/CXCR4 axis toward enhancing the efficacy of stem cells’ homing. Cell Biol Int 2016;40:730–41. [DOI] [PubMed] [Google Scholar]
- 28. Liu H, Liu S, Li Y. et al. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One 2012;7:e34608.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang SJ, Song XY, He M. et al. Effect of TGF-beta1/SDF-1/CXCR4 signal on BM-MSCs homing in rat heart of ischemia/perfusion injury. Eur Rev Med Pharmacol Sci 2016;20:899–905. [PubMed] [Google Scholar]
- 30. Li L, Wu S, Liu Z. et al. Ultrasound-targeted microbubble destruction improves the migration and homing of mesenchymal stem cells after myocardial infarction by upregulating SDF-1/CXCR4: a pilot study. Stem Cells Int 2015;2015:691310.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soleimani M, Nadri S.. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc 2009;4:102–6. [DOI] [PubMed] [Google Scholar]
- 32. Yuan R, Ke J, Sun L. et al. HES1 promotes metastasis and predicts poor survival in patients with colorectal cancer. Clin Exp Metastasis 2015;32:169–79. [DOI] [PubMed] [Google Scholar]
- 33. Horwitz EM, Le Blanc K, Dominici M. et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy 2005;7:393–5. [DOI] [PubMed] [Google Scholar]
- 34. Lee RH, Kim B, Choi I. et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem 2004;14:311–24. [DOI] [PubMed] [Google Scholar]
- 35. Kern S, Eichler H, Stoeve J. et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294–301. [DOI] [PubMed] [Google Scholar]
- 36. Dowling CM, Herranz Ors C, Kiely PA.. Using real-time impedance-based assays to monitor the effects of fibroblast-derived media on the adhesion, proliferation, migration and invasion of colon cancer cells. Biosci Rep 2014;34:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang X, Zhang F, Wang Y. et al. Oroxylin A inhibits colitis-associated carcinogenesis through modulating the IL-6/STAT3 signaling pathway. Inflamm Bowel Dis 2013;19:1990–2000. [DOI] [PubMed] [Google Scholar]
- 38. Squillaro T, Peluso G, Galderisi U.. Clinical trials with mesenchymal stem cells: an update. Cell Transplant 2016;25:829–48. [DOI] [PubMed] [Google Scholar]
- 39. Wei X, Yang X, Han ZP. et al. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin 2013;34:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lourenco S, Teixeira VH, Kalber T. et al. Macrophage migration inhibitory factor-CXCR4 is the dominant chemotactic axis in human mesenchymal stem cell recruitment to tumors. J Immunol 2015;194:3463–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hall B, Andreeff M, Marini F.. The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. Handb Exp Pharmacol 2007;180:263–83. [DOI] [PubMed] [Google Scholar]
- 42. Shahrokhi S, Daneshmandi S, Menaa F.. Tumor necrosis factor-alpha/CD40 ligand-engineered mesenchymal stem cells greatly enhanced the antitumor immune response and lifespan in mice. Hum Gene Ther 2014;25:240–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dulai PS, Sandborn WJ, Gupta S.. Colorectal cancer and dysplasia in inflammatory bowel disease: a review of disease epidemiology, pathophysiology, and management. Cancer Prev Res (Phila) 2016;9:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yashiro M. Molecular alterations of colorectal cancer with inflammatory bowel disease. Dig Dis Sci 2015;60:2251–63. [DOI] [PubMed] [Google Scholar]
- 45. Bruck F, Belle L, Lechanteur C. et al. Impact of bone marrow-derived mesenchymal stromal cells on experimental xenogeneic graft-versus-host disease. Cytotherapy 2013;15:267–79. [DOI] [PubMed] [Google Scholar]
- 46. Li M, Zhang YX, Zhang Z. et al. Endomicroscopy will track injected mesenchymal stem cells in rat colitis models. Inflamm Bowel Dis 2015;21:2068–77. [DOI] [PubMed] [Google Scholar]
- 47. Dave M, Hayashi Y, Gajdos GB. et al. Stem cells for murine interstitial cells of cajal suppress cellular immunity and colitis via prostaglandin E2 secretion. Gastroenterology 2015;148:978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Castelo-Branco MT, Soares ID, Lopes DV. et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One 2012;7:e33360.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karp JM, Leng Teo GS.. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009;4:206–16. [DOI] [PubMed] [Google Scholar]
