Abstract
Beckwith-Wiedemann syndrome (BWS) is the most common epigenetic overgrowth disorder and presents with patients affected by a variety of clinical features. Although genotype-phenotype correlations have been demonstrated in BWS and although BWS has been reported to occur equally among racial and ethnic backgrounds, no study to date has evaluated the frequency of findings in different backgrounds. In this study, we evaluated the incidence of clinical features and molecular diagnoses among patients with BWS in Caucasian, Mixed, and non-Caucasian groups. These results suggest that clinical features and molecular diagnoses differ between race/ethnicity groups and raise the possibility of race and ethnicity effects on genotype-phenotype correlations in BWS.
Keywords: Beckwith-Wiedemann Syndrome, Race, Ethnicity, Diverse populations, Overgrowth, Methylation, Imprinting
INTRODUCTION
Beckwith-Wiedemann syndrome (BWS) (OMIM #130650) is the most common epigenetic overgrowth disorder, affecting at least 1 in 10,540 live births (Mussa, Di Candia, et al., 2016). Patients with BWS can present with a spectrum of clinical features, including macroglossia, abdominal wall defects (omphalocele, umbilical hernia, diastasis recti), ear creases/pits, organomegaly, macrosomia, and lateralized overgrowth (a more accurate term than the previously used “hemihypertrophy”) (Brioude et al., 2018; Kalish et al., 2017). Patients also have a predisposition to embryonal tumors, most commonly Wilms tumor and hepatoblastoma.
Molecular testing has led to the classification of subtypes of patients with BWS due to alterations on chromosome 11: loss of methylation at KCNQ1OT1:TSS differentially methylated region (DMR; IC2 LOM), gain of methylation at H19/IGF2:IG-DMR (IC1 GOM), paternal uniparental isodisomy of chromosome 11 (pUPD11), CDKN1C loss of function mutations, and chromosome abnormalities altering copy number or structure of 11p15.5. In some patients, a clinical diagnosis is made, but a molecular subtype is not identified. The role of these molecular subtypes has been evaluated in regard to clinical features and tumor formation, leading to clinically significant (epi)genotype-phenotype correlations (Brioude et al., 2013; Ibrahim et al., 2014; Maas et al., 2016; Mussa et al., 2016a; Weksberg et al., 2001).
It has been reported that BWS occurs regardless of race and ethnicity (Mussa, Di Candia, et al., 2016; Weksberg, Shuman, & Beckwith, 2010); however, demographic information is rarely noted in the literature. The potential for race and ethnicity to influence the molecular subtype and clinical features of BWS patients has previously been suggested in small cohorts of patients from Japan (Sasaki et al., 2007) and China (Luk, 2017). Nevertheless, to our knowledge, no dedicated analysis of patients of varying racial/ethnic backgrounds has been performed.
The importance of diversity in malformation syndromes has recently arisen as an important topic for both diagnosis and management of patients. Studies using facial analysis technology have revealed subtle differences in the facial appearance between racial groups in patients with Down syndrome, 22q syndrome, Noonan syndrome and Williams-Beuren syndrome (Kruszka, Addissie, et al., 2017; Kruszka, Porras, Addissie, et al., 2017; Kruszka et al., 2018; Kruszka, Porras, Sobering, et al., 2017).
As patients with BWS present with a wide range of clinical features, we sought to investigate whether clinical presentation and molecular subtypes varied across different race/ethnicity groups. We report differences observed within the current cohort as well as differences between these patients and previously reported European/North American and Asian groups. These findings indicate that (epi)genotype-phenotype differences between patients of various racial/ethnic backgrounds are likely, and further analysis with larger datasets is needed to determine how race/ethnicity plays a role in the presentation of clinical features and molecular diagnosis of patients affected by BWS.
MATERIALS AND METHODS
Editorial Policies and Ethical Considerations
Children’s Hospital of Philadelphia Institutional Board Review approval and patient and/or guardian consent was obtained for the study (IRB 13-010658).
Participants
Patients enrolled in an international growth registry at Children’s Hospital of Philadelphia (IRB 13-010658) prior to July 2017 were reviewed for inclusion in the study. Eligible patients included those with a molecularly confirmed diagnosis of BWS in one or more tissues, as well as available medical records and demographic information as defined in Brioude et al 2018 (Brioude et al., 2018). Patients with missing information about three or more of the surveyed clinical features were excluded. Additionally, patients with negative molecular testing were excluded as these patients without a clear molecular result may represent a less well-defined group beyond the scope of this analysis. For monozygotic twins with positive molecular testing and phenotypic discordance, only the more affected twin was included. All families provided consent for the study.
Medical records were reviewed for information on diagnosis, characteristics of pregnancy and birth, as well as presence of clinical features. Demographic information regarding race and ethnicity was ascertained from available pedigrees and medical records, which in turn were obtained by family report. Patients were clustered into three groups based on race and ethnicity: Caucasian, Mixed, non-Caucasian. For the purposes of this paper, Hispanic ethnicity was defined based on the U.S. Office of Management and Budget (OMB) as non-Hispanic/Latino or Hispanic/Latino. The Caucasian group was defined as patients who identified as Caucasian and were non-Hispanic/Latino. The Mixed group was defined as patients who identified as two or more races, or those who identified as one race in addition to Hispanic/Latino ethnicity. The non-Caucasian designation was defined as patients who identified as one race other than Caucasian only, or those who defined themselves as Hispanic/Latino only.
Literature review
A literature search was conducted in PubMed to identify patient cohorts in which (epi)genotype-phenotype correlations were evaluated in patients with BWS between January 2000 and July 2017. Studies with patient cohorts in which the incidence of clinical features in molecularly-confirmed patients and/or the molecular subtypes of patients were reported were included, and ten cohorts were identified (Brioude et al., 2013; DeBaun et al., 2002; Ibrahim et al., 2014; Lin et al., 2016; Luk, 2017; Maas et al., 2016; Mussa et al., 2016a, 2016b; Sasaki et al., 2007; Weksberg et al., 2001). Several additional studies were identified that included data from earlier publications, so the earlier publications were excluded to avoid duplicating patients.
