Abstract
The function of ligaments and tendons is to support and transmit loads applied to the musculoskeletal system. These tissues are often able to perform their function for many decades; however, connective tissue disease and injury can compromise ligament and tendon integrity. A range of protein and non-protein constituents, combined in a complex structural hierarchy from the collagen molecule to the tissue and covering nanometer to centimeter length scales, govern tissue function and impart characteristic non-linear material behavior. This review summarizes the structure of ligaments and tendons, the roles of their constituent components for load transfer across the hierarchy of structure, and the current understanding of how damage occurs in these tissues. Disease and injury can alter the constituent make-up and structural organization of ligaments and tendons, affecting tissue function, while also providing insight to the role and interactions of individual constituents. The studies and techniques presented here have helped to understand the relationship between tissue constituents and the physical mechanisms (e.g. stretching, sliding) that govern material behavior at and between length scales. In recent years, new techniques have been developed to probe ever smaller length scales and may help to elucidate mechanisms of load transfer and damage and the molecular constituents involved in the in the earliest stages of ligament and tendon damage. A detailed understanding of load transfer and damage from the molecular to the tissue level may elucidate targets for the treatment of connective tissue diseases and inform practice to prevent and rehabilitate ligament and tendon injuries.
Keywords: ligament, tendon, injury, damage, failure
INTRODUCTION
Connective tissue injury and disease affect millions of people in the United States each year,1, 2 dramatically reducing quality of life for these patients. As collagen is the primary structural constituent in all connective tissues, injury and disease involve changes and/or damage to collagen across its hierarchy of organization. In the case of ligaments and tendons, injury can be acute, resulting from a single traumatic event, or chronic, resulting from overuse. Acute injuries that result in complete tissue rupture often must be surgically repaired, while “subfailure” acute injuries, such as sprains, can often be treated successfully with temporary activity modification and physical therapy. However, the increased risk of recurrent injury and symptoms of chronicpain, chronic joint instability, and recurrent swelling following ligament sprain3 suggests that damage to ligaments or tendons may persist following functional recovery.
In addition to the effects of acute and chronic injury, many diseases, both heritable and acquired, are associated with pathologies of ligament and tendon constituents, structure and function. As an example, Marfan syndrome is caused by mutations to the fibrillin 1 gene,4 causing many symptoms including increased joint laxity. This increased joint laxity is likely related to observed lower density of elastic fibers along with the presence of a disrupted elastic network of mutations to the colVα1 gene, resulting in decreased production of Type V collagen heterotrimers that co-assemble with Type I collagen and are involved regulating Type I collagen with discontinuous fiber aggregates.5, 6 Classical Ehlers-Danlos syndrome (EDS) is the result of abnormal fibril growth, causing both increased and decreased fibril diameter, irregular transverse fibril shape, and altered fibril packing density.7, 8 Although EDS has different manifestations, many forms involve mutations to collagen genes, producing increased joint laxity, skin hypermobility, and weakened blood vessels.9 Other forms of EDS are also characterized by disrupted fibril structure due to mutations to other collagen types (e.g. Type-V collagen mutation), disruption of enzymatic crosslinking, and other unknown causes. Additional connective tissue diseases affecting ligaments and tendons are acquired or are due to a combination of genetic and environmental factors, such as scleroderma, rheumatoid arthritis and lupus.
It is clear that disease and injury directly affect ligament and tendon structure and composition, which in turn impacts their mechanical behavior and thus function. A vast body of research has addressed the intricate structure of ligament and tendon, mechanisms of force and strain transfer across physical scales, and mechanisms of damage to this structure. This article reviews the most germane results related to the constituents and hierarchical structural features of ligament and tendon, the current understanding of their role in load transfer between physical scales, and their relation to tissue damage and failure.
CONSTITUENTS AND STRUCTURE OF LIGAMENTS AND TENDONS
The behavior of ligament and tendon is governed by its constituents, both protein and non-protein components, and their structural organization. Protein constituents are the primary determinants of connective tissue mechanical function, while non-protein and cellular constituents are critical for the regulation of development, growth, and repair in ligament and tendon.
Collagens are the primary structural component of ligament and tendon, with total collagen content accounting for >70% of the dry weight in most healthy, mature ligaments and tendons.10 The functional molecular unit of all collagens is the triple helix. Three α-chain peptides containing a Gly-X-Y amino acid repeat structure, where approximately 30% of the X and Y positions are occupied by proline and hydroxyproline, respectively, assemble to form the right-handed triple helix. Presently, 28 collagen types have been identified, which differ by their amino acid and alpha-chain structure, as well as their function (e.g. fibrillar versus basement membrane collagens). The collagens in ligament and tendon are fibrillar collagens and predominantly Types I, III, and V.
Collagen type-I is a heterotrimeric molecule, with each triple helix containing two α1(I) chains and one α2(I) chain. This collagen is the primary determinant of mechanical behavior in healthy ligament and tendon and it is present through all stages of development, growth, remodeling, and healing. Collagen type-III is a homotrimeric molecule of α1(III) chains, involved mostly during ligament and tendon development and healing. Studies of tendon development in the chick embryo have revealed that type-III collagen is initially co-distributed with type-I collagen, but by tendon maturity the type-III collagen is confined to the surface of Type-I collagen fibrils.11 Collagen type-V is a heterotrimer containing α1(V), α2(V), and α3(V) chains with various triple helix stiochometries observed in healthy tissue12. Type-V collagen is a quantitatively minor component of type I collagen fibrils that has a regulatory role during fibril formation, with in vivo and in vitro evidence indicating a role in type-I fibril nucleation and regulation of fibril diameter. Mouse models of α1(V) genetic knockout are lethal at day ten of embryonic development, with a lack of fibril formation observed.13 Studies of collagen fibrillogenesis in vitro have demonstrated that increasing amounts of type-V collagen results in decreased mean fibril diameter and variance, demonstrating the role of type-V collagen in modulating fibril size.14
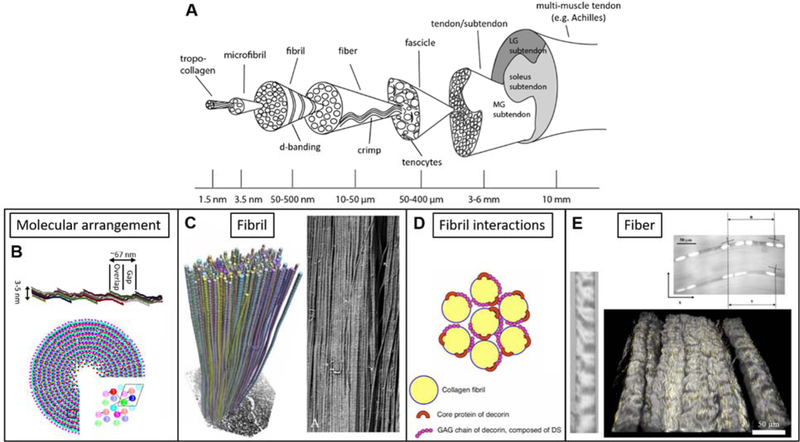
Collagen exists in a hierarchy of organization, with distinct organizational motifs at the nano-, micro- and millimeter length scales, as demonstrated in the classic structural schematic developed by Kastelic (Fig. 1).15 Since its publication, this structural model has remained relatively consistent through nearly four decades of research on ligament and tendon structure-function relationships. At the molecular scale, tropocollagen molecules self-assemble into fibrils (50–500 nm), with molecules arranged in a quarter-stager pattern16 and chemically crosslinked via enzymatic aldehyde bond formation by lysyl oxidase+ and non-enzymatic glycation.18 This staggered molecular organization is responsible for the characteristic 67 nm d-banding pattern that can be observed by electron microscopy (Fig. 1C), AFM, and x-ray scattering.19–22 Recent investigations have extended the 2D Hodge-Petruska model of tropocollagen organization to 3 dimensions, preserving the basic staggered overlap organization of tropocollagen molecules, while revealing a lateral arrangement of molecules into a quasi-hexagonal lattice with regions of amorphous and crystalline order (Fig. 1B).23–25 Collagen fibrils assemble to form fibers (10–50 µm) and fibers assemble to form fascicles (100–500 µm), which are the last distinct structural scale below the level of the tissue and visible to the eye. While this definition of the hierarchy is in agreement with newly proposed nomenclature, it has been suggested that the number of hierarchical levels present or their physical size may vary between tissue source and species.26 A crimp pattern is observed at the fiber and fascicle levels (Fig. 1A, E), with the amplitude and period of crimp variable between tissue sources.15 In many tendons commonly used for research such as tail tendons, the fascicle is smallest level in the collagen structural hierarchy that is easily distinguished by eye and can be isolated without the need for optical magnification. However, in many functional tendons the fascicle can be difficult to observe or isolate without additional tissue processing, and in especially small tendons (e.g. mouse tail) the fascicle may not exist as a distinct level separate from the tendon.
