Abstract
Aim:
To achieve durable and broad protection against human papillomaviruses by vaccination with multimers of minor capsid antigen L2 using self-adjuvanting fusions with the toll-like receptor-5 (TLR5) ligand bacterial flagellin (Fla) instead of co-formulation with alum.
Methods:
Fla fusions with L2 protective epitopes comprising residues 11–200, 11–88 and/or 17–38 of a single or multiple HPV types were produced in E. coli and their capacity to activate TLR5 signaling was assessed. Immunogenicity was evaluated serially following administration of 3 intramuscular doses of Fla-L2 multimer without exogenous adjuvant, followed by challenge 1, 3, 6 or 12 months later, and efficacy compared to vaccination with human doses of L1 VLP vaccines (Gardasil and Cervarix) or L2 multimer formulated in alum. Serum antibody responses were assessed by peptide ELISA, in vitro neutralization assays and passive transfer to naïve rabbits in which End-Point Protection Titers (EPPT) were determined using serial dilutions of pooled immune sera collected 1, 3, 6 or 12 months after completing active immunization. Efficacy was assessed by determining wart volume following concurrent challenge at different sites with HPV6/16/18/31/45/58 ‘quasivirions’ containing cottontail rabbit papillomavirus (CRPV) genomes.
Results:
Vaccination in the absence of exogenous adjuvant with Fla-HPV16 L2 11–200 fusion protein elicited durable protection against HPV16, but limited cross-protection against other HPV types. Peptide mapping data suggested the importance of the 17–38 aa region in conferring immunity. Indeed, addition of L2 residues 17–38 of HPV6/18/31/39/52 to a Fla-HPV16 L2 11–200 or 11–88 elicited broader protection via active or passive immunization, similar to that seen with vaccination with an alum-adjuvanted L2 multimer comprising the aa 11–88 peptides of five or eight genital HPV types.
Conclusions:
Vaccination with flagellin fused L2 multimers provided lasting (>1 year) immunity without the need for an exogenous adjuvant. Inclusion of the L2 amino acid 17–38 region in such multi-HPV type fusions expanded the spectrum of protection.
Keywords: Human papillomavirus, Flagellin, Cutaneous challenge, L2, HPV, Prophylactic vaccine, Neutralizing antibody, Protective efficacy
1. Introduction
There is considerable interest in the conserved protective epitopes of L2 as a potential single antigen-based broad spectrum HPV vaccine [1]. There are several cross-protective epitopes at the amino terminus of L2 [2–4] but it is not clear which are immunodominant and optimal to achieve the broadest spectrum of protection. We previously described two candidate broad spectrum HPV vaccines comprising concatamers of L2 residues ~11–88 encoded by either 5 or 8 different mucosal α HPV genotypes (termed α11–88×5 and α11–88×8 respectively, see Fig. 1) [5–7]. These L2 multimers were purified after recombinant expression in E. coli and formulated in alum, an expression system and adjuvant chosen to drive down manufacturing cost. Vaccination of mice with either construct induced robust neutralizing serum antibody responses in mice, although peak titers were significantly lower than for homologous type L1 VLP vaccination. Importantly, mice that had been immunized three times with either L2 multimer formulated with alum were strongly protected against intravaginal pseudovirion challenge with all six α species (α10, α7, α9, α5, α6, and α11) tested, including low risk type HPV6, or high risk types HPV16, HPV26, HPV31, HPV33, HPV35, HPV45, HPV51, HPV56, HPV58, or HPV59 [6]. These findings suggested that immunologic competition between units is not a significant issue and that it is not necessary to include a unit of L2 derived from each species to achieve broader protection against diverse medically significant mucosal HPV types than is observed with the licensed HPV vaccines.
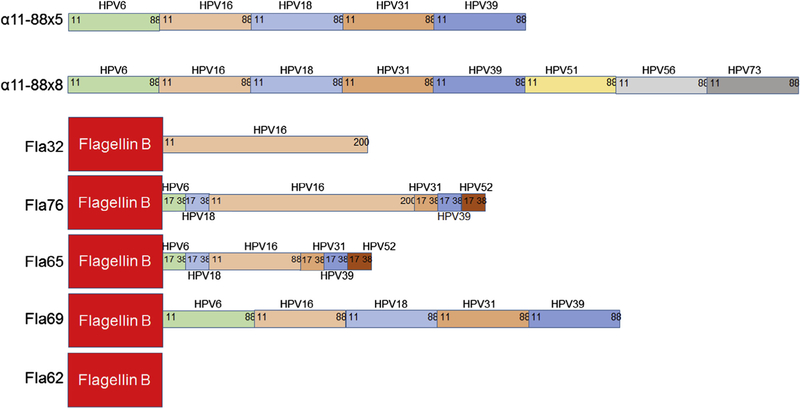
Fig. 1.
Summary of constructs. The following recombinant concatamers of L2 epitopes were purified from E. coli and formulated at 125 µg/dose with aluminum phosphate adjuvant: α11–88×5 (comprising L2 amino acids 11–88 of HPV6, 16, 18, 31 and 39) and α11–88×8 (L2 amino acids 11–88 of HPV6, 16, 18, 31, 39, 51, 56 and 73). In addition, Flagellin B (ΔD3) fusions with L2 multimers were produced in E. coli and used without addition of adjuvant. Fla32 (flagellin fused to HPV16 L2 amino acids 11–200), Fla76 (flagellin fused to HPV6 L2 17–38, HPV18 L2 17–38, HPV16 L2 11–200, HPV31 L2 17–38, HPV39 L2 17–36 and HPV52 17–36), Fla65 (flagellin fused to HPV6 L2 17–38, HPV18 L2 17–38, HPV16 L2 11–88, HPV31 L2 17–38, HPV39 L2 17–36 and HPV52 17–36), and Fla69 (flagellin fused to L2 11–88 of HPV6, 16, 18, 31 and 39), or Fla62 which lacks any L2.