The included references were reviewed for information about molecular diagnosis and clinical features in patients and were grouped based on the location of the reporting institutions to develop two cohorts: the European/North American cohort and the Asian cohort. The number of patients presenting with each clinical feature and the total number of patients evaluated for the presence of each feature was recorded from each source as available. If the source included data from other included sources or from patients with negative molecular testing, these patients were not included in the analysis. The total percentage of each feature was calculated to determine the expected incidence for patients from the European/North American cohort and the Asian cohort and used for clinical feature comparisons. The number of patients presenting with each molecular subtype were also recorded from each source and combined to calculate the incidence within the European/North American cohort and the Asian cohort and used for molecular subtype comparisons. Based on the available information, not all ten references were used for the clinical feature and/or molecular subtype comparisons and the specific references included for each are described in the Results section.
Statistical Analysis
Data were analyzed using SPSS Statistics (version 24.0). Descriptive statistics were used to summarize the mean and standard deviation for continuous variables and the frequencies for nominal/categorical variables. Differences between the clinical features in race/ethnicity groups within the cohort were tested with Chi-squared test statistics and Fisher’s exact test when appropriate. One-way t testing was performed to compare the incidence of clinical features in the overall current cohort and within subgroups to expected values based on the literature cohorts. Pearson chi-squared testing was used to evaluate overall differences in molecular subtype incidence between the cohorts and column proportion testing (z-test) with adjusted p-values using the Bonferroni method was used to evaluate for differences between the cohorts in regard to each molecular subtype. Significance was set at p < 0.05.
RESULTS
Characteristics of the Current BWS Cohort
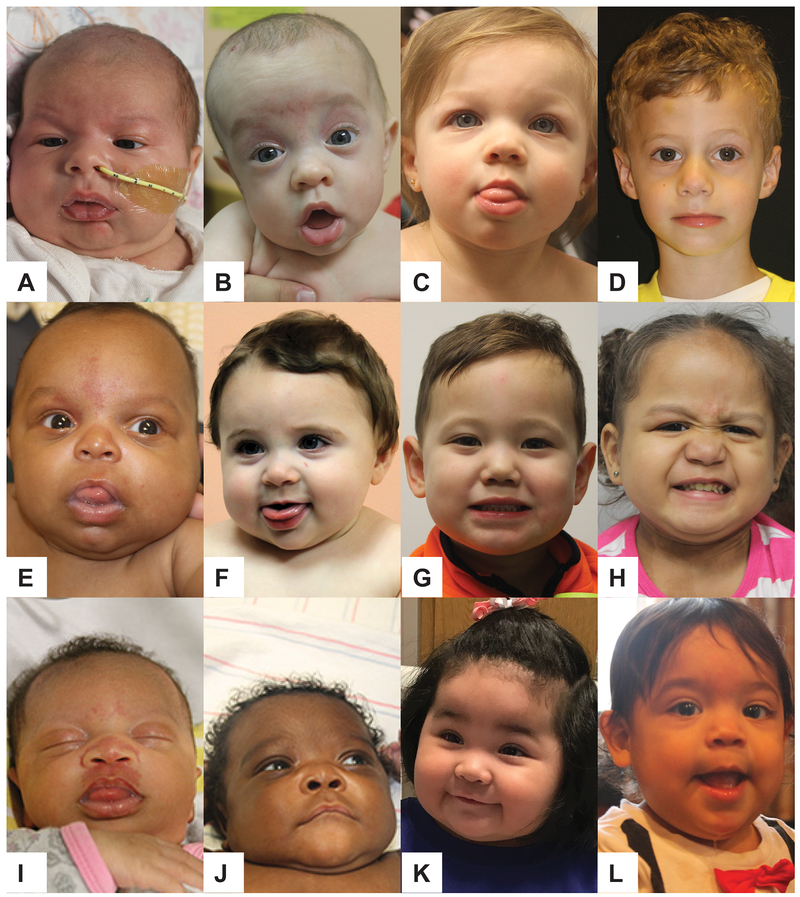
A total of 139 patients were included for analysis. To our knowledge, only 32 of these patients have previously been reported (Kalish et al., 2016; MacFarland et al., 2018; MacFarland et al., 2017; Peterson et al., 2016; Tong et al., 2017). Characteristics about diagnosis including the age at diagnosis and indications for diagnosis were evaluated for differences between the three groups (Caucasian, Mixed, non-Caucasian) within the current cohort. Facial photographs of representative patients within the cohort are shown in Fig 1.
Figure 1 –
Facial photographs of patients with BWS demonstrating features in Caucasian, Mixed, and Non-Caucasian patients. Abbreviations: IC2 (imprinting center 2 loss of methylation); pUPD (paternal uniparental disomy of chromosome 11); GWpUPD (genome wide paternal uniparental disomy)
Top Row Caucasian patients: A) pUPD, 1 month old B) IC2, 5 months old C) pUPD, 15 months old D) IC2, 3 years old
Middle Row Mixed patients: E) IC2, 2 months old F) pUPD, 9 months old G) IC2, 2 years old H) GWpUPD, 3 years old
Bottom Row Non-Caucasian patients: I) IC2, 1 month old J) pUPD, 3 months old K) pUPD, 8 months old L) IC2, 14 months old
Age at Diagnosis
The age at diagnosis was grouped into three categories: Prenatally confirmed (9.5%); Diagnosed in the neonatal period (less than 28 days) (45.3%); and Diagnosed after the neonatal period (45.3%). No significant difference was found in the age categories between the race/ethnicity groups by Pearson chi-square analysis (p=0.377), however a trend was observed with Caucasian patients being diagnosed younger than Mixed and non-Caucasian patients. More than half of the patients in the Mixed and non-Caucasian groups were diagnosed after the neonatal period (52.2% and 54.5% respectively), while more than half of the Caucasian patients were diagnosed either prenatally (12.0%) or in the neonatal period (46.7%). Among patients diagnosed after the neonatal period, no significant differences were found between the groups in the mean or median age at diagnosis (months) by Kruskal-Wallis (p=0.545) and median (p=0.913) testing.
