Abstract
Rationale
Humans typically self-administer cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC) together repeatedly (as in cannabis, cannabis extract, or Sativex®) to relieve pain. It has been suggested that one benefit of the drug combination may be decreased tolerance development.
Objective
The present study compared the development of tolerance to the antinociceptive effects of THC given alone versus combined with CBD, in rats.
Methods
THC dose-effect curves on tail withdrawal and paw pressure tests were obtained before and after twice-daily treatment with vehicle or CBD (10 mg/kg), plus vehicle or THC (3.6 mg/kg females; 9.3 mg/kg males) for 4 days.
Results
On the first day, THC was more potent in females than males on both nociceptive tests. From pre- to post-chronic (Day 1 to Day 6), THC potency on the tail withdrawal test decreased more in females than males, and rats that had been treated with CBD+THC repeatedly showed greater rightward/downward shifts of the THC dose-effect curve than rats that had been treated with THC alone. Analysis of blood samples taken after Day 6 testing showed that serum THC levels were higher in CBD+THC-treated females than in vehicle+THC-treated females, and THC’s active metabolite 11-OH-THC and its inactive metabolite THC-COOH were lower in CBD+THC-treated rats than in vehicle+THC-treated rats of both sexes. CBD also increased serum levels of the active metabolite cannabinol in both sexes.
Conclusion
The decrease in THC’s antinociceptive effects after repeated CBD exposure may be due to CBD-induced inhibition of THC metabolism, and/or antagonism of THC effects that emerges with repeated CBD treatment.
Keywords: Sex Differences, Cannabinoids, Pain
Investigations into the therapeutic utility of cannabis have largely focused on Δ9-tetrahydrocannabinol (THC). Another major phytocannabinoid, cannabidiol (CBD), has recently received more attention as a potential therapeutic compound for multiple disorders, in part because CBD does not produce the cannabinoid receptor type 1 (CB1)-mediated side effects of THC (Russo and Guy 2006). There is some evidence that CBD and THC interact in a therapeutically beneficial manner (Russo and Guy 2006), but highly variable outcomes across studies suggest that CBD-THC interactions are complex. As such, investigators have hypothesized that experimental parameters can significantly influence CBD-THC interactions, such as the route of administration (Nadulski et al. 2005), the time interval between CBD and THC dosing (Dalton et al. 1976; Zuardi et al. 2012), the THC to CBD dosing ratio (Ilan et al. 2005; Zuardi et al. 2012; Casey et al. 2017; King et al. 2017), and the specific endpoint examined (Casey et al. 2017). For example, CBD attenuated subjective euphoria from THC when the drugs were co-administered to humans in a single cigarette, while this effect was not observed with CBD pretreatment (Dalton et al. 1976; Haney et al. 2016). In contrast, another human study found that CBD-THC co-administration, compared to THC alone, increased subjective reports of pleasurable effects by reducing THC-induced anxiety (Karniol et al. 1974), while a third study found no impact of CBD on THC-induced feelings of “stoned”, although CBD blocked THC’s detrimental effect on facial emotion recognition (Hindocha et al. 2015). Similar discrepancies in CBD-THC interactions are found in the animal literature. For example, on tests of acute pain, two studies have reported a synergistic antinociceptive effect from certain dose combinations of THC and CBD (Karniol and Carlini 1973; Varvel et al. 2006), while other studies report limited or no antinociceptive synergy with various CBD-THC dose combinations administered acutely (Britch et al. 2017; Finn et al. 2004). In regard to rodent models of chronic pain, two recent studies showed that analgesic synergy occurred between CBD and THC, but biphasic or even triphasic drug interactions were observed (Casey et al. 2017; King et al. 2017). Further characterization of CBD-THC interactions on various pain-related measures has important implications for the clinical utility of cannabinoid treatments.
Inhibition of hepatic THC metabolism by CBD is one mechanism underlying CBD enhancement of THC effects (Jaeger et al. 1996). CBD is a potent inhibitor of hepatic drug metabolism via inhibition of multiple cytochrome P450 (CYP) isozymes (Bornheim and Grillo 1998; Jiang et al. 2013; Narimatsu et al. 1990; Watanabe et al. 2007; Yamaori et al. 2010; 2011). Both animal (Britch et al. 2017; Bornheim et al. 1995; Klein et al. 2011; Hložek et al. 2017) and human (Nadulski et al. 2005) studies have demonstrated that CBD inhibits hydroxylation of THC to its primary psychoactive metabolite, 11-hydroxy-tetrahydrocannabinol (11-OH-THC). However, this pharmacokinetic mechanism alone cannot explain enhancement of some THC effects but suppression of other THC effects by CBD. Several investigators have also questioned the relevance of metabolic interactions between CBD and THC to the therapeutic benefit of the drug combination (Karshner et al. 2011; Nadulski et al. 2005), especially considering the significant individual variability in THC metabolism and CYP enzyme polymorphisms (Nadulski et al. 2005). Cellular and molecular mechanisms of CBD-THC interaction, such as negative allosteric modulation of CB1 receptors (Laprairie et al. 2015), cross-talk between CB1 receptors and transient receptor potential vanilloid 1 (TRPV1) receptors (Amaya et al. 2006), and CBD-induced inhibition of ATP-binding cassette (ABC) transporters (Holland et al. 2007; Zhu et al. 2006) may also contribute to CBD-THC interactions observed on behavioral tests.
Given that medical marijuana users typically use drug on a daily basis, and pain is one of the most commonly given reasons for using medical marijuana (Cuttler et al. 2016), the purpose of the present study was to determine whether repeated co-administration of CBD with THC modulates THC-induced antinociception. Given the impact of CBD on THC metabolism documented in the acute drug administration studies noted above, we also determined whether repeated CBD treatment alters the metabolism of THC. Although we have previously used a 9- to 9.5-day THC dosing regimen to induce tolerance in female and male rats (Wiley et al. 2007; Wakley et al. 2014; 2015), a pilot study revealed that 4 days of twice-daily treatment with THC was sufficient to produce robust tolerance to the antinociceptive effects of THC, while being less likely to completely eliminate its effects (i.e., flatten the dose-effect function) than a longer regimen. Thus in the present study we employed a 4-day repeated dosing regimen to induce significant tolerance but avoid a floor effect. Because CBD has been shown to acutely inhibit THC metabolism (Britch et al. 2017; Jones and Pertwee 1972; Klein et al. 2011), we predicted that 4 days of repeated CBD co-administration with THC would promote the development of tolerance to THC by maintaining higher circulating levels of THC during the 4-day exposure regimen.
Sex differences in the acute behavioral effects of cannabinoids and in the development of tolerance to THC have been reported in rodents. For example, female rats are more sensitive than males to the reinforcing, anxiogenic, antinociceptive, and sedative effects of cannabinoids (Craft et al. 2012; Fattore et al. 2007; Harte-Hargrove and Dow-Edwards 2012; Tseng and Craft 2001). Profound tolerance to THC-induced antinociception occurs in both sexes following repeated THC administration (Wiley et al. 2007), and even when sex differences in acute THC potency are adjusted for, female rats develop greater tolerance than males to the antinociceptive effects of THC (Wakley et al. 2014; 2015). Considering these sex differences in THC’s effects on behavior as well as sex differences in the effect of CBD pretreatment on THC metabolism (Britch et al. 2017), we assessed CBD-THC interactions in both sexes.
Methods
Subjects.
Gonadally intact, male and female Sprague-Dawley rats (61–100 days old at the start of the 6-day protocol) were used (bred in-house from rats purchased from Harlan, Livermore, CA). Ad libitum access to food and water was provided except during testing. The vivarium was maintained at 21±2°C on a 12:12 h light:dark cycle with lights on at 0700 h. Rats used in this study were treated in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011), and all procedures were approved by the Institutional Animal Care and Use Committee at Washington State University.
Apparatus.
Tail withdrawal antinociception was assessed using a 2.5-L water bath (Precision Scientifics Inc., Winchester, VA) maintained at 50±0.5°C. This relatively low water bath temperature was chosen so that the effects of THC, a partial agonist, could be readily assessed (Cook et al. 2000; Terner et al. 2003). Paw pressure antinociception was assessed using an analgesy-meter (Randall-Selitto test: Ugo-Basile, Varese, Italy). The pressure on the paw increased at a constant rate of 48 g/s to a maximum of 720 g. Horizontal locomotor activity was measured using a photobeam apparatus (Opto-Varimex, Columbus Instruments, Columbus, OH) in which 15 photobeams cross the width of a 20 X 40 X 23-cm clear Plexiglas rodent cage. Photobeams were spaced 2.5 cm apart and 5.5 cm above the cage floor.