Figure 1.
A) Collagen structural hierarchy in ligament and tendon from the molecule to the tissue. The collagen triple-helical molecule, or tropocollagen, is assembled of three alpha-chains and forms the fundamental component of ligament and tendon (reprinted with permission, from Handsfield et al.26) B) Collagen molecules arrange longitudinally and laterally in a quarter-stagger pattern to form collagen micro-fibrils (top, length compressed 5 times for display). Transverse organization follows a quasihexagonal packing (bottom), with groups of 5 molecules forming the lattice-like structure (inset; adapted with permission, from Orgel27). C) The quarter-stagger arrangement is responsible for the characteristic d-banding pattern of collagen fibrils, which is observed in electron microscopy (3D reconstruction from serial transverse SEM images – left; SEM backscatter image - right). Fibril-level SEM imaging demonstrates high level of alignment, high aspect ratio, and continuity of fibrils along the length of ligament and tendons (left adapted with permission, from Svensson et al.28; right adapted with permission, from Provenzano et al.29). D) Small leucine rich proteoglycans such as decorin proteoglycan interact with collagen at the fibril level, playing an important role in regulating fibril growth (reprinted from Kosho30). E) Collagen fibrils assemble to form collagen fibers, which contain a characteristic crimp pattern that is observed by many optical techniques including brightfield microscopy (left, adapted with permission, from Legerlotz et al.31), fluorescence microscopy (top, reproduced with permission, from Screen et al.32) and second harmonic generation imaging (bottom). Tenocytes (top) and elastin (bottom, yellow) reside between and in register with collagen fibers. Groups of fibers are enclosed by a fascia-like layer of endotenon to form fascicles, with groups of fascicles comprising the ligament or tendon. Some tendons, such as the Achilles tendon, originate from multiple muscle bellies with a single bone insertion point; in these multi-muscle tendons, each muscle belly contributes a subtendon.
At every scale of the organizational hierarchy there are non-collagenous constituents located in the inter fibril, fiber, and fascicular spaces that are important for ligament and tendon function, such as other structural proteins, non-collagenous matrix material, and cells. Elastin is the primary non-collagenous protein constituent of ligament and tendon, accounting for approximately 1–4% of the dry weight in tendons,33, 34 4–9% of the dry weight in typical ligaments,34, 35 but in excess of 70% of the dry weight in some highly extensible ligaments.34, 36 The base molecular unit of elastin is tropoelastin, a linear peptide chain rich in glycine, alanine, and valine, containing α-helical regions and randomly coiled regions that contribute to elastic behavior and high extensibility.37, 38 In mature tissue, elastin forms a highly crosslinked network (Fig. 2A-D).38 Recent imaging studies have demonstrated that elastin fibers reside between and along collagen fibers, in-register with collagen fiber crimp both in ligament (porcine MCL) and tendon (rabbit Achilles tendon) (Figs. 1E, 2E-F).39–41
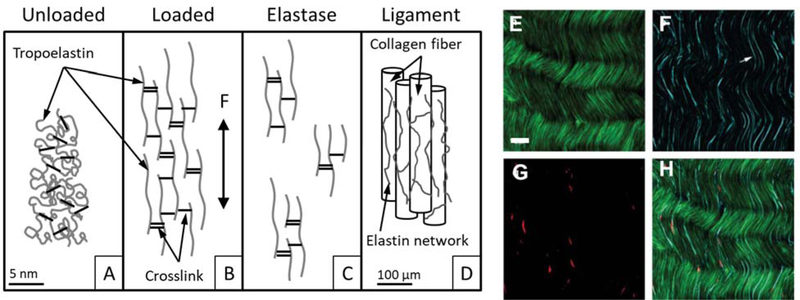
Figure 2.
(A-D) Representation of the elastin network in ligament tissue. (A) In an unloaded state, the crosslinked elastin network exists as an unorganized network of randomly coiled elastin fibers. (B) Under applied load, the elastin network elongates and (C) following selective digestion with elastase the network is disrupted and only fragments remain. (D) Representation of elastin residing along and between collagen fibers in ligament (A-D reprinted with permission, from Henninger et al.35). (E-H) Multiphoton microscopy of collagen (E, green), elastin (F, cyan), and cells (G, red) in unloaded tendon/ligament. These images reveal the presence of elastin between collagen fibers and in register with fiber crimp. Scale bar, 30 µm (E-H adapted with permission, from Pang et al.41).
Fibrillin is a structural glycoprotein that is not known to directly contribute to ligament and tendon mechanics, but it is involved in the development of the elastin network. Immunohistochemical studies of elastic fiber growth during development indicate that growth of a fibrillin microfibril network precedes elastin deposition and acts as a scaffold for elastin network development.42, 43 The importance of fibrillin 1 for elastic fiber formation has been demonstrated in mouse models, where Fbn1 heterozygous knockout mice die soon after birth due to vascular and pulmonary failure, due to an abnormally thin and fragmented elastic fiber network.44
A number of non-fibrillar constituents play an important role in regulating collagen fibril assembly and make up the inter fibril, fiber, and fascicle matrix. Small leucine rich proteoglycans (SLRPs) decorin, biglycan, fibromodulin, and lumican make up 0.1–5% of ligament and tendon dry weight10, 45, 46 and regulate collagen fibril assembly. SLRPs consist of a core protein and covalently attached glycosaminoglycan (GAG) side chains, which can be sulfated.47 Binding of SLRPs to collagen molecules and fibrils may occur via the horseshoe shaped core protein, as most common for decorin (Fig. 1C), or through interaction of fibrils with the GAG side chains, which has been demonstrated for biglycan.48 The expression and role of these SLRPs occurs in linked pairs with decorin/biglycan and fibromodulin/lumican performing similar functions and having inversely related expression in genetic knockout animal models.49, 50 Mouse models of decorin knockout result in irregularly shaped collagen fibrils and increased mean fibril diameter throughout development and at maturity.50 While decorin is the most abundant SLRP, knockout of biglycan, fibromodulin, and lumican result in similar type and extent of fibril abnormalities.51 This regulatory role has also been demonstrated during in vitro self-assembly of collagen fibrils, with collagen fibrils assembled in the presence of decorin exhibiting smaller mean fibril diameter52 and delayed onset and slower rate of fibrillogenesis.53 Large proteoglycans, such as aggrecan, are present in the inter fiber and fascicular matrix, occurring in healthy tissue predominantly in fibrocartilaginous regions of pulley tendons that are subject to compressive loading.54–56 Accumulation of large proteoglycans in tensile loaded regions of tendon is a hallmark of tendinopathy57–59 and these proteoglycan-rich regions alter the loading environment at the level of the cell.60, 61
The final constituent of ligament and tendon discussed here may be argued by some as the most important constituent: cells, with fibroblasts in ligament and tenocytes in tendon comprising the primary cell populations. Cells manufacture all of the structural constituents discussed above and are the means by which the tissue forms during development, is remodeled to adapt to loading, or is repaired following injury. Although the direct contribution of ligament and tendon cells to tissue-level mechanics is still under consideration, these cells are functionally responsive to the local loading environment. We refer the reader to recent reviews and research focused on defining ligament and tendon cell populations and their function during tissue development, maintenance, repair, and regeneration.62–65
MATERIAL BEHAVIOR ACROSS DIFFERENT PHYSICAL SCALES
The variety of constituents and their intricate structural organization are important for normal mechanical function of ligament and tendon. Significant effort has been expended to understand the material behavior across these different physical scales, from the molecule to the whole tissue, to elucidate the contribution of each to overall tissue behavior. In this review, we highlight a recent emphasis in the literature to investigate the mechanical differences of functionally distinct tendons. Tendons are sometimes classified as “energy-storing”, which store a significant amount of elastic energy during loading and typically experience high in vivo strain (e.g. Achilles tendon), or “positional”, which function primarily in limb positioning and typically experience lower in vivo strain (e.g. digital extensor tendons). Table 1 provides a summary of material properties across the ligament and tendon hierarchy, from the molecular level to the tissue level.