Indications for Diagnosis
The indications for diagnosis, representative of what brought these patients to medical attention, were grouped into four categories: typical BWS clinical features (79.1%); first presenting with hyperinsulinism (10.1%); first presenting with tumor (9.4%); and incidentally diagnosed (1.4%). The indications for diagnosis did not differ significantly between the race/ethnicity groups by Pearson chi-square analysis (p=0.352). A trend was observed with a higher incidence of patients diagnosed after first presenting with hyperinsulinism among the Mixed and non-Caucasian patients (12.5% and 18.2% respectively) compared to the Caucasian patients (7.5%).
Clinical Feature Comparisons in the Current Cohort and in Literature Cohorts
Seven literature cohorts were included for clinical feature analysis. Among the ten identified cohorts, some were excluded if no clinical features were reported (Sasaki et al., 2007), or if it was not possible to determine the incidence of clinical features in the patients with positive molecular testing (DeBaun et al., 2002; Lin et al., 2016). In the Mussa et al. (2016a) cohort, reported incidence of features as percentages, and it was assumed that all patients were evaluated and the percentage was used to calculate the number of patients affected by each feature (Mussa et al., 2016a). Although overlapping cohorts existed between the Mussa et al. (2016a) and (2016b) cohorts, only the data about birth was included from the Mussa et al. (2016b) reference and these features were not recorded from the Mussa et al. (2016a) reference. For this analysis, the European/North American cohort was composed of patients reported from the Netherlands (Maas et al., 2016), France (Brioude et al., 2013), Italy (Mussa et al., 2016a, 2016b), United Kingdom (Ibrahim et al., 2014), and Canada (Weksberg et al., 2001). The Asian cohort was composed of patients reported from China (Luk, 2017) and Korea (Lee et al., 2013).
Significant differences and trends in the incidence of clinical features were found between the subgroups within the current cohort (Table I) and between the current cohort compared to the European/North American cohort (Table II) and Asian cohort (Table III). Notable differences and trends are summarized by clinical feature below:
Table I.
Incidence of Clinical Features in the Current Cohort and Comparisons Between Race/Ethnicity Groups
| Feature | Total (n=139) | Caucasian (n=93) | Mixed (n=24) | Non-Caucasian (n=22) | p-valuea |
|---|---|---|---|---|---|
| Macroglossia | 72.7% | 71.0% | 79.2% | 72.7% | 0.724 |
| Tongue Reductionb | 48.4% | 56.5% | 42.1% | 21.4% | 0.050 |
| Abdominal Wall Defects | 65.5% | 66.7% | 62.5% | 63.6% | 0.912 |
| Omphalocele | 20.9% | 26.9% | 4.2% | 13.6% | 0.034* |
| Umbilical Hernia | 37.7% | 31.5% | 50.0% | 50.0% | 0.108 |
| Diastasis Recti | 18.4% | 18.7% | 26.1% | 9.1% | 0.336 |
| Organomegaly | 45.3% | 41.9% | 36.4% | 68.2% | 0.055 |
| Hepatomegaly | 24.6% | 22.8% | 27.3% | 30.0% | 0.758 |
| Nephromegaly | 26.9% | 22.8% | 22.7% | 50.0% | 0.041* |
| Splenomegaly | 13.4% | 13.0% | 9.1% | 20.0% | 0.574 |
| Lateralized Overgrowth | 82.5% | 82.4% | 79.2% | 86.4% | 0.814 |
| Hypoglycemia | 64.5% | 67.4% | 58.3% | 59.1% | 0.602 |
| Transient hypoglycemiac | 62.9% | 71.0% | 57.1% | 30.8% | 0.021* |
| Hyperinsulinismc | 38.6% | 31.1% | 42.9% | 69.2% | 0.035* |
| Pancreatectomyd | 47.1% | 36.8% | 66.7% | 55.6% | 0.371 |
| Ear creases/pits | 70.4% | 71.1% | 69.6% | 68.2% | 0.960 |
| Facial nevus simplex | 49.3% | 52.7% | 47.8% | 36.4% | 0.383 |
| Tumor | 23.4% | 22.8% | 17.4% | 31.8% | 0.509 |
| Placentomegaly | 17.7% | 18.2% | 14.3% | 19.0% | 0.901 |
| Polyhydramnios | 25.4% | 27.3% | 9.5% | 33.3% | 0.161 |
| Preterm Birth (<37 weeks) | 44.2% | 48.4% | 33.3% | 38.1% | 0.345 |
| LGAe | 65.2% | 60.0% | 79.2% | 71.4% | 0.174 |
significant at p < .05
P values refer to the frequency of each feature between the three subgroups (Caucasian, Mixed, non-Caucasian), so all subgroups are compared at the same time
Percentages reported among just those patients with macroglossia
Percentages reported among just those patients with hypoglycemia
Percentages reported among just those patients with hyperinsulinism
Large for gestational age (>90%ile)
Table II.
