Abstract
Introduction
Discrepancies between diffusion tensor imaging (DTI) findings and functional rating scales in amyotrophic lateral sclerosis (ALS) may be due to symptom heterogeneity, particularly coexisting cognitive-behavioural dysfunction affecting non-motor regions of the brain. Carriers of expansion mutations in the C9orf72 gene, whose motor and cognitive-behavioural symptoms span a range from ALS to frontotemporal dementia, present an opportunity to evaluate the relationship between symptom heterogeneity and DTI changes.
Methods
Twenty-eight C9orf72 mutation carriers with varied cognitive and motor symptoms underwent clinical evaluation and DTI imaging. Twenty returned for two or more follow-up evaluations. Each evaluation included motor, executive, and behavioural scales and disease staging using the King’s college staging system (Balendra, 2014).
Results
Widespread reduction of white matter integrity occurred in C9orf72 mutation carriers compared to 28 controls. The ALS Functional Rating Scale (ALSFRS-R) and King’s stage correlated with DTI measures of the corticospinal tract and mid-callosum. Cognitive and behavioural scores correlated with diffusion measures of frontal white matter. King’s stage, but not ALSFRS-R, correlated with anterior callosum DTI measures. Over a 6-month follow-up, DTI changes spread from anterior to posterior, and from deep to superficial subcortical white matter. In C9orf72 carriers with ALS or ALS-FTD, changes in corticospinal tractography measures correlated with changes in ALSFRS-R.
Conclusion
Discrepancies between DTI findings and clinical measures of disease severity in ALS may partly be accounted for by cognitive-behavioural deficits affecting extramotor white matter tracts. Both ALSFRS-R and King’s stage correlated with corticospinal DTI measures. Group-level DTI changes could be detected over six months.
Keywords: C9orf72, Amyotrophic lateral sclerosis, Frontotemporal dementia, Diffusion Tensor Imaging, Disease Staging, Presymptomatic carriers
INTRODUCTION
Neuroimaging has been proposed as a biomarker of the progression of upper motor neuron degeneration in ALS,1,2 but brain imaging findings do not always mirror clinical measures. Such discrepancies may result from clinical scales lacking specificity for decline related to upper, as opposed to lower, motor neuron degeneration. Limitations of the ALS functional rating scale (ALSFRS-R3), the most commonly used scale in ALS clinical trials, have led to alternative ALS disease staging systems,4 such as the “King’s college” staging system, which was found to be sensitive to disease severity in the early stages of ALS.5 Heterogeneity of the ALS patient population, particularly for cognitive dysfunction, may also produce neuroimaging changes unrelated to motor dysfunction. The growing recognition of concurrent cognitive dysfunction in ALS patients has led to calls for incorporating cognitive dysfunction into ALS severity scores.6,7
The need for including a measure of cognitive impairment is particularly acute in ALS patients with expansion mutations in the C9orf72 gene. Carriers of mutations in C9orf72 can present with ALS, frontotemporal dementia (FTD), or a mixture of motor and cognitive symptoms.8–11 ALS patients who carry the C9orf72 mutation have more cortical and subcortical brain atrophy and more diffuse involvement of white matter pathways than patients with sporadic ALS.12–16 Some imaging changes resemble those found in FTD patients17,18 and in cognitively impaired ALS patients without C9orf72 mutations,15 and thus are likely to reflect concurrent cognitive-behavioural impairment.12,14 To capture decline in clinical function, we have used a combination of three brief scales – one each for motor, cognitive, and behavioural impairment – that we found useful for following progression of disease longitudinally in C9orf72 mutation carriers.19
Because carriers of the C9orf72 mutation have varied presentations across these clinical domains, they offer an opportunity to determine whether integrity of specific white matter tracts is associated with function in particular clinical domains. The first goal of this study was to explore the relationship between white matter diffusion measures and the clinical scales for motor, cognitive, and behavioural impairment. The second goal was to understand the trajectory of white matter changes over time by following patients longitudinally. Most patients were able to undergo repeat clinical and imaging evaluations six months later. Patients whose clinical status permitted had follow-up clinical and imaging evaluations one year later (18-month follow-up). The third goal was to evaluate whether DTI measures of the corticospinal tract correlated with the King’s disease stage.