Table 1.
Material properties across the structural hierarchy in ligament and tendon. Properties reported from multiple studies are listed as a range of the reported values, while properties reported from a single study are reported as mean ± SD.
| Tissue or hierarchical level | Tensile tangent modulus (MPa) | Tensile strength (MPa) | Transverse modulus (MPa) | Transverse strength (MPa) | Shear modulus (MPa) |
|---|---|---|---|---|---|
| Type-I collagen molecule 66–69 |
350–12000 | 11000* | N/A | N/A | N/A |
| Type-I collagen fibril72,73 |
300–1500 | 70–200 | N/A | N/A | N/A |
| Rat tail tendon fascicle72,31 |
480–1400 | 38–110 | N/A | N/A | N/A |
| Porcine MCL35,40 | 267.0 ± 123.6 | 9.1 ±4.1 | 0.84 ±0.22 | N/A | 0.66 ±0.37 |
| Human MCL 82,83 | 332.15 ±58.27 | 38.56 ±4.76 | 11.02 ± 3.57 | 1.69 ± 0.53 | 1.72 ± 0.49 |
estimate from computational modeling
Collagen Molecule.
Relatively few experiments have attempted to directly determine the material behavior of collagen molecules. Most of the published data on the material properties of the collagen molecule are estimates based on tests at higher length scales or computational simulation. Individual type-I collagen molecules have been directly stretched using optical tweezers, demonstrating a highly non-linear force-displacement relationship (Fig. 3A) and providing an estimate of the linear elastic modulus from 0.35–12 GPa.66 A few investigations have measured collagen molecule deformation due to loading applied to fascicles or tendon using x-ray diffraction67 and Brillouin light scattering,68 providing estimates for the linear elastic modulus of the molecule in the range 3–9 GPa. Atomistic scale modeling of the collagen triple helix has recreated the experimentally observed non-linear force-displacement relationship, while estimating the elastic modulus in the range of 2–7 GPa.69–71
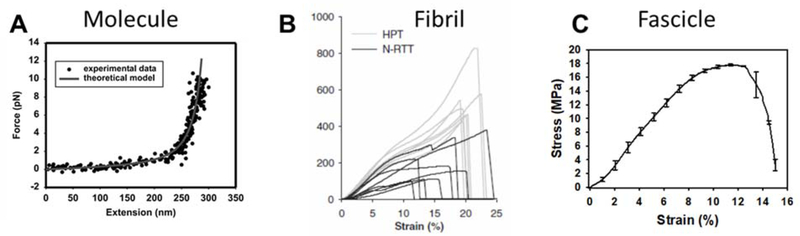
Figure 3.
Force-displacement and stress-strain relationships at different levels of the ligament and tendon hierarchy. Each scale exhibits non-linear behavior and the behavior of higher level structures is due to a combination of the behavior of subscale features directly and their interactions. A) force-extension of a single tropocollagen molecule, stretched by optical tweezers. Since, the optical tweezer method is not capable of achieving failure loads for a collagen molecule, this curve only displays behavior of the molecule at low strains. The elongated toe region is a result of stretching the molecule to its contour length, estimated at 315 ± 44nm. While this toe region appears long with respect to the molecule force-extension curve, it is still short compared to the micron to millimeter scale toe regions observed for higher level structures (reprinted with permission, from Sun et al.66). B) Stress-strain curves from human patellar tendon (HPT) and native rat tail tendon (N-RTT) fibrils, loaded in tension using an atomic force microscope (reprinted with permission, from Svensson et al.72). C) Stress-strain curve of a single fascicle from rat tail tendon, loaded by an electro-mechanical test system. This shape of this curve holds strong similarity to that observed for collagen fibrils. The toe region at the fascicle level is likely the result of molecules stretching to their contour length and uncrimping at the fiber level.
Collagen Fibril.
Until recently, there was very limited reliable data on the material behavior of the collagen fibril as well. Early experiments measured collagen fibril deformations by x-ray scattering, while applying load at the fascicle or tissue scale.20 Just within the last decade, investigators have been able to isolate individual mammalian collagen fibrils and perform mechanical testing using atomic force microscopy72 or a novel micro-electromechanical system.73 These experiments have demonstrated differences in the stress-strain behavior of type-I collagen fibrils from tissues with different levels of intermolecular crosslinking and from different tissue sources (e.g. differences in species and/or tendon function). Non-linear stress-strain behavior is also present at the fibril scale (Fig. 3B) and published estimates of the fibril elastic modulus range from 0.3–1.5 GPa.72, 73
To the authors’ knowledge, while fiber-level kinematics have been measured during loading applied to higher structural scales, no direct material tests have been reported at the scale of the collagen fiber.
Fascicle.
The tendon fascicle has been a common choice for studies of mechanics due to the ease of isolation and the ideal geometry for uniaxial tensile testing. Fascicles exhibit non-linear force-displacement behavior, a result of the complex interaction of its constituent structures. The characteristic fascicle stress-strain curve contains a soft toe region at small strains, due to uncrimping of the fascicle,74, 75 that transitions to a stiff linear region (Fig. 3C). Depending on tissue physiological function and species, fascicles may rupture shortly after departure from linear stress-strain behavior or exhibit an extended region of soft material behavior (Fig. 3C).72, 76 Material properties of the fascicle vary with the physiological function of the tendon; bovine digital extensor tendon (positional) exhibits higher ultimate tensile strength, but increased stress relaxation and hysteresis during fatigue loading compared to deep digital flexor tendon (energy storing).77 While positional and energy storing tendons are often presented as distinct populations, the energy storing behavior of tendons likely exists as a continuum governed by the physiological requirements of the tissue.
Tissue Level.
Mechanical testing at the tissue scale best probes the material behavior at a physiologically relevant scale for musculoskeletal function. Additionally, the larger size test samples from whole tissue enable a wider range of loading mechanisms to be investigated, including transverse,40, 78 biaxial,79 and shear loading.40, 56, 78, 80 While not all ligaments and tendons experience significant transverse and biaxial loading in vivo, these test methods provide valuable insight to the anisotropy of the tissue, mechanisms of load transfer between scales, and the structural organization of tissue constituents. Similar to the fascicle level, whole ligament and tendon exhibit non-linear force-displacement behavior in the longitudinal direction, with a soft toe region at low strains followed by a stiff linear region.81, 82 Conversely, stress-strain behavior transverse to the fiber orientation is relatively linear and typically exhibits tensile strength and tangent modulus an order of magnitude less than the longitudinal direction.82 While shear moduli are more than an order of magnitude lower than in the longitudinal and even transverse directions, shear stress-strain behavior is highly non-linear.83 Material behavior at the tissue level also remains viscoelastic, with tensile strength and strain at failure increasing by approximately 1.5 to 4 fold due to a 200 fold increase in strain rate.81 Strain rate has a small effect on phase shift during longitudinal viscoelastic testing84 indicating increased energy dissipation at higher strain rates, but the linear region of the stress-strain curve during monotonic tensile testing and the dynamic modulus from harmonic oscillation testing do not exhibit significant changes.78, 81, 84–86 Viscoelasticity at the tissue level has been attributed both to the biphasic nature of the tissue, whereby time-dependent behavior is the result of fluid flow through the solid phase of the tissue, and solid phase viscoelasticity, the result of intrinsic viscoelasticity of individual hierarchical structures and the interactions between levels.
LOAD TRANSFER ACROSS PHYSICAL SCALES AND BETWEEN CONSTITUENTS
The complex material behavior of ligaments and tendons is not completely explained by the behavior at each structural level of the collagen hierarchy; it is also influenced by molecular-scale chemical interactions and the non-collagenous constituents in the inter-fibril, fiber, and fascicular spaces, which mediate load and strain transfer between each level. Although many gaps remain in our knowledge of how load is transferred within and between structural scales from the molecule to the tissue level, recent research has helped to elucidate several relationships, and these new findings are changing how we interpret the origins of ligament and tendon material behavior and changes that occur due to aging, injury and disease.
Load Transfer between Collagen Molecules.