Comparison of Clinical Features in Current Cohort to the European/North American Cohort
| Feature | European/North American Cohort | Current Cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Caucasian | Mixed | Non-Caucasian | ||||||
| % | % | p-value | % | p-value | % | p-value | % | p-value | |
| LGAa | 53.6% | 65.2% | 0.006** | 60.0% | 0.221 | 79.2% | 0.006** | 71.4% | 0.093 |
| Preterm Birth (<37 weeks) | 32.0% | 44.2% | 0.005** | 48.4% | 0.002** | 33.3% | 0.893 | 38.1% | 0.581 |
| Polyhydramnios | 30.7% | 25.4% | 0.168 | 27.3% | 0.475 | 9.52% | 0.004** | 33.3% | 0.805 |
| Macroglossia | 83.4% | 72.7% | 0.005** | 71.0% | 0.010** | 79.2% | 0.622 | 72.7% | 0.285 |
| Ear creases/pits | 51.0% | 70.4% | <0.001*** | 71.1% | <0.001*** | 69.6% | 0.072 | 68.2% | 0.106 |
| Facial Nevus Simplex | 51.0% | 49.3% | 0.687 | 52.7% | 0.746 | 47.8% | 0.768 | 36.4% | 0.178 |
| Abdominal Wall Defect | 62.3% | 65.5% | 0.435 | 66.7% | 0.377 | 62.5% | 0.984 | 63.6% | 0.900 |
| Omphalocele | 37.7% | 20.9% | <0.001*** | 26.9% | 0.021* | 4.17% | <0.001*** | 13.6% | 0.004** |
| Umbilical Hernia | 32.5% | 37.7% | 0.213 | 31.5% | 0.841 | 50.0% | 0.107 | 50.0% | 0.124 |
| Diastasis Recti | 29.4% | 18.4% | 0.001** | 18.7% | 0.011* | 26.1% | 0.727 | 9.1% | 0.004** |
| Organomegaly | 46.2% | 45.3% | 0.825 | 41.9% | 0.409 | 36.4% | 0.359 | 68.2% | 0.042* |
| Nephromegaly | 23.8% | 26.9% | 0.427 | 22.8% | 0.825 | 22.7% | 0.908 | 50.0% | 0.034* |
| Hepatomegaly | 18.4% | 24.6% | 0.098 | 22.8% | 0.317 | 27.3% | 0.372 | 30.0% | 0.284 |
| Splenomegaly | 9.5% | 13.4% | 0.186 | 13.0% | 0.318 | 9.1% | 0.949 | 20.0% | 0.267 |
| Hypoglycemia | 47.6% | 64.5% | <0.001*** | 67.4% | <0.001*** | 58.3% | 0.307 | 59.1% | 0.296 |
| Lateralized Overgrowth | 40.8% | 82.5% | <0.001*** | 82.4% | <0.001*** | 79.2% | <0.001*** | 86.4% | <0.001*** |
| Tumor | 10.3% | 23.4% | <0.001*** | 22.8% | 0.005** | 17.4% | 0.390 | 31.8% | 0.046* |
significant at p < .05
significant at p < .01
significant at p < .001
Large for gestational age (>90%ile)
Table III.
Comparison of Clinical Features in Current Cohort to the Asian Cohort
| Feature | Asian Cohort | Current Cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Caucasian | Mixed | Non-Caucasian | ||||||
| % | % | p-value | % | p-value | % | p-value | % | p-value | |
| LGAa | 38.6% | 65.2% | <0.001*** | 60.0% | <0.001*** | 79.2% | <0.001*** | 71.4% | 0.004** |
| Preterm | 54.5% | 44.2% | 0.017 | 48.4% | 0.244 | 33.3% | 0.042* | 38.1% | 0.146 |
| Polyhydramniosb | 18.5% | 25.4% | 0.075 | 27.3% | 0.070 | 9.5% | 0.187 | 33.3% | 0.175 |
| Macroglossia | 79.5% | 72.7% | 0.074 | 71.0% | 0.075 | 79.2% | 0.969 | 72.7% | 0.494 |
| Ear Creases/Pits | 61.4% | 70.4% | 0.025* | 71.1% | 0.046* | 69.6% | 0.414 | 68.2% | 0.512 |
| Facial Nevus Simplexb | 59.3% | 49.3% | 0.020* | 52.7% | 0.207 | 47.8% | 0.293 | 36.4% | 0.040* |
| Omphaloceleb | 14.8% | 20.9% | 0.082 | 26.9% | 0.010* | 4.2% | 0.018* | 13.6% | 0.878 |
| Umbilical Hernia | 52.3% | 37.7% | 0.001** | 31.5% | <0.001*** | 50.0% | 0.827 | 50.0% | 0.835 |
| Organomegalyb | 77.8% | 45.3% | <0.001*** | 41.9% | <0.001*** | 36.4% | 0.001** | 68.2% | 0.355 |
| Hypogylcemia | 45.5% | 64.5% | <0.001*** | 67.4% | <0.001*** | 58.3% | 0.224 | 59.1% | 0.219 |
| Lateralized Overgrowth | 45.5% | 82.5% | <0.001*** | 82.4% | <0.001*** | 79.2% | 0.001** | 86.4% | <0.001*** |
| Tumor | 9.1% | 23.4% | <0.001*** | 22.8% | 0.002** | 17.4% | 0.316 | 31.8% | 0.036* |
significant at p < .05
significant at p < .01
significant at p < .001
Large for gestational age (>90%ile)
Data only from Chinese cohort (not included in Korean)
Macroglossia
Macroglossia incidence did not differ significantly between the groups within our cohort. A trend towards Caucasian patients receiving a tongue reduction more frequently than Mixed and non-Caucasian patients was observed. Macroglossia occurred significantly more frequently in patients from the European/North American cohort compared to patients in our overall cohort and the Caucasian group within our cohort.
Omphalocele
A significant increase in the incidence of omphalocele was observed for Caucasian patients compared to the Mixed and non-Caucasian patients in the current cohort. A significantly lower omphalocele incidence was observed for our overall cohort and within each subgroup compared to the European/North American cohort, although this difference was less significant for the Caucasian patients. A significantly higher incidence in Caucasian patients and significantly lower incidence in Mixed patients from this cohort was found compared to the Asian cohort, however no significant difference was found overall between the literature cohorts.
Organomegaly/Nephromegaly
The overall incidence of organomegaly did not differ significantly between subgroups within our cohort. Compared to the European/North American cohort, our non-Caucasian patients had a significantly higher frequency of organomegaly. The Asian cohort had a significantly higher frequency of organomegaly compared to our overall cohort and the Caucasian and Mixed groups, but not compared to the non-Caucasian patients in our cohort. Nephromegaly was significantly increased in the non-Caucasian patients within our cohort and compared to the European/North American cohort.