Drugs.
THC and CBD (National Institute on Drug Abuse, Bethesda, MD) were dissolved in 1:1:18 parts ethanol:cremophor:saline solution, which also served as the vehicle. Vehicle, CBD, and THC were administered i.p. in a volume of 1 ml/kg, except high THC doses. Due to solubility limitations, THC doses > 10 mg/kg were administered in larger volumes of a 10 mg/ml THC solution.
Behavioral procedure.
To determine THC doses for chronic administration, THC dose-effect curves were initially obtained in 7 male and 8 female rats, and the ED50 was calculated via log-linear interpolation for each rat on each of the two nociceptive tests. Sex-specific ED50 values then were calculated as the mean of each rat’s average ED50 value (average of the ED50 on each test); these group means were 9.3 mg/kg (males) and 3.6 mg/kg (females). The protocol for the tolerance experiment is illustrated in Figure 1. Twelve rats/sex were assigned to each treatment group (vehicle+vehicle, CBD+vehicle, vehicle+THC, CBD+THC); assignment was random except care was taken to avoid assigning same-sex siblings to the same treatment group. Rats were tested in cohorts of 5–7 rats, with at least 2 rats/sex in each cohort, and 2–3 of the 4 treatment groups (vehicle+vehicle, CBD+vehicle, vehicle+THC, CBD+THC) represented in each cohort. The experimenter (N. Greene) was not blinded to treatment group assignment. Body weight for each rat was measured prior to testing on the pre-chronic (Day 1) and post-chronic (Day 6) test days, as well as in the morning of Day 3, so that injection volumes could be adjusted if body weight had changed after the first two days of the experiment. On the pre- and post-chronic test days, testing began between 1000–1100 h. On the pre-chronic test day, three baselines were obtained on each nociceptive test. For the tail withdrawal assay, the rat was wrapped in a small towel and held vertically while the distal 5 cm of the tail was submerged in the warm water bath, and latency to withdraw the tail was measured to the nearest 0.1 s with a cutoff of 15 s. For the paw pressure test, the rat was lightly restrained with a small towel with one hindpaw placed under the probe; latency to withdraw the hindpaw from under the probe or attempt to withdraw it (i.e., backwards jerk of the hindpaw) was measured to the nearest 0.1 s, with a cutoff of 15 s (720 g). After the third baseline test, THC dose-effect curves were obtained using a cumulative dosing procedure: using ¼-log unit dose intervals, the following THC doses were injected at 15-min intervals: 1.0, 0.8, 1.4, 2.4, 4.4 and in some cases 8.0 mg/kg, reaching a total cumulative dose of 10–18 mg/kg THC. In a pilot study we tested 15-, 20-, and 30-min inter-injection intervals with i.p. THC, and found that the 15-minute interval yielded the most orderly cumulative dose-effect curves (unpublished data). Fourteen min after each injection, rats were tested on both nociceptive tests, starting with the tail withdrawal assay (the two tests together taking approximately 1 min to complete). Once the rat had reached cutoff on both nociceptive tests, locomotor activity was assessed as a measure of THC-induced sedation. Locomotor activity was measured as the number of photobeams broken in 10 min. Rats were placed on a twice-daily drug treatment regimen the next morning.
Fig. 1.
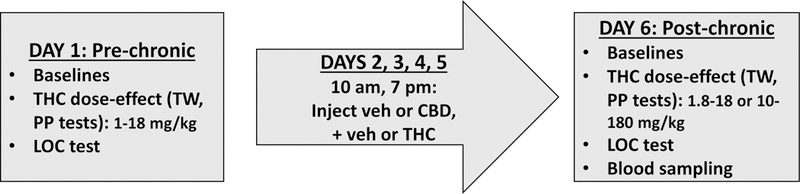
Experimental timeline
To induce tolerance to THC, vehicle or CBD 10 mg/kg, and vehicle or the predetermined ED50 for THC were administered i.p., twice-daily (morning injections at 0930–1030 h and evening injections at 1830–1930 h) for 4 days. THC-treated females received 3.6 mg/kg/injection and THC-treated males received 9.3 mg/kg/injection. Injection volumes were adjusted on the second day of chronic drug treatment, to adjust for changes in body weight; these adjusted volumes were used for the remainder of chronic drug treatment. On the morning of the post-chronic test day (Day 6, starting approximately 15 h after the last chronic injection), three baseline tests were conducted on each of the nociceptive tests, and then the THC dose-effect curve was re-determined using the cumulative dosing procedure, but starting at a higher dose. Specifically, vehicle+vehicle and CBD+vehicle groups were injected with 1.8–18.0 mg/kg THC, and vehicle+THC and CBD+THC groups were injected with 10–180 mg/kg THC, and behavioral testing was conducted as described above.
A separate group of control rats (8/sex) was tested to determine locomotor activity in the absence of THC on the pre- and post-chronic days. On the pre-chronic test day (Day 1), baseline tail withdrawal and paw pressure trials were conducted as described above, followed by a 10-min test of locomotor activity. Rats were then injected with vehicle+vehicle twice-daily for 4 days, as described above. On the post-chronic test day (Day 6), tail withdrawal, paw pressure, and locomotor tests were repeated.
Serum cannabinoid analysis.
Upon completion of behavioral testing on the post-chronic day, rats were euthanized via rapid decapitation, and trunk blood was collected. Trunk blood samples were centrifuged for 20 min at 2000 x g at 4°C; serum was removed and stored at −80°C until analysis. Concentrations of THC, 11-OH-THC, 11-nor-9-carboxy-THC (THC-COOH), CBD, and cannabinol (CBN) in the serum were determined for 9 rats/sex/treatment group, to assess potential effects of repeated CBD administration on THC metabolism.
To determine whether there were any residual cannabinoids in the bloodstream on the post-chronic test day (before obtaining the post-chronic THC dose-effect curve), a separate group of rats (3–6/sex/treatment group) was subjected to the same chronic drug treatment regimen described above, but on the morning of Day 6 these rats were euthanized via rapid decapitation, and trunk blood was collected for later analysis of serum cannabinoids.
Quantitation of cannabinoids was achieved using an ultra-performance liquid chromatography system (Waters Acquity I-Class UPLC, Milford, MA, USA) coupled with a quadrupole time of flight mass spectrometer (QTOF, Waters Xevo G2, Manchester, UK). The first step of sample preparation was sample centrifugation at 5900 x g for 10 min to remove any remaining cells. 185 uL of the resulting supernatant was spiked with 15 uL of solution containing 200 ppb each of the deuterated standards (THC-d3, OH-THC-d3, COOH-THC-d3, CBD-d3 and CBN-d3, Cerilliant, Round Rock, TX). Combined with the high resolution and accurate mass of the QTOF platform, these internal standards can minimize contributions from non-ideal metabolite extraction and instrumental variability and allow for direct quantitation of each targeted analyte. Following the internal standard addition, protein precipitation was promoted by adding 400 uL of cold acetonitrile (ACN) dropwise while vortexing. Immediately the samples were centrifuged at 1500 x g for 10 min at 25˚C. 0.6 mL of 1% ammonium hydroxide was added to the obtained sample supernatant and vortexed before solid phase extraction (SPE). A mixed mode SPE cartridge (OAXIS Max 1 cc, Waters, Ireland) was used for cannabinoid isolation. Each SPE cartridge was conditioned with 1 mL methanol followed by 1 mL 1% ammonium hydroxide. After the cartridge conditioning, the newly prepared sample was loaded onto the SPE cartridge and pulled through the system using a light vacuum (~1–2 psi). Then 0.5 mL of 35% ACN was added and allowed to dry under full vacuum for 10 min. 1.5 mL of a hexane/ethyl acetate/acetic acid (49:49:2, v/v/v) mixture was used to elute the samples. The eluent was then evaporated under nitrogen at room temperature, and 100 uL of a methanol:water solution (80:20, v/v) was used for final sample and transferred to an autosampler vial. Analyte separation was achieved using a 50-mm C18 BEH UPLC column (Waters, Milford, MA, USA) kept at 40°C. The mobile phases were high purity water (Fisher Scientific Co., Fair Lawn, NJ) with 0.1% formic acid (A) and pure acetonitrile (Fisher Scientific Co., Fair Lawn, NJ) with 0.1% formic acid (B), respectively. Initially mobile phase B was increased from 5% to 60% in 0.2 min, and kept increasing to 90% at 3.5 min. This level was held for an additional 0.5 min. At 4 min, mobile B was decreased to its initial condition of 5% within 0.1 min and was held static for 0.9 min for column re-equilibration. With an operational flow rate of 0.3 mL/min, a total of 10 uL of each prepared sample was injected onto the column. The obtained experimental data were analyzed by TargetLynx (Waters, Milford, MA), software used to generate quantitative results. Briefly, the parameters used for TargetLynx were: Retention time window: ± 0.2 min, Response use: integrated area, Polynomial Type: linear, Weighting function: 1/X.
