Abstract
Economical and environmentally-friendly routes to convert feedstock chemicals like acetate into valuable chiral products such as (R)-3-hydroxybutyrate are in demand. Here, seven enzymes (CoaA, CoaD, CoaE, ACS, BktB, PhaB, and GDH) are employed in a one-pot, in vitro, biocatalytic synthesis of (3R)-3-hydroxybutyryl-CoA, which was readily isolated. This platform generates not only chiral diketide building blocks but also desirable CoA derivatives.
Better methods are needed to generate chiral products from abundant feedstock chemicals. Biosynthetic pathways naturally generate such compounds in vivo within the one-pot environment of the cytosol, and synthetic biologists can often steer the carbon flux towards a desired product. However, manipulating such pathways in vitro using cell-free extracts confers several advantages – the substrates and enzymes as well as their concentrations can be controlled, molecules in the pathway are not metabolized by organisms, and diffusion of the substrates and products are not impeded by cellular membranes. Indeed, multienzyme cascade reactions with cell-free extracts are increasingly being employed in the asymmetric synthesis of chiral alcohols, amines, and amino acids.1,2 Impressive feats of carbon–carbon formation (even converting CO2 into malate through a 17-enzyme system) have been achieved.3,4
A long-term goal of ours is to employ modular polyketide synthase (PKS) ketoreductases (KRs) that can set two stereocenters during a reduction reaction in the conversion of molecules like acetate and propionate into chiral diketide products.5,6 Some of these KRs display good stereocontrol even toward α-alkyl, β-ketoacyl-S-N-Acetylcysteamine (SNAC) thioesters, truncated versions of their natural substrates.7 We have been inspired by the engineering of Escherichia coli to exclusively produce either the chiral diketide (R)-3-hydroxybutyrate or its enantiomer (S)-3-hydroxybutyrate with the help of enzymes from the polyhydroxyalkanoate pathway.8-10 In these biosynthetic schemes, acetoacetyl-CoA is generated from two molecules of acetyl-CoA (one CoA being recycled in the process) by a biosynthetic thiolase (e.g., BktB from Cupriavidus necator, formerly Ralstonia eutropha), reduced by a stereoselective reductase (e.g., PhaB from C. necator), and cleaved from CoA as 3-hydroxybutyrate by a thioesterase (e.g., TesB from E. coli).
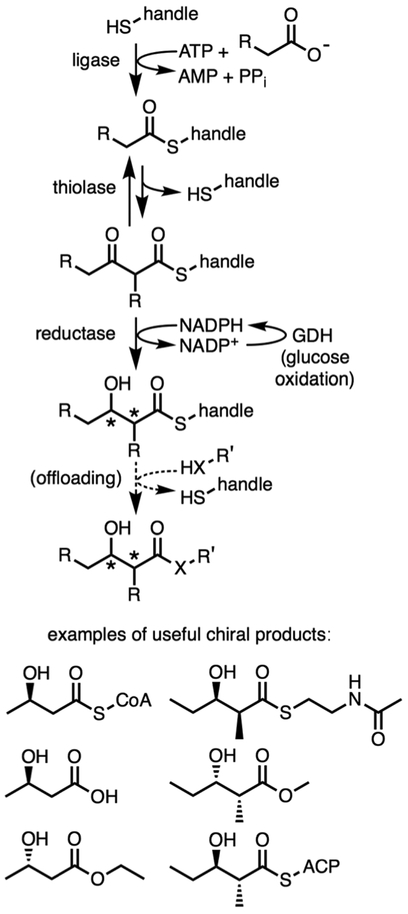
We sought to generate the 3R-hydroxybutyryl fragment in vitro from acetate through a multi-enzyme cascade. This would serve as proof of principle that more complex chiral molecules can be generated through this route utilizing PKS KRs (Fig. 1). The primary concern was whether the unfavorable thermodynamics of the BktB thiolase reaction (Keq = 1.1 × 10−5) could be overcome by exergonic reactions in the designed pathway.11,12 Our previous studies established that BktB operates on acyl-SNAC substrates, but how active and stereocontrolled PhaB would be toward a truncated acyl thio-ester was unknown.13 An acetyl-CoA synthetase (ACS, Streptomyces coelicolor) that can ligate acetate with diverse thiol acceptors, and a glucose dehydrogenase (GDH, Bacillus subtilis) that oxidizes D-glucose to regenerate NADPH from NADP+ were also employed.7
Fig. 1.