Passive transfer of α11–88×8 antisera protected naïve mice from experimental vaginal challenge with HPV pseudovirions, suggesting that neutralizing antibodies are sufficient to mediate this protection, and therefore their measurement is likely to correlate with protective efficacy [6]. Concern has been raised that the neutralization of native HPV virions may subtly differ from pseudovirions, although rabbit antisera to α11–88×8 and α11–88×5 similarly neutralized native HPV18 virions [6]. Interestingly, α11–88×8 antisera also neutralized HPV pseudovirions of not only all 22 a types examined, but also key cutaneous β HPV that are associated with non-melanoma skin cancer [8]. Since currently licensed HPV vaccines do not target cutaneous HPV genotypes, here we sought to explore in a second challenge model the durability and breadth of protection elicited by the α11–88×8 and α11–88×5 vaccines, the possibility of protection from cutaneous challenge with HPV pseudovirion or native virions of the highly divergent cottontail rabbit papillomavirus (CRPV).
The licensed L1 VLP vaccines have demonstrated efficacy over a decade and once stabilized, the levels of serum neutralizing antibodies in vaccinated patients have remained durable. However, all are formulated on alum, precluding frozen storage and limiting their shelf life. The potent immunogenicity of L1 VLP is believed to reflect their particulate nature [9]. Since L2 does not form VLP alone [10], and is less immunogenic than L1 VLP, several groups have explored virus-display of L2 [11–14], or the use of adjuvants mixed with linear L2 antigen [15,16], spanning use with conventional alum, with or without toll-like receptor (TLR) agonists such as monophosphoryl lipid A (MPL) and CpG, saponins and even Freund’s adjuvant [1].
Direct coupling of TLR agonists to antigens has emerged as a promising approach to enhancing immune responses and provide dose sparing [17]. We previously demonstrated that vaccination with as little as 1 µg of a fusion protein comprising the TLR5 agonist flagellin linked to HPV16 L2 11–200 (termed Fla32, Fig. 1) could provide complete protection from experimental HPV16 challenge without co-formulation with an adjuvant, although it was less effective against other genotypes [18]. Here we explored new fusions of flagellin that incorporate multiple copies of the L2 neutralization epitope 17–36 as a potential approach to broaden cross-protection elicited by vaccination in the absence of exogenous adjuvant [19]. These flagellin fusions (Fig. 1)were compared to alum-formulated L2 multimers and licensed HPV vaccines for the induction of neutralizing antibody responses, epitope recognition and durability (passive and active immunity) against challenge with diverse HPV types. The vaccines were tested in rabbits using HPV quazivirions containing CRPV genomes as a cutaneous challenge model because it has a disease endpoint and permits parallel study of multiple genotypes [20].
2. Results
2.1. Broad and durable immunity with self or alum-adjuvant L2 multimers
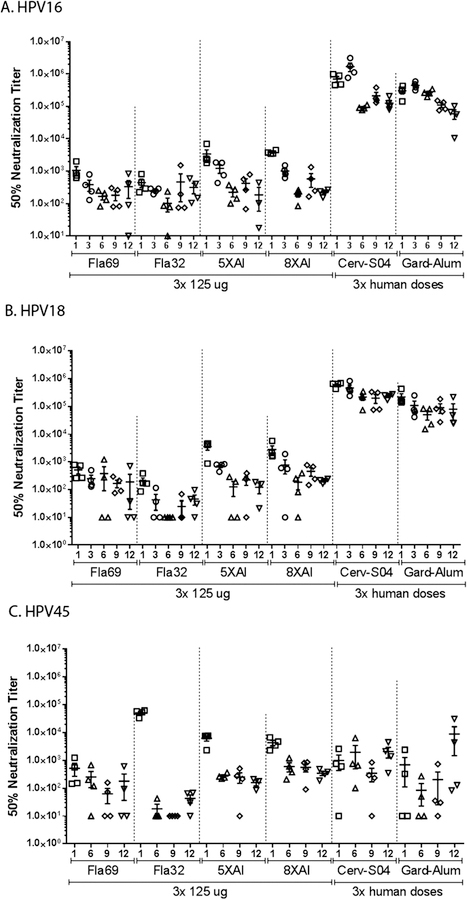
Groups of rabbits were immunized intramuscularly three times monthly with 125 µg of either the α11–88×8 or the α11–88×5 L2 multimer vaccines formulated in alum, or a flagellin fusion with α11–88×5 (Fla69) or flagellin fused to HPV16 11–200 (Fla32) formulated in buffer alone [18] (see Fig. 1 for summary of vaccine constructs). Positive control groups, vaccinated three times at monthly intervals with human doses of either quadrivalent Gardasil or Cervarix were included as well as negative controls. Serum samples were harvested prior to, and 1, 3, 6, 9 and 12 months after completion of vaccination and were initially assessed in vitro for HPV16, HPV18 and HPV45 neutralizing antibody titer (Fig. 2) [21]. In all cases, the highest titers occurred 1 month post vaccination, and waned approximately 10-fold by month 6, and thereafter remained stable through month 12. Peak titers generated by the aluminum phosphate-adjuvanted L2 multimers were initially slightly higher than for the self-adjuvanted flagellin-L2 fusions, but by month 6 both waned to similar levels and thereafter stabilized.
Fig. 2.
Kinetics of serum neutralizing antibody response through 12 months. Rabbits were immunized i.m. three times with placebo (not shown) or 125 µg of the following antigen preparations: Fla69, Fla32, α11–88×5 formulated with aluminum phosphate (5XAl); α11–88×8 formulated with aluminum phosphate (8XAl). As a positive control, additional rabbits were vaccinated with 3 full human doses of Cervarix® which includes HPV 16 and 18 L1 VLP formulated with the ASO4 adjuvant (Cerv-SO4), or GARDASIL® comprising HPV6, 11, 16 and 18 L1 VLP formulated on a proprietary alum-based adjuvant (Gard-Alum). The sera were collected and tested using the in vitro HPV16 (A), HPV18 (B) or HPV45 (C) PBNA and reciprocal titers to achieve 50% neutralization are presented.