Data analysis.
Baseline nociceptive latencies for each rat on the tail withdrawal and paw pressure tests were calculated as the mean of the three pre-injection trials. Because baseline responding changed from pre- to post-chronic testing, individual response latencies following drug were converted to % Maximum Possible Effect (MPE) on each day: (drug latency – baseline latency)/(cutoff latency – baseline latency) x 100, using the baseline latency on the same day on which drug was tested. On the pre-chronic test day, THC potency (ED50 value) was estimated for each treatment group via log-linear regression, and dose-effect curves were compared between groups by ANOVA, using PharmToolsPro (version 1.27; PharmSoft.Net, Wynnewood, PA); these values and statistical comparisons are shown in Table 1. On the post-chronic test day, THC-induced antinociception did not reach 50% MPE in some rats, particularly those that had received THC chronically. Additionally, the post-chronic dose-effect curves typically had shallower slopes than the pre-chronic dose-effect curves; thus, in many cases THC potency could not be estimated accurately or compared between pre- and post-chronic tests. Therefore, pre- vs. post-chronic dose-effect data (% MPE values) were compared by ANOVA using 5 doses of each dose-effect curve, with additional factors of treatment group (4 levels), sex (2 levels) and time (pre- vs. post-chronic, repeated). For all rats at the pre-chronic test as well as for rats in vehicle+vehicle and CBD+vehicle groups at the post-chronic test, 1.8–18 mg/kg (five) doses were included in the analysis. Because an equivalent number of doses of THC from the post-chronic curves in THC-treated rats needed to be included in the analysis, data for 56 mg/kg THC was not included in the initial ANOVA comparing all four treatment groups. However, subsequent post-hoc analyses in THC-treated rats included data from all 6 post-chronic THC doses.
Table 1.
Antinociceptive potency of THC (ED50 values (95% C.L.), in mg/kg) before (pre-chronic) and after (post-chronic) 4 days of twice-daily vehicle and/or cannabinoid administration, in male and female rats (12/sex/treatment group).
| Treatment Group | Tail Withdrawal | Paw Pressure | ||
|---|---|---|---|---|
| Pre-Chronic (Day 1) | Post-Chronic (Day 6) | Pre-Chronic (Day 1) | Post-Chronic (Day 6) | |
| Veh + Veh | ||||
| Males | 7.10 (5.32, 10.35) | 12.02 (9.04, 18.71) | 4.86 (3.71, 7.05) | 17.34 (11.99, 33.27) # |
| Females | 2.83 (2.40, 3.31) | 8.83 (5.75, 18.92) # | 2.05 (1.57, 2.56) | --a |
| CBD + Veh | ||||
| Males | 5.83 (4.86, 7.16) | 15.00 (11.14, 26.00) # | 4.36 (3.50, 5.43) | --a |
| Females | 2.74 (2.22, 3.31) | 9.82 (6.67, 24.83) # | 1.65 (1.14, 2.21) | --a |
| Veh + THC | ||||
| Males | 7.74 (6.24, 10.12) | --a | 6.27 (4.57, 8.89) | --a |
| Females | 3.07 (2.65, 3.55) | --a | 1.66 (0.89, 2.59) | --a |
| CBD + THC | ||||
| Males | 8.07 (6.46, 10.69) | 171.4 (118.3, 330.4) # | 4.06 (3.23, 5.36) | --a |
| Females | 2.67 (2.21, 3.18) | --a | 2.30 (1.56, 3.08) | --a |
| All Groups | ||||
| Males | 7.16 (6.35, 8.17) | 4.93 (4.33, 5.64) | ||
| Females | 2.90 (2.65, 3.16)* | 1.92 (1.50, 2.34)* | ||
ED50 value could not be calculated (few or no values > 50% MPE)
significantly different from males on same test, p<0.05
significantly higher than Pre-Chronic ED50 value, p<0.05
Baseline latencies to respond on pre- and post-chronic test days, as well as changes in body weight during chronic drug administration, were also compared among treatment groups using ANOVA. Considering that adult male Sprague-Dawley rats typically weigh 50–60% more than adult Sprague-Dawley females, body weight on Days 2 and 5 was converted to percent of baseline weight on the pre-chronic test day for each rat. Locomotor activity (number of photobeam breaks) was analyzed using three-way ANOVA, with factors of sex, chronic treatment group (vehicle+vehicle, CBD+vehicle, vehicle+THC, CBD+THC), and time (pre-chronic vs. post-chronic, repeated). Group differences were considered significant if P≤0.05.
Results
Prior to analysis, data from one male rat from the CBD+vehicle group was dropped because withdrawal latencies for this rat were more than 2 standard deviations below the same-sex mean on both nociceptive tests, after multiple THC doses on the pre-chronic test day. This rat also repeatedly vocalized (even before THC injection), and showed considerably more hyperlocomotion and thigmotaxis than all other male rats.
Nociceptive baselines.
Figure 2 shows baseline latency to respond on the tail withdrawal and paw pressure tests on the pre- vs. post-chronic days (Day 1 vs. Day 6) in each treatment group. On the tail withdrawal test (Fig. 2, top panel), baseline latency to respond lengthened from pre- to post-chronic tests in all groups, but the magnitude of this effect was sex- and treatment group-dependent (Day x Sex x CBD x THC: F(1,88)=9.16, p=0.003). Analysis within each sex revealed that the treatment group-dependent effect was limited to males (Males, Day x CBD x THC: F(1,44)=10.92, p=0.002; Females, Day: F(1,44)=59.52, p<0.001; no interaction with CBD or THC). Post-hoc analysis in males showed that there were no significant differences in baseline latency among the four treatment groups on the pre-chronic day, but on the post-chronic day, the CBD+vehicle group had longer latencies than other groups (CBD x THC: F(1,44)=6.58, p=0.014). The change in baseline from pre- to post-chronic test days was significantly longer in the CBD+vehicle group than in the vehicle+vehicle group (p=0.003) and CBD+THC group (p=0.016), but not the vehicle+THC group (p=0.11), On the paw pressure test (Fig. 2, bottom panel), baseline latency to respond shortened from pre- to post-chronic tests in most groups, but the magnitude of this effect was treatment group-dependent (Day x CBD x THC: F(1,88)=4.16, p=0.044). Additionally, there were group differences among the males but not the females (Sex x CBD x THC: F(1,88)=6.53, p=0.012). Specifically, baseline paw pressure latencies shortened significantly from pre- to post-chronic tests only in the male CBD+vehicle and vehicle+THC groups.
Fig. 2.
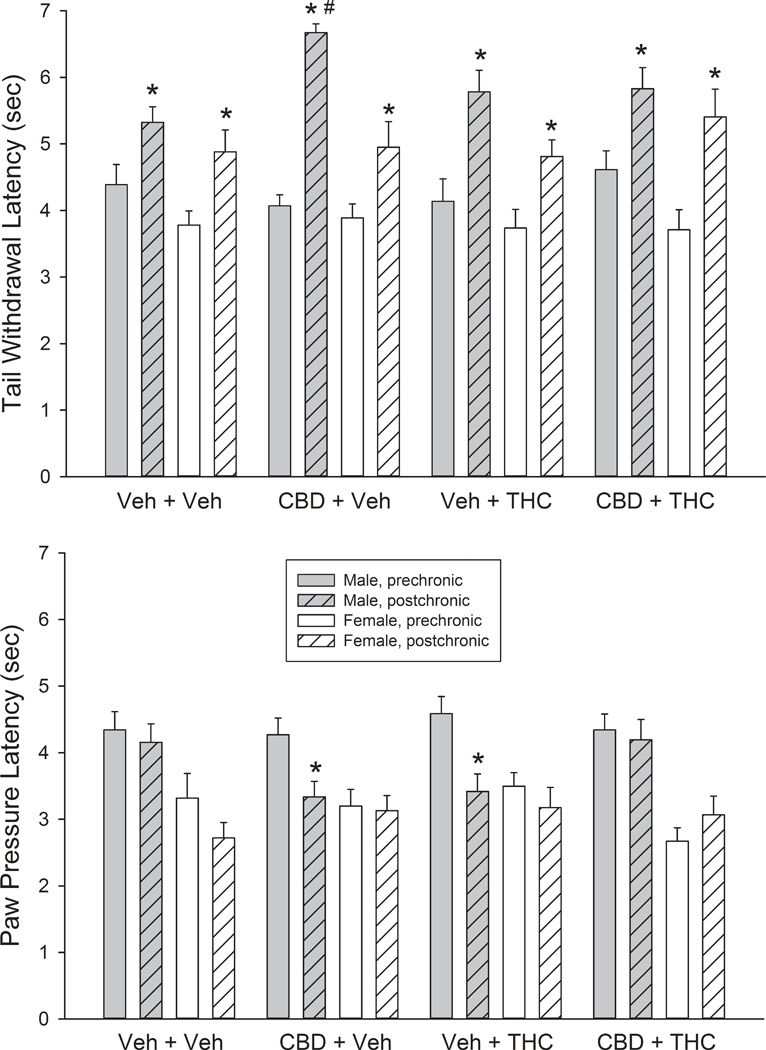
Baseline latency to respond on the tail withdrawal (top panel) and paw pressure tests (bottom panel) in male and female rats in each of the four treatment groups, on the pre-chronic test day vs. the post-chronic test day. Each bar is the mean ± 1 S.E.M. of 12 rats. *Significantly different from pre-chronic test p<0.05. #Significantly different from same-sex vehicle+vehicle group, p<0.05
Body weight.