METHODS
Subjects
Twenty-eight unrelated C9orf72 gene mutation carriers (hereafter referred to as C9+ subjects) and twenty-eight healthy controls gave written, informed consent for protocols that were approved by the NIH Combined Neuroscience Institutional Review Board. The C9+ subjects were participating in a prospective natural history and biomarker study with evaluations that specified MRI imaging at 0, 6, 18, and 30 months (NCT01925196). All C9+ subjects had > 30 repeats in the C9orf72 gene by testing in clinical laboratories. The number of repeats was not further quantified. All C9+ subjects were examined by an experienced neurologist and underwent electromyography and a battery of cognitive testing to determine their clinical diagnosis. The 2015 revised El Escorial criteria20 was used to diagnose ALS. The Rascovsky criteria was used for diagnosis of possible or greater behavioural variant FTD (bvFTD).21 Because some subjects met criteria for both ALS and bvFTD, C9+ subjects were classified into four groups: 1) C9+ asymptomatic, 2) C9+ ALS, 3) C9+ bvFTD, and 4) C9+ ALS-FTD. All healthy controls had a normal neurological examination and normal scores on cognitive screening tests.
Clinical Measures of Disease Severity
Three clinical scores, the ALSFRS-R, letter fluency, and Frontal Behavioural inventory (FBI) were used to grade severity of dysfunction in motor, executive, and behavioural domains, as described in our previous study.19 The ALSFRS-R is a 48-point scale assessing bulbar, fine motor, gross motor, and respiratory function.3 The FBI is an examiner-delivered 36-item caregiver questionnaire.22 Items affected by a patient’s motor dysfunction were excluded, and thus the FBI scores are reported as the percent of the total possible score for each patient. Higher FBI scores indicate greater behavioural impairment. Letter fluency, a measure of executive function, was scored as the total number of correct, non-repeated words generated to three letters, either written or spoken, using different letter sets at follow-up visits. Letter fluency was not tested in patients unable to speak or write.
ALS Disease Staging
The ALS disease stage at each visit was determined according to the King’s staging system,4,5 incorporating the following modifications. A stage 0 was added for asymptomatic carriers and stage 2 was divided into stages 2a (newly diagnosed ALS, value 2.0) and 2b (a second CNS region involved, value of 2.5). As described in the original publication, the onset of symptoms in the first CNS segment is Stage 1; symptoms in three CNS segments is Stage 3, and need for gastrostomy or non-invasive ventilation is Stage 4.
Imaging Methods
MRI Acquisition
Multi-slice diffusion weighted images were acquired on a 3T MRI scanner (GE HDX, GE Medical Systems, Milwaukee, WI) with a receive-only, eight-channel head coil using a single-shot spin-echo echo-planar sequence with 64 contiguous axial slices (FOV 240X240, Resolution 96X96, 64 axial slices, 2.5mm slice thickness). A total of 80 diffusion volumes were acquired. Diffusion weighting was applied in 70 directions with 10 volumes at b=300 s/mm2 and 60 volumes at b=1100 s/mm2. Ten additional volumes were collected without gradients applied (b0). The b0 and b=300 s/mm2 volumes were distributed evenly throughout the acquisition, and the DTI protocol was broken up into four scans, each lasting approximately 5 minutes long.23 A high-resolution T2-weighted image was also acquired for anatomical registration and echo-planar distortion correction (fast spin echo, TE/TR 100ms/7100ms, Resolution 256X256, FOV 240X240mm, 100 axial slices, 1.7mm slice thickness). All patients undergoing scanning had a vital capacity of at least 60% predicted; a pulse oximeter was used to monitor patients with vital capacity below 70%, and all had greater than 90% oxygen saturation throughout the scanning session.
Image processing
The TORTOISE software package (https://science.nichd.nih.gov/confluence/display/nihpd/TORTOISE) was used for processing raw diffusion-weighted images, including correcting motion artifacts, eddy current distortion, calculation of the tensor and DTI metrics of fractional anisotropy (FA), mean diffusivity (MD).