Hypoglycemia
The overall incidence of hypoglycemia did not significantly differ between the groups within the current cohort, however incidence was significantly increased for our overall cohort and Caucasian patients compared to the European/North American and Asian cohorts. Among patients with hypoglycemia in our cohort, the degree of hypoglycemia (transient versus hyperinsulinism) was found to differ significantly between the groups. Caucasian patients had a significantly higher frequency of transient hypoglycemia, while non-Caucasian patients had a significantly higher frequency of hypersinsulinism. Although not significant, a trend in hypersinulinism requiring a partial pancreatectomy was found to be higher in patients in the Mixed and non-Caucasian groups compared to the Caucasian group.
Facial Nevus Simplex
No significant difference in the incidence of facial nevus simplex (FNS) was found between subgroups within the current cohort, although a trend was observed in that Caucasian patients were noted to present with FNS more frequently than the non-Caucasian patients and the frequency in the Mixed group was between these two groups.
Compared to the Asian cohort, the entire current cohort and the non-Caucasian subgroup had a significantly lower incidence of FNS. No other significant differences were found between the European/North American cohort and within our own cohort. Although not significant, a lower incidence was observed in our non-Caucasian patients compared to the European/North American cohort, while the other groups had similar frequencies.
Tumor Development
No significant difference in tumor development was found between the race/ethnicity groups in the current cohort. A significantly higher incidence of tumors was demonstrated in our overall cohort compared to both the European/North American cohort and the Asian cohort. Caucasian and non-Caucasian patients in our cohort similarly had a significantly higher incidence compared to the European/North American and Asian cohorts, and no significant difference was demonstrated in Mixed patients compared to these cohorts.
Birth Characteristics
No significant difference in prematurity (defined as <37 weeks) was found between the groups within our cohort, although Caucasian patients tended to be premature more often than the Mixed or non-Caucasian patients. Caucasian patients were found to be premature significantly more frequently than the European/North American cohort, and the incidence of prematurity was significantly higher in our overall cohort compared to the European/North American cohort. Patients in the Asian cohort were premature more frequently than the Mixed patients in our cohort.
Within our cohort, no significant differences in regard to large for gestational age (LGA), defined as >90%ile, were found, although frequency was higher in the Mixed and non-Caucasian patients compared to the Caucasian patients. Our cohort had a significantly higher incidence of LGA overall and within each group compared to the Asian cohort. Compared to the European/North American cohort, only the Mixed patients had a significantly higher incidence, and all other comparisons were not significant.
Molecular Subtype Comparisons in Our Cohort and in Literature Cohorts
No significant differences in molecular etiology between the race/ethnicity groups within the current cohort were found by Pearson chi-square analysis (p=0.693). The most frequent molecular subtype in the Caucasian and Mixed groups was IC2 LOM, while the most frequent subtype in the non-Caucasian group was pUPD11 (Table IV). IC1 GOM was observed slightly more frequently in the Mixed and non-Caucasian patients.
Table IV.
Incidence of Molecular Subtypes within Our Cohort
| Molecular Type | Total | Caucasian | Mixed | Non-Caucasian |
|---|---|---|---|---|
| % | % | % | % | |
| IC1 GOM1 | 10.8% | 9.7% | 12.5% | 13.6% |
| IC2 LOM2 | 48.2% | 51.6% | 50.0% | 31.8% |
| pUPD113 | 29.5% | 25.8% | 29.2% | 45.5% |
| CDKN1C mutation | 3.6% | 4.3% | 4.2% | 0% |
| Chromosome 11p15 abnormality | 7.9% | 8.6% | 4.2% | 9.1% |
Imprinting center 1 gain of methylation
Imprinting center 2 loss of methylation
Paternal uniparental disomy of chromosome 11
Nine patient cohorts reporting the molecular subtypes of BWS patients were compared. The cohort reported by (Mussa et al., 2016b) was not included, as it overlapped with the included cohort by (Mussa et al., 2016a). The European/North American cohort was composed of patients reported from the Netherlands (Maas et al., 2016), France (Brioude et al., 2013), Italy (Mussa et al., 2016a, 2016b), United Kingdom (Ibrahim et al., 2014), Canada (Weksberg et al., 2001), and the United States (DeBaun et al., 2002). The Asian cohort was composed of patients reported from China (Luk, 2017), Japan (Sasaki et al., 2007), Korea (Lee et al., 2013), and Taiwan (Lin et al., 2016).
There was a significant difference between molecular subtypes between our overall cohort, the European/North American cohort, and the Asian cohort by Pearson chi-square analysis (p<0.001) (Table V). Column proportion testing demonstrated a higher incidence of 11p15.5 anomalies in our cohort and the Asian cohort compared to the North American/European cohort and a lower incidence of CDKN1C mutations in the North American/European cohort compared to the Asian cohort. There was a significantly lower incidence of IC2 LOM in our cohort compared to the North American/European cohort.
Table V.
Incidence of Molecular Subtypes between Cohorts
| Molecular Type | Our Cohort | European/North American Cohort | Asian Cohort |
|---|---|---|---|
| % | % | % | |
| IC1 GOM1 | 10.8%a | 8.6%a | 6.1%a |
| IC2 LOM2 | 48.2%a | 62.2%b | 60.6%a,b |
| pUPD113 | 29.5%a | 24.4%a | 20.2%a |
| CDKN1C mutation | 3.6%a,b | 3.2%a | 7.1%b |
| Chromosome 11p15 abnormality | 7.9%a | 1.7%b | 6.1%a |
Imprinting center 1 gain of methylation
Imprinting center 2 loss of methylation
Paternal uniparental disomy of chromosome 11
Subset of cohort categories whose column proportions do not differ significantly from each other at the 0.05 level
There was a significant difference (p<0.001) in the molecular subtypes between the race/ethnicity groups within our cohort, the European/North American cohort, and the Asian cohort (Table VI). Column proportion testing demonstrated a significantly higher frequency of pUPD11 and lower frequency of IC2 LOM among non-Caucasian patients in our cohort compared to the North American/European and Asian cohorts. Patients in the Caucasian group also had a significantly lower frequency of IC2 LOM compared to the European/North American cohort. There was a significantly higher incidence of 11p15.5 anomalies among Caucasian patients in our cohort and Asian patients compared to the European/North American cohort. Patients in the Asian cohort had a significantly higher incidence of CDKN1C mutations compared to the groups within our cohort and the European/North American cohort.