Figure 3 shows that THC-treated males, but not females, lost a significant percentage of their body weight compared to same-sex controls during the experiment (Sex x THC x Day: F(2,176)=7.93, p=0.001). Analysis within each sex revealed that females lost approximately 4–5% of their body weight regardless of treatment group (Day: F(2,88)=117.41, p<0.001; no interaction with CBD or THC), and all body weight loss occurred within the first two days of the study. In contrast, vehicle-treated males lost 3–4% of their body weight (primarily within the first two days of the study), whereas THC-treated males lost 5–6% of their body weight by Day 2, and approximately 8% of their body weight by Day 6, the post-chronic test day (Day x THC: F(2,88)=13.55, p<0.001).
Fig. 3.
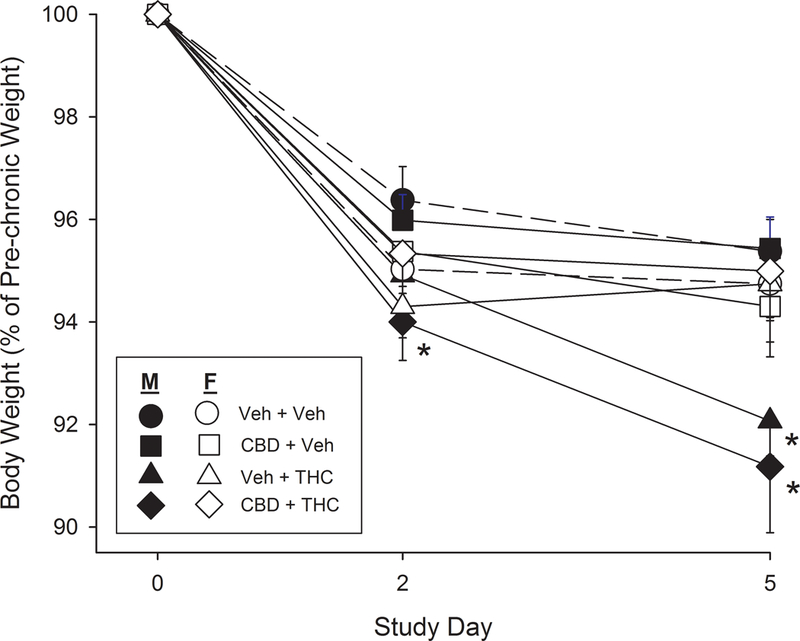
Body weight loss during the study in female vs. male rats in each of the four treatment groups. Each point is the mean ± 1 S.E.M. of 12 rats. *Significantly different from same-sex vehicle+vehicle group on same day, Dunnett’s test, p<0.05
Antinociceptive tolerance to THC.
Figure 4 shows the THC dose-effect curves for each treatment group on the pre- vs. post-chronic test days (Day 1 vs. Day 6). ED50 values derived from these dose-effect curves (when they could be calculated) are shown for each group in Table 1. On the pre-chronic test day, THC was more potent in females compared to males (N=48 rats/sex) on both nociceptive tests (Table 1). There were no significant within-sex differences in THC potency on the pre-chronic test among males assigned to the four treatment groups, and among females assigned to the four treatment groups (Table 1).
Fig. 4.
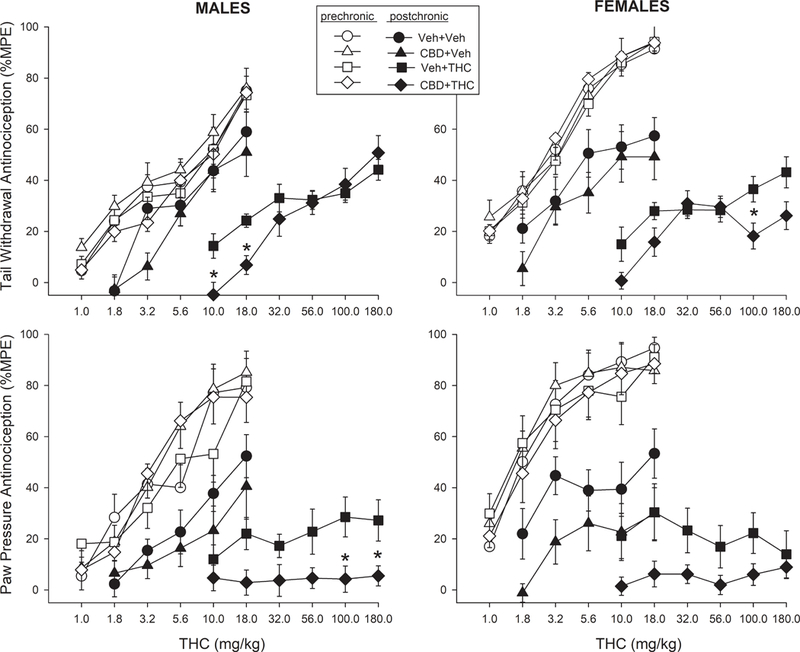
Antinociceptive tolerance to THC in male and female rats treated with vehicle or CBD. Top panels: THC dose-effect curves on the tail withdrawal test on pre-chronic vs. post-chronic test days (Days 1 vs. 6), in males (left) and females (right). Bottom panel: THC dose-effect curves on the paw pressure test on pre-chronic vs. post-chronic test days, in males (left) and females (right). Each point is the mean ± 1 S.E.M. of 12 rats. *CBD+THC-treated group significantly different from same-sex, vehicle+THC group, t-test with Bonferroni correction, p<0.05
Figure 4 (top panels) shows that on the tail withdrawal test, the rightward shift in the THC dose-effect curve from pre- to post-chronic was greater in THC-treated rats compared to vehicle-treated rats in both sexes, and tolerance development was greater in females than males (Sex x THC x Day: F(1,352)=5.77, p=0.018; Sex x Day x Dose: F(4,352)=10.22, p<0.001; Day x THC x Dose: F(4,352)=6.53, p<0.001). CBD increased the development of tolerance to THC (CBD x Day: F(1,352)=7.99, p=0.006). This CBD effect was not significant in the chronic vehicle-treated groups, but in chronic THC-treated rats, CBD altered THC’s effect in a sex- and THC dose-dependent manner (CBD x Sex x Dose: F(3.74, 4.09)=180.05, p=0.006). Specifically, CBD tended to decrease THC effect at the lowest THC doses in both sexes but also at the highest THC doses in females (Fig. 4 top panels).
On the paw pressure test (Fig. 4, bottom panels), the rightward shift in the THC dose-effect curve from pre- to post-chronic was greater in THC-treated rats compared to vehicle-treated rats in both sexes (Day x THC x Dose: F(2.92,256.88)=4.73, p=0.003). CBD increased the development of tolerance to THC (CBD x Day: F(1,88)=10.05, p=0.002). However, this CBD effect was significant in both the chronic vehicle-treated groups (CBD: F(1,44)=4.44, p=0.041) and in the chronic THC-treated groups (CBD: F(1,44)=10.57, p=0.002). CBD decreased THC effect similarly in both sexes, although this effect was only statistically significant in males (males: F(1,22)=7.27, p=0.013; females: F(1,22)=4.03, p=0.057).
Tolerance to THC-induced sedation.
