Abstract
Objective:
X-linked adrenoleukodystrophy (ALD) is a neurodegenerative disorder due to mutations in the peroxisomal very long-chain fatty acyl-CoA transporter, ABCD1, with limited therapeutic options. ALD may manifest in a slowly progressive adrenomyeloneuropathy (AMN) phenotype, or switch to rapid inflammatory demyelinating cerebral disease (cALD), in which microglia have been shown to play a pathophysiological role. The aim of this study was to determine the role of patient phenotype in the immune response of ex vivo monophagocytic cells to stimulation, and to evaluate the efficacy of polyamidoamine dendrimer conjugated to the antioxidant precursor N-acetyl-cysteine (NAC) in modulating this immune response.
Methods:
Human monophagocytic cells were derived from fresh whole blood, from healthy (n = 4), heterozygote carrier (n = 4), AMN (n = 7), and cALD (n = 4) patients. Cells were exposed to very long-chain fatty acids (VLCFAs; C24:0 and C26:0) and treated with dendrimer-NAC (D-NAC).
Results:
Ex vivo exposure to VLCFAs significantly increased tumor necrosis factor α (TNFα) and glutamate secretion from cALD patient macrophages. Additionally, a significant reduction in total intracellular glutathione was observed in cALD patient cells. D-NAC treatment dose-dependently reduced TNFα and glutamate secretion and replenished total intracellular glutathione levels in cALD patient macrophages, more efficiently than NAC. Similarly, D-NAC treatment decreased glutamate secretion in AMN patient cells.
Interpretation:
ALD phenotypes display unique inflammatory profiles in response to VLCFA stimulation, and therefore ex vivo monophagocytic cells may provide a novel test bed for therapeutic agents. Based on our findings, D-NAC may be a viable therapeutic strategy for the treatment of cALD.
X-linked adrenoleukodystrophy (ALD) is a neurode-generative disorder caused by mutations in the ABCD1 gene, which encodes the peroxisomal ALD protein (ALDP). ALDP is thought to import coenzyme A derivatives of very long-chain fatty acids (VLCFAs), facilitating degradation of excess VLCFAs.1
Affected males do not exhibit neurological symptoms in the first few years of life but will go on to develop a slow dying back axonopathy of spinal cord neurons, adrenomyeloneuropathy (AMN), typically in early adulthood. In addition, up to 60% of affected males are at risk for developing the rapidly progressive inflammatory and demyelinating cerebral adrenoleukodystrophy (cALD) phenotype. About 35% of patients manifest cerebral disease in childhood, usually between 5 and 7 years. The course of cALD in childhood is typically fatal or results in complete disability within 4 years of diagnosis. A similar rapid clinical course is seen in at least half of those adult males who develop inflammatory cerebral demyelination.2 It is not clear why some patients with ABCD1 deficiency develop the cerebral disease whereas others do not. It is also important to note that the ABCD1-deficient mouse does not exhibit inflammatory demyelination as seen in cALD, but only exhibits myelopathy and peripheral neuropathy in old age,3,4 hampering its utility in assessing therapeutics targeting cALD.
Allogeneic hematopoietic stem cell transplantation (HSCT) for cALD can be lifesaving when performed in the early stages of disease and prior to onset of neurological symptoms. The presumed mechanism of action is replenishment of the monophagocytic cell population by healthy donor cells. Monophagocytic cells are thought to play a key role in both cALD and AMN. First, demyelinating lesions from patient autopsies have shown an immediate surrounding perilesional ring of apoptotic microglia, followed further outward by a zone characterized by a host of activated microglia, that precedes demyelination.5 Second, HSCT has been shown to halt cerebral disease progress only some months after intervention. Transplantation effectively replaces the ABCD1, and thereby ALDP function, in donor-derived cells. The delay between intervention and arrest of progression may be due to the time that metabolically capable donor-derived monophagocytic cells require to mature, differentiate, enter the brain, and take over physiological metabolic function.6 Third, in the ABCD1− mouse and human AMN spinal cord, excessive synaptic pruning and impairment in microglial phagocytosis has been shown.3
Antioxidants have also been attempted for therapy both in the mouse model of ALD and in patients with ALD.4 Among these, N-acetyl-L-cysteine (NAC) has been studied the most in patients.7 NAC is an L-cysteine derivative that replenishes glutathione, a potent antioxidant that also has anti-inflammatory effects. In childhood cALD patients with advanced disease, NAC administered at doses of 140mg/kg per day experienced prolonged survival when used in combination with HSCT; however, there was no impact on neurological function endpoints.8 Potential reasons for this include decreased bioavailability of NAC due to increased protein-binding capacity, decreased brain permeability of NAC, and increased glutamate levels in the brain due to intracellular transport of L-cysteine, the active agent in NAC, through the system Xc− or cystine/glutamate antiporter.9
These limitations can be overcome by the use of hydroxyl-terminated polyamidoamine (PAMAM) dendrimers, which have recently been investigated as a drug delivery system to target cells involved in neuroinflammation.10 We have previously shown that such dendrimers traverse the blood–brain barrier and localize specifically in activated microglia and astrocytes; this occurs only in the presence of injury, and the extent of uptake correlates with the extent of injury.11–13 In a rabbit model of cerebral palsy, intravenous administration of dendrimer-NAC (D-NAC) to affected newborn kits showed decreased neuroinflammation, and improved myelination and motor function.14,15
In this study, we hypothesized that the immunological response of monophagocytic cells isolated from ALD patients to extracellular free VLCFAs is altered in a phenotype-specific manner. The rationale for this hypothesis is based on the previous observations that (1) the cerebral form of the disease appears to be associated with a neuroinflammatory response5,16,17 and (2) HSCT highlights the role of monophagocytic cells in restoring ABCD1 function and thereby downstream metabolic capabilities. We also aimed to determine the effect of D-NAC therapy on inflammation, glutathione, and glutamate levels of patient-derived peripheral monophagocytic cells.
Subjects and Methods
Patient Recruitment and Inclusion
All patients were seen at the Kennedy Krieger Institute and had a confirmed molecular and biochemical diagnosis of ALD. The patients or their guardians gave informed consent prior to inclusion. The study was approved by the Johns Hopkins Institutional Review Board (protocol NA_00045735). Patients were recruited consecutively over a 7-month period between January 2016 and July 2016. Patients with a diagnosis of ALD (n = 11), including AMN (n = 7) and childhood and adult cALD (n = 4), were included in the study as well as symptomatic heterozygote female ALD carriers (n = 4). Patient age, diagnosis, and mutation are listed in Table 1. Healthy control samples (age = 20–51 years, mean age = 32.8 years; 2 males, 2 females) were run in parallel to all ALD samples. Patient data and blood samples were collected during routine physician visits in tandem with healthy sample collection.