Whole-brain analysis of participant groups
A voxel-wise comparison of whole-brain FA skeletons of the C9+ and control groups was carried out in Tract-Based Spatial Statistics (TBSS v1.2, http://www.fmrib.ox.ac.uk/fsl/) using the methods of Smith.24 The most “typical” subject in the study was chosen by TBSS as the target image for registration. A threshold of 0.2 was used to create the mean FA skeleton. Contrasts were performed using the Randomise tool in FSL (v2.9, 5000 permutations), with gender and age as covariates. Thresholding for significant clusters was determined using Threshold-Free Cluster Enhancement, corrected for multiple comparisons across space (FWE). Threshold for significance was p < 0.001 for group comparisons, and p < 0.05 for correlation analyses.
Tract-of-Interest analysis
Fiber tracking was carried out in DTI Studio 25 using methods previously shown to have longitudinal reliability on individual subjects.26,27 The average FA and MD for whole tracts were obtained for eight tracts of interest: right and left corticospinal tracts (CST), right and left uncinate fasciculus (UNC) and four anatomical segments of the corpus callosum – genu (CCg), pre-motor (CCpm), motor (CCm), and splenium (CCsp).27,28
Statistics
Demographic and clinical results are given as means ± standard deviations (SD) in tables and text. The Shapiro-Wilk test was used to test normality. Box-Cox transformation was applied to fiber tracking measures.
Analysis of baseline data
ANOVA with post-hoc Dunnett’s test was used to compare clinical measures between asymptomatic and symptomatic C9+ diagnostic subgroups with threshold of p < 0.05. ANCOVA was used to compare FA and MD between controls and the C9+ groups with age as a covariate. Dunnett’s test was used to compare each C9+ diagnostic subgroup to the healthy control group. Pearson’s correlation was used to assess the relationship between tractography measures and clinical scores. Spearman’s correlation was used to assess relationship of tractography measures to King’s stage.
Analysis of longitudinal data
The changes in tractography measures between diagnosis groups (4 C9+ diagnoses and controls) were examined using a random coefficient model with intercept and time as random effects. The model was also tested with and without age as a covariate to assess the interaction between diagnosis and time. A significant interaction would indicate that the diagnosis groups had different slopes. The relationship between changes in (or within-subject correlation between) ALSFRS-R and CST diffusion measures was assessed using a mixed model to remove the differences between subjects.29
RESULTS
Demographic and Clinical Measures
At the baseline visit, seven C9+ subjects were asymptomatic, eleven had C9+ ALS, seven had C9+ ALS-FTD, and three had C9+ bvFTD (table 1). The asymptomatic C9+ carriers were younger than C9+ ALS-FTD and bvFTD and healthy controls. Among symptomatic subgroups, the age of symptom onset and disease duration did not differ, but C9+ ALS-FTD and bvFTD patients were all male. C9+ subjects and controls did not differ in years of education. The ALSFRS-R score was significantly lower in C9+ ALS subjects compared to other C9+ subjects. The C9+ bvFTD and ALS-FTD subjects had lower scores on the MMSE, Letter Fluency, and FBI, consistent with cognitive-behavioural impairment.
Table 1.