The HPV16 neutralizing antibody responses to L2-based vaccination were two orders of magnitude lower in this assay as compared to the L1 VLP-based vaccines [22] (p < 0.001). However, there was no clear difference in the HPV16 neutralizing antibody response elicited by the four L2-based vaccines tested. For HPV18, the neutralizing antibody titers were again 2-orders of magnitude higher in rabbits immunized with L1 VLP as compared to the L2-based vaccines (p < 0.001). The α11–88×8 and α11–88×5 and Fla69, which each contain HPV18 L2 sequences, were similarly immunogenic, but the response to Fla32, which only contains HPV16 L2 sequences, trended lower (p = 0.006 vs. α11–88×8 at month 12).
HPV45 in vitro neutralizing antibody titers for the sera of rabbits vaccinated with Cervarix and Gardasil were substantially lower than for HPV16 and HPV16. Indeed, they were not significantly different to the responses induced by the α11–88×8, α11–88×5 and Fla69 vaccines (which contain highly related HPV18 and HPV39 L2 sequences, two viruses within the same α9 species as HPV45). Again, the response to Fla32 was weaker than the other L2-based vaccines (p = 0.02 vs. α11–88×5, and p = 0.003 vs. α11–88×8 at month 12) suggesting the value of using a mutimeric L2 protein comprising protective epitopes of multiple different L2 types to broaden the genotype spectrum of the neutralizing antibody response.
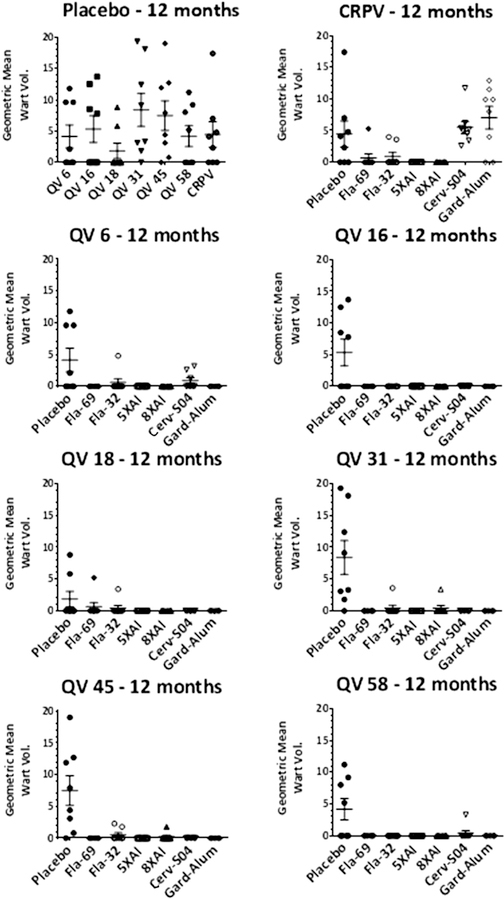
At months 1, 3, 6, 9 and 12, 5 animals in each group were challenged with HPV6, 16, 18, 31, 45 and 58 quazivirions (QV) and CRPV (Fig. 3 shows data for the month 12 challenge; data not shown for challenge of separate cohorts of animals at months 1, 3, 6, 9 was consistent) [20]. Neither L1 VLP vaccine protected rabbits from CRPV challenge, but as expected both protected fully against challenge with the HPV QV directly targeted by each vaccine. Cervarix did not fully protect against HPV6 or HPV58, but fully prevented warts after challenge with HPV31 and HPV45, a similar profile to that described clinically. Vaccination with three full human doses of Gardasil protected against HPV challenge with all types targeted by the vaccine and HPV31, but not HPV58. We note that in a similar study with one tenth human doses of Gardasil, the protection in this model was more type restricted [14].
Fig. 3.
Skin challenge of rabbits at 12 months post vaccination with HPV6, 16, 18, 31, 45 and 58 quazivirions and CRPV. Rabbits were immunized i.m. three times with placebo (not shown) or 125 µg of the following antigen preparations: Fla69, Fla32, α11–88×5 formulated with aluminum phosphate (5XAl); α11–88×8 formulated with aluminum phosphate (8XAl). As a positive control, additional rabbits were vaccinated with 3 full human doses of Cervarix® which includes HPV 16 and 18 L1 VLP formulated with the ASO4 adjuvant (Cerv-SO4), or GARDASIL® comprising HPV6, 11, 16 and 18 L1 VLP formulated on a proprietary alum-based adjuvant (Gard-Alum). One year later the rabbits were challenged with HPV6, 16, 18, 31, 45 and 58 quazivirions and CRPV and followed for 8 weeks for papilloma growth.
Vaccination with Fla32 (HPV16 L2 11–200 fused to flagellin) completely protected against HPV16 challenge, and reduced papilloma produced by QV challenge with all other types and CRPV. Fla69 (α11–88×5 fused to flagellin) provided broader protection gainst the HPV QV and partial protection against CRPV challenge. Interestingly, α11–88×5 formulated in alum provided complete protection against all of the HPV QV and CRPV. Surprisingly, the α11–88×8 formulated in alum was slightly less effective, with papilloma observed in one animal challenged with HPV18 QV and one with HPV31 QV. Similar data were obtained upon challenge of separate cohorts of animals at months 1, 3, 6, 9 post-vaccination (not shown), suggesting that the level and breadth of protection was stable over one year after active vaccination.