TABLE 1.
Patient Phenotype, Age, and ABCD1 Mutation
| Patient Group | Subject | Age, yr | Mutation |
|---|---|---|---|
| Heterozygote | 1 | 65 | c.1832A>G (p.Gln611Arg) |
| 2 | 57 | c.760A>G (p.Thr254Ala) | |
| 3 | 53 | c.463_47delGAGGGCCAACTinsA (p.E155fs*36) | |
| 4 | 51 | c.1679C>T (p.Pro560Leu) | |
| AMN | 5 | 37 | c.565C>T (p.Arg189Trp) |
| 6 | 25 | c.760A>G (p.Thr254Ala) | |
| 7 | 44 | c.1224G>A | |
| 8 | 52 | c.253incC (p.Arg85Profs*110) | |
| 9 | 19 | c.225-245del21 (p.Leu76_Leu82del) | |
| 10 | 43 | c.1772G>A (p.R591Q) | |
| 11 | 33 | c.1772G>A (p.R591Q) | |
| cALD | 12 | 41 | c.1832A>G ; p.Gln611Arg |
| 13 | 10 | c.225-245del21 (p.Leu76_Leu82del) | |
| 14 | 44 | c.1850G>A (p.Arg617His) | |
| 15 | 58 | c.1771C>T (p.Arg591Trp) |
Phenotype, age, and ABCD1 mutation of monophagocytic cell donors.
AMN, adrenomyeloneuropathy; cALD, cerebral adrenoleukodystrophy.
Cell Derivation and Culture
Human monocytes were derived from fresh whole blood within 1 hour of venipuncture via double Ficoll (Sigma-Aldrich, St Louis, MO) and Percoll (Sigma-Aldrich) centrifugation as described in Turk et al.18 Monocytes were cultured in MEM (Thermo Fisher Scientific, Waltham, MA) with NEAA (Gibco, Grand Island, NY; Thermo Fisher Scientific), L-glutamine (Gibco, Thermo Fisher Scientific), 1% Pen/Strep (Gibco, Thermo Fisher Scientific), OPI Media Supplement (Sigma-Aldrich), 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific), IL-4 (Sigma-Aldrich; 20ng/ml), and GM-CSF (Sigma-Aldrich; 50ng/ml). Forty-eight–well plates were seeded with 200,000 cells per well to ensure adherence and cultured for 7 days prior to VLCFA exposure.
VLCFA C24:0 C26:0 Solubilization
For cell stimulation, 200mg of delipidated bovine serum albumin (BSA; Sigma-Aldrich) was solubilized in 10ml of 1mM sterile HEPES (Thermo Fisher Scientific) at 48°C. Stock solutions (10mg/ml) of lignoceric acid (C24:0) and hexacosanoic acid (C26:0) in chloroform/methanol (2:1) were prepared. Four hundred microliters of C24:0 and 40μl of C26:0 were put in a glass tube and evaporated under a stream of nitrogen. Five milliliters of sterile, warm 1mM HEPES was added to the dried fatty acids and sonicated to a fine suspension. This was followed by the dropwise addition of 5ml warm BSA solution. The solution was vortexed, sonicated, and heated as the drops were gradually added until the fatty acids were adsorbed to the BSA. The pH was adjusted to 7.4 by addition of 0.6N NaOH. This final solution is approximately 1mM C24:0 and 100μM C26:0. Solution concentration was confirmed by quantitative gas chromatography/mass spectrometry and routinely had 880–950μM C24:0 and 80–90μM C26:0. Aliquots were stored at −30°C and thawed and sonicated for 2 minutes before use.
D-NAC Synthesis and Characterization
D-NAC conjugates were designed and synthesized to avoid premature drug release and enable intracellular release of NAC via glutathione (GSH). We utilized disulfide linkage (-S-S-) to conjugate NAC molecules to the dendrimer surface. The D-NAC conjugates were synthesized using a multistep reaction protocol previously established and reported by our laboratory.15,19 The purity of the product was evaluated using HPLC using previously established protocols and was estimated to be >95%.15
The NAC loading was evaluated using 1H NMR. Using the proton integration method, we estimated that ~18 molecules of NAC were conjugated to the dendrimer surface. D-NAC conjugates were stable in phosphate-buffered saline (PBS) at 37°C for >3 days and released NAC only at intracellular GSH concentrations (>250μM).15
Fluorescently Labeled Dendrimer (D-Cy5)
To evaluate the cellular uptake kinetics and localization of dendrimers in human monophagocytic cells, dendrimers were labeled with Cy5 using NHS-NH2 click chemistry as previously described by our group.20 Fluorescently labeled dendrimers (D-Cy5) were synthesized and characterized using a previously reported 2-step reaction procedure.21 Briefly, in the first step, bifunctional dendrimers were synthesized using tert-butyloxycarbonyl (BOC) protection/deprotection chemistry resulting in 4 to 5 -NH2 groups on the dendrimer surface. In the second step, Cy5-NHS dye was reacted with NH2 groups on the bifunctional dendrimer in the presence of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC-HCL) and 4-(dimethylamino)pyridine (DMAP) to obtain the D-Cy5 conjugates. A bifunctional dendrimer with primary -NH2 groups on the dendrimer surface to enable Cy5 conjugation was synthesized by conjugating ~5 to 6 molecules of (BOC-amino) caproic acid in the presence of EDC-HCL as coupling agent, anhydrous DMF as solvent, and DMAP as base under N2 atmosphere. The BOC groups were deprotected using trifluoroacetic acid in dichloromethane (1:3) resulting in a bifunctional dendrimer with 3 NH2 groups on its surface (evaluated using 1H NMR). In the second step, Cy5-NHS was conjugated to the primary -NH2 groups under N2 atmosphere. The D-Cy5 was purified by dialysis against DMF to remove unreacted Cy5 followed by dialysis against H2O to remove residual DMF. D-Cy5 was characterized for Cy5 loading using 1H NMR. Using proton integration methods, we estimated that ~1.2 molecules of Cy5 were conjugated to each dendrimer. HPLC analysis using our previously established protocol14 confirmed formation of D-Cy5 conjugates with a purity of ~98%. D-Cy5 conjugates were stable in pooled human plasma (in vitro) for >48 hours and only released ~4% of its loading intracellularly until 6 hours. The crude product was purified by dialysis for 24 hours against anhydrous DMF followed by dialysis against H2O for 6 hours. The purified product was lyophilized to obtain an off-blue solid D-Cy5 conjugate. The D-Cy5 conjugates were characterized using 1H NMR, HPLC, and fluorescence spectroscopy.