Demographics and clinical features of C9+ and control groups at baseline visit
| Healthy Controls | C9+ diagnosis subgroups | ||||
|---|---|---|---|---|---|
| Asymptomatic | ALS | ALS-FTD | bvFTD | ||
| Number at baseline | 28 | 7 | 11 | 7 | 3 |
| Age (years) | 52.8 ± 9.1 | 42.8 ± 10.1* | 52.4 ± 8.8 | 61.9 ± 9.8† | 60.6 ± 5.1† |
| Male: Female | 18:10 | 2:5 | 5:6 | 7:0 | 3:0 |
| Education (yrs.) | 16.3 ± 2.8 | 15.4 ± 2.8 | 14.8 ± 2.4 | 17.7 ± 1.4 | 14.7 ± 2.3 |
| Disease Duration (months) | - | - | 30.7 ± 23.9 | 37.5 ± 28.6 | 29.4 ± 26.5 |
| MMSE | 29.0 ± 1.2 | 29.0 ± 1.0 | 28.1 ± 2.1 | 25.4 ± 4.1*† | 25.0 ± 4.4*† |
| ALSFRS-R | - | 48 ± 0 | 36.0 ± 8.7† | 41.1 ± 5.1 | 46.0 ± 2.6 |
| FBI Score (%) | - | 3.2 ± 3.3 | 6.7 ± 6.5 | 28.4 ± 11.0† | 36.1 ± 24.2† |
| Letter Fluency Score | - | 34.4 ± 16.6 | 32.6 ± 13.1 | 27.9 ± 16.8† | 17.7 ± 5.5† |
| Follow-up scan interval (mos. from baseline) | 3.0 ± 3.0 (n=23) | 5.3 ± 0.5 (n=5) | 5.6 ± 0.8 (n=6) | 6.3 ± 0.5 (n=5) | 6.2 ± 1.0 (n=3) |
| 2nd follow-up scan interval (mos. from baseline) | 16.3 ± 3.8 (n=4) | 17.7 ± 0.4 (n=4) | 18.9 ± 0.5 (n=2) | 18.4 ± 2.1 (n=2) | |
differs from healthy controls (p < 0.05)
differs from C9+ asymptomatic subgroup (p < 0.05)
MMSE – mini-mental state exam; ALSFRS-R – revised ALS Functional Rating Scale
FBI –Frontal Behavioural Inventory, scored as % of possible
The cohort that had longitudinal scans consisted of twenty C9+ subjects who had two or more follow-up scans. The first follow-up scan was approximately six months later, obtained in 5 asymptomatic, 6 ALS, 5 ALS-FTD, and 3 bvFTD C9+ subjects; and 18 months later in one C9+ asymptomatic subject. Of the 19 who had a 6-month follow-up scan, 11 had an 18-month scan. Twenty-three of the 28 healthy controls had a follow-up scan. The number of participants and inter-scan intervals are shown in table 1.
Diffusion Tensor Imaging Findings
Whole-brain analysis – group differences
At baseline, C9+ subjects had widespread reduction of FA in white matter compared to controls, with a frontal predominance (figure 1A). The affected white matter tracts included the rostral and mid-portions of the corpus callosum, cingulum, superior longitudinal fasciculus, uncinate fasciculi, anterior limb of the internal capsule, and portions of the CST. MD was increased in a more extensive distribution than the changes in FA (figure 1B). However, the splenium of the corpus callosum and occipital subcortical white matter were mostly spared. For the twenty-three healthy controls with longitudinal scans, the whole-brain analysis found no change in FA and MD between scan sessions. In contrast, in the twenty C9+ subjects, white matter regions with reduced FA extended both more posteriorly and more superficially into subcortical white matter at the second scan compared to controls (figure 2A, red = scan 1; orange = scan 2). As at baseline, regions of white matter with increased MD had a broader distribution than the regions with increased FA, extending posteriorly and into temporal lobes (figure 2B, blue=scan 1; green=scan 2). To determine which subgroups accounted for the group changes, the change in FA and MD of the white matter skeletons in the expanded regions between the two scans was analyzed for individual subjects by ANOVA, with Dunnett’s posthoc test. The change in FA was significantly greater in C9+ ALS and ALS-FTD than other C9+ subgroups and controls (p <0.001). Changes in MD in the expanded area were not different across the C9+ diagnostic subgroups (p=0.09).
Figure 1.