2.2. Assessment of humoral immunity by passive transfer
Passive transfer of titrated doses of immune serum to naïve animals prior to viral challenge provides a robust and sensitive approach to quantitate protective humoral responses to vaccination, including both direct antibody mediated neutralization and potential Fc-dependent effects [23,24]. To address whether the level of protective serum antibodies waned over the study period, sera from each vaccination group, at each time point (1, 3, 6, and 12 months post-vaccination), were pooled for passive transfer studies. Groups of 2 naïve rabbits were infused with the pooled immune serum in volumes corresponding to a dilution of 20, 100, 500, 2500 or 12,500 of the recipient’s blood volume for each vaccination cohort. These animals were then simultaneously challenged with HPV6, 16, 18, 31, 45 and 58 quazivirions (QV) and CRPV on different sites and papilloma size then scored 8 weeks later. These data are summarized in Table 1. The data suggest that the L1 VLP vaccines elicit a durable and type restricted protective antibody response. Very broad immunity was seen with all three multimeric L2 vaccines (α11–88×8 and α11–88×5 and Fla69 vaccines), whereas the antiserum to Fla32 provided protection against a more limited range of HPV genotypes, notably HPV16 from which Fla32 was derived. These findings are consistent with the in vitro neutralizing antibody data in some respects. The in vitro neutralizing data suggest that the highest titer response was detected at month 1, and that it wanes ~10-fold by month 3–6 and a similar pattern is observed by passive transfer. However, there were some striking differences observed. The protective titers for L1 and L2-based vaccines are quite similar by passive transfer, but differ by 2 orders of magnitude by in vitro neutralization titer. Furthermore, protection by passive transfer wanes to below detection (EPPT < 20), yet the actively vaccinated animals are completely protected at these time points. We note that the requirement of complete protection in the EPPT assay is very stringent, and the sera exhibit clear protection, although incomplete, in many cases.
Table 1.
Passive transfer studies. A. Groups of 2 naïve rabbits were infused with the pooled immune serum (collected either 1, 3, 6 and 12 months post vaccination as described in Fig. 2) in volumes corresponding to a dilution of 20, 100, 500, 2500 or 12500 of the recipient’s blood volume for each vaccination cohort and compared to placebo. These animals were then challenged with HPV6, 16, 18, 31, 45 and 58 quazivirions (QV) and CRPV and papilloma size then scored 8 weeks later. The maximum dilution that shows complete protection is shown (EPPT). (nr) = not reliable.
| Vaccine | Month | CRPV | HPV6 | HPV16 | HPV18 | HPV31 | HPV45 | HPV58 |
|---|---|---|---|---|---|---|---|---|
| Placebo | 1 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| Placebo | 3 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| Placebo | 6 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| Placebo | 12 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| Fla69 | 1 | <20 | 100 | 500 | 500 | 20 | <20 | 20 |
| Fla69 | 3 | <20 | <20 | 20 | 20 | <20 | <20 | 20 |
| Fla69 | 6 | <20 | 20 | 20 | 20 | <20 | <20 | 20 |
| Fla69 | 12 | <20 | <20 | <20 | 20 | <20 | <20 | 20 |
| 5XAL | 1 | <20 | 500 | 2500 | 500 | 20 | 20 | 100 |
| 5XAL | 3 | <20 | 100 | 20 | 100 | <20 | 20 | 20 |
| 5XAL | 6 | <20 | 20 | 20 | <20 | <20 | <20 | 20 |
| 5XAL | 12 | <20 | 20 | <20 | <20 | <20 | <20 | <20 |
| Fla32 | 6 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| Fla32 | 12 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| 8XAL | 6 | <20 | 20 | 500 | 20 | 20 | <20 | 20 |
| 8XAL | 12 | <20 | 20 | <20 | 100 | <20 | <20 | <20 |
| Cervarix | 6 | <20 | <20 | 2500 | 500 | 20 | <20 | 20 |
| Cervarix | 12 | <20 | 20 | 20 | nr | <20 | <20 | <20 |
| Gardasil | 6 | <20 | 20 | 100 | 100 | <20 | <20 | <20 |
| Gardasil | 12 | <20 | 20 | 20 | nr | <20 | <20 | <20 |
suggests that the RG1 epitope-specific response may contribute to broad immunity.
2.3. Preferential antibody response to L2 17–38 associated with broader protection
Given the broader spectrum of protection observed after vaccination with the multimeric versus the monomeric Fla fusion protein (Fla69 vs. Fla32), we sought to determine which epitope(s) might contribute to this difference. Sera from animals within each group were combined and the pools analyzed on an overlapping set of peptides, derived from L2 of many medically relevant HPV types, ROPV and CRPV, spanning L2 residues 11–200 for Fla 32 and 11–88 for Fla69 (Supplementary Figs. S1–2). Notably, the more broadly cross-reactive response to Fla69 appeared to be focused upon two peptides that correspond to the previously described epitope (aa 17–36) that is bound by the cross-neutralizing and protective monoclonal antibody RG1 [19]. The response to the RG1 epitope was present, but less pronounced in the Fla32 antiserum. Some reactivity to a second neutralizing epitope in the 65–81 region of HPV16 L2 was also seen [25]. The wider spectrum of protection elicited by the Fla69 construct as compared to Fla32
2.4. Anamnestic response after cutaneous challenge
The protection elicited by active vaccination appeared to be stronger than that conferred by passive transfer. This might reflect either the 20-fold difference in antibody concentration between active vs. passively immunized animals, or that viral challenge stimulates an anamnestic response that rapidly raises the neutralizing antibody response after it has waned. To assess this, animals were vaccinated three times with an L2 vaccine, and sera was harvested one month post vaccination, and then both 2 days before and 7 days after a cutaneous challenge with HPV16/18/31/45/58 PsV and CRPV at 9 months after the completion of vaccination. The RG1 peptide-specific serum antibody response waned between 1 month and 9 months post vaccination (Fig. 4A). However, no significant change in titer was seen when comparing 7 days post challenge to 2 days prior to challenge suggesting the lack of an anamnestic response after cutaneous challenge. In contrast, an anamnestic response after cutaneous challenge was seen in animals vaccinated with Cervarix (p = 0.001) or Gardasil (p = 0.06) (Fig. 4B).