At the level of the collagen molecule, load transfer between collagen molecules occurs primarily through though chemical crosslinks. Crosslinks form by the natural conversion of amino acid side chains to reactive aldehydes, which react with adjacent amino acids to form a covalently bonded structure between collagen α-chains.17, 87–89 These aldehyde-based crosslinks form by enzymatic activity, via lysyl oxidase, or by the non-enzymatic formation of advanced glycation end-products, and crosslinks are known to form both between α-chains of the same triple helix (intramolecular) and between adjacent triple-helices (intermolecular). The critical role of crosslinks for tissue integrity and function is revealed by the perinatal lethality of lysyl oxidase genetic knockout in mice.90, 91 Administration of the lysyl oxidase inhibitor β-aminopropionitrile,92 which causes enzymatic crosslink deficiency in developing animals, has been used extensively to understand the role of enzymatic crosslinks. These studies demonstrated that crosslink deficiency reduced the stress, strain, and energy at failure of rat tail tendons over the entire course of development.76, 92 Atomic-scale computational simulation of crosslinked collagen molecules support these experimental results and have helped elucidate the role of different enzymatic crosslinks in tissue behavior93, 94. Simulation of collagen fibrils containing increasing concentrations of intermolecular crosslinks demonstrated a transition from dissipative deformation behavior with large yield regions to brittle deformation behavior with increasing crosslink density.93 This transition from dissipative to brittle deformation behavior was shown to depend on the crosslink type (transition at lower crosslink density for trivalent vs. divalent).94
Load Transfer from Fascicle to Fibril Scale.
The effect of strain attenuation from the fascicle scale to the fibril has been demonstrated by x-ray diffraction imaging of tendon fascicles under tensile load, revealing that fibril strain, as measured by the change in d-period, is always smaller than the fascicle-level applied strain.76 While the mechanism of load transfer from the tissue to the fibril remains unknown, load transfer between collagen fibrils was a topic of great debate over the last 10 years. SLRPs have been considered a primary candidate mechanism for load transfer between fibrils and tissue viscoelasticity,95, 96 due to their structural organization, where GAG side chains from SLRPs on adjacent fibrils are observed to interact.47 Experiments using decorin genetic knockout have demonstrated altered tensile and viscoelastic properties of tendons from knockout animals.96, 97 However, a series of experiments in which porcine medial collateral ligaments underwent selective removal of dermatan sulfate, the primary GAG side chain associated with decorin, demonstrated that these GAG side chains do not contribute to ligament material behavior under quasistatic tensile or shear loading,80 viscoelastic tensile loading (Fig. 4),84 or incremental stress relaxation.46 Together, these experiments emphasize the developmental importance of SLRPs for normally functioning mature tissues. It has also been proposed that the collagen fibril itself is the structural mechanism for fibril-to-fibril and tissue-to-fibril load transfer. Transmission and scanning electron microscopy studies have been unable to detect a significant number of collagen fibril ends in mature ligament and tendon, with only a single end identified in nearly 70 mm of combined tissue length.28, 29 These studies provide strong evidence that fibrils are either structurally or functionally continuous throughout the tissue length and support the notion that load is transferred directly through collagen fibrils rather than via interfibrillar coupling.29 A recent study using serial scanning electron microscopy along the length of rat tail tendon fascicles demonstrated the existence of small diameter fibrils that branch between larger diameter collagen fibrils,98 which may facilitate load transfer between adjacent fibrils and serve as a mechanism for shear load transfer between the continuous fibrils.
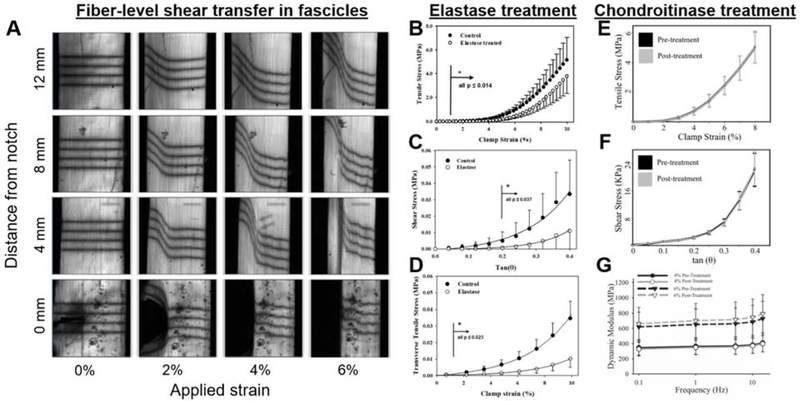
Figure 4.
Mechanisms of load transfer at the fiber and fibril scale. (A) Deformation of photobleached lines at increasing tensile strain and along the length of a tendon fascicle during tensile testing of a notched specimen. The continuity and increased deflection of the photobleached lines reveal shear transfer across the strain localization caused by the notch (adapted from Szczesny et al.98). (B-D) Elastin contribution to the tensile, shear, and transverse behavior of porcine MCL. While removal of elastin by elastase treatment reveals a contribution to tensile behavior, namely an extension in the toe-region, elastin is the primary determinant of shear and transverse mechanics suggesting it is a likely candidate mechanism for fiber-level shear load transfer (B adapted with permission, from Henninger et al.35; C-D adapted with permission, from Henninger et al.40). (E-G) Decorin does not contribute to the quasistatic or viscoelastic behavior of porcine MCL, demonstrated by unchanged material response following selective removal of the chondroitin and dermatan sulfate side chains. Thus, while proteoglycans have demonstrated an important role during tissue development, they do not contribute to the mechanics of mature tissues (E-F adapted with permission, from Lujan et al.80; G adapted from Lujan et al.84).
Load Transfer from Fascicle to Fiber Scale.
Adding to the debate regarding load transfer at the fibril scale, there is additional disagreement regarding which observed features and behaviors are associated with the fibril and fiber scales. Based on the definitions used for the ligament and tendon structural hierarchy, which are in agreement with the nomenclature proposed by Handsfield et al.,26 observations at micron length scale are referred to hereinafter as occurring at the level of the fiber. While an argument may be made that collagen second harmonic generation (SHG) imaging of collagen provides fibril-level results since SHG signal is due to the organization of collagen molecules within fibrils, these optical microscopy observations are still diffraction-limited and limited by the pixel resolution of the imaging system, thus they may be most appropriately described as fiber-level features imaged by fibril-level optical phenomena. Notch testing of rat tail tendon fascicles has revealed that fiber-level tensile strain is continuous throughout the fascicle, demonstrating that a mechanism for inter-fiber shear transfer must exist.98 In these experiments, a partial thickness cut (notch) created a material discontinuity and caused a strain localization at the notch tip to propagate along the length of the fascicle.98 The continuity of strain across the fascicle width was monitored using a series of photobleached lines, which continued to deflect toward the fascicle axis at increasing applied tensile strain (Fig. 4).98 One candidate for inter-fiber load transfer is the elastin network, which resides between and along collagen fibers.39–41 Experiments using selective elastin degradation with elastase have demonstrated that elastin is an important contributor to tensile strength,35, 99 is the primary determinant of transverse ligament behavior,40 and a major contributor to ligament and tendon shear properties (Fig. 4).40, 100 Interestingly, hysteresis during quasistatic tensile loading was unaltered between control and elastase treated tendon,99 suggesting that other components of the tissue are responsible for energy dissipation.