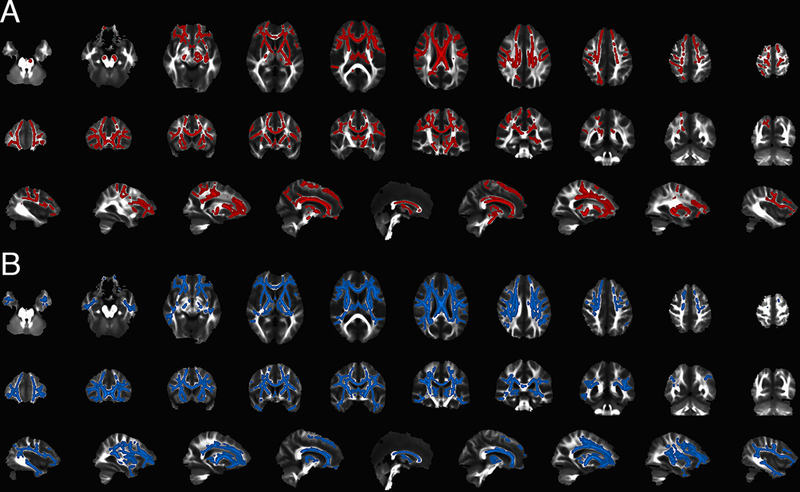
Distribution of white matter alterations in subjects with C9orf72 mutations by whole-brain analysis (TBSS, p <0.001, FWE correction). White matter regions that differ between twenty-eight healthy controls and twenty-eight C9+ subjects are more pronounced frontally, with relative sparing of splenium and occipital white matter. A) Areas with reduced fractional anisotropy (FA) in C9+ subjects are shown in red in axial (top row), coronal (middle row) and sagittal (bottom row) views. B) Areas with increased mean diffusivity (MD) are shown in blue in axial (top row), coronal (middle row) and sagittal (bottom row) views. Images shown with right brain on the left side.
Figure 2.
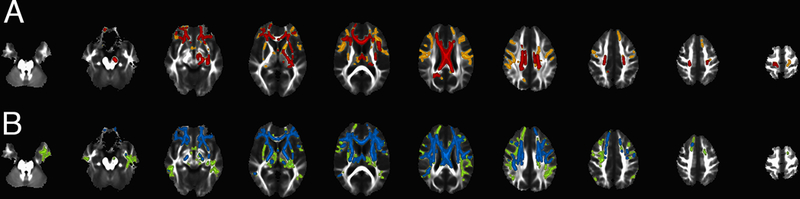
Spread of white matter alterations over time. The distribution of white matter changes at baseline and follow-up scan is shown for 20 C9+ subjects (interval 6.4 ± 2.9 months). (A) Regions of reduced FA compared to healthy controls are shown at baseline in red. At follow-up, additional regions with reduced FA are shown in orange. These regions extend further into fronto-parietal subcortical white matter and anterior limbs of the internal capsule. (B) Regions with increased MD at baseline are shown in blue. At follow-up, additional regions with increased MD, shown in green, extend more posteriorly into parietal lobes and temporal lobes.
Tracts-of-interest analysis
The pattern of white matter tracts with altered whole-tract diffusion measures differed among the symptomatic C9+ subgroups at baseline compared to controls (table 2). Tracts with a reduction of FA mostly corresponded to the particular clinical impairment of the C9+ subgroup, whereas increased MD was more extensive. The CST FA was significantly reduced only in C9+ ALS patients. CST DTI measures in the C9+ bvFTD patients did not differ from controls. All symptomatic subgroups had reduced FA in the motor segment of the corpus callosum. FA of the premotor segment of the callosum was reduced in C9+ ALS and bvFTD patients. Asymptomatic C9+ carriers were not significantly different from controls.
Table 2.