Fig. 4.
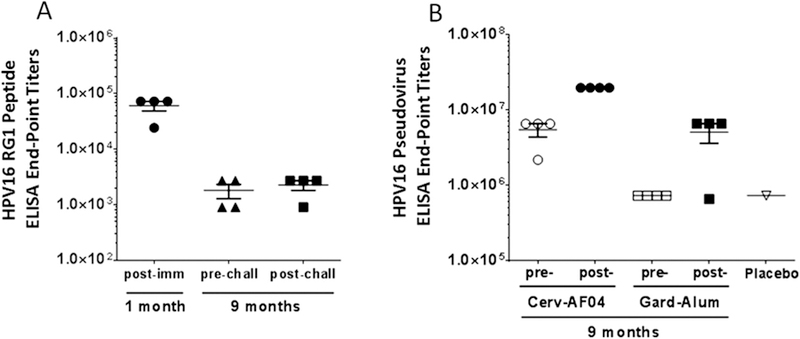
Assessment of anamnestic response upon challenge 9 months post-immunization with either Fla69, or Gardasil (Gard-Alum) or Cervarix (Cerv-AS04) immunized i.m. once a month for three months. (A) HPV16 RG1 (aa 17–38) peptide ELISA with rabbit serum from 1 month (study day 28) post-vaccination with Fla69 and 9 months (study day 310) post-vaccination with Fla69 and 1 week post-cutaneous challenge with HPV6, 16, 18, 31, 45 and 58 quazivirions and CRPV (study day 317). Since there was no significant difference in titer one week after challenge, which suggests no detectable systemic anamnestic response to this epitope after skin challenge. (B) HPV16 pseudovirus ELISA with rabbit serum from 9 months (study day 310) post-vaccination with Cervarix and Gardasil and post-challenge (study day 317) demonstrating an anamnestic response to HPV16 L1 VLP.
2.5. A fourth dose is not beneficial
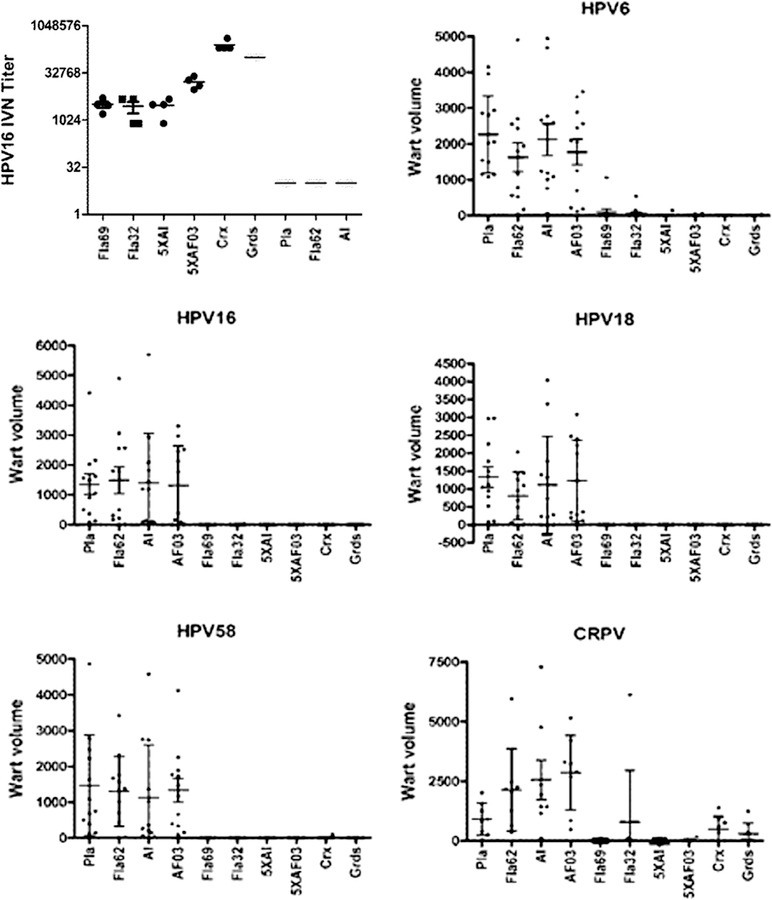
Since the in vitro neutralizing antibody titers were lower for L2-based vaccines as compared with the homologous responses to L1 VLP vaccination, a pilot experiment was performed to examine the benefit of an additional vaccination dose (a total of 4) and an alternative and potentially more potent oil-in-water adjuvant containing a TLR4 agonist (AF03), with α11–88×5 (Fig. 5). Significantly higher titers could be elicited by use of AF03 as compared with alum to adjuvant the L2 multimer vaccine, although these titers still remained well below those produced by the commercial L1 VLP vaccines (Fig. 5). Interestingly, the use of the AF03 adjuvant appeared to broaden the epitope specificity of the antibody response (Supplemental Fig. S1). A fourth vaccination did not further boost the titers significantly for the L2 multimer vaccine. Nevertheless, the L2-based vaccines provided broad protection against challenge with either HPV QV or CRPV (Fig. 5). The adjuvants alone, including flagellin, did not provide protection, and the L1 VLP vaccines protected against the HPV PsV but not CRPV challenge.
Fig. 5.
Impact of adjuvant on protection. Groups of 4 rabbits were immunized four times with placebo (Pla), 125 µg of flagellin only (Fla62), aluminum phosphate adjuvant only (Al), AF03 adjuvant only (AF03), or 125 µg Fla32, Fla69, or 125 µg α11–88×5 formulated in aluminum phosphate (5XAl), or with AF03 adjuvant (5XAF03), or full human doses of Cervarix or Gardasil on days 0, 21, 35 and 56. On day 77 serum was harvested and tested for HPV16 neutralizing antibody titer (top left panel). The rabbits were challenged on the skin with quazivirus of HPV types 6, 16, 18, 58 and CRPV. Graphs show wart volume readings 8 weeks post-challenge.