VLCFA, Lipopolysaccharide, and Free NAC Exposure
The D-NAC dose indicated is the dose of NAC administered as D-NAC. Therefore, equivalent doses of NAC are used as free NAC or dendrimer-conjugated NAC. Cell cultures were exposed to VLCFAs (30μM C24:0 and 3μM C26:0) for 6 hours. D-NAC treatment was initiated simultaneously for 6 hours at 30μM, 100μM, and 300μM doses. Treatment doses were diluted from a 1M stock solution using PBS. Free NAC (not conjugated to dendrimer) was used at 300μM. Every control, exposure, and treatment cell culture well was processed in duplicate. Treatment with lipopolysaccharide (LPS) was performed at a dose of 30ng/ml, and LPS exposure time was 6 hours. LPS (Sigma-Aldrich) used was generated from Escherichia coli.
Culture medium was collected prior to cell detachment. Cells were detached using 0.05% trypsin (Sigma-Aldrich), centrifuged at 1,500 × g, and washed twice with PBS. Cell pellets were frozen and stored at −80°C prior to assay. Collected cell media was frozen and stored at −30°C prior to assay.
Just prior to assay, cells and media were thawed, cells were lysed using probe sonication for 10 seconds per sample, and samples were run in duplicate. Human tumor necrosis factor α (TNFα) enzyme-linked immunosorbent assay assay (Cayman Chemical, Ann Arbor, MI) and fluorometric glutamate assay (Abcam, Cambridge, MA) kits were used to determine levels in media. Intracellular glutathione was measured using a glutathione assay kit (Cell Bio-Labs, San Diego, CA). Spectrophotometric measurement was performed using a Spectramax M5 plate reader from Molecular Devices (Sunnyvale, CA).
Evaluation of Dendrimer Uptake by Human Monophagocytic Cells
Fluorescently labeled dendrimer (D-Cy5) was used to evaluate the cellular localization and uptake by human monophagocytic cells. For immunocytochemistry, cells were seeded in a 48-well plate at 500,000 cells per well to assure adherence and cultured for 7 days as described above and by Turk et al.18 The cultured cells (healthy control, n = 4; AMN, n = 3; cALD, n = 3) were exposed to D-Cy5 (40μg/ml) and VLCFAs (C24:0 30μmol, C26:0 3μmol) 24 hours prior to fixation. At 24 hours, cells were rinsed, fixed with 4% paraformaldehyde, and stained for macrophage marker CD11b (Abcam) and counterstained with 4,6-diamidino-2-phenylindole.
Kinetics of cellular D-Cy5 uptake were measured via flow cytometry using our previously established protocol.21 The cells (healthy control, AMN, and cALD) were seeded as above and allowed to attach to the plate surface for 24 hours. After 24 hours, macrophages were stimulated with VLCFAs for 6 hours and incubated with D-Cy5 (40μg/ml) for 3 or 6 hours. At these time points, the cells were detached using 0.05% trypsin, centrifuged at 1,500 × g for 5 minutes, and resuspended in 100μl of buffer (1 × PBS with 10% fetal bovine serum). The cellular uptake of D-Cy5 was measured using a BD Accuri C6 Flow Cytometer (BD Biosciences, San Jose, CA) with an FL4 bandpass filter with emission detection wavelength of 675/625nm. Data were analyzed using the BD Accuri C6 software. Thresholds were set using untreated control samples.
Statistics
Baseline and VLCFA-stimulated macrophages were separately compared using a linear mixed model comparison across diagnoses. For both baseline and VLCFA-stimulated comparisons, cALD was used as the reference group. Similarly, for efficacy of D-NAC therapy and measurements of TNFα, glutamate, and glutathione responses, multiple doses of D-NAC were tested in VLCFA-stimulated cells across different ALD phenotypes and data were log transformed and compared using linear mixed models with the VLCFA-stimulated group serving as the reference category. Plot error bars show standard error of the mean. Comparisons were considered significant when p ≤ 0.05.
Results
Ex Vivo cALD Cell Culture Response to Free VLCFAs and LPS
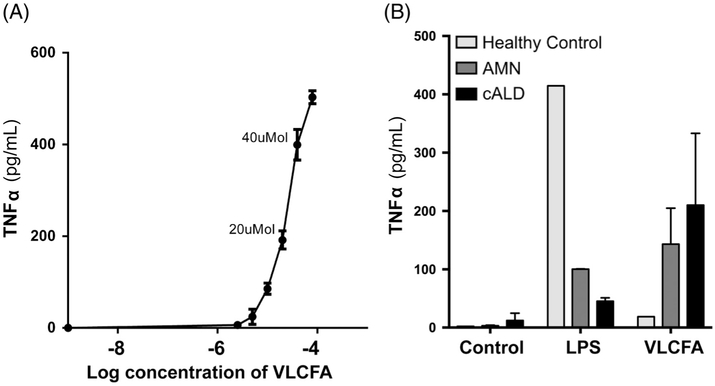
To determine the effect of VLCFAs on ALD monophagocytic cells, cALD patient cells were cultured for 7 days and then exposed to VLCFAs for 6 hours. TNFα was assayed in media and showed increased levels in a dose-response manner (Fig 1A). Based on these data, 30μM of C24:0 and 3μM of C26:0, which was the midpoint on the dose–response curve, was used for all subsequent studies. Next, in a pilot experiment of 2 patients, 1 AMN, 1 cALD, and 1 healthy control, stimulation with VLCFAs was compared to stimulation with LPS (see Fig 1B). Interestingly, LPS-induced TNFα secretion was lower in AMN and cALD than in healthy controls; however, as monophagocytic cell response to LPS-induced stimulation is highly variable and only 1 patient was included in this preliminary experiment, this finding should be assessed with caution. VLCFA stimulation, however, mirrored the initial dose–response study, and the difference between AMN and cALD suggested an altered immune response. Based on these initial data, we aimed to prospectively determine the phenotype-specific proinflammatory response to free VLCFA stimulation.