Diffusion tensor imaging measures in white matter tracts of interest at baseline visit
| C9+ Diagnosis subgroups | |||||
|---|---|---|---|---|---|
| Tract | Healthy controls N=28 | Asymptomatic N=7 | ALS N=11 | ALS-FTD N=7 | bvFTD N=3 |
| R CST FA | 0.600 ± 0.023 | 0.604 ± 0.031 | 0.555 ± 0.034* | 0.580 ± 0.055 | 0.603 ± 0.033 |
| R CST MD | 0.703 ± 0.022 | 0.701 ± 0.011 | 0.750 ± 0.035* | 0.753 ± 0.031* | 0.724 ± 0.019 |
| L CST FA | 0.593 ± 0.028 | 0.590 ± 0.020 | 0.549 ± 0.030* | 0.585 ± 0.032 | 0.584 ± 0.018 |
| L CST MD | 0.716 ± 0.021 | 0.711 ± 0.010 | 0.754 ± 0.029* | 0.768 ± 0.026* | 0.741 ± 0.020 |
| R UNC FA | 0.435 ± 0.023 | 0.426 ± 0.009 | 0.424 ± 0.026 | 0.414 ± 0.027 | 0.403 ± 0.018 |
| R UNC MD | 0.810 ± 0.022 | 0.820 ± 0.009 | 0.836 ± 0.024* | 0.876 ± 0.054* | 0.883 ± 0.052* |
| L UNC FA | 0.432 ± 0.023 | 0.428 ± 0.019 | 0.424 ± 0.028 | 0.405 ± 0.038 | 0.412 ± 0.018 |
| L UNC MD | 0.814 ± 0.027 | 0.824 ± 0.013 | 0.848 ± 0.034* | 0.875 ± 0.037* | 0.888 ± 0.041* |
| CCg FA | 0.516 ± 0.032 | 0.525 ± 0.016 | 0.490 ± 0.025 | 0.481 ± 0.043 | 0.484 ± 0.053 |
| CCg MD | 0.860 ± 0.039 | 0.852 ± 0.030 | 0.914 ± 0.029* | 0.991 ± 0.061* | 1.002 ± 0.140* |
| CCpm FA | 0.542 ± 0.024 | 0.540 ± 0.007 | 0.514 ± 0.024* | 0.509 ± 0.037 | 0.488 ± 0.044* |
| CCpm MD | 0.796 ± 0.036 | 0.796 ± 0.022 | 0.840 ± 0.046* | 0.906 ± 0.051* | 0.965 ± 0.187* |
| CCm FA | 0.582 ± 0.017 | 0.578 ± 0.020 | 0.526 ± 0.032* | 0.535 ± 0.033* | 0.531 ± 0.031* |
| CCm MD | 0.755 ± 0.029 | 0.766 ± 0.027 | 0.819 ± 0.052* | 0.849 ± 0.037* | 0.903 ± 0.162* |
| CCsp FA | 0.611 ± 0.017 | 0.616 ± 0.013 | 0.602 ± 0.023 | 0.608 ± 0.014 | 0.609 ± 0.013 |
| CCsp MD | 0.804 ± 0.031 | 0.803 ± 0.014 | 0.836 ± 0.034* | 0.869 ± 0.023* | 0.826 ± 0.038 |
Differs from healthy controls by ANCOVA (age as covariate), Dunnett’s test, adjusted p < 0.05.
ALS, amyotrophic lateral sclerosis; bvFTD, behavioural variant FTD; CCg, corpus callosum—genu; CCm, corpus callosum—motor; CCpm, corpus callosum—pre-motor; CCsp, corpus callosum—splenium; CST, corticospinal tract; FA, fractional anisotropy; L, left; MD, mean diffusivity; R, right; UNC, uncinate fasciculus.
For the longitudinal whole-tract measures of FA and MD of the eight tracts-of-interest in the 20 C9+ and 23 control subjects, the random coefficient model analysis indicated that intercepts differed between groups, reflecting the differences at baseline, but the coefficients for change over time (slopes) were not significantly different between groups. In C9+ ALS patients the CST FA was reduced at baseline and remained low and CST MD was increased at baseline and remained high over follow-up. In C9+ bvFTD and ALS-FTD patients, the UNC FA was reduced and UNC MD was increased at baseline, and at follow-up. In C9+ asymptomatic subjects values of FA and MD for tracts-of-interest were in the range of healthy controls at both time points (supplementary figure).