Figure 5 shows locomotor activity measured in control rats that did not receive any THC at all, as well as rats in the tolerance study that were tested for locomotor activity after completion of THC dose-effect curve determination on the pre- vs. post-chronic test days (Day 1 vs. Day 6). Control females were more active than control males (Sex: F(1,36)=8.30, p=0.007), but there was no significant change in locomotor activity from pre- to post-chronic in the control male or female groups. Compared to Controls, THC treatment on the pre- and post-chronic days (up to 18 mg/kg) reduced locomotor activity (Control vs. vehicle+vehicle group: F(1,36)=95.02, p<0.001). In all groups of rats tested with THC on the pre- and post-chronic test days (vehicle+vehicle, CBD+vehicle, vehicle+THC, CBD+THC groups), locomotor activity increased from the pre-chronic to post-chronic day, despite the fact that all chronic THC-treated rats were tested after a 10X higher terminal dose of THC on the post-chronic day compared to the pre-chronic day (Day: F(1,88)=23.99, p<0.001). Females tended to locomote more than males in vehicle- but not THC-treated groups (Sex x THC: F(1,88)=4.14, p=0.045). Further analysis within each sex revealed that in males, the significant increase in locomotor activity from pre- to post-chronic was limited to the vehicle+THC group (Day x THC: F(1,44)=4.26, p=0.045), whereas in females, both vehicle+THC and CBD+vehicle groups showed significant increases in activity from pre- to post-chronic tests (Day: F(1,44)=15.01, p<0.001; Fig. 5).
Fig. 5.
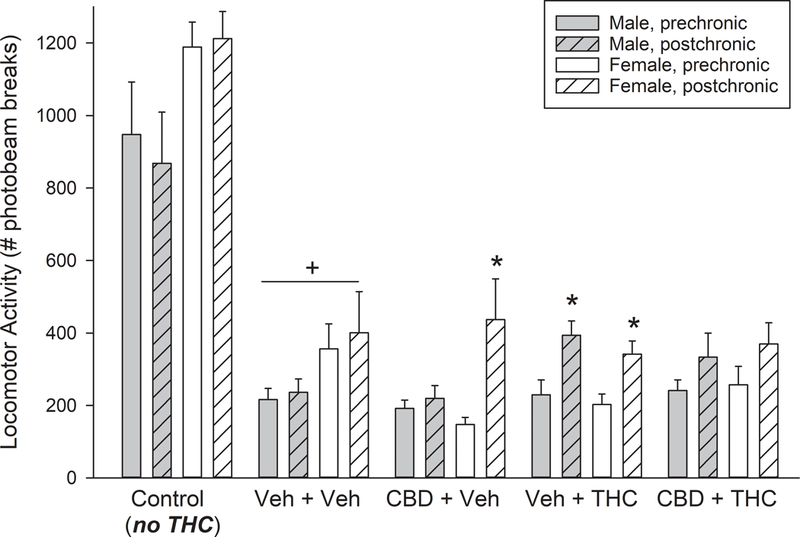
Locomotor activity measured after vehicle treatment only (“Control”) and immediately after completion of THC dose-effect curve determinations on pre- vs. post-chronic test days (Days 1 vs. 6), in vehicle+vehicle, CBD+vehicle, vehicle+THC, and CBD+THC tolerance groups. On the pre-chronic test day, all tolerance groups had received a total cumulative dose of up to 18 mg/kg THC, whereas the Control group received vehicle. On the post-chronic test day, vehicle+vehicle and CBD+vehicle groups received a total cumulative dose of 18 mg/kg THC, vehicle+THC and CBD+THC groups received a total cumulative dose of 180 mg/kg, and Control groups received vehicle. Each bar is the mean ± 1 S.E.M. of 8 rats (Control group) or 12 rats (all other groups). +Significant suppression of locomotor activity by THC in the vehicle+vehicle group, compared to Controls that did not receive THC; *Post-chronic day activity significantly higher than pre-chronic day activity, within same sex/treatment group, p<0.05
Serum levels of CBD, THC and metabolites.
Figure 6 shows serum levels of cannabinoids after completion of the post-chronic (Day 6) THC dose-effect curve determination, in each treatment group. As expected, levels of THC and its major metabolites 11-OH-THC and THC-COOH, as well as the minor metabolite CBN, were higher in rats tested with high THC doses on the post-chronic day (those in the vehicle+THC and CBD+THC groups, which had received 180 mg/kg THC) compared to rats tested with lower THC doses on the post-chronic test day (those in the vehicle+vehicle and CBD+vehicle groups, which had received 18 mg/kg) (main effect of THC treatment on serum THC: F(1,62)= 87.47, p<0.001; serum 11-OH-THC: F(1,62)=76.45, p<0.001; serum THC-COOH: F(1,62)=222.71, p<0.001; serum CBN: F(1,62)=36.84, p<0.001).
Fig. 6.
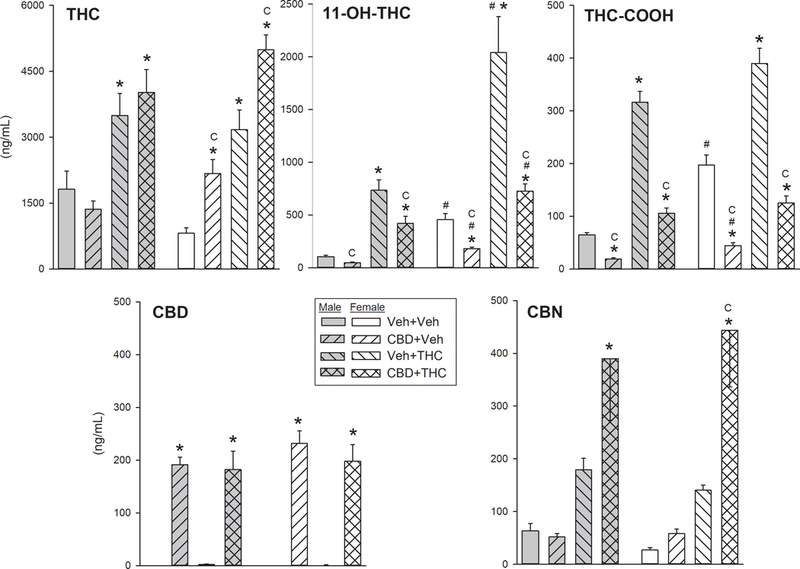
THC, CBD and metabolite concentrations in blood samples taken on the post-chronic day (Day 6) after completion of THC injections and behavioral tests. Note that y-axis scales are not the same for all cannabinoids. Vehicle+vehicle and CBD+vehicle groups had received a total cumulative dose of 18 mg/kg THC, and vehicle+THC and CBD+THC groups had received a total cumulative dose of 180 mg/kg. Each bar is the mean ± 1 S.E.M. of 8–9 rats. *Significantly different from same-sex vehicle+vehicle group, Dunnett’s test, p<0.05; #Significant sex difference, within the same treatment group; CSignificant CBD effect, t-test with Bonferroni correction, p<0.05
After testing with THC on the post-chronic day, serum CBD levels were approximately 200 ng/mL in all CBD-treated groups even though the last CBD injection had been given approximately 15 h before blood samples were taken (Fig. 6, bottom left panel; F(1,62)=231.09, p<0.001). CBD treatment increased serum THC in both chronic vehicle- and chronic THC-treated females but not males (Fig. 6, top left panel; Sex x CBD: F(1,62)=9.29, p=0.003). CBD treatment also increased serum CBN in chronic THC-treated rats of both sexes (Fig. 6, bottom right panel; CBD x THC: F(1,62)=9.94, p=0.002), although the post-hoc test was significant only in females. CBD treatment decreased serum 11-OH-THC (F(1,62)=13.10, p=0.001) and serum THC-COOH (F(1,62)=45.49, p<0.001) in both sexes (Fig.6, top middle and right panels). However, 11-OH-THC levels were significantly higher in females than males, and CBD suppression of serum 11-OH-THC was greater in females than males (Sex x CBD x THC: F(1,62)=4.74, p=0.033), indicating that CBD suppressed the transformation of THC to its major active metabolite more in females than males.