- 50. Sohni A, Verfaillie CM.. Mesenchymal stem cells migration homing and tracking. Stem Cells Int 2013;2013:130763.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nasuno M, Arimura Y, Nagaishi K. et al. Mesenchymal stem cells cancel azoxymethane-induced tumor initiation. Stem Cells 2014;32:913–25. [DOI] [PubMed] [Google Scholar]
- 52. Greten FR, Eckmann L, Greten TF. et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004;118:285–96. [DOI] [PubMed] [Google Scholar]
- 53. Xie J, Wang W, Si JW. et al. Notch signaling regulates CXCR4 expression and the migration of mesenchymal stem cells. Cell Immunol 2013;281:68–75. [DOI] [PubMed] [Google Scholar]
- 54. Endaya B, Guan SP, Newman JP. et al. Human mesenchymal stem cells preferentially migrate toward highly oncogenic human hepatocellular carcinoma cells with activated EpCAM signaling. Oncotarget 2017;8:54629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pourjafar M, Saidijam M, Etemadi K. et al. All-trans retinoic acid enhances in vitro mesenchymal stem cells migration by targeting matrix metalloproteinases 2 and 9. Biotechnol Lett 2017;39:1263–8. [DOI] [PubMed] [Google Scholar]
- 56. Yang Z, Wu B, Jia S. et al. The mechanically activated p38/MMP-2 signaling pathway promotes bone marrow mesenchymal stem cell migration in rats. Arch Oral Biol 2017;76:55–60. [DOI] [PubMed] [Google Scholar]
- 57. Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol 2013;35:229–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Horvath B, Liu G, Wu X. et al. Overexpression of p53 predicts colorectal neoplasia risk in patients with inflammatory bowel disease and mucosa changes indefinite for dysplasia. Gastroenterol Rep (Oxf) 2015;3:344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Popivanova BK, Kitamura K, Wu Y. et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 2008;118:560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Azer SA. Overview of molecular pathways in inflammatory bowel disease associated with colorectal cancer development. Eur J Gastroenterol Hepatol 2013;25:271–81. [DOI] [PubMed] [Google Scholar]
- 61. Taniguchi K, Karin M.. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol 2014;26:54–74. [DOI] [PubMed] [Google Scholar]
- 62. Yu H, Lee H, Herrmann A. et al. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 2014;14:736–46. [DOI] [PubMed] [Google Scholar]
- 63. Taniguchi K, Wu LW, Grivennikov SI. et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 2015;519:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Waldner MJ, Neurath MF.. Master regulator of intestinal disease: IL-6 in chronic inflammation and cancer development. Semin Immunol 2014;26:75–9. [DOI] [PubMed] [Google Scholar]
- 65. Wang L, Zhao M, Guo C. et al. PDCD4 deficiency aggravated colitis and colitis-associated colorectal cancer via promoting IL-6/STAT3 pathway in mice. Inflamm Bowel Dis 2016;22:1107–18. [DOI] [PubMed] [Google Scholar]
- 66. Bandzar S, Gupta S, Platt MO.. Crohn’s disease: a review of treatment options and current research. Cell Immunol 2013;286:45–52. [DOI] [PubMed] [Google Scholar]
- 67. He XW, He XS, Lian L. et al. Systemic infusion of bone marrow-derived mesenchymal stem cells for treatment of experimental colitis in mice. Dig Dis Sci 2012;57:3136–44. [DOI] [PubMed] [Google Scholar]
- 68. Kraus S, Arber N.. Inflammation and colorectal cancer. Curr Opin Pharmacol 2009;9:405–10. [DOI] [PubMed] [Google Scholar]
- 69. Parekkadan B, Fletcher AL, Li M. et al. Aire controls mesenchymal stem cell-mediated suppression in chronic colitis. Mol Ther 2012;20:178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen Y, Song Y, Miao H. et al. Gene delivery with IFN-gamma-expression plasmids enhances the therapeutic effects of MSCs on DSS-induced mouse colitis. Inflamm Res 2015;64:671–81. [DOI] [PubMed] [Google Scholar]