Optical tracking of fiber scale deformations has also been used understand the relationship between applied fascicle-level strain and deformations at the collagen fiber.32, 101 Photobleached lines or grids can be used to track general fiber-level matrix strain and cell nuclei can be used as fiducial markers to distinguish inter and intra-fiber strain, as cells reside in the inter-fiber space. These experiments have demonstrated significant strain attenuation from the fascicle to the fiber scale, with fiber-level matrix strain measured from the deformation of photobleached grids typically 10–35% of the tissue-level tensile strain or applied grip strain.60, 101 Incremental stress relaxation experiments utilizing cell tracking have demonstrated significant strain attenuation at the fiber level with fiber strain reaching only 1.2% for an 8% applied tensile strain to the fascicle.32 Stress relaxation experiments using this cell tracking strain measurement revealed significant relaxation by inter-fiber sliding, with the inter-fiber relaxation nearly 20× greater than intra-fibril relaxation under the same loading, as measured by x-ray scattering.102 Fiber-level kinematics are different between functionally distinct tendons, with porcine digital flexor tendon (energy storing) exhibiting greater inter-fiber sliding and less intra-fiber relaxation compared to common digital extensor tendon (positional) under stress relaxation, though the primary mechanism of fiber-level relaxation remained inter-fiber sliding for both tissues.103
DAMAGE AND FAILURE
Just as the material behavior at each structural scale and the interaction between scales are important determinants of tissue-level behavior, tissue-level damage is manifested across the hierarchy of structure. It is important to define damage as it applies to soft biological tissues, as a broadly accepted definition does not exist in the literature. Conventionally, damage has often been described as an unrecoverable change in material behavior, which has been defined in terms of different material parameters including elastic modulus or stiffness,74, 104, 105 toe-to-linear region transition strain,104, 106 and failure strength.77, 104 However, these definitions do not require detection of temporary or permanent changes in tissue structure. In this paper, we propose a working definition of mechanical damage to ligaments and tendons as an unrecoverable change to the structure or material behavior of the tissue or its constituents within at least one level the tissue structural hierarchy. This definition of damage includes changes in the hierarchical structure that alter mechanics at the scale of interest, but may not be detectable at the tissue level, while requiring that observed changes in material behavior are permanent. Failure of ligament and tendon at the tissue level can be more easily defined as the inability to carry a load. This definition is complicated by the existence of partial tendon and ligament tears, but under the definitions of damage and failure used here, these partial tears can be considered material damage as the tear causes permanent change in the material structure and function but does not result in a complete loss of function.
Damage to the Collagen Molecule.
Several computational studies have investigated the failure behavior or individual collagen molecules, and recent experimental studies have demonstrated that molecular-level damage and/or failure of collagen occurs due to mechanical loading. Steered molecular dynamics simulations of the collagen triple helix under applied tensile load have predicted that the fracture stress of a collagen molecule is 11.2 GPa (~40% strain), due to rupture of covalent bonds in the peptide backbone.69 More recent simulations investigating additional molecular-level damage mechanisms have demonstrated that collagen triple-helices are capable of permanent unfolding under tensile loading, due to shear load transfer through intermolecular crosslinks, at much lower stress and strain than required for molecule fracture.107
Experimentally, molecular-level changes due to mechanical loading have been demonstrated in bovine tail tendon repeatedly stretched beyond the linear region of the stress-strain curve.108–110 These overloaded tissues exhibited decreased thermodynamic and mechanical stability at the molecular level, as evidenced by reduced enthalpy of denaturation and increased susceptibility to trypsin degradation.108–110 Most recently, our laboratory demonstrated that these molecular-level changes are the direct result of mechanical unfolding of the collagen triple-helix. Rat tail tendon fascicles subjected to increasing strain exhibited increased molecular-level damage as indicated by binding of collagen hybridizing peptide (CHP), which specifically hybridizes unfolded α-chains with triple-helical folding propensity (Fig. 5).107 Furthermore, the mechanical damage detected using CHP corresponded with increased susceptibility to trypsin digestion107 (Fig. 5c), suggesting that previously observed thermodynamic and mechanical instability following tensile overload was the result of mechanical unfolding of the collagen molecule. An important finding of this study was that molecular-level collagen damage occurred at subfailure levels of strain and correlated with changes in tissue mechanics107, suggesting that molecular-level collagen damage is critical to the initiation and progression of tendon mechanical damage.
Figure 5.
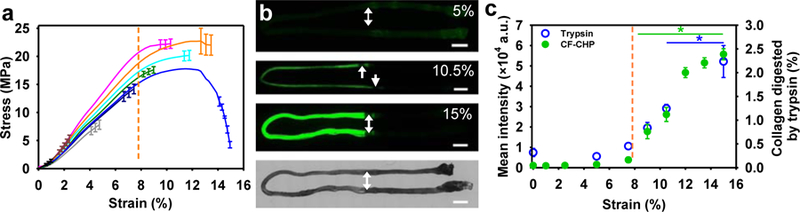
Collagen molecular damage due to incrementally applied tensile strain. A) Average stress-strain curves for fascicles stretched to incremental levels of strain. B) Fluorescence images of rat tail tendon fascicles stretched to 5, 10.5, and 15 % strain, then stained with fluorescent collagen hybridizing peptide, which detects unfolded collagen α-chains. On the bottom, a brightfield image of a fascicle shows the orientation of the fascicles in the fluorescence images. Scale bars, 2 mm. C) Incremental molecular damage with increasing strain quantified by intensity of fluorescent-CHP staining (green) and trypsin digestion (blue). Correspondence between the two techniques confirms that CHP binding detects mechanically unfolded collagen. The orange dotted lines in (a) and (c) indicate the approximate transition strain from the linear region to the onset of damage as identified by deviation of the stress-strain curve from linearity, which correlates with the onset of fluorescent-CHP intensity (reprinted from Zitnay et al.107).
Damage and Failure of Fibrils.
Very few studies have been able to produce failure in collagen fibrils due to direct fibril-scale tensile loading. Atomic force microscopy has been used to apply tensile loading to individual fibrils until failure.72 These experiments examined the failure behavior of native rat tail tendon fibrils with or without crosslink stabilization by sodium borohydride reduction, tendon fibrils from Zucker diabetic fat rats, and human patellar tendon fibrils. Interestingly, material and failure parameters for native, crosslink stabilized, or diabetic rat tail tendon fibrils were similar, indicating that stabilization of immature crosslinks did not alter material or failure behavior of fibrils.72 Uniaxial tensile failure tests of rat tail tendon fibrils using a MEMS device resulted in a sharp fracture interface, indicative of a brittle type failure behavior.73 Interestingly, brittle failure is rarely observed in samples loaded at the fascicle and tissue scales, suggesting that catastrophic, brittle failure of substructural levels (i.e. molecule and fibril) happens in a progressive manner during loading at higher levels (i.e. fascicle and tissue) causing the observed tearing type of failure. Other experiments have sought to investigate mechanisms of fibril-level damage due to loading at a higher structural level. Veres et al. demonstrated that kinks form along the length of collagen fibrils due to fascicle scale tensile loading.111, 112 At the location of the kinks, collagen fibrils exhibit increased susceptibility to trypsin degradation, and the spacing of kinks along fibrils seems to be dependent on both loading condition and physiological tendon function.108, 112, 113
Damage at the Fiber Level.
Fiber-level damage has been investigated through the use of optical microscopy techniques on samples loaded at the fascicle and tissue scales. Following subrupture creep fatigue loading of rat flexor digitorum longus tendon, kinked fiber deformations were observed, indicating structural damage at the fiber level.105 These structural changes were associated with permanent mechanical changes, as a diagnostic tensile test to half the fatigue load, performed after an 80 minute unloaded recovery period, resulted in increased clamp strain compared to the pre-fatigue diagnostic loading.105 At the highest level of fatigue loading, authors noted fiber dissociation and rupture, along with decreased tissue modulus and increased hysteresis.105 Similar morphological changes have been observed in fatigue loaded rat tail tendons,114 bovine extensor and flexor tendon fascicles,115 and in an in vivo model of rat patellar tendon fatigue.116 The rat patellar tendon in vivo fatigue model has also been used to monitor the effect of fatigue loading on cellular function and material behavior following short-term repair out to 7 days. These studies found suppression of inflammatory markers and proteinases at low-level fatigue loading, with expression of these same genes increased 5–6 fold following moderate fatigue loading117 and increased cell apoptosis at 3 and 7 days following fatigue loading.118 Clearly, mechanically induced tissue damage causes a cascade of altered cellular activity along with altered material behavior and tissue structure.
Damage at the Fascicle Scale.
Relatively few studies have investigated damage occurring specifically within the fascicle or tissue level, with most studies focusing on damage within the lower structural levels under fascicle-level loading. A study investigating the effects of tissue crosslinking in rat tail tendon found that while crosslink stabilized fascicles lacked the stress plateau characteristic of the native fascicles, they exhibited similar linear modulus resulting in decreased strain and increased stress at failure.72 Most recently, one group has focused on the function of the inter-fascicular matrix in tendon damage, demonstrating that the inter-fascicular matrix of bovine superficial digital flexor tendon, a model energy storing tendon, can withstand significantly more loading cycles to failure than bovine common digital extensor tendon, a model positional tendon.119 Previously, this group reported that the inter-fascicular matrix of positional tendons was stiffer, restricting fascicle sliding and possibly accounting for observed differences between fascicle and tissue failure strains.89 These studies on the inter-fascicular matrix demonstrate that fascicle-level constituents may play an important role in the damage and failure of ligaments and tendons, which is worthy of additional study.