Long-term goal of generating useful chiral products from feedstocks like acetate and propionate (* = chiral center).
We tested the pathway composed of ACS, BktB, PhaB, and GDH with the short -SNAC handle but did not detect the expected 3-hydroxybutyryl-SNAC product by liquid chromatography/mass spectroscopy (LC/MS). However, using the longer handle pantetheine, prepared by reducing the abundant compound pantethine with dithiothreitol (DTT), 3-hydroxybutyryl-S-pantetheine was detected. A comparison of crystal structures of the C. necator PhaB (the Burkholderia pseudomallei PhaB employed in the cascade reaction is 56% identical) in the presence and absence of acetoacetyl-CoA (PDB Codes: 4N5M & 4N5L) offers an explanation for these results.14,15 The natural substrate induces a conformational change of PhaB, including a 4.6 Å shift of the “Clamp-lid”, into its catalytically competent form. The interactions with PhaB extend beyond the acetoacetyl-SNAC and even past the acetoacetyl-pantetheine portion of the substrate, with contacts to the diphosphate and the adenine base. Thus, to obtain full activity and stereocontrol from PhaB, the entire CoA handle might be necessary.
As CoA can be readily generated from pantetheine through the action of CoaA (Staphylococcus aureus), CoaD (E. coli), and CoaE (E. coli), these enzymes were added to the multienzyme cascade reaction (Fig. 2).16 The seven-enzyme reaction consisting of CoaA, CoaD, CoaE, ACS, BktB, PhaB, and GDH (using crude ammonium sulfate precipitates from overexpressing E. coli) was monitored in stages by LC/MS. First, the formation of CoA through incubating pantethine with DTT, CoaA, CoaD, CoaE, and ATP was ensured. Next, the formation of acetyl-CoA through the addition of ACS was checked. Finally, the formation of (3R)-3-hydroxybutyryl-CoA through the addition of BktB, PhaB, GDH, NADP+, and D-glucose was observed, confirming that the one-pot scheme was working. The (3R)-3-hydroxybutyryl-CoA product was purified by diluting the 100 mL cascade reaction 20-fold, passing it through a Q-Sepharose ion exchange column, washing it with 50 mM HCl, and eluting with 100 mM HCl. Mass spectral analysis is consistent with (3R)-3-hydroxybutyryl-CoA, and absorbance measurements reveal that the yield approaches 70% (120 mg) (Fig. 3A, B and ESI†). HPLC as well as 1H and 31P NMR analysis indicate the eluted (3R)-3-hydroxybutyryl-CoA is at least 90% pure. The peaks in the NMR spectrum are consistent with acyl-CoAs, and the methyl group of the 3-hydroxybutyryl fragment (1.08 ppm, d, J = 6.3 Hz, 3H) shows the same parameters as the methyl group of authentic (R)-3-hydroxybutyric acid.17 Eluted (3R)-3-hydroxybutyryl-CoA can be brought to neutral pH with aqueous lithium hydroxide and precipitated in 95% (v/v) acetone.
Fig. 2.
Seven-enzyme cascade reaction yielding (3R)-3-hydroxybutyryl-CoA from pantethine, ATP, and acetate. BktB fuses acetyl groups from two molecules of acetyl-CoA and allows one CoA to be recycled.
Fig. 3.
Authentication of (3R)-3-hydroxybutyryl-CoA. (A) Negativemode low-resolution LC/MS. (B) High-resolution MS yielded the anticipated molecular formula. (C) The (R)-3-hydroxybutyryl group generated through the cascade reaction was transferred to a -SNAC handle, and a comparison with synthetic standards by chiral chromatography indicates its high level of diastereomeric purity.
To determine the diastereomeric purity of (3R)-3-hydroxybutyryl-CoA, the 3-hydroxybutyryl fragment was moved onto a-SNAC handle through thiol-thioester exchange and compared with a standard synthesized from authentic (R)-3-hydroxybutyric acid (Fig. 3C). The resulting 3-hydroxybutyryl-SNAC was analyzed with a chiral column (ChiralCel OC-H). It matched the retention time of authentic (R)-3-hydroxybutyryl-SNAC. As no (S)-3-hydroxybutyryl-SNAC was detected, the generated (3R)-3-hydroxybutyryl-CoA appears to be of high diastereomeric purity.