FIGURE 1:
(A) The tumor necrosis factor α (TNFα) secretion in ex vivo human cerebral adrenoleukodystrophy (cALD) monophagocytic cells in dose response to very long-chain fatty acid (VLCFA) stimulation. The labeled doses show C24:0 levels. The C26:0 level is 1/10th of the C24:0 dose. (B) Healthy control, adrenomyeloneuropathy (AMN), and cerebral response to lipopolysaccharide (LPS) and VLCFA stimulation.
VLCFA Response of Cultured Monophagocytic Cells from Multiple ALD Phenotypes
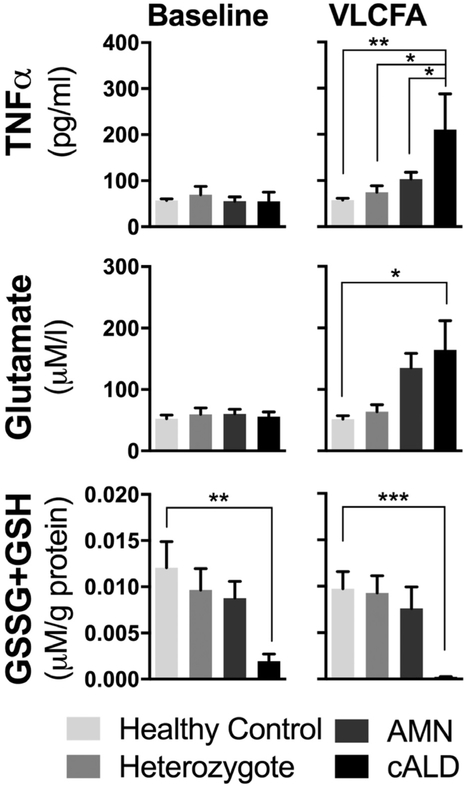
The effect of VLCFA stimulation on the secretion of TNFα and glutamate, as well as total intracellular glutathione, was measured in healthy control, AMN, and cALD monophagocytic cells. TNFα in cell culture medium was significantly increased in cALD compared to stimulated healthy control (p < 0.01), heterozygote (p < 0.01), and AMN (p < 0.05; Fig 2). Similarly, VLCFA stimulation led to significantly increased release of glutamate from cALD patient cells, compared to healthy controls (p < 0.05). Finally, total intracellular glutathione (oxidized glutathione [GSSG] + GSH) was significantly lower in cALD cells (p < 0.001) at baseline and when stimulated (p < 0.0001).
FIGURE 2:
VLCFA stimulation of ex vivo human healthy control, heterozygote female, AMN, and cALD monophagocytic cells’ (A) TNFα secretion, (B) glutamate secretion, and (C) total intracellular glutathione. GSH = reduced glutathione; GSSG = oxidized glutathione. (*) p < 0.05; (**) p < 0.01; (***) p < 0.001.
Dendrimer Uptake in Cultured Monophagocytic Cells
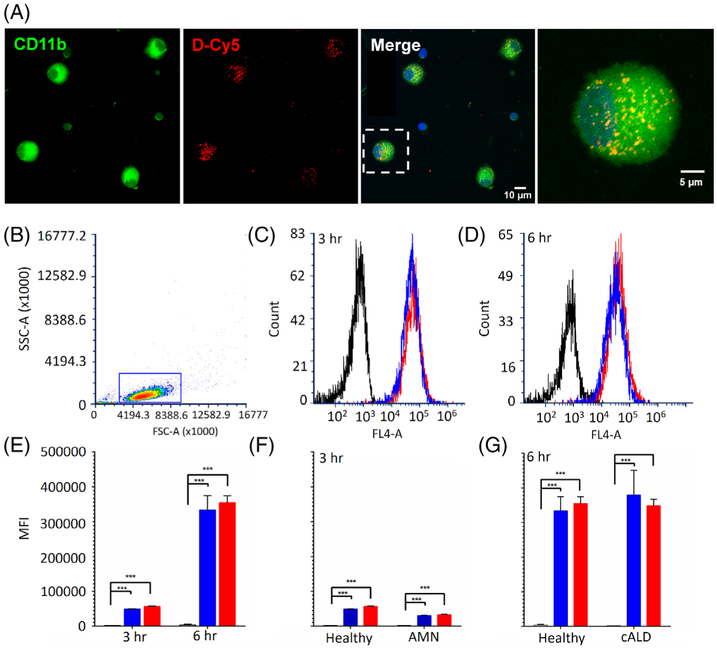
We proposed that dendrimer may serve as a viable antioxidant drug-delivery system in the ex vivo model, ameliorating the posited VLCFA-stimulated oxidative stress burden, which may contribute to the proinflammatory cytokine secretion. To determine whether the PAMAM dendrimer is taken up by human monophagocytic cells, fluorescently labeled dendrimers (D-Cy5) were employed. Confocal microscopy and flow cytometry were used to image the internalized dendrimers and determine the differences in dendrimer uptake upon stimulation.
Healthy human monophagocytic cells were cultured, then stimulated both with and without VLCFA stimulation as described above, while simultaneously exposed to D-Cy5 for 6 hours. Under confocal microscopy, monophagocytic cell marker CD-11b staining showed colocalization with Cy5 signals at 6 hours, both with and without VLCFA stimulation, indicating dendrimer uptake in all instances (Fig 3A).
FIGURE 3:
Immunofluorescent and flow cytometry analysis of dendrimer uptake. (A) D-Cy5 exposure to healthy human monophagocytic cells with and without VLCFA stimulation. D-Cy5 (red), CD11b (green), and 4,6-diamidino-2-phenylindole (blue) in both VLCFA-stimulated and nonstimulated states. (B) Representative gating of flow cytometry D-Cy5 exposure in healthy, AMN, and cALD ex vivo monophagocytic cells both with and without VLCFA stimulation. Representative gating of cell population is shown and illustrates side-scattered light area (SSC-A) versus forward-scattered light area (FSC-A). (C) Representative fluorescence intensity of healthy monophagocytic cells without D-Cy5 (black), with D-Cy5 (blue), and D-Cy5 + VLCFAs (red) after 3-hour exposure and (D) 6-hour exposure. Dendrimer was measured via FL4-A detector (640 nm). (E) Mean fluorescence intensity (MFI) of healthy control monophagocytic cells (black) after 3-hour and 6-hour exposure to D-Cy5 (blue) and D-Cy5 + VLCFAs (red). (F) MFI of AMN cells after 3-h exposure. (G) MFI of cALD cells after 6-hour exposure. (*) p < 0.05; (**) p < 0.01; (***) p < 0.001.