2.6. Addition of RG1 epitopes of multiple HPV to broaden immunity
The flagellin fusions show promise as vaccine candidates that do not need an exogenous adjuvant [26–28]. However, to achieve broad immunity, it is beneficial to include protective epitopes of multiple diverse and medically significant HPV genotypes. As the sequences of more types are included, the size of the fusion product increases such that expression levels are reduced and the purification becomes more challenging. Furthermore, we observed some evidence for competition between epitopes. Therefore we examined whether simply fusing multiple RG1 epitopes derived from several other HPV types to flagellin fused HPV16 11–200 (see Fla76) could provide similarly broad immunity as Fla69 (Fig. 6). From this construct, residues 89–200 of HPV16 L2 were also removed to produce Fla65 [29], because prior data suggested that this region was weakly immunogenic. Fla65 retains residues 11–88 of HPV16 since we recently showed that the presence of the putative transmembrane domain (45–67) is important for immunogenicity and there is a broad neutralization epitope described for HPV16 L2 65–81 [7]. These flagellin fusion proteins were purified and used to vaccinate rabbits four times (Fig. 6).
Fig. 6.
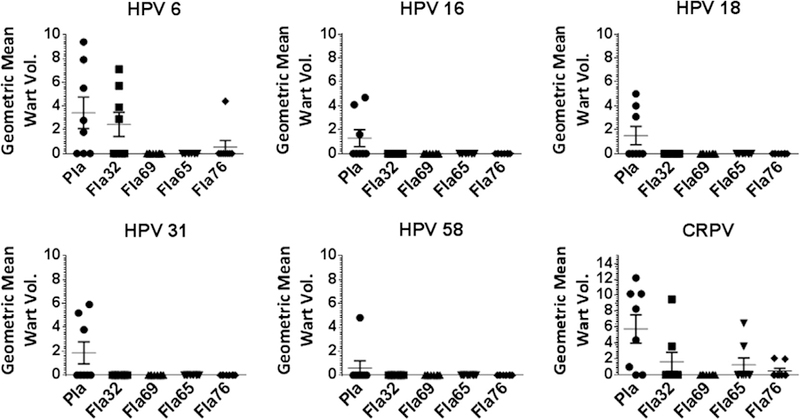
Multi-RG1 epitope fusions elicit broadly protection against skin challenge with HPV quazivirions. Groups of 4 rabbits were immunized four times with 125 µg of Fla69, Fla32, Fla65, or Fla76 or placebo (Pla) on days 0, 21, 42 and 63. On day 91 the rabbits were challenged on the skin with quazivirus of HPV types 6, 16, 18, 31, 58 as well as CRPV. Graphs show wart volume readings 8 weeks post-challenge.
The immune sera were then tested for in vitro neutralizing antibody titer against several HPV types. These data supported the broader reactivity of the Fla fusions containing multiple RG1 peptides as compared to Fla32 without compromising the HPV16-specific response. Further, upon active immunization, there was broad protection afforded by all of these constructs against HPV QV (Fig. 6).
The immune serum was tested for reactivity with peptide arrays corresponding to the amino termini of multiple HPV types as well as CRPV and ROPV. The Fla65 and Fla76 antisera exhibited an RG1 peptide focused response similar to that seen for Fla69, but minimal response to other regions of HPV16 L2 (Supplementary Figs. S1–2).
To address whether the serum antibody responses to the mutli-RG1 epitope fusions also confers broad immunity, the antisera to Fla32, Fla76, Fla65 and Fla69 were tested by passive transfer (Table 2A). Animals that received passive transfer of Fla76 and Fla65 antisera were similarly protected from challenge with a broad range of HPV QV as seen for the Fla69, yet broader than that observed for Fla32. These results were in accord with the in vitro neutralization titers (Table 2B).
Table 2.
To generate antiserum for passive transfer, groups of 4 rabbits were immunized with 125 µg of Fla69, Fla32, Fla65, or Fla76 or placebo four times (on days 0, 21, 42 and 63) and sera was collected at 1 month post final vaccination and pooled for each group of rabbits. A. To determine the end-point protection titer (EPPT) by passive transfer, groups of 4 naïve rabbits were infused with the pooled immune serum in volumes corresponding to a dilution of 20, 100, 500, 2500 or 12,500 of the recipient’s blood volume for each vaccination cohort and compared to placebo. These animals were then challenged with HPV6, 16, 18, 31, 45 and 58 quazivirions (QV) and CRPV and papilloma size then scored 8 weeks later. The maximum dilution that showed complete protection is presented (EPPT). B. The in vitro neutralization titer of the pooled sera utilized was determined. (nd = not done).
| Vaccine | Month | CRPV | HPV6 | HPV16 | HPV18 | HPV31 | HPV45 | HPV58 |
|---|---|---|---|---|---|---|---|---|
| A | ||||||||
| Fla32 | 1 | <20 | 20 | 500 | 20 | 20 | <20 | 100 |
| Fla65 | 1 | <20 | 100 | 2500 | 500 | 500 | 100 | 500 |
| Fla69 | 1 | 20 | 100 | 100 | 100 | 100 | 20 | 100 |
| Fla76 | 1 | 20 | 2500 | 500 | 500 | 12,500 | 20 | 12,500 |
| Placebo | 1 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| B | ||||||||
| Fla32 | 1 | nd | 152 | 14,960 | 767 | 1113 | 219 | nd |
| Fla65 | 1 | nd | 1781 | 16,494 | 3295 | 3470 | 7323 | nd |
| Fla69 | 1 | nd | 12,361 | 15,846 | 20,106 | 2684 | 14,744 | nd |
| Fla76 | 1 | nd | 9661 | 38,958 | 2286 | 7283 | 5390 | nd |
3. Discussion
Multi-RG1 peptides fused to flagellin show comparably broad protection against diverse HPVs as compared to longer L2 concatemers (aa11–88), and is superior to monomeric Fla32 construct containing only HPV16L2 aa11–200. Notably, vaccination with a fusion protein comprising 20 copies of the RG1 region, each derived from a different HPV type, was very poorly immunogenic even in Freund’s adjuvant [5], suggesting the value of the fusion to provide T help [17].