CONCLUSION AND FUTURE DIRECTIONS
There has been a focus in recent years to understand the inter-connection and relationship between each of the constituent scales in collagenous connective tissues; however, this research area is still in its infancy. As described in this article, a number of investigations have helped establish the relationship between tissue/fascicle-level strain and fiber or fibril-level strain. While evidence of damage in ligament and tendon can be observed across the entire hierarchy of structure, it remains unclear how strain or load are transmitted between these structural scales or the order in which structural damage progresses. New tools to investigate molecular-level damage should be employed and integrated with existing techniques to detect structural and mechanical damage at higher length scales. Combined with advancements in multiscale computational simulation, we are at an opportune time to bridge length scales and elucidate the multiscale nature of load transfer and damage in ligament and tendon.
Answering basic questions about load transfer between structural scales and developing an understanding for the mechanisms by which it occurs will have significant clinical impact. This information will help elucidate the mechanisms at play in heritable and acquired connective tissue diseases and reveal potential targets for treatment. This knowledge will also help determine the mechanisms and progression of mechanical injury to ligaments and tendon, providing new insight on injury prevention and rehabilitation.
ACKNOWLEDGMENTS
Financial support from NIH #AR047369 is gratefully acknowledged.
Footnotes
The authors have no professional or financial conflicts of interest to disclose.
REFERENCES
- 1.2014. What Are Heritable Disorders of Connective Tissue? Bethesda, MD: NIAMS Information Clearinghouse. [Google Scholar]
- 2.2014. National Inpatient Sample HCUP Healthcare Cost and Utilization Project: Agency for Healthcare Research and Quality, Rockville, MD. [Google Scholar]
- 3.Petersen W, Rembitzki IV, Koppenburg AG, et al. 2013. Treatment of acute ankle ligament injuries: a systematic review. Arch Orthop Trauma Surg 133:1129–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietz HC, Cutting GR, Pyeritz RE, et al. 1991. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352:337–339. [DOI] [PubMed] [Google Scholar]
- 5.Hollister DW, Godfrey M, Sakai LY, et al. 1990. Immunohistologic abnormalities of the icrofibrillar-fiber system in the Marfan syndrome. N Engl J Med 323:152–159. [DOI] [PubMed] [Google Scholar]
- 6.Gigante A, Chillemi C, Greco F. 1999. Changes of elastic fibers in musculoskeletal tissues of Marfan syndrome: a possible mechanism of joint laxity and skeletal overgrowth. J Pediatr Orthop 19:283–288. [PubMed] [Google Scholar]
- 7.Holbrook KA, Byers PH. 1982. Structural abnormalities in the dermal collagen and elastic matrix from the skin of patients with inherited connective tissue disorders. J Invest Dermatol 79 Suppl 1:7s–16s. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen RH, Couppe C, Jensen JK, et al. 2014. Low tendon stiffness and abnormal ultrastructure distinguish classic Ehlers-Danlos syndrome from benign joint hypermobility syndrome in patients. Faseb J 28:4668–4676. [DOI] [PubMed] [Google Scholar]
- 9.Mao JR, Bristow J. 2001. The Ehlers-Danlos syndrome: on beyond collagens. J Clin Invest 107:1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amiel D, Frank C, Harwood F, et al. 1984. Tendons and ligaments: a morphological and biochemical comparison. J Orthop Res 1:257–265. [DOI] [PubMed] [Google Scholar]
- 11.Birk DE, Mayne R. 1997. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur J Cell Biol 72:352–361. [PubMed] [Google Scholar]
- 12.Fratzl P 2008. Collagen : structure and mechanics; xviii, 506 pages p. [Google Scholar]
- 13.Wenstrup RJ, Florer JB, Brunskill EW, et al. 2004. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem 279:53331–53337. [DOI] [PubMed] [Google Scholar]
- 14.Birk DE, Fitch JM, Babiarz JP, et al. 1990. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci 95 (Pt 4):649–657. [DOI] [PubMed] [Google Scholar]
- 15.Kastelic J, Galeski A, Baer E. 1978. The multicomposite structure of tendon. Connect Tissue Res 6:11–23. [DOI] [PubMed] [Google Scholar]
- 16.Hodge AJ, Petruska JA. 1963. Recent Studies with the Electron Microscope on Ordered Aggregates of the Tropocollagen Macromolecule. In: Ramachandran GN editor. Aspects of protein structure London, New York,: Academic Press; pp. 289–300. [Google Scholar]
- 17.Eyre DR, Paz MA, Gallop PM. 1984. Cross-linking in collagen and elastin. Annu Rev Biochem 53:717–748. [DOI] [PubMed] [Google Scholar]
- 18.Robins SP, Bailey AJ. 1972. Age-related changes in collagen: the identification of reducible lysine-carbohydrate condensation products. Biochem Biophys Res Commun 48:76–84. [DOI] [PubMed] [Google Scholar]
- 19.Bear RS. 1952. The structure of collagen fibrils. Adv Protein Chem 7:69–160. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki N, Odajima S. 1996. Elongation mechanism of collagen fibrils and force-strain relations of tendon at each level of structural hierarchy. J Biomech 29:1131–1136. [DOI] [PubMed] [Google Scholar]
- 21.Baselt DR, Revel JP, Baldeschwieler JD. 1993. Subfibrillar structure of type I collagen observed by atomic force microscopy. Biophys J 65:2644–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bozec L, van der Heijden G, Horton M. 2007. Collagen fibrils: nanoscale ropes. Biophys J 92:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hulmes DJ, Wess TJ, Prockop DJ, et al. 1995. Radial packing, order, and disorder in collagen fibrils. Biophys J 68:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wess TJ, Hammersley AP, Wess L, et al. 1998. Molecular packing of type I collagen in tendon. J Mol Biol 275:255–267. [DOI] [PubMed] [Google Scholar]
- 25.Orgel JP, Irving TC, Miller A, et al. 2006. Microfibrillar structure of type I collagen in situ. Proc Natl Acad Sci U S A 103:9001–9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handsfield GG, Slane LC, Screen HRC. 2016. Nomenclature of the tendon hierarchy: An overview of inconsistent terminology and a proposed size-based naming scheme with terminology for multi-muscle tendons. J Biomech 49:3122–3124. [DOI] [PubMed] [Google Scholar]
- 27.Orgel JP, San Antonio JD, Antipova O. 2011. Molecular and structural mapping of collagen fibril interactions. Connect Tissue Res 52:2–17. [DOI] [PubMed] [Google Scholar]
- 28.Svensson RB, Herchenhan A, Starborg T, et al. 2017. Evidence of structurally continuous collagen fibrils in tendons. Acta Biomater 50:293–301. [DOI] [PubMed] [Google Scholar]
- 29.Provenzano PP, Vanderby R Jr. 2006. Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol 25:71–84. [DOI] [PubMed] [Google Scholar]
- 30.Kosho T 2013. Discovery and Delineation of Dermatan 4-O-Sulfotransferase-1 (D4ST1)-Deficient Ehlers-Danlos Syndrome. In: Oiso N editor. Current Genetics in Dermatology Rijeka: InTech; p. Ch. 06. [Google Scholar]
- 31.Legerlotz K, Riley GP, Screen HR. 2010. Specimen dimensions influence the measurement of material properties in tendon fascicles. J Biomech 43:2274–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Screen HRC, Bader DL, Lee DA, et al. 2004. Local Strain Measurement within Tendon. Strain 40:157–163. [Google Scholar]
- 33.Jozsa L, Lehto M, Kvist M, et al. 1989. Alterations in dry mass content of collagen fibers in degenerative tendinopathy and tendon-rupture. Matrix 9:140–146. [DOI] [PubMed] [Google Scholar]
- 34.Lowry OH, Gilligan DR, Katersky EM. 1941. The determination of collagen and elastin in tissues, with results obtained in various normal tissues from different species. Journal of Biological Chemistry 139:795–804. [Google Scholar]
- 35.Henninger HB, Underwood CJ, Romney SJ, et al. 2013. Effect of elastin digestion on the quasi-static tensile response of medial collateral ligament. J Orthop Res 31:1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagawa H, Mikawa Y, Watanabe R. 1994. Elastin in the human posterior longitudinal ligament and spinal dura. A histologic and biochemical study. Spine (Phila Pa 1976) 19:2164–2169. [DOI] [PubMed] [Google Scholar]