Figure 7 shows serum cannabinoid levels in a separate group of rats that were treated chronically with vehicle or CBD and vehicle or THC, but were not given any THC before blood collection on the post-chronic test day. These data therefore show residual blood levels of cannabinoids before THC injection and testing on the final day, the last previous drug injection having occurred approximately 15 h before blood sampling. In these rats, THC and its metabolites were detectable but low compared to levels observed in rats given THC on the post-chronic day (compare Figs. 6 and 7). Levels of THC and its metabolites were slightly to significantly higher in males compared to females, presumably because males had received 9.3 mg/kg THC/injection, whereas females had received 3.6 mg/kg THC/injection during the chronic treatment period (Sex x THC, serum THC: F(1,33)=44.12, p<0.001; serum 11-OH-THC: F(1,33)=6.78, p=0.014; serum THC-COOH: F(1,33)=2.63, p=0.11; serum CBN: F(1,33)=26.63, p<0.001). CBD levels were approximately 140–200 ng/ml in all CBD-treated groups that received their last CBD injection 15 h before blood sampling (Fig. 7, bottom left panel), indicating that CBD was present in the bloodstream before THC was administered on Day 6 (and still present after testing: Fig. 6). Similar to the results from the first cohort of rats (Fig. 6), in rats that were not tested on Day 6 (Fig. 7), CBD increased serum THC, while decreasing serum 11-OH-THC and THC-COOH (CBD x THC, serum THC: F(1,33)=30.47, p<0.001; serum 11-OH-THC: F(1,33)=4.30, p=0.046; serum THC-COOH: F(1,33)=8.50, p=0.006). Specific blood levels of THC in THC-treated rats were: vehicle+THC group, 142±12 ng/ml in males, 31±3 ng/ml in females; CBD+THC group, 253±21 ng/ml in males, 120±15 ng/ml in females. Specific blood levels of 11-OH-THC in THC-treated rats were: vehicle+THC group, 41±12 ng/ml in males, 8±6 ng/ml in females; CBD+THC group, 12±7 in males, 0±0 in females. However, the substantial increases in serum CBN that were observed in the chronically CBD-treated rats given high doses of THC on the post-chronic test day (Fig. 6) were not observed in rats that received no THC on the post-chronic day (Fig. 7, lower right panel).
Fig. 7.
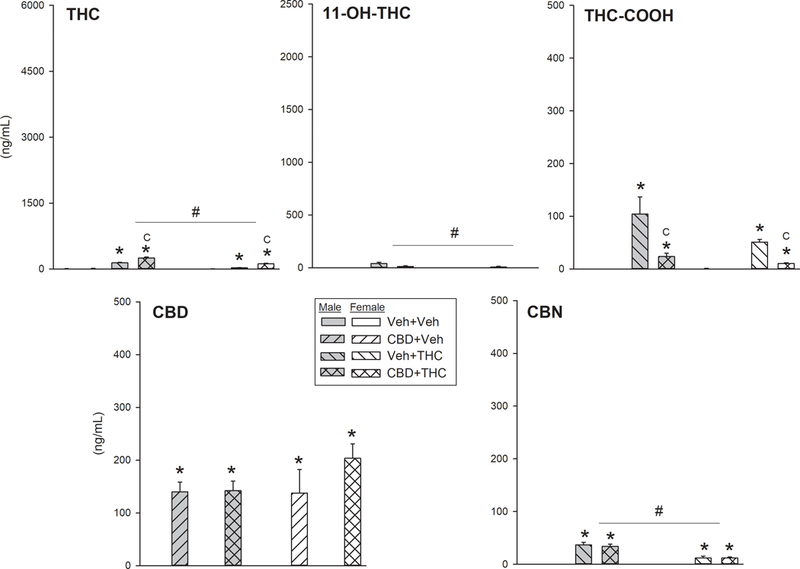
THC, CBD and metabolite concentrations in blood samples taken on the post-chronic day (Day 6) before any injections/tests (15 h after the last injection on the evening of Day 5). Note that y-axis scales are not the same for all cannabinoids, but scaling is the same in Figs. 6 and7 for each cannabinoid. Each bar is the mean ± 1 S.E.M. of 3–6 rats. *Significantly different from same-sex vehicle+vehicle group, Dunnett’s test, p<0.05; #Significant sex difference; CSignificant CBD effect, t-test with Bonferroni correction, p<0.05
Discussion
Several findings in the present study replicate those of previous studies. First, initial antinociceptive potencies of THC on tests of acute heat and pressure pain were greater in female compared to male rats (Craft et al. 2012; Romero et al. 2002; Tseng and Craft 2001). Second, females developed more tolerance than males to the antinociceptive effects of THC, even though females received only ~40% as much THC as males during the chronic treatment period (Wakley et al. 2014; 2015). Third, females had higher blood levels of the major active metabolite, 11-OH-THC, relative to males (Tseng et al. 2004; Wiley and Burston 2014). Fourth, inhibition of THC metabolism by CBD observed in the present study has been reported previously in rats (Britch et al. 2017; Klein et al. 2011; Hložek et al. 2017), mice (Varvel et al. 2006), and humans (Nadulski et al. 2005). The present results therefore suggest that these four findings are reliable.
The novel finding is that CBD appeared to enhance the development of tolerance to THC-induced antinociception in both nociceptive tests. That is, THC-induced antinociception decreased significantly more in rats that received twice-daily treatment with CBD in combination with THC than in rats that received THC alone. One possible mechanism underlying this drug interaction is inhibition of THC metabolism by CBD: consistently higher blood levels of THC during the chronic THC treatment period would result in greater CB1 receptor down-regulation and/or desensitization, two known mechanisms of cannabinoid tolerance in rats (Sim-Selley 2003; Burston et al. 2010) and in humans (Villares 2007). Although CBD significantly increased blood levels of THC only in female rats, this sex difference was likely due to the single time point assessed after completion of THC dose-effect curve determination, since CBD has been shown previously to increase blood and brain levels of THC in male rats, at 0.5–1 h post-injection (Klein et al. 2011; Hložek et al. 2017). In the present study, repeated CBD treatment also increased blood levels of CBN, an active metabolite, in chronic THC-treated rats of both sexes. CBN is known to produce CB1 receptor-mediated, THC-like effects, and so would also be expected to down-regulate and desensitize CB1 receptors, although perhaps with lower potency and efficacy than THC (Booker et al. 2009; Hiltunen et al. 1988; Sanders et al. 1979; Sofia et al. 1975).
CBD also decreased blood levels of 11-OH-THC and THC-COOH, the primary metabolites of THC, in both sexes, compared to rats that received THC alone. Acute suppression of 11-OH-THC production by CBD would be expected to decrease THC’s effects particularly in females, because they produce significantly more 11-OH-THC than males do (Wiley & Burston 2014; Britch et al. 2017; present study), and because greater 11-OH-THC production contributes to greater acute THC-induced antinociception in female compared to male rats (Tseng et al. 2004). Acute suppression of 11-OH-THC production by CBD also may explain why females chronically treated with CBD+vehicle showed significant decreases in THC effect on the post-chronic test day (paw pressure test). Thus, chronic CBD-induced changes in THC effect on the final day of testing are likely due, at least in part, to CBD-induced changes in THC metabolism.
An alternative explanation for the observed decrease in THC potency and efficacy in chronic CBD-treated rats is that CBD antagonized THC’s effects on the post-chronic test day. CBD was not administered on the last day, but was present in the bloodstream in chronic CBD-treated rats (Figs. 6 and 7). Furthermore, even in rats that had not received THC twice-daily (i.e., the CBD+Veh group), THC potency/efficacy decreased from the pre- to post-chronic tests. That is, twice-daily CBD treatment for 4 days in the absence of THC also reduced THC effect assessed on the final day. A single injection of CBD enhanced or did not alter THC’s antinociceptive effect on the tail withdrawal or paw pressure tests, when CBD was given either 15 min or 13 h before THC (Britch et al. 2017). Therefore, the present results suggest that CBD-induced reduction of THC effect on the post-chronic day resulted from repeated rather than acute CBD treatment. Todd and colleagues (2017) recently reported a similar phenomenon: when CBD and THC (10 mg/kg of each) were co-administered daily to male mice, no drug interaction was observed on the first day, yet CBD “modestly inhibited” (p. 132) THC-induced hyperlocomotion on days 3, 5 and 7 of chronic treatment. This emergent drug interaction was not observed on other behavioral tests (e.g., of anxiety; antinociception was not tested) (Todd et al. 2017). Using schizophrenia- and anxiety-related tests in male mice, Long and colleagues (2010) also observed effects of chronic CBD that were not observed after acute CBD. Finally, when CBD and THC were co-administered acutely to monkeys, no interaction was observed on a cognitive test (stop signal reaction time) at a 1:1 dose combination, although a 1:3 THC:CBD dose combination produced less suppression of “go-trial success” than THC alone, at one dose of THC (Jacobs et al., 2016). When the drugs were co-administered chronically, CBD appeared to enhance the development of tolerance to THC’s disruptive effect (Jacobs et al. 2016) – or chronic CBD was more effective than acute CBD in antagonizing THC’s effect. Taken together, these studies demonstrate that CBD-THC interactions are complex, and may change with repeated exposure. Instead of (or in addition to) promoting the development of tolerance to THC, chronic CBD treatment may attenuate THC effects even when no interactions occur after acute administration.