Flow cytometry was employed to assess D-Cy5 uptake in healthy control, AMN, and cALD patient-derived cells, which were subject to the same paradigm. A homogenous population with forward and side scatter dispersion typical of monophagocytic cells was identified, and the gating used is shown in a representative plot (see Fig 3). Uptake of D-Cy5 did not differ between VLCFA-stimulated and nonstimulated cells, regardless of phenotype. In both cases, significant dendrimer uptake (p < 0.0001), as determined by fluorescent intensity, was observed by as early as 3 hours and increased by 6 hours. These findings were replicated in both AMN and cALD cell populations.
D-NAC Efficacy Ex Vivo
Having established dendrimer uptake in monophagocytic cells, the effect of D-NAC was assessed in the VLCFA-stimulated healthy control and ALD cells. Following derivation and 7-day culture as described previously, cells were simultaneously VLCFA-stimulated and treated with increasing doses of D-NAC. TNFα, glutamate, and total intracellular glutathione levels were measured.
TNFα in Culture Medium
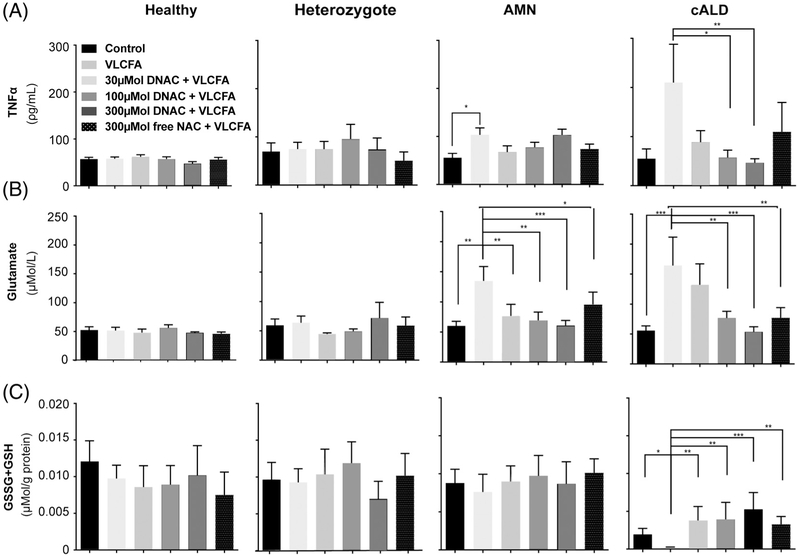
Medium TNFα levels showed a 2-fold increase in AMN (p < 0.01) and a 4-fold increase in cALD (p < 0.01) compared to healthy controls in VLCFA-stimulated patient-derived cells (Fig 4A). No changes to TNFα levels in culture medium following VLCFA stimulation or D-NAC were observed across healthy control and heterozygote samples.
FIGURE 4:
Dendrimer–N-acetyl-L-cysteine (D-NAC) and free NAC treatment of healthy control, heterozygote female, AMN, and cALD ex vivo human monophagocytic cells stimulated with VLCFA. (A) TNFα secretion, (B) glutamate secretion, and (C) total intracellular glutathione. The D-NAC dose indicated is the dose of NAC administered as D-NAC. GSH = reduced glutathione; GSSG = oxidized glutathione.
In VLCFA-stimulated cALD cells, cotreatment with D-NAC reduced TNFα levels in a dose–response manner (see Fig 4A). TNFα levels were normalized to prestimulation levels by 100μM D-NAC (p < 0.01) and 300μM D-NAC (p = 0.01) doses. Free NAC, without dendrimer conjugation, did not significantly affect TNFα levels. These data demonstrate that D-NAC treatment in cALD cells can reduce TNFα levels to baseline.
Glutamate in Culture Medium
Similarly, VLCFA stimulation increased glutamate secretion into culture medium from AMN and cALD cells (see Fig 4B). In both populations, D-NAC treatment reduced medium glutamate in a dose-dependent manner, back down to prestimulation baseline levels.
In AMN cells, treatment with D-NAC reduced glutamate release at 30μM D-NAC (p < 0.005), 100μM D-NAC (p < 0.005), and 300μM D-NAC (p < 0.001). Additionally, 300μM free NAC (p < 0.05) showed a reduction in glutamate levels in the medium.
In cALD cells, glutamate levels were significantly reduced by 100μM D-NAC (p < 0.005), reduced and normalized to baseline by 300μM D-NAC (p < 0.001), and additionally by 300μM free NAC (p < 0.005).
Intracellular Glutathione Levels
Results shown in Figure 4A and B are consistent with D-NAC having anti-inflammatory properties. Thus, we sought to assess the antioxidant replenishment effect of D-NAC by measuring intracellular glutathione. Both GSSG and GSH were measured from cell lysates and reported as total glutathione. Although no significant changes were detected among healthy control, heterozygote, or AMN patient-derived cells, VLCFA stimulation significantly reduced total glutathione in cALD patient-derived cells when compared to unstimulated cells (p < 0.05; see Fig 4C). Cotreatment with D-NAC dose-dependently restored total glutathione with a significant increase observed at 300μM D-NAC (p < 0.001), 100μM D-NAC (p <0.005), 30μM D-NAC (p < 0.005), and 300μM free NAC (p < 0.005).
In Vitro Effect of Dendrimer Alone
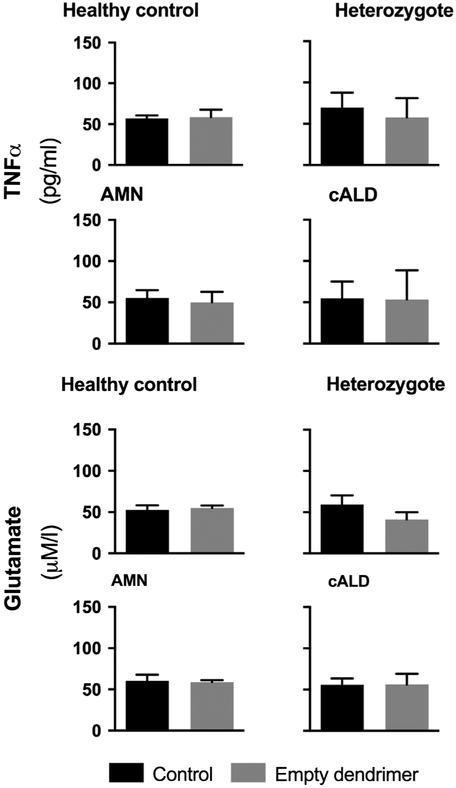
To rule out an immunomodulatory effect of the dendrimer particle alone, during each VLCFA stimulation and D-NAC treatment paradigm, healthy control, heterozygote female, AMN, and cALD cells were treated with unconjugated dendrimer. The treatment dose was equivalent to that present in the highest dose of dendrimer-drug conjugate (300μmol of D-NAC). The dendrimer alone had no effect on TNFα or glutamate levels in the culture medium (p > 0.05; Fig 5).