- 37.Foster JA, Shapiro R, Voynow P, et al. 1975. Isolation of soluble elastin from lathyritic chicks. Comparison to tropoelastin from copper deficient pigs. Biochemistry 14:5343–5347. [DOI] [PubMed] [Google Scholar]
- 38.Gray WR, Sandberg LB, Foster JA. 1973. Molecular model for elastin structure and function. Nature 246:461–466. [DOI] [PubMed] [Google Scholar]
- 39.Grant TM, Thompson MS, Urban J, et al. 2013. Elastic fibres are broadly distributed in tendon and highly localized around tenocytes. J Anat 222:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henninger HB, Valdez WR, Scott SA, et al. 2015. Elastin governs the mechanical response of medial collateral ligament under shear and transverse tensile loading. Acta 13 Biomater 25:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pang X, Wu JP, Allison GT, et al. 2016. The three dimensional microstructural network of elastin, collagen and cells in Achilles tendons. J Orthop Res [DOI] [PubMed]
- 42.Rosenbloom J, Abrams WR, Mecham R. 1993. Extracellular matrix 4: the elastic fiber. Faseb J 7:1208–1218. [PubMed] [Google Scholar]
- 43.Raghunath M, Bachi T, Meuli M, et al. 1996. Fibrillin and elastin expression in skin regenerating from cultured keratinocyte autografts: morphogenesis of microfibrils begins at the dermo-epidermal junction and precedes elastic fiber formation. J Invest Dermatol 106:1090–1095. [DOI] [PubMed] [Google Scholar]
- 44.Carta L, Pereira L, Arteaga-Solis E, et al. 2006. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J Biol Chem 281:8016–8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillard GC, Merrilees MJ, Bell-Booth PG, et al. 1977. The proteoglycan content and the axial periodicity of collagen in tendon. Biochem J 163:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henninger HB, Underwood CJ, Ateshian GA, et al. 2010. Effect of sulfated glycosaminoglycan digestion on the transverse permeability of medial collateral ligament. J Biomech 43:2567–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott JE. 1996. Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen. Biochemistry 35:8795–8799. [DOI] [PubMed] [Google Scholar]
- 48.Pogany G, Hernandez DJ, Vogel KG. 1994. The in vitro interaction of proteoglycans with type I collagen is modulated by phosphate. Arch Biochem Biophys 313:102–111. [DOI] [PubMed] [Google Scholar]
- 49.Svensson L, Aszodi A, Reinholt FP, et al. 1999. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem 274:9636–9647. [DOI] [PubMed] [Google Scholar]
- 50.Zhang G, Ezura Y, Chervoneva I, et al. 2006. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem 98:1436–1449. [DOI] [PubMed] [Google Scholar]
- 51.Young MF, Bi Y, Ameye L, et al. 2002. Biglycan knockout mice: new models for musculoskeletal diseases. Glycoconj J 19:257–262. [DOI] [PubMed] [Google Scholar]
- 52.Pins GD, Christiansen DL, Patel R, et al. 1997. Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys J 73:2164–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neame PJ, Kay CJ, McQuillan DJ, et al. 2000. Independent modulation of collagen fibrillogenesis by decorin and lumican. Cell Mol Life Sci 57:859–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benjamin M, Ralphs JR. 1998. Fibrocartilage in tendons and ligaments--an adaptation to compressive load. J Anat 193 ( Pt 4):481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feitosa VL, Reis FP, Esquisatto MA, et al. 2006. Comparative ultrastructural analysis of different regions of two digital flexor tendons of pigs. Micron 37:518–525. [DOI] [PubMed] [Google Scholar]
- 56.Fang F, Sawhney AS, Lake SP. 2014. Different regions of bovine deep digital flexor tendon exhibit distinct elastic, but not viscous, mechanical properties under both compression and shear loading. J Biomech 47:2869–2877. [DOI] [PubMed] [Google Scholar]
- 57.Samiric T, Parkinson J, Ilic MZ, et al. 2009. Changes in the composition of the extracellular matrix in patellar tendinopathy. Matrix Biol 28:230–236. [DOI] [PubMed] [Google Scholar]
- 58.Corps AN, Robinson AH, Harrall RL, et al. 2012. Changes in matrix protein biochemistry and the expression of mRNA encoding matrix proteins and metalloproteinases in posterior tibialis tendinopathy. Ann Rheum Dis 71:746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Attia M, Scott A, Duchesnay A, et al. 2012. Alterations of overused supraspinatus tendon: a possible role of glycosaminoglycans and HARP/pleiotrophin in early tendon pathology. J Orthop Res 30:61–71. [DOI] [PubMed] [Google Scholar]
- 60.Han WM, Heo SJ, Driscoll TP, et al. 2013. Macro- to microscale strain transfer in fibrous tissues is heterogeneous and tissue-specific. Biophys J 105:807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han WM, Heo SJ, Driscoll TP, et al. 2016. Microstructural heterogeneity directs micromechanics and mechanobiology in native and engineered fibrocartilage. Nat Mater 15:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Snedeker JG, Foolen J. 2017. Tendon injury and repair - A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater 63:18–36. [DOI] [PubMed] [Google Scholar]
- 63.Howell K, Chien C, Bell R, et al. 2017. Novel Model of Tendon Regeneration Reveals Distinct Cell Mechanisms Underlying Regenerative and Fibrotic Tendon Healing. Sci Rep 7:45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lavagnino M, Wall ME, Little D, et al. 2015. Tendon mechanobiology: Current knowledge and future research opportunities. J Orthop Res 33:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun HB, Schaniel C, Leong DJ, et al. 2015. Biology and mechano-response of tendon cells: Progress overview and perspectives. J Orthop Res 33:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun YL, Luo ZP, Fertala A, et al. 2002. Direct quantification of the flexibility of type I collagen monomer. Biochem Biophys Res Commun 295:382–386. [DOI] [PubMed] [Google Scholar]
- 67.Sasaki N, Odajima S. 1996. Stress-strain curve and Young’s modulus of a collagen molecule as determined by the X-ray diffraction technique. J Biomech 29:655–658. [DOI] [PubMed] [Google Scholar]
- 68.Harley R, James D, Miller A, et al. 1977. Phonons and the elastic moduli of collagen and muscle. Nature 267:285–287. [DOI] [PubMed] [Google Scholar]
- 69.Buehler MJ. 2006. Atomistic and continuum modeling of mechanical properties of collagen: elasticity, fracture, and self-assembly. Journal of Materials Research 21:1947–1961. [Google Scholar]
- 70.Gautieri A, Buehler MJ, Redaelli A. 2009. Deformation rate controls elasticity and unfolding pathway of single tropocollagen molecules. J Mech Behav Biomed Mater 2:130–137. [DOI] [PubMed] [Google Scholar]
- 71.Gautieri A, Vesentini S, Redaelli A, et al. 2011. Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nano Lett 11:757–766. [DOI] [PubMed] [Google Scholar]
- 72.Svensson RB, Mulder H, Kovanen V, et al. 2013. Fracture mechanics of collagen fibrils: influence of natural cross-links. Biophys J 104:2476–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y, Ballarini R, Eppell SJ. 2016. Tension tests on mammalian collagen fibrils. Interface Focus 6:20150080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rigby BJ, Hirai N, Spikes JD, et al. 1959. The Mechanical Properties of Rat Tail Tendon. J Gen Physiol 43:265–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hansen KA, Weiss JA, Barton JK. 2002. Recruitment of tendon crimp with applied tensile strain. J Biomech Eng 124:72–77. [DOI] [PubMed] [Google Scholar]
- 76.Puxkandl R, Zizak I, Paris O, et al. 2002. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos Trans R Soc Lond B Biol Sci 357:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shepherd JH, Legerlotz K, Demirci T, et al. 2014. Functionally distinct tendon fascicles exhibit different creep and stress relaxation behaviour. Proc Inst Mech Eng H 228:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bonifasi-Lista C, Lake SP, Small MS, et al. 2005. Viscoelastic properties of the human medial collateral ligament under longitudinal, transverse and shear loading. J Orthop Res 23:67–76. [DOI] [PubMed] [Google Scholar]