The data presented here supports 17–38 as the dominant linear neutralizing B-cell epitope within L2 across ‘high-risk’ HPV oncogenic types and likely the corresponding epitopes from many cutaneous HPV types could readily be incorporated [4,19]. While there has been focus on addressing the HPV types associated with anogenital cancers, it is also reasonable to consider targeting the HPV types potentially associated with non-melanoma skin cancer or even benign foot and hand warts, especially in immunocompromised patients [30–32]. Vaccination early in life against the cutaneous HPV types would presumably be optimal, but in the cutaneous rodent Mastomys natalensis papillomavirus (MnPV) model L1 VLP vaccination of animals already-infected perinatally can prevent the onset of disease, including malignant skin cancer, likely by controlling viral load and spread within the host [32].
The commercial vaccines Cervarix and Gardasil showed unexpected cross-protection against several non-targeted HPV types in the rabbit challenge model that is not consistent with clinical experience [33]. This likely reflects the high doses used (full human doses) as similar studies by us using lower doses resulted in type restricted protection in this rabbit model [14].
A major question for L2-based vaccines is the longevity of the immunity, especially given the lower titers of neutralizing antibody elicited as compared with L1 VLP vaccines. Our data suggest that strong and broad immunity lasts at least one year, and that even low neutralizing antibody titers are still associated with protection. While there is an initial waning of the serum antibody titers in the first 3 months (which was more pronounced for the alum-formulated L2 multimers), the levels appear to stabilize over the next 6–9 months. This trend was evident by ELISA, in vitro neutralization titer and EPPT via passive transfer.
The titers of neutralizing antibodies elicited by L2 vaccines were 2-orders of magnitude lower than for the L1 VLP vaccines when measured by in vitro neutralization assays or ELISA, but serum is undiluted in the host, and thus this difference in titer may be less relevant [34]. Indeed, in the EPPT assay the differences in protective titers between L1 and L2-based antisera was much less pronounced, and there was no clear difference in the active vaccination data.
The in vitro neutralization assay and EPPT by passive transfer used in this study correlated reasonably with protection in immunized rabbits, but was not always predictive of active immunity [24]. This suggests either a lack of assay sensitivity or that perhaps a rabbit anamnestic response may contribute to protection. While an anamnestic response was triggered by viral challenge in L1 VLP vaccinated animals [35], this was not observed in L2 vaccinated rabbits after challenge. These findings, together with the demonstration of protection via passive transfer, suggest that sterilizing immunity is responsible for protection. Several improvements to the in vitro neutralization assay have recently been made to better detect L2-specific neutralizing antibodies, and they may better correlate with active immunity [36]. However, unlike passive transfer studies, in vitro neutralization assays do not measure Fc-dependent effects such as opsonization or complement-mediated cell killing [37].
4. Conclusion
A concatemer of protective epitopes from L2 of several ‘high-risk’ oncogenic HPV types fused in tandem to the TLR5 agonist flagellin is a single, self-adjuvanting antigen candidate with promise as a next generation HPV vaccine [18]. Based on studies with other antigens such as influenza HA and M2e [28,38], this fusion flagellin format is feasible to manufacture and can be stored frozen or possibly lyophilized for extended shelf-life.
Supplementary Material
Acknowledgments
Financial disclosure
This study was funded by Sanofi Pasteur.
Competing Interests
SJ and RBSR are co-inventors on L2 patents that were licensed to Shantha Biotechnics Ltd., GlaxoSmithKline, PaxVax, Inc. and Acambis, Inc, and have received grant support from Sanofi Pasteur. The terms of these arrangements are managed by Johns Hopkins University in accordance with its conflict of interest policies.
Abbreviations:
- CRPV
cottontail rabbit papillomavirus
- EDV
epidermodysplasia verruciformis
- EPPT
endpoint protection titer
- Fla
flagellin
- HPV
human papillomavirus
- MnPV
Mastomys natalensis papillomavirus
- MPL
monophosphoryl lipid A
- NMSC
non-melanoma skin cancer
- TLR5
toll-like receptor-5
- VLP
virus-like particle
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2017.07.086.