Table VI.
Incidence of Molecular Subtypes within our Cohort and Literature Cohorts
| Molecular Type | Our Cohort | European/North American Cohort | Asian | ||
|---|---|---|---|---|---|
| Caucasian | Mixed | Non-Caucasian | |||
| % | % | % | % | % | |
| IC1 GOM1 | 9.7%a | 12.5%a | 13.6%a | 8.6%a | 6.1%a |
| IC2 LOM2 | 51.6%a,b | 50.0%a,b,c | 31.8%b | 62.2%c | 60.6%a,c |
| pUPD113 | 25.8%a,b | 29.2%a,b | 45.5%b | 24.4%a | 20.2%a |
| CDKN1C mutation | 4.3%a | 4.2%a | 0%a | 3.2%a | 8.5%b |
| Chromosome 11p15 abnormality | 8.6%a | 4.2%a,b | 9.1%a,b | 1.7%b | 7.3%a |
Imprinting center 1 gain of methylation
Imprinting center 2 loss of methylation
Paternal uniparental disomy of chromosome 11
Subset of cohort categories whose column proportions do not differ significantly from each other at the 0.05 level
DISCUSSION
In this work, we reviewed differences in clinical features and molecular subtypes in racial and ethnic subgroups using a cohort from an emerging registry as well as several previously published cohorts. We noted significant differences and trends between the race/ethnicity groups in regard to both clinical features and molecular subtypes. Caucasian patients appear to be more frequently diagnosed with outwardly recognizable features of BWS (omphalocele, macroglossia, FNS) while non-Caucasian patients appear to be more prone to less visually apparent features (hyperinsulinism, nephromegaly). This difference in phenotype may be due to a combination of recognition bias and referral bias. Recognition bias is due to the possibility that facial features are distinct in different populations as has been demonstrated in other syndromes [Kruszka et al., 2017a; Kruszka et al., 2017b; Kruszka et al., 2017c; Kruszka et al., 2018] and as such the facial gestalt in BWS may be under-diagnosed in non-Caucasian groups. Secondly, referral bias may exist in that non-Caucasian patients with BWS may be less frequently referred to a tertiary care center due to socioeconomic stratification and/or other barriers to care and follow-up, resulting in only the extreme cases such as hyperinsulinism being referred. Unfortunately, our data do not dissect the severity of each finding, just its presence. With regard to molecular subtype differences, in the current cohort, non-Caucasian patients had a remarkably lower incidence of IC2 LOM and higher incidence of pUPD11 compared to literature cohorts. In addition, the trends noted in our data suggest that IC1 GOM is more common in Mixed and non-Caucasian patients. It may be possible as well, that these findings are reflective of socioeconomic status, in that IC2 LOM is seen more commonly in IVF pregnancies, which often incur significant out of pocket expenses. Nonetheless, these correlations are consistent with the fact that classic BWS features of omphalocele and macroglossia, which most often correlate with IC2 LOM, are more common in Caucasian patients while severe HI more common in non-Caucasian patients, most often correlates with pUPD11. Finally, these findings may in fact reflect bonafide background variation and there may be specific racial or ethnic differences or modifiers that predispose to different epigenetic/genetic alterations in BWS.
The current cohort had a significantly higher incidence of hypoglycemia and tumors compared to both the European/North American and Asian cohorts. Approximately one-fifth of the patients included in this cohort first presented with hyperinsulinism or tumors. As a result, the patients included in this cohort are likely more representative of the broader Beckwith-Wiedemann spectrum (Brioude et al., 2018).
These differences in molecular subtypes may reflect the differences in clinical features observed between the groups. Caucasian patients were found to have a higher frequency of omphalocele, which is most common in the IC2 group (Brioude et al., 2013; Ibrahim et al., 2014; Mussa et al., 2016a). Similarly, among patients with omphalocele in the general pediatric population, Caucasian was found to be the most common race/ethnicity (Corey et al., 2014), suggesting a background predisposition whereas umbilical hernias are more common in African American patients (Marinkovic & Bukarica, 2003). The non-Caucasian patients were found to have a higher frequency of nephromegaly and hyperinsulinism, which is some cases required partial pancreatectomy. (Epi)genotype-phenotype correlations found nephromegaly to be most common in IC1 GOM and pUPD11 (Goldman et al., 2002; Ibrahim et al., 2014; Mussa et al., 2012). In addition, hyperinsulinism and partial pancreatectomies are most common in patients with pUPD11 (Flanagan, Kapoor, Smith, Hussain, & Ellard, 2011; Hussain et al., 2005; Kalish et al., 2016; Laje et al., 2013; Meissner et al., 2001). There is a trend in IC1 GOM, which can also account for the trend in LGA being observed in Mixed and non-Caucasian patients (Brioude et al., 2013; Mussa et al., 2016b).
These findings raise the question of whether race/ethnicity affects the molecular type of BWS or whether it affects phenotypic features that result from each molecular etiology. We propose that if (epi)genotype-phenotype correlations predominate, then it is likely the molecular subtype affecting the various race groups is responsible for the differences in clinical features observed. If the race/ethnicity of the patient is driving the clinical features, then currently understood (epi)genotype-phenotype correlations may not be entirely accurate, but may be confounded by race/ethnicity. A larger dataset is required to investigate for (epi)genotype-phenotype correlations within racial groups.
Limitations
Certain differences in the current cohort compared to the literature cohorts can be explained by patient ascertainment. Due to the tertiary referral nature of our institution, approximately one-fifth of patients in this cohort were diagnosed with BWS after presenting with hyperinsulinism or a tumor, instead of the classic BWS features. Although these patients had positive molecular testing in one or more tissues, they did not always exhibit some of the classic BWS features, thus affecting the results. These patients highlight the need to detect more subtle BWS features and perform testing in affected tissues when available (Brioude et al., 2018).