FIGURE 5:
Unconjugated dendrimer treatment of ex vivo human monophagocytic cells’ TNFα secretion and glutamate secretion.
D-NAC Effect over Multiple Time Points
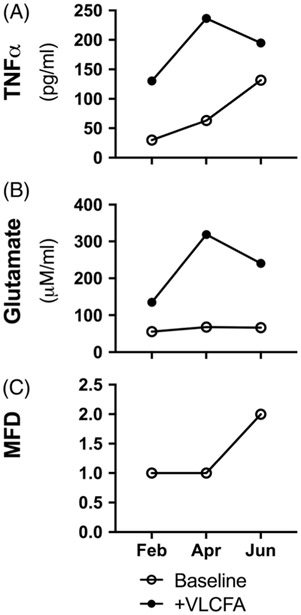
As one cALD patient made 3 visits within a 6-month period, his monophagocytic cell response to VLCFA stimulation and D-NAC treatment was measured and results plotted for each time point and compared to cells from a healthy control. The patient’s clinical status rapidly declined over the 6-month period (Fig 6). Interestingly, baseline TNFα but not glutamate secretion was higher at later time points. However, as symptoms progressed in this patient, a simultaneous increase in VLCFA-stimulated supernatant TNFα and glutamate levels was not observed.
FIGURE 6:
Ex vivo monophagocytic cells stimulated with VLCFA and clinical neurological function from one cALD patient over 3 time points. (A) TNFα secretion, (B) glutamate secretion, and (C) major functional disability (MFD) score.
Discussion
In this study, we describe a novel, C26:0 and C24:0 stimulated ex vivo human monophagocytic cell model for ALD and demonstrate its use as a test bed for compounds that modulate a proinflammatory immune response to free VLCFA stimulation and in the rescue of antioxidant levels. Using this model, we show that proinflammatory cytokine secretion and intracellular antioxidant levels vary based on the patient’s disease phenotype. This phenotype-specific response is seen in response both to free VLCFA stimulation and to treatment with the dendrimer-NAC conjugate.
Importantly, we demonstrate that D-NAC is effective in an anti-inflammatory manner and exhibits an antioxidant replenishing effect. These effects are shown in a dose–response manner in cALD and AMN monophagocytic cells. We also demonstrate that D-NAC is more effective than NAC alone. Additionally, we show D-NAC uptake in these cells is not affected by VLCFA levels or ALD phenotype.
Initially, we performed a pilot experiment to validate the inflammatory propensity of the ex vivo cells. Here, the monophagocytic healthy control, AMN, and cALD cells were stimulated with LPS and free VLCFAs. As well described in literature, we showed that LPS induced a significant increase in TNFα secretion in healthy control cells.22 In our initial data, LPS stimulation greatly increased TNFα secretion in healthy control, and interestingly to a lesser extent in AMN and cALD cells. Gong et al recently showed that (1) phagocytosis markers and not the inflammatory profile of ABCD1− mouse and human AMN spinal cord microglia are upregulated in vivo; and (2) in vitro, in response to LPS stimulation, these cells show a greater proinflammatory response in IL1b, NOS2, and COX2 than wild type; however, there was no increase in TNFα mRNA and an actual decrease in phagocytic protein gene expression.3 Furthermore, Gong et al observed a significant response to LPC-VLCFA stimulation compared to free VLCFA stimulation, whereas we show a phenotypic-specific response to VLCFA stimulation on human macrophages.
These differences observed within our study may reflect a critical interspecies difference (mouse microglia are primed to an increase in inflammatory response, whereas human microglia may see a decreased response) and/or a phenotype-specific response as seen between AMN (decreased response) and cALD (further decreased response). As the ABCD1− mouse does not develop cerebral disease, this primed response to LPS stimulation observed in Gong et al3 may represent one of the mechanisms of cerebral protection in the mouse model. In our human data, the decreased TNFα response to LPS seen in AMN, and further decreased in cALD, may suggest that this aberrant immune response is a variable contributing to the phenotypes in ALD. However, the LPS experiment conducted in our study was an exploratory attempt to develop an in vitro paradigm as a test bed for therapeutics against the inflammatory response in ALD. Further systemic studies are needed to determine important mechanistic processes involved in the aberrant immune response to LPS and the impaired phagocytic response caused by LPC-VLCFAs in human ALD macrophages.
In the ex vivo model, monophagocytic cell exposure to patient serum levels of free VLCFAs simulates one aspect of the ALD pathology. Our primary hypothesis was validated, as a phenotypic-specific immune response to VLCFA stimulation was shown; cALD patient cells responded with a 4-fold increased TNFα and increased glutamate secretion, whereas AMN cells responded with increased glutamate but only 2-fold increased TNFα secretion. Heterozygote females showed no response to VLCFA stimulation.
The significantly stronger proinflammatory response in cALD versus AMN monophagocytes supports the hypothesis of the monophagocytic cells’ altered inflammatory propensity. In these data, we show that a phenotype-specific proinflammatory response is triggered by VLCFAs in cALD. An increased inflammatory propensity in cALD (vs AMN) was shown recently in increased proinflammatory cytokine generation in cALD astrocytes derived from induced pluripotent stem cells.23 Macrophages and astrocytes in the demyelinating lesions of patients with cALD have been shown to be positive for TNFα.16 These data are also in concordance with previous observations where inflammatory markers have been noted predominantly in the cerebral phenotype.
It is important to point out that VLCFA levels used in our study are similar to patient plasma levels, and that these C24:0/C26:0 levels induce a proinflammatory response in monophagocytic cells in cALD. Although plasma VLCFA levels have not been shown to be predictive of phenotype in ALD, these results suggest that elevated VLCFA levels may contribute to the inflammatory response in cALD patients.