- 79.Claeson AA, Barocas VH. 2017. Planar biaxial extension of the lumbar facet capsular ligament reveals significant in-plane shear forces. J Mech Behav Biomed Mater 65:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lujan TJ, Underwood CJ, Henninger HB, et al. 2007. Effect of dermatan sulfate glycosaminoglycans on the quasi-static material properties of the human medial collateral ligament. J Orthop Res 25:894–903. [DOI] [PubMed] [Google Scholar]
- 81.Haut RC. 1983. Age-dependent influence of strain rate on the tensile failure of rat-tail tendon. J Biomech Eng 105:296–299. [DOI] [PubMed] [Google Scholar]
- 82.Quapp KM, Weiss JA. 1998. Material characterization of human medial collateral ligament. J Biomech Eng 120:757–763. [DOI] [PubMed] [Google Scholar]
- 83.Weiss JA, Gardiner JC, Bonifasi-Lista C. 2002. Ligament material behavior is nonlinear, viscoelastic and rate-independent under shear loading. J Biomech 35:943–950. [DOI] [PubMed] [Google Scholar]
- 84.Lujan TJ, Underwood CJ, Jacobs NT, et al. 2009. Contribution of glycosaminoglycans to viscoelastic tensile behavior of human ligament. J Appl Physiol (1985) 106:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lynch HA, Johannessen W, Wu JP, et al. 2003. Effect of fiber orientation and strain rate on the nonlinear uniaxial tensile material properties of tendon. J Biomech Eng 125:726–731. [DOI] [PubMed] [Google Scholar]
- 86.Wren TA, Yerby SA, Beaupre GS, et al. 2001. Mechanical properties of the human achilles tendon. Clin Biomech (Bristol, Avon) 16:245–251. [DOI] [PubMed] [Google Scholar]
- 87.Light ND, Bailey AJ. 1982. Covalent cross-links in collagen. Methods Enzymol 82 Pt A:360–372. [DOI] [PubMed] [Google Scholar]
- 88.Piez KA. 1968. Cross-linking of collagen and elastin. Annu Rev Biochem 37:547–570. [DOI] [PubMed] [Google Scholar]
- 89.Thorpe CT, Udeze CP, Birch HL, et al. 2012. Specialization of tendon mechanical properties results from interfascicular differences. J R Soc Interface 9:3108–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maki JM, Rasanen J, Tikkanen H, et al. 2002. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106:2503–2509. [DOI] [PubMed] [Google Scholar]
- 91.Hornstra IK, Birge S, Starcher B, et al. 2003. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem 278:14387–14393. [DOI] [PubMed] [Google Scholar]
- 92.Haut RC. 1985. The effect of a lathyritic diet on the sensitivity of tendon to strain rate. J Biomech Eng 107:166–174. [DOI] [PubMed] [Google Scholar]
- 93.Buehler MJ. 2008. Nanomechanics of collagen fibrils under varying cross-link densities: atomistic and continuum studies. J Mech Behav Biomed Mater 1:59–67. [DOI] [PubMed] [Google Scholar]
- 94.Depalle B, Qin Z, Shefelbine SJ, et al. 2014. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J Mech Behav Biomed Mater [DOI] [PMC free article] [PubMed]
- 95.Cribb AM, Scott JE. 1995. Tendon response to tensile stress: an ultrastructural investigation of collagen:proteoglycan interactions in stressed tendon. J Anat 187 ( Pt 2):423–428. [PMC free article] [PubMed] [Google Scholar]
- 96.Robinson PS, Lin TW, Reynolds PR, et al. 2004. Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J Biomech Eng 126:252–257. [DOI] [PubMed] [Google Scholar]
- 97.Elliott DM, Robinson PS, Gimbel JA, et al. 2003. Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Ann Biomed Eng 31:599–605. [DOI] [PubMed] [Google Scholar]
- 98.Szczesny SE, Caplan JL, Pedersen P, et al. 2015. Quantification of Interfibrillar Shear Stress in Aligned Soft Collagenous Tissues via Notch Tension Testing. Sci Rep 5:14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grant TM, Yapp C, Chen Q, et al. 2015. The Mechanical, Structural, and Compositional Changes of Tendon Exposed to Elastase. Ann Biomed Eng 43:2477–2486. [DOI] [PubMed] [Google Scholar]
- 100.Fang F, Lake SP. 2016. Multiscale mechanical integrity of human supraspinatus tendon in shear after elastin depletion. J Mech Behav Biomed Mater 63:443–455. [DOI] [PubMed] [Google Scholar]
- 101.Cheng VWT, Screen HRC. 2007. The micro-structural strain response of tendon. J Mater Sci 42:8957–8965. [Google Scholar]
- 102.Gupta HS, Seto J, Krauss S, et al. 2010. In situ multi-level analysis of viscoelastic deformation mechanisms in tendon collagen. J Struct Biol 169:183–191. [DOI] [PubMed] [Google Scholar]
- 103.Screen HR, Toorani S, Shelton JC. 2013. Microstructural stress relaxation mechanics in functionally different tendons. Med Eng Phys 35:96–102. [DOI] [PubMed] [Google Scholar]
- 104.Provenzano PP, Heisey D, Hayashi K, et al. 2002. Subfailure damage in ligament: a structural and cellular evaluation. J Appl Physiol (1985) 92:362–371. [DOI] [PubMed] [Google Scholar]
- 105.Fung DT, Wang VM, Laudier DM, et al. 2009. Subrupture tendon fatigue damage. J Orthop Res 27:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Andarawis-Puri N, Sereysky JB, Jepsen KJ, et al. 2012. The relationships between cyclic fatigue loading, changes in initial mechanical properties, and the in vivo temporal mechanical response of the rat patellar tendon. J Biomech 45:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zitnay JL, Li Y, Qin Z, et al. 2017. Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides. Nature communications 8:14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Veres SP, Harrison JM, Lee JM. 2014. Mechanically overloading collagen fibrils uncoils collagen molecules, placing them in a stable, denatured state. Matrix Biol 33:54–59. [DOI] [PubMed] [Google Scholar]
- 109.Willett TL, Labow RS, Avery NC, et al. 2007. Increased proteolysis of collagen in an in vitro tensile overload tendon model. Ann Biomed Eng 35:1961–1972. [DOI] [PubMed] [Google Scholar]
- 110.Willett TL, Labow RS, Lee JM. 2008. Mechanical overload decreases the thermal stability of collagen in an in vitro tensile overload tendon model. J Orthop Res 26:1605–1610. [DOI] [PubMed] [Google Scholar]
- 111.Veres SP, Lee JM. 2012. Designed to fail: a novel mode of collagen fibril disruption and its relevance to tissue toughness. Biophys J 102:2876–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Veres SP, Harrison JM, Lee JM. 2013. Repeated subrupture overload causes progression of nanoscaled discrete plasticity damage in tendon collagen fibrils. J Orthop Res 31:731–737. [DOI] [PubMed] [Google Scholar]
- 113.Herod TW, Chambers NC, Veres SP. 2016. Collagen fibrils in functionally distinct tendons have differing structural responses to tendon rupture and fatigue loading. Acta Biomater 42:296–307. [DOI] [PubMed] [Google Scholar]
- 114.Parent G, Huppe N, Langelier E. 2011. Low stress tendon fatigue is a relatively rapid process in the context of overuse injuries. Ann Biomed Eng 39:1535–1545. [DOI] [PubMed] [Google Scholar]
- 115.Shepherd JH, Riley GP, Screen HR. 2014. Early stage fatigue damage occurs in bovine tendon fascicles in the absence of changes in mechanics at either the gross or microstructural level. J Mech Behav Biomed Mater 38:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fung DT, Wang VM, Andarawis-Puri N, et al. 2010. Early response to tendon fatigue damage accumulation in a novel in vivo model. J Biomech 43:274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun HB, Li Y, Fung DT, et al. 2008. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin Orthop Relat Res 466:1555–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Andarawis-Puri N, Philip A, Laudier D, et al. 2014. Temporal effect of in vivo tendon fatigue loading on the apoptotic response explained in the context of number of fatigue loading cycles and initial damage parameters. J Orthop Res 32:1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thorpe CT, Riley GP, Birch HL, et al. 2016. Fascicles and the interfascicular matrix show adaptation for fatigue resistance in energy storing tendons. Acta Biomater [DOI] [PMC free article] [PubMed]