An unexpected finding in the present study was tolerance development in the vehicle+vehicle group, which received THC only on Day 1. Although THC dose-effect curves in the vehicle+vehicle group shifted less than those in the other three treatment groups, the decrease in THC potency was statistically significant in vehicle+vehicle males (paw pressure test) and females (both tests) (Table 1). We conducted a subsequent experiment to compare THC potency in rats that were simply handled/injected with vehicle for 5 days vs. rats that were not handled/injected before the test day, and there were no significant differences in THC potency between the two groups, on either nociceptive test (unpublished data). This result rules out handling/injection as a cause of tolerance development in the vehicle+vehicle group, and suggests that single-day exposure to THC – up to 18 mg/kg on Day 1 – was enough to produce some pharmacological or behavioral (learned) tolerance assessed 5 days later. In previous tolerance studies we observed smaller, non-significant decreases in THC potency in vehicle-treated groups (Wakley et al. 2014; 2015), but the length of time between the pre- and post-chronic tests was 9 days in those studies, compared to 5 days in the present study. Thus, the short period between pre- and post-tests may have contributed to the greater tolerance development in vehicle+vehicle groups in the present compared to previous studies.
In the present study, tolerance also developed to THC-induced decreases in locomotor activity in both sexes, evidenced by the fact that chronic THC-treated rats locomoted more on the post-chronic test than on the pre-chronic test, despite receiving a ~10X higher dose of THC on the post-chronic test than on the pre-chronic test (180 mg/kg vs. 10–18 mg/kg, respectively). This increase in locomotor activity from the pre- to post- chronic test day was significant in the vehicle+THC group but not in the CBD+THC group, in both sexes. It was also significant in CBD+vehicle-treated females but not males, which may again reflect CBD-induced decreases in THC metabolism to its active metabolite, 11-OH-THC, which is responsible for greater THC effect in female rats (Tseng et al. 2004). If inhibition of THC metabolism by CBD is solely responsible for the greater tolerance to THC-induced antinociception in the CBD+THC group relative to the vehicle+THC group, we would expect similarly greater tolerance to THC-induced inhibition of locomotor activity in the CBD+THC group relative to the vehicle+THC group. This did not occur, suggesting that mechanisms underlying this CBD-THC interaction may be endpoint-dependent.
In the present study, baseline nociceptive latencies changed significantly from the pre- to post-chronic test day. On the tail withdrawal test, these changes were consistent: latency was significantly longer on the post-chronic test day than on the pre-chronic test day, in both sexes and all treatment groups. Lengthening of tail withdrawal latency over time is not likely due to chronic drug exposure per se, since it occurred even in the vehicle+vehicle group. One exception may be the male CBD+vehicle group: baseline tail withdrawal latency lengthened an average of 2.60 sec from pre- to post-chronic tests in this group, whereas the change in other groups averaged 0.94–1.65 sec. CBD is known to desensitize TRPV1 receptors (De Petrocellis et al. 2011), which play a crucial role in heat pain sensitivity (Brederson et al. 2013; Costa et al. 2007); additionally, there may be sex differences in TRPV1 gene expression (Kim et al. 2004). These mechanisms may explain the fact that latencies to respond to noxious heat were significantly and substantially longer after 5 days of chronic treatment with CBD in males.
One additional sex difference warrants mention. When treated chronically with THC, males lost more body weight than females. Previous studies show that chronic THC administration can cause weight loss and/or hypophagia in humans (Le Foll et al. 2013) and in animals (Drewnowski and Grinker 1978; Klein et al. 2011; Levendal et al. 2012). We are not aware of any studies that have statistically compared the effects of chronic THC treatment on weight loss or feeding in males vs. females, but this sex difference has been observed (Marusich et al. 2014; Wakley et al. 2015). Weight loss during chronic THC administration may be caused by THC-induced sedation, although studies showing that THC was approximately twice as potent in reducing locomotion in female compared to male rats (e.g., Tseng and Craft 2001) do not support this hypothesis. Alternatively, the hypothalamic endocannabinoid system is known to be an important regulator of food consumption and energy balance (Cristino et al. 2014), hypothalamic CB1 receptor density is higher in male than female rats (Riebe et al. 2010), and hypothalamic neurons respond to cannabinoids in a sex-dependent manner (Corchero et al. 2002; Tang et al. 2005). Thus, repeated THC treatment may induce sexually dimorphic weight loss by differentially altering the hypothalamic endocannabinoid system in males versus females.
In summary, the present results show that repeated CBD treatment decreased rats’ sensitivity to THC’s antinociceptive effects on the final test day, which may reflect enhanced development of tolerance to THC-induced antinociception, and/or emergent antagonism of THC-induced antinociception by repeated exposure to CBD. Mechanisms that may contribute to CBD modulation of THC effect include CBD-induced inhibition of THC metabolism and negative allosteric modulation of CB1 receptors by CBD, among others. Although the relatively short drug exposure regimen used in the present study (4 days) may limit generalizability to long-term drug use in humans, these results demonstrate the potential impact that non-THC constituents of cannabis may have on THC’s pharmacological effects, suggesting the need for additional chronic drug interaction studies in both sexes.
Acknowledgements
The authors thank Kelly Hewitt and Abby Pondelick for excellent technical assistance. This research was funded by NIDA DA016644 (J. Wiley, PI), by a Diversity Supplement to DA016644 (to support N. Greene), and by funds dedicated for marijuana research by the State of Washington Initiative Measure 502.
Footnotes
Conflict of Interest: N. Greene, J. Wiley, Z. Yu, B. Clowers, and R. Craft declare that they have no conflicts of interest.
References
- Amaya F, Shimosato G, Kawasaki Y, Hashimoto S, Tanaka Y, Ji RR, Tanaka M (2006) Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain 124: 175–83. [DOI] [PubMed] [Google Scholar]
- Booker L, Nauidu PS, Razadan RK, Mahadevan A, Lichtman AH (2009) Evaluation of prevalent phytocannabinoids in the acetic acid model of visceral nociception. Drug Alcohol Depend 105: 42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornheim LM, Grillo MP (1998) Characterization of cytochrome P450 3A inactivation by cannabidiol: possible involvement of cannabidiol-hydroxyquinone as a P450 inactivator. Chem Res Toxicol 11: 1209–16. [DOI] [PubMed] [Google Scholar]
- Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ (1995) Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain. Drug Metab Dispos 23: 825–31. [PubMed] [Google Scholar]
- Brederson J-D, Kym PR, Szallasi A (2013) Targeting TRP channels for pain relief. Eur J Pharmacol 716: 61–76. [DOI] [PubMed] [Google Scholar]
- Britch SC, Wiley JL, Yu Z, Clowers BH, Craft RM (2017) Cannabidiol-delta-9-tetrahydrocannbinol interactions on acute pain and locomotor activity. Drug Alcohol Depend 175: 187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ (2010) Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Δ9-tetrahydrocannabinol exposure. Brit J Pharmacol 161: 103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ (2000) Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the mu opioid receptor. Psychopharmacology 150: 430–42. [DOI] [PubMed] [Google Scholar]
- Corchero J, Fuentes JA, Manzanares J (2002) Gender differences in proenkephalin gene expression response to Δ9-tetrahydrocannabinol in the hypothalamus of the rat. J Psychopharm 16: 283–9. [DOI] [PubMed] [Google Scholar]
- Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M (2007) The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol 556: 75–83. [DOI] [PubMed] [Google Scholar]
- Craft RM, Wakley AA, Tsutsui KT, Laggart JD (2012) Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by delta9-tetrahydrocannabinol and CP55,940 in the rat. J Pharmacol Exp Ther 340: 787–800. [DOI] [PubMed] [Google Scholar]
- Cristino L, Palomba L, Di Marzo V (2014) New horizons on the role of cannabinoid CB1 receptors in palatable food intake, obesity and related dysmetabolism. Int J Obesity Supple 4:S26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttler C, Mischley LK, Sexton M (2016) Sex differences in cannabis use and effects: A cross-sectional survey of cannabis users. Cannabis Cannabinoid Res 1: 166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton WS, Martz R, Lemberger L, Rodda BE, Forney RB (1976) Influence of cannabidiol on delta-9-tetrahydrocannabinol effects. Clin Pharmacol Ther 19: 300–9. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Moriello AS, Allara M, Bisogno T, Petrosino S, Stott CG, Di Marzo V (2011) Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 163: 1479–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, Grinker JA (1978) Food and water intake, meal patterns and activity of obese and lean Zucker rats following chronic and acute treatment with delta9-tetrahydrocannabinol. Pharmacol Biochem Behav 9: 619–30. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W (2007) Cannabinoid self-administration in rats: Sex differences and the influence of ovarian function. Brit J Pharmacol 152: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DP, Beckett SR, Roe CH, Madjd A, Fone KC, Kendall DA, Marsden CA, Chapman V (2004) Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. Eur J Neurosci 19: 678–86. [DOI] [PubMed] [Google Scholar]
- Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, Gray KM, McRae-Clark A, Lofwall MR, Sparenborg S, Walsh SL (2016) Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology 41: 1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Dow-Edwards DL (2012) Withdrawal from THC during adolescence: Sex differences in locomotor activity and anxiety. Behav Brain Res 231: 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen AJ, Jarbe TUC, Wangdahl K (1988) Cannabinol and cannabidiol in combination: Temperature, open-field activity, and vocalization. Pharmacol Biochem Behav 30: 675–8. [DOI] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJ, Curran HV (2015). Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur Neuropsychopharmacol 25:325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hložek T, Uttl L, Kadeřábek L, Balíková M, Lhotková E, Horsley RR, Nováková P, Šichová K, Štefková K, Tylš F, Kuchař M, Páleníček T (2017) Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacology 27: 1223–37. [DOI] [PubMed] [Google Scholar]
- Holland ML, Lau DT, Allen JD, Arnold JC (2007) The multidrug transporter ABCG2 (BCRP) is inhibited by plant-derived cannabinoids. Brit J Pharmacol 152: 815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan AB, Gevins A, Coleman M, ElSohly MA, de Wit H (2005) Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav Pharmacol 16: 487–96. [DOI] [PubMed] [Google Scholar]
- Jacobs DS, Kohut SJ, Jiang S, Nikas SP, Makriyannis A, Bergman J (2016) Acute and chronic effects of cannabidiol and Δ9-tetrahydrocannabinol (Δ9-THC)-induced disruption in stop signal task performance. Exp Clin Psychopharmacol 24: 320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger W, Benet LZ, Bornheim LM (1996) Inhibition of cyclosporine and tetrahydrocannabinol metabolism by cannabidiol in mouse and human microsomes. Xenobiotica 26: 275–84. [DOI] [PubMed] [Google Scholar]
- Jiang R, Yamaori S, Okamoto Y, Yamamoto I, Watanabe K (2013) Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab Pharmacokinet 28: 332–8. [DOI] [PubMed] [Google Scholar]
- Jones G, Pertwee RG (1972) A metabolic interaction in vivo between cannabidiol and 1 -tetrahydrocannabinol. Brit J Pharmacol 45: 375–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karniol IG, Carlini EA (1973) Pharmacological interaction between cannabidiol and delta 9-tetrahydrocannabinol. Psychopharmacologia 33: 53–70. [DOI] [PubMed] [Google Scholar]
- Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA (1974) Cannabidiol interferes with the effects of Δ⁹-tetrahydrocannabinol in man. Eur J Pharmacol 28: 172–7. [DOI] [PubMed] [Google Scholar]
- Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA (2011) Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem 57: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Neubert JK, San Miguel A, Xu K, Krishnaraju RK, Iadarola MJ, Goldman D, Dionne RA (2004) Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain 109: 488–96. [DOI] [PubMed] [Google Scholar]
- Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, Gunasekaran N, Karl T, Long LE, Huang XF, Liu K, Arnold JC, McGregor IS (2011) Cannabidiol potentiates Δ⁹-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology 218: 443–57. [DOI] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM (2015) Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Brit J Pharmacol 172: 4790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Trigo JM, Sharkey KA, Le Strat Y (2013) Cannabis and Δ9-tetrahydrocannabinol (THC) for weight loss? Med Hypotheses 80: 564–7. [DOI] [PubMed] [Google Scholar]
- Levendal RA, Schumann D, Donath M, Frost CL (2012) Cannabis exposure associated with weight reduction and β-cell protection in an obese rat model. Phytomedicine 19: 575–82. [DOI] [PubMed] [Google Scholar]
- Long LE, Chesworth R, Huang X, McGregor IS, Arnold JC, Karl T (2010) A behavioural comparison of acute and chronic Δ9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int J Neuropsychopharm 13: 861–76. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Antonazzo KR, Craft RM, Wiley JL (2014) Evaluation of sex differences in cannabinoid dependence. Drug Alcohol Depend 137: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, Stadelmann AM (2005) Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit 27: 799–810. [DOI] [PubMed] [Google Scholar]
- Narimatsu S, Watanabe K, Matsunaga T, Yamamoto I, Imaoka S, Funae Y, Yoshimura H (1990) Inhibition of hepatic microsomal cytochrome P450 by cannabidiol in adult male rats. Chem Pharm Bull 38: 1365–8. [DOI] [PubMed] [Google Scholar]
- Romero EM, Fernández B, Sagredo O, Gomez N, Urigüen L, Guaza C, De Miguel R, Ramos JA, Viveros MP (2002) Antinociceptive, behavioural and neuroendocrine effects of CP 55,940 in young rats. Brain Res Dev Brain Res 136: 85–92. [DOI] [PubMed] [Google Scholar]
- Riebe CJN, Hill MN, Lee TTY, Hillard CJ, Gorzalka BB (2010) Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology 35: 1265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E, Guy GW (2006) A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses 66: 234–46. [DOI] [PubMed] [Google Scholar]
- Sanders J, Jackson DM, Starmer GA (1979) Interactions among the cannabinoids in the antagonism of the abdominal constriction response in the mouse. Psychopharmacology 61: 281–5. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ (2003) Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol 15: 91–119. [DOI] [PubMed] [Google Scholar]
- Sofia RD, Vassar HB, Knobloch LC (1975) Comparing analgesic activity of various naturally occurring cannabinoids in rats and mice. Psychopharmacologia 40: 285–95. [DOI] [PubMed] [Google Scholar]
- Tang SL, Tran V, Wagner EJ (2005) Sex differences in the cannabinoid modulation of an A-Type K+ current in neurons of the mammalian hypothalamus. J Neurophysiol 94: 2983–6. [DOI] [PubMed] [Google Scholar]
- Terner JM, Lomas LM, Smith ES, Barrett AC, Picker MJ (2003) Pharmacogenetic analysis of sex differences in opioid antinociception in rats. Pain 106: 381–91. [DOI] [PubMed] [Google Scholar]
- Todd SM, Zhou C, Clarke DJ, Chohan TW, Bahceci D, Arnold JC (2017) Interactions between cannabidiol and Δ9-THC following acute and repeated dosing: Rebound hyperactivity, sensorimotor gating and epigenetic and neuroadaptive changes in the mesolimbic pathway. Eur Neuropsychopharmacology 27: 132–45. [DOI] [PubMed] [Google Scholar]
- Tseng AH, Craft RM (2001) Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol 430: 41–7. [DOI] [PubMed] [Google Scholar]
- Tseng AH, Harding JW, Craft RM (2004) Pharmacokinetic factors in sex differences in delta 9-tetrahydrocannabinol-induced behavioral effects in rats. Behav Brain Res 154: 77–83. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH, Martin BR (2006) Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology 186: 226–34. [DOI] [PubMed] [Google Scholar]
- Villares J (2007) Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience 145: 323–34. [DOI] [PubMed] [Google Scholar]
- Wakley AA, Wiley JL, Craft RM (2014) Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alcohol Depend 143: 22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakley AA, Wiley JL, Craft RM (2015) Gonadal hormones do not alter the development of antinociceptive tolerance to delta-9-tetrahydrocannabinol in adult rats. Pharmacol Biochem Behav 133: 111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I (2007) Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci 80: 1415–9. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ (2014) Sex differences in Δ(9)-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci Lett 576: 51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, O’Connell MM, Tokarz ME, Wright MJ (2007) Pharmacological effects of acute and repeated administration of delta(9)-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther 320: 1097–105. [DOI] [PubMed] [Google Scholar]
- Yamaori S, Kushihara M, Yamamoto I, Watanabe K (2010) Characterization of major phytocannabinoids, cannabidiol and cannabinol, as isoform-selective and potent inhibitors of human CYP1 enzymes. Biochem Pharmacol 79: 1691–8. [DOI] [PubMed] [Google Scholar]
- Yamaori S, Okamoto Y, Yamamoto I, Watanabe K (2011) Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab Dispos 39: 2049–56. [DOI] [PubMed] [Google Scholar]
- Zhu HJ, Wang JS, Markowitz JS, Donovan JL, Gibson BB, Gefroh HA, Devane CL (2006) Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. J Pharmacol Exp Ther 317: 850–7. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Hallak JE, Crippa JA (2012) Interaction between cannabidiol (CBD) and ∆(9)-tetrahydrocannabinol (THC): influence of administration interval and dose ratio between the cannabinoids. Psychopharmacology 219: 247–9. [DOI] [PubMed] [Google Scholar]