A phenotype-specific immune response may support the notion of 2 distinct pathological mechanisms. In cALD, the higher TNFα secretion and depleted antioxidant capacity highlight an active inflammatory disease component. However, in AMN cells, the inflammatory response was much less pronounced.
Although preliminary, we find it very interesting that in the cALD patient with repeated assessments, the baseline TNFα secretion increased as he began to rapidly decline neurologically. However, the increase in VLCFA-stimulated cytokine secretion was not shown to correlate with the progression in disease severity. Therefore, although the VLCFA-stimulated ex vivo model may show a phenotype-specific immune response and predict the presence of cALD, it may not serve as a biomarker of disease severity within the cALD phenotype.
Another critical hallmark of ALD pathology is oxidative stress. Excess hexacosanoic acid (C26:0), the main accumulating VLCFAs in ALD, has been shown to generate reactive oxygen species, decrease mitochondrial membrane potential, and induce endoplasmic reticulum stress.24,25 Oxidized proteins have also been shown to decrease ubiquitin-proteasome function and impair misfolded protein clearance.26 Evidence of oxidative injury and decrease in antioxidant reserve has been found in blood, fibroblasts, and postmortem brains in ALD patients.27 Additionally, a decreased antioxidant capacity in peripheral blood monocytes and a reduction in the antioxidant superoxide dismutase in AMN and cALD patient blood plasma have been reported previously by our group.18 In our data, the antioxidant capacity of cALD monophagocytic cells shows significantly lower, seemingly depleted levels of total glutathione in comparison to other phenotypes, that were replenished by D-NAC. These data concur with findings presented by Lopez-Erauskin et al,4 describing increased radical generation and rescue by antioxidant therapy. We additionally show that antioxidant depletion and rescue are phenotype-specific in our cell model.
However, typically, increased free radical generation results in less antioxidant-capable GSH, and more GSSG, with little effect on the total glutathione. We therefore hypothesize that other antioxidant capacity–depleting mechanisms may be in effect and warrant fUrther investigation. The depletion in baseline total glutathione in cALD monophagocytic cells further supports a phenotype-specific reduced antioxidant capacity, as we previously demonstrated by showing low superoxide dismutase levels in cALD blood plasma,18 and a reduction in total glutathione in the lymphocytes and granulocytes of cALD and AMN patients.28 A significant decrease in total glutathione and GSH was previously reported in lymphocytes and erythrocytes of ALD patients, associated with high levels of all GSSG forms.28 That D-NAC therapy attenuated the proinflammatory cytokine response in cALD while restoring total glutathione levels suggests that the oxidative stress response may potentially drive the neuroinflammation.
In further translating these findings in an animal model and potential clinical trial, the time point of D-NAC administration in relationship to HSCT will play an especially important role. NAC administered in very high doses at 140mg/kg when used in combination with HSCT, starting before and continuing after HSCT, was found to be effective in increasing survival, although it did not improve neurologic outcomes in these patients.7 However, NAC has poor brain penetration due to its high protein-binding capacity. NAC is transported into the cell as cysteine, or its dimer cystine through the system Xc− or cystine/glutamate antiporter.9
Dendrimer is transported by fluid phase endocytosis,29 and each molecule of the dendrimer can transport 16 to 20 molecules of NAC into the cell, ensuring an increased intracellular concentration of NAC. We have also previously shown that D-NAC bypasses the system Xc− cysteine/glutamate antiporter and thereby prevents the increase in extracellular glutamate associated with L-cysteine uptake.30 We have previously shown specific targeting of the dendrimer and D-NAC in “activated” microglia and astrocytes only in the presence of injury/inflammation.12,15,20
In this study, the cells were exposed to free NAC for 6 hours and NAC at 300μmol also showed efficacy in suppressing TNFα and glutamate levels and in improving glutathione levels. However, D-NAC even at lower doses was as effective as free NAC. Additionally, the specific in vivo targeting seen with D-NAC would deliver a much higher concentration of the drug to the activated microglia/macrophages in the brain. The effect of D-NAC is similar to those seen in previous studies where it is most effective in the presence of inflammation as seen by its greater effect on the cALD cells when compared to AMN and normal monophagocytic cells.15 Similarly, we also demonstrate that dendrimer alone has no effect on the cells, indicating that it only acts as a specific carrier. This indicates that D-NAC may be an effective mechanism of arresting neuroinflammation in patients with cALD.
Ex vivo monophagocytic cells may function as a unique test bed for evaluating promising, novel therapeutics for the cALD phenotype. As no existing mouse models have been shown to emulate cerebral disease, ameliorating inflammatory processes seen in cerebral demyelination in the ex vivo monophagocytic model may provide a much-needed stepping stone. Ex vivo cultured monophagocytic cells from cALD patients exhibit unique propensities for inflammation and oxidative stress compared to other phenotypes, making it an ideal model for testing and discovery in a precision medicine manner.
These results allow insight into the differences in immune function of a free VLCFA-stimulated model of ALD and demonstrate the potential benefit of D-NAC in attenuating an induced proinflammatory response. The limited survivability of human monophagocytic derived cells required fresh blood samples to be drawn from patients. Additionally, as only a small blood volume could be drawn from each child, the number of experiments we were able to perform was strictly limited, thereby restricting the scope of our investigation. In this study, we are demonstrating that there is a difference in the immune response of monophagocytic cells to free VLCFAs based on the patient phenotype. We believe this is a crucial step in developing an understanding as to why certain patients develop the cerebral form of the disease whereas others do not. In our future studies, we will further evaluate the different mechanisms that lead to this exaggerated immune response to VLCFAs and LPC-VLCFAs in patients with cerebral ALD when compared to AMN and heterozygote patients.
Acknowledgment
This project received funding from the NIH National Institute of Neurological Disorders and Stroke (R01NS097511, A.F.), National Institute of Biomedical Imaging and Bioengineering (R01EB018306, R.K.), and National Institute of Child Health and Human Development (R01HD069562, S.Kan.), the Brian’s Hope Foundation, and the Run for ALD Foundation.
We thank K. Hollandsworth for her contributions and the Johns Hopkins University Department of Anesthesiology and Critical Care Medicine Clinical Research Core for biostatistical support.
Footnotes
Potential Conflicts of Interest
B.Tu., S.Kan., R.K., and A.F. report a patent application US20170119899 issued October 16, 2018 for the use of dendrimer technologies described in this paper. The patent will be owned by those authors. At the time of publication, the patent is licensed to Ashvattha. S.Kan. and R.K. are the cofounders of the companies Ashvattha and Orpheris, which focus on therapies with the dendrimer platform. S.Kan. and R.K. are cofounders and members of the board of directors and own shares in Ashvattha and Orpheris, companies that are translating and commercializing the dendrimer platform.