Due to the heterogeneity of this group and small numbers of specific racial groups, we were unable to further subclassify the Mixed and non-Caucasian groups. To achieve this, larger data sets are needed to group patients by geographic origin (African, Asian, Latin American, etc.) and to evaluate whether significant differences occur within groups. Additionally, for the literature cohorts, we made assumptions that patients from the European/North American cohort were predominantly Caucasian as no demographic information was reported. This provides a limitation in that the cohort may be more diverse than was assumed in this analysis.
CONCLUSION
In summary, BWS is a heterogeneous syndrome with a spectrum of clinical features. While a racial/ethnic predisposition has not been previously reported, these data suggest that race/ethnicity may play a role in both phenotypic manifestation of BWS and epigenetic/genetic cause. This leads to a potentially distinct phenotype-(epi)genetic correlation within different races or ethnic groups which need to be considered in making the diagnosis.
ACKNOWLEDGEMENTS
We acknowledge the patients with BWS who participated in this study and the funding supporting this work including the National Institutes of Health (K08 CA193915), Alex’s Lemonade Stand Foundation, St. Baldrick’s Foundation, and the University of Pennsylvania Orphan Disease Center (J.M.K.).
Grant numbers:
NCI K08 CA193915; St. Baldrick’s Foundation for Childhood Cancer Research; Alex’s Lemonade Stand Foundation; Orphan Disease Center at the University of Pennsylvania
References
- Brioude F, Kalish JM, Mussa A, Foster AC, Bliek J, Ferrero GB, Maher ER (2018). Expert consensus document: Clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: an international consensus statement. Nat Rev Endocrinol, 14(4), 229–249. doi: 10.1038/nrendo.2017.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioude F, Lacoste A, Netchine I, Vazquez MP, Auber F, Audry G... Rossignol S. (2013). Beckwith-Wiedemann syndrome: growth pattern and tumor risk according to molecular mechanism, and guidelines for tumor surveillance. Horm Res Paediatr, 80(6), 457–465. doi: 10.1159/000355544 [DOI] [PubMed] [Google Scholar]
- Corey KM, Hornik CP, Laughon MM, McHutchison K, Clark RH, & Smith PB (2014). Frequency of anomalies and hospital outcomes in infants with gastroschisis and omphalocele. Early Hum Dev, 90(8), 421–424. doi: 10.1016/j.earlhumdev.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun MR, Niemitz EL, McNeil DE, Brandenburg SA, Lee MP, & Feinberg AP (2002). Epigenetic alterations of H19 and LIT1 distinguish patients with Beckwith-Wiedemann syndrome with cancer and birth defects. Am J Hum Genet, 70(3), 604–611. doi: 10.1086/338934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan SE, Kapoor RR, Smith VV, Hussain K, & Ellard S (2011). Paternal Uniparental Isodisomy of Chromosome 11p15.5 within the Pancreas Causes Isolated Hyperinsulinemic Hypoglycemia. Front Endocrinol (Lausanne), 2, 66. doi: 10.3389/fendo.2011.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M, Smith A, Shuman C, Caluseriu O, Wei C, Steele L... Rosenblum ND. (2002). Renal abnormalities in beckwith-wiedemann syndrome are associated with 11p15.5 uniparental disomy. J Am Soc Nephrol, 13(8), 2077–2084. doi: 10.1097/01 [DOI] [PubMed] [Google Scholar]
- Hussain K, Cosgrove KE, Shepherd RM, Luharia A, Smith VV, Kassem S... Dunne MJ. (2005). Hyperinsulinemic hypoglycemia in Beckwith-Wiedemann syndrome due to defects in the function of pancreatic beta-cell adenosine triphosphate-sensitive potassium channels. J Clin Endocrinol Metab, 90(7), 4376–4382. doi: 10.1210/jc.2005-0158 [DOI] [PubMed] [Google Scholar]
- Ibrahim A, Kirby G, Hardy C, Dias RP, Tee L, Lim D... Maher ER. (2014). Methylation analysis and diagnostics of Beckwith-Wiedemann syndrome in 1,000 subjects. Clin Epigenetics, 6(1), 11. doi: 10.1186/1868-7083-6-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish JM, Biesecker LG, Brioude F, Deardorff MA, Di Cesare-Merlone A, Druley T, Hennekam RC (2017). Nomenclature and definition in asymmetric regional body overgrowth. Am J Med Genet A. doi: 10.1002/ajmg.a.38266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish JM, Boodhansingh KE, Bhatti TR, Ganguly A, Conlin LK, Becker SA, Deardorff MA (2016). Congenital hyperinsulinism in children with paternal 11p uniparental isodisomy and Beckwith-Wiedemann syndrome. J Med Genet, 53(1), 53–61. doi: 10.1136/jmedgenet-2015-103394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszka P, Addissie YA, McGinn DE, Porras AR, Biggs E, Share M, Muenke M (2017). 22q11.2 deletion syndrome in diverse populations. Am J Med Genet A, 173(4), 879–888. doi: 10.1002/ajmg.a.38199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszka P, Porras AR, Addissie YA, Moresco A, Medrano S, Mok GTK... Muenke M. (2017). Noonan syndrome in diverse populations. Am J Med Genet A, 173(9), 2323–2334. doi: 10.1002/ajmg.a.38362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszka P, Porras AR, de Souza DH, Moresco A, Huckstadt V, Gill AD... Muenke M. (2018). Williams-Beuren syndrome in diverse populations. Am J Med Genet A, 176(5), 1128–1136. doi: 10.1002/ajmg.a.38672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszka P, Porras AR, Sobering AK, Ikolo FA, La Qua S, Shotelersuk V... Muenke M. (2017). Down syndrome in diverse populations. Am J Med Genet A, 173(1), 42–53. doi: 10.1002/ajmg.a.38043 [DOI] [PubMed] [Google Scholar]