The in vitro method described here has its limitations. It may not be possible to outcompete the in vivo routes to preparing industrial quantities of (R)-3-hydroxybutyric acid or (S)-3-hydroxybutyric acid. While none of the reagents used (ATP, NADP+, D-glucose, pantethine, DTT, precipitated enzyme) are costly and the enzymes can be immobilized so they can be used again, the reagents used for growing engineered E. coli are less expensive. Also, it may not be trivial to ligate propionate fragments together since BktB naturally generates α-unsubstituted products. However, the M290A point mutant of BktB mutant catalyzes the thiolysis of α-alkyl-β-ketoacyl NAC thioesters and could possess the desired biosynthetic activity.13 Other thiolases (e.g., Erg10 from Saccharomyces cerevisiae) are also being engineered to generate α-substituted β-ketoacyl thioesters.18
The cascade platform also holds several advantages. It is greener than chemical processes, which rely on metal catalysts and high pressures.19 In contrast to in vivo methods, which molecules are available to the enzymes can be controlled (e.g., to substitute other substrates for acetyl-CoA). The cascade reaction also provides an opportunity to isolate acyl-CoA products. These compounds are precious themselves but can be used as donors of the chiral fragment to yield desired small molecules (amides, esters, and thioesters) as well as acyl–acyl carrier proteins (ACPs) used in biosynthetic studies (through thiol-thioester exchange to holo-ACPs or apo-ACP phosphopantetheinylation).20
Offloading schemes such as enzymatic cleavage by TesB and PKS thioesterases or transfer through thiol-thioester exchange are being explored to enhance production of chiral fragments. This would allow the use of catalytic quantities of valuable handles (e.g., CoA, ACP), which provide optimal kinetics and stereocontrol.
In conclusion, a multi-enzyme cascade reaction has been developed that yields 3R-hydroxybutyryl groups from acetate. The isolated (3R)-3-hydroxybutyryl-CoA provides an example for the types of chiral products that can be obtained from such reactions. Further development employing PKS KRs should enable the generation of needed two-stereocenter chiral buiding blocks from feedstock chemicals.
Supplementary Material
Acknowledgements
This work was supported by NIH (GM106112) and the Welch Foundation (F-1712). We thank Josh Beckham (UT Austin Freshman Research Initiative Virtual Cures Stream) for the pNIC-Bsa4-PhaB plasmid.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ob02858c
Notes and references
- 1.Ricca E, Brucher B and Schrittwieser JH, Multi-Enzymatic Cascade Reactions: Overview and Perspectives, Adv. Synth. Catal, 2011, 353, 2239–2262. [Google Scholar]
- 2.Sperl JM and Sieber V, Multienzyme Cascade Reactions-Status and Recent Advances, ACS Catal., 2018, 8, 2385–2396. [Google Scholar]
- 3.Schwander T, Schada von Borzyskowski L, Burgener S, Cortina NS and Erb TJ, A Synthetic Pathway for the Fixation of Carbon Dioxide in Vitro, Science, 2016, 354, 900–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt NG, Eger E and Kroutil W, Building Bridges: Biocatalytic C-C-Bond Formation toward Multifunctional Products, ACS Catal., 2016, 6, 4286–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keatinge-Clay AT, The Structures of Type I Polyketide Synthases, Nat. Prod. Rep, 2012, 29, 1050–1073. [DOI] [PubMed] [Google Scholar]
- 6.Zheng JT and Keatinge-Clay AT, The Status of Type I Polyketide Synthase Ketoreductases, MedChemComm, 2013, 4, 34–40. [Google Scholar]