References
- 1.Moser HW, Mahmood A, Raymond GV. X-linked adrenoleukodystrophy. Nat Clin Pract Neurol 2007;3:140–151. [DOI] [PubMed] [Google Scholar]
- 2.Kemp S, Huffnagel IC, Linthorst GE, et al. Adrenoleukodystrophy—neuroendocrine pathogenesis and redefinition of natural history. Nat Rev Endocrinol 2016;12:606–615. [DOI] [PubMed] [Google Scholar]
- 3.Gong Y, Sasidharan N, Laheji F, et al. Microglial dysfunction as a key pathological change in adrenomyeloneuropathy. Ann Neurol 2017;82:813–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez-Erauskin J, Fourcade S, Galino J, et al. Antioxidants halt axonal degeneration in a mouse model of X-adrenoleukodystrophy. Ann Neurol 2011;70:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichler FS, Ren J-Q, Cossoy M, et al. Is microglial apoptosis an early pathogenic change in cerebral X-linked adrenoleukodystrophy? Ann Neurol 2008;63:729–742. [DOI] [PubMed] [Google Scholar]
- 6.Cartier N, Aubourg P. Hematopoietic stem cell transplantation and hematopoietic stem cell gene therapy in X-linked adrenoleukodystrophy. Brain Pathol 2010;20:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tolar J, Orchard PJ, Bjoraker KJ, et al. N-acetyl-L-cysteine improves outcome of advanced cerebral adrenoleukodystrophy. Bone Marrow Transplant 2007;39:211–215. [DOI] [PubMed] [Google Scholar]
- 8.Miller WP, Rothman SM, Nascene D, et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood 2011;118:1971–1978. [DOI] [PubMed] [Google Scholar]
- 9.Bridges RJ, Natale NR, Patel SA. System xc− cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol 2012;165:20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balakrishnan B, Nance E, Johnston MV, et al. Nanomedicine in cerebral palsy. Int J Nanomedicine 2013;8:4183–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nance E, Zhang F, Mishra MK, et al. Nanoscale effects in dendrimer-mediated targeting of neuroinflammation. Biomaterials 2016;101:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemeth CL, Drummond GT, Mishra MK, et al. Uptake of dendrimer-drug by different cell types in the hippocampus after hypoxic-ischemic insult in neonatal mice: effects of injury, microglial activation and hypothermia. Nanomedicine 2017;13:2359–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F, Nance E, Alnasser Y, et al. Microglial migration and interactions with dendrimer nanoparticles are altered in the presence of neuroinflammation. J Neuroinflammation 2016;13:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesniak WG, Mishra MK, Jyoti A, et al. Biodistribution of fluorescently labeled PAMAM dendrimers in neonatal rabbits: effect of neuroinflammation. Mol Pharm 2013;10:4560–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannan S, Dai H, Navath RS, et al. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med 2012;4:130ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powers JM, Liu Y, Moser AB, Moser HW. The inflammatory myelinopathy of adreno-leukodystrophy: cells, effector molecules, and pathogenetic implications. J Neuropathol Exp Neurol 1992;51:630–643. [DOI] [PubMed] [Google Scholar]
- 17.Gilg AG, Singh AK, Singh I. Inducible nitric oxide synthase in the central nervous system of patients with X-adrenoleukodystrophy. J Neuropathol Exp Neurol 2000;59:1063–1069. [DOI] [PubMed] [Google Scholar]
- 18.Turk BR, Theisen BE, Nemeth CL, et al. Antioxidant capacity and superoxide dismutase activity in adrenoleukodystrophy. JAMA Neurol 2017;74:519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nance E, Porambo M, Zhang F, et al. Systemic dendrimer-drug treatment of ischemia-induced neonatal white matter injury. J Control Release 2015;214:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra MK, Beaty CA, Lesniak WG, et al. Dendrimer brain uptake and targeted therapy for brain injury in a large animal model of hypothermic circulatory arrest. 2014;8:2134–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannan G, Kambhampati SP, Kudchadkar SR. Effect of anesthetics on microglial activation and nanoparticle uptake: implications for drug delivery in traumatic brain injury. J Control Release 2017;263:192–199. [DOI] [PubMed] [Google Scholar]
- 22.Agbanoma G, Li C, Ennis D, et al. Production of TNF-alpha in macrophages activated by T cells, compared with lipopolysaccharide, uses distinct IL-10-dependent regulatory mechanism. J Immunol 2012;188:1307–1317. [DOI] [PubMed] [Google Scholar]
- 23.Baarine M, Khan M, Singh A, Singh I. Functional characterization of IPSC-derived brain cells as a model for X-linked adrenoleukodystrophy. PLoS One 2015;10:e0143238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Launay N, Ruiz M, Grau L, et al. Tauroursodeoxycholic bile acid arrests axonal degeneration by inhibiting the unfolded protein response in X-linked adrenoleukodystrophy. Acta Neuropathol 2017;133:283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Beek M-C, Ofman R, Dijkstra I, et al. Lipid-induced endoplasmic reticulum stress in X-linked adrenoleukodystrophy. Biochim Biophys Acta 2017;1863:2255–2265. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Erauskin J, Galino J, Ruiz M, et al. Impaired mitochondrial oxidative phosphorylation in the peroxisomal disease X-linked adrenoleukodystrophy. Hum Mol Genet 2013;22:3296–3305. [DOI] [PubMed] [Google Scholar]
- 27.Deon M, Marchetti DP, Donida B, et al. Oxidative stress in patients with X-linked adrenoleukodystrophy. Cell Mol Neurobiol 2016;36:497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrillo S, Piemonte F, Pastore A, et al. Glutathione imbalance in patients with X-linked adrenoleukodystrophy. Mol Genet Metab 2013;109:366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perumal OP, Inapagolla R, Kannan S, Kannan RM. The effect of surface functionality on cellular trafficking of dendrimers. Biomaterials 2008;29:3469–3476. [DOI] [PubMed] [Google Scholar]
- 30.Nance E, Kambhampati SP, Smith ES, et al. Dendrimer-mediated delivery of N-acetyl cysteine to microglia in a mouse model of Rett syndrome. J Neuroinflammation 2017;14:252. [DOI] [PMC free article] [PubMed] [Google Scholar]