- Laje P, Palladino AA, Bhatti TR, States LJ, Stanley CA, & Adzick NS (2013). Pancreatic surgery in infants with Beckwith-Wiedemann syndrome and hyperinsulinism. J Pediatr Surg, 48(12), 2511–2516. doi: 10.1016/j.jpedsurg.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Kim GH, Oh TJ, Kim JH, Lee JJ, Choi SH... Yoo, H. W. (2013). Quantitative analysis of methylation status at 11p15 and 7q21 for the genetic diagnosis of Beckwith-Wiedemann syndrome and Silver-Russell syndrome. J Hum Genet, 58(9), 604–610. doi: 10.1038/jhg.2013.67 [DOI] [PubMed] [Google Scholar]
- Lin HY, Chuang CK, Tu RY, Fang YY, Su YN, Chen CP, Lin SP (2016). Epigenotype, genotype, and phenotype analysis of patients in Taiwan with Beckwith-Wiedemann syndrome. Mol Genet Metab, 119(1–2), 8–13. doi: 10.1016/j.ymgme.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Luk HM (2017). Clinical and molecular characterization of Beckwith-Wiedemann syndrome in a Chinese population. J Pediatr Endocrinol Metab, 30(1), 89–95. doi: 10.1515/jpem-2016-0094 [DOI] [PubMed] [Google Scholar]
- Maas SM, Vansenne F, Kadouch DJ, Ibrahim A, Bliek J, Hopman S, Hennekam RC (2016). Phenotype, cancer risk, and surveillance in Beckwith-Wiedemann syndrome depending on molecular genetic subgroups. Am J Med Genet A, 170(9), 2248–2260. doi: 10.1002/ajmg.a.37801 [DOI] [PubMed] [Google Scholar]
- MacFarland SP, Duffy KA, Bhatti TR, Bagatell R, Balamuth NJ, Brodeur GM, Kalish JM (2018). Diagnosis of Beckwith-Wiedemann syndrome in children presenting with Wilms tumor. Pediatr Blood Cancer, 65(10), e27296. doi: 10.1002/pbc.27296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarland SP, Mostoufi-Moab S, Zelley K, Mattei PA, States LJ, Bhatti TR, Kalish JM (2017). Management of adrenal masses in patients with Beckwith-Wiedemann syndrome. Pediatr Blood Cancer, 64(8). doi: 10.1002/pbc.26432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic S, & Bukarica S (2003). [Umbilical hernia in children]. Med Pregl, 56(5–6), 291–294. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed [DOI] [PubMed] [Google Scholar]
- Meissner T, Rabl W, Mohnike K, Scholl S, Santer R, & Mayatepek E (2001). Hyperinsulinism in syndromal disorders. Acta Paediatr, 90(8), 856–859. doi: 10.1111/j.1651-2227.2001.tb02445.x [DOI] [PubMed] [Google Scholar]
- Mussa A, Di Candia S, Russo S, Catania S, De Pellegrin M, Di Luzio L, Ferrero GB (2016). Recommendations of the Scientific Committee of the Italian Beckwith-Wiedemann Syndrome Association on the diagnosis, management and follow-up of the syndrome. Eur J Med Genet, 59(1), 52–64. doi: 10.1016/j.ejmg.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Mussa A, Peruzzi L, Chiesa N, De Crescenzo A, Russo S, Melis D, Ferrero GB (2012). Nephrological findings and genotype-phenotype correlation in Beckwith-Wiedemann syndrome. Pediatr Nephrol, 27(3), 397–406. doi: 10.1007/s00467-011-2009-4 [DOI] [PubMed] [Google Scholar]
- Mussa A, Russo S, De Crescenzo A, Freschi A, Calzari L, Maitz S, Ferrero GB (2016a). (Epi)genotype-phenotype correlations in Beckwith-Wiedemann syndrome. Eur J Hum Genet, 24(2), 183–190. doi: 10.1038/ejhg.2015.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussa A, Russo S, de Crescenzo A, Freschi A, Calzari L, Maitz S, Ferrero GB (2016b). Fetal growth patterns in Beckwith-Wiedemann syndrome. Clin Genet, 90(1), 21–27. doi: 10.1111/cge.12759 [DOI] [PubMed] [Google Scholar]
- Peterson JF, Bick DP, Geddes GC, McCarrier J, Grignon JW Jr., Chirempes B, vanTuinen P (2016). Concomitant 11p15.4-p15.5 duplication and terminal 22q13.33 deletion in a patient with features of Beckwith-Wiedemann syndrome. Am J Med Genet A, 170(12), 3348–3351. doi: 10.1002/ajmg.a.37939 [DOI] [PubMed] [Google Scholar]
- Sasaki K, Soejima H, Higashimoto K, Yatsuki H, Ohashi H, Yakabe S, Mukai T (2007). Japanese and North American/European patients with Beckwith-Wiedemann syndrome have different frequencies of some epigenetic and genetic alterations. Eur J Hum Genet, 15(12), 1205–1210. doi: 10.1038/sj.ejhg.5201912 [DOI] [PubMed] [Google Scholar]
- Tong CC, Duffy KA, Chu DI, Weiss DA, Srinivasan AK, Canning DA, Kalish JM (2017). Urological Findings in Beckwith-Wiedemann Syndrome With Chromosomal Duplications of 11p15.5: Evaluation and Management. Urology, 100, 224–227. doi: 10.1016/j.urology.2016.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksberg R, Nishikawa J, Caluseriu O, Fei YL, Shuman C, Wei C, Squire J (2001). Tumor development in the Beckwith-Wiedemann syndrome is associated with a variety of constitutional molecular 11p15 alterations including imprinting defects of KCNQ1OT1. Hum Mol Genet, 10(26), 2989–3000. doi: 10.1093/hmg/10.26.2989 [DOI] [PubMed] [Google Scholar]
- Weksberg R, Shuman C, & Beckwith JB (2010). Beckwith-Wiedemann syndrome. Eur J Hum Genet, 18(1), 8–14. doi: 10.1038/ejhg.2009.106 [DOI] [PMC free article] [PubMed] [Google Scholar]