References
- [1].Jiang RT, Schellenbacher C, Chackerian B, Roden RB. Progress and prospects for L2-based human papillomavirus vaccines. Expert Rev Vaccines 2016;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Roden RB, Yutzy WHT, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology 2000;270:254–7. [DOI] [PubMed] [Google Scholar]
- [3].Kawana K, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. Common neutralization epitope in minor capsid protein L2 of human papillomavirus types 16 and 6. J Virol 1999;73:6188–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rubio I, Seitz H, Canali E, Sehr P, Bolchi A, Tommasino M, et al. The N-terminal region of the human papillomavirus L2 protein contains overlapping binding sites for neutralizing, cross-neutralizing and non-neutralizing antibodies. Virology 2011;409:348–59. [DOI] [PubMed] [Google Scholar]
- [5].Jagu S, Karanam B, Gambhira R, Chivukula SV, Chaganti RJ, Lowy DR, et al. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst 2009;101:782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jagu S, Kwak K, Schiller JT, Lowy DR, Kleanthous H, Kalnin K, et al. Phylogenetic considerations in designing a broadly protective multimeric L2 vaccine. J Virol 2013;87:6127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jagu S, Kwak K, Karanam B, Huh WK, Damotharan V, Chivukula SV, et al. Optimization of multimeric human papillomavirus L2 vaccines. PLoS One 2013;8:e55538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kwak K, Jiang R, Wang JW, Jagu S, Kirnbauer R, Roden RB. Impact of inhibitors and L2 antibodies upon the infectivity of diverse alpha and beta human papillomavirus types. PLoS One 2014;9:e97232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA 1992;89:12180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kirnbauer R, Taub J, Greenstone H, Roden R, Durst M, Gissmann L, et al. Efficient self-assembly of human papillomavirus type 16 L1 and L1–L2 into virus-like particles. J Virol 1993;67:6929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Canali E, Bolchi A, Spagnoli G, Seitz H, Rubio I, Pertinhez TA, et al. A high-performance thioredoxin-based scaffold for peptide immunogen construction: proof-of-concept testing with a human papillomavirus epitope. Sci Rep 2014;4:4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tumban E, Peabody J, Tyler M, Peabody DS, Chackerian B. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PLoS One 2012;7:e49751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schellenbacher C, Kwak K, Fink D, Shafti-Keramat S, Huber B, Jindra C, et al. Efficacy of RG1-VLP vaccination against infections with genital and cutaneous human papillomaviruses. J Invest Dermatol 2013;133:2706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jagu S, Karanam B, Wang JW, Zayed H, Weghofer M, Brendle SA, et al. Durable immunity to oncogenic human papillomaviruses elicited by adjuvanted recombinant Adeno-associated virus-like particle immunogen displaying L2 17–36 epitopes. Vaccine 2015;33:5553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chandrachud LM, Grindlay GJ, McGarvie GM, O’Neil BW, Wagner ER, Jarrett WF, et al. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology 1995;211:204–8. [DOI] [PubMed] [Google Scholar]
- [16].Gambhira R, Jagu S, Karanam B, Gravitt PE, Culp TD, Christensen ND, et al. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N-terminus of HPV16 minor capsid antigen L2. J Virol 2007;81:13927–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Alphs HH, Gambhira R, Karanam B, Roberts JN, Jagu S, Schiller JT, et al. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc Natl Acad Sci USA 2008;105:5850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kalnin K, Tibbitts T, Yan Y, Stegalkina S, Shen L, Costa V, et al. Low doses of flagellin-L2 multimer vaccines protect against challenge with diverse papillomavirus genotypes. Vaccine 2014;32:3540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, et al. A protective and broadly cross-neutralizing epitope of Human Papillomavirus L2. J Virol 2007;81:13927–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mejia AF, Culp TD, Cladel NM, Balogh KK, Budgeon LR, Buck CB, et al. Preclinical model to test HPV capsid vaccines in vivo using infectious HPV/CRPV chimeric papillomavirus particles. J Virol 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 2004;321:205–16. [DOI] [PubMed] [Google Scholar]
- [22].Jagu S, Kwak K, Garcea RL, Roden RB. Vaccination with multimeric L2 fusion protein and L1 VLP or capsomeres to broaden protection against HPV infection. Vaccine 2010;28:4478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, et al. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol 1995;69:3959–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Longet S, Schiller JT, Bobst M, Jichlinski P, Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol 2011;85:13253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nakao S, Mori S, Kondo K, Matsumoto K, Yoshikawa H, Kanda T. Monoclonal antibodies recognizing cross-neutralization epitopes in human papillomavirus 16 minor capsid protein L2. Virology 2012;434:110–7. [DOI] [PubMed] [Google Scholar]
- [26].Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001;410:1099–103. [DOI] [PubMed] [Google Scholar]
- [27].Huleatt JW, Jacobs AR, Tang J, Desai P, Kopp EB, Huang Y, et al. Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine 2007;25:763–75. [DOI] [PubMed] [Google Scholar]
- [28].Tussey L, Strout C, Davis M, Johnson C, Lucksinger G, Umlauf S, et al. Phase 1 Safety and Immunogenicity Study of a Quadrivalent Seasonal Flu Vaccine Comprising Recombinant Hemagglutinin-Flagellin Fusion Proteins. Open Forum Infect Dis 2016;3:ofw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, et al. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology 2005;337:365–72. [DOI] [PubMed] [Google Scholar]
- [30].McLaughlin-Drubin ME. Human papillomaviruses and non-melanoma skin cancer. Semin Oncol 2015;42:284–90. [DOI] [PubMed] [Google Scholar]
- [31].Vinzon SE, Rosl F. HPV vaccination for prevention of skin cancer. Hum Vaccin Immunother 2015;11:353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vinzon SE, Braspenning-Wesch I, Muller M, Geissler EK, Nindl I, Grone HJ, et al. Protective vaccination against papillomavirus-induced skin tumors under immunocompetent and immunosuppressive conditions: a preclinical study using a natural outbred animal model. PLoS Pathog 2014;10:e1003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Malagon T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. The Lancet Infectious Diseases 2012;12:781–9. [DOI] [PubMed] [Google Scholar]
- [34].Bachmann MF, Kalinke U, Althage A, Freer G, Burkhart C, Roost H, et al. The role of antibody concentration and avidity in antiviral protection. Science 1997;276:2024–7. [DOI] [PubMed] [Google Scholar]
- [35].Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 2007;25:4931–9. [DOI] [PubMed] [Google Scholar]
- [36].Day PM, Pang YY, Kines RC, Thompson CD, Lowy DR, Schiller JT. An HPV in vitro neutralization assay that recapitulates the in vivo process of infection provides a sensitive measure of L2 infection-inhibiting antibodies. Clin Vaccine Immunol 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Day PM, Kines RC, Thompson CD, Jagu S, Roden RB, Lowy DR, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 2010;8:260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, et al. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine 2011;29:5145–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.