- 7.Piasecki SK, Taylor CA, Detelich JF, Liu J, Zheng J, Komsoukaniants A, Siegel DR and Keatinge-Clay AT, Employing Modular Polyketide Synthase Ketoreductases as Biocatalysts in the Preparative Chemoenzymatic Syntheses of Diketide Chiral Building Blocks, Chem. Biol, 2011, 18, 1331–1340. [DOI] [PubMed] [Google Scholar]
- 8.Tseng HC, Martin CH, Nielsen DR and Prather KLJ, Metabolic Engineering of Escherichia Coli for Enhanced Production of (R)- and (S)-3-Hydroxybutyrate, Appl. Environ. Microbiol, 2009, 75, 3137–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SH, Park SJ, Lee SY and Hong SH, Biosynthesis of Enantiopure (S)-3-Hydroxybutyric Acid in Metabolically Engineered Escherichia Coli, Appl. Microbiol. Biotechnol, 2008, 79, 633–641. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH and Park OJ, Uses and Production of Chiral 3-Hydroxy-Gamma-Butyrolactones and Structurally Related Chemicals, Appl. Microbiol. Biotechnol, 2009, 84, 817–828. [DOI] [PubMed] [Google Scholar]
- 11.Masamune S, Walsh CT, Sinskey AJ and Peoples OP, Poly-(R)-3-Hydroxybutyrate (Phb) Biosynthesis - Mechanistic Studies on the Biological Claisen Condensation Catalyzed by Beta-Ketoacyl Thiolase, Pure Appl. Chem, 1989, 61, 303–312. [Google Scholar]
- 12.Lan EI and Liao JC, ATP Drives Direct Photosynthetic Production of 1-Butanol in Cyanobacteria, Proc. Natl. Acad. Sci. U. S. A, 2012, 109, 6018–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fage CD, Meinke JL and Keatinge-Clay AT, Coenzyme A-Free Activity, Crystal Structure, and Rational Engineering of a Promiscuous Beta-Ketoacyl Thiolase from Ralstonia Eutropha, J. Mol. Catal. B: Enzym, 2015, 121, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baugh L, Gallagher LA, Patrapuvich R, Clifton MC, Gardberg AS, Edwards TE, Armour B, Begley DW, Dieterich SH, Dranow DM, Abendroth J, Fairman JW, Fox D 3rd, Staker BL, Phan I, Gillespie A, Choi R, Nakazawa-Hewitt S, Nguyen MT, Napuli A, Barrett L, Buchko GW, Stacy R, Myler PJ, Stewart LJ, Manoil C and Van Voorhis WC, Combining Functional and Structural Genomics to Sample the Essential Burkholderia Structome, PLoS One, 2013, 8, e53851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Chang JH, Kim EJ and Kim KJ, Crystal Structure of (R)-3-hydroxybutyryl-CoA Dehydrogenase PhaB from Ralstonia Eutropha, Biochem. Biophys. Res. Commun, 2014, 443, 783–788. [DOI] [PubMed] [Google Scholar]
- 16.van Wyk M and Strauss E, One-Pot Preparation of Coenzyme A Analogues via an Improved Chemo-Enzymatic Synthesis of Pre-CoA Thioester Synthons, Chem. Commun, 2007, 398–400. [DOI] [PubMed] [Google Scholar]
- 17.PaI M and Bearne SL, Synthesis of Coenzyme A Thioesters Using Methyl acyl Phosphates in an Aqueous Medium, Org. Biomol. Chem, 2014, 12, 9760–9763. [DOI] [PubMed] [Google Scholar]
- 18.Torres-Salas P, Bernal V, Lopez-Gallego F, Martinez-Crespo J, Sanchez-Murcia PA, Barrera V, Morales-Jimenez R, Garcia-Sanchez A, Manas-Fernandez A, Seoane JM, Sagrera Polo M, Miranda JD, Calvo J, Huertas S, Torres JL, Alcalde-Bascones A, Gonzalez-Barrera S, Gago F, Morreale A and Gonzalez-Barroso MDM, Engineering Erg10 Thiolase from Saccharomyces Cerevisiae as a Synthetic Toolkit for the Production of Branched-Chain Alcohols, Biochemistry, 2018, 57, 1338–1348. [DOI] [PubMed] [Google Scholar]
- 19.Noyori R, Ohkuma T, Kitamura M, Takaya H, Sayo N, Kumobayashi H and Akutagawa S, Asymmetric Hydrogenation of Beta-Keto Carboxylic Esters - a Practical, Purely Chemical Access to Beta-Hydroxy Esters in High Enantiomeric Purity, J. Am. Chem. Soc, 1987, 109, 5856–5858. [Google Scholar]
- 20.Walsh CT, Gehring AM, Weinreb PH, Quadri LE and Flugel RS, Post-Translational Modification of Polyketide and Nonribosomal Peptide Synthases, Curr. Opin. Chem. Biol, 1997, 1, 309–315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.