Clinical Correlations
Imaging Correlations with ALSFRS-R, Fluency, and Behavioural Scale
The correlations between whole-brain white matter measures of FA and MD and ALSFRS-R, letter fluency, and the FBI scores in all C9+ subjects at baseline were evaluated with TBSS. The ALSFRS-R score was correlated with FA values in portions of the CST extending from subcortical white matter to the pons, with a right sided predominance (figure 3A, upper panel). Smaller regions of the right CST MD were correlated with ALSFRS-R. Letter fluency (figure 3B) and FBI scores (figure 3C) correlated with FA and MD values in diffuse white matter regions, largely with a frontal predominance. Affected tracts included the genu of the callosum, the anterior limb of the internal capsule, external capsule and anterior thalamic radiations.
Figure 3.
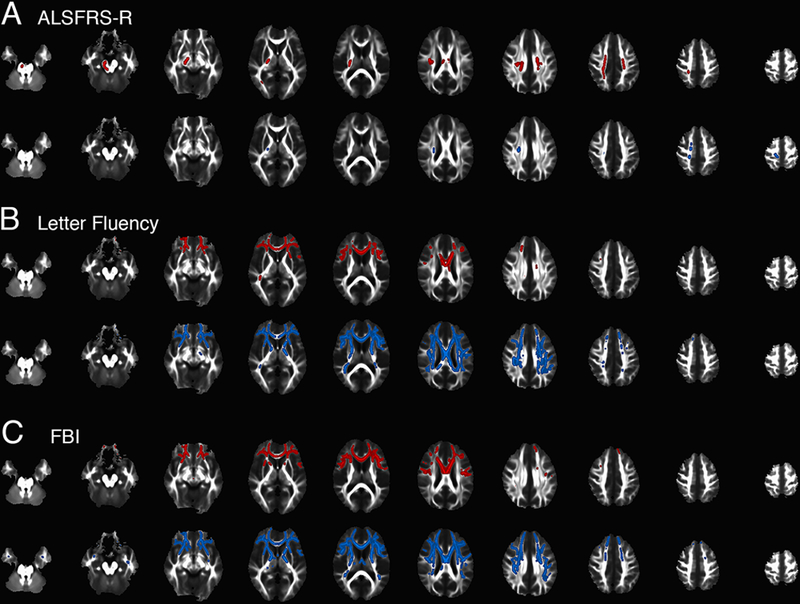
Correlation between FA (red) or MD (blue) in whole-brain white matter analysis and scores on clinical scales at the baseline visit in 28 subjects with C9orf72 mutations. A) Regions of FA (top row, red) correlated with the ALSFRS-R are largely confined to portions of the CST from subcortical white matter to pons. There are small regions of correlated MD in the right CST (blue, bottom row). B) Letter fluency scores are correlated with FA (top row, red) and MD (bottom row, blue) predominantly in frontal white matter and C) Frontal Behavioural Inventory scores are correlated with FA (top row, red) and MD (bottom row, blue) in similar frontal regions.
Whole-tract measures for tracts-of-interest and clinical scores for individual patients, pooling observations over all visits, showed that the ALSFRS-R had moderately strong correlations with CST FA and CST MD values and with FA of the motor segment of the callosum. The FBI score was moderately strongly correlated with FA of the right uncinate fasciculus, genu, and premotor segments of the callosum, and with MD of the uncinate and callosal segments (table 3).
Table 3.
Correlations between DTI measures, ALS disease stage, and clinical scores in 28 C9+ subjects, observations from all visits
| King’s disease stage† | ALSFRS-R | Letter Fluency | FBI | |
|---|---|---|---|---|
| Number of observations | 61 | 61 | 59 | 59 |
| R CST FA | −0.47 | 0.60 | n.s. | n.s. |
| R CST MD | 0.59 | −0.64 | n.s. | n.s. |
| L CST FA | −0.39 | 0.60 | n.s. | n.s. |
| L CST MD | 0.61 | −0.56 | n.s. | 0.48 |
| R UNC FA | n.s. | n.s. | n.s. | −0.51 |
| R UNC MD | n.s. | n.s. | n.s. | 0.81 |
| L UNC FA | n.s. | n.s. | n.s. | n.s. |
| L UNC MD | n.s. | n.s. | n.s. | 0.73 |
| CCg FA | −0.26 | n.s. | n.s. | −0.58 |
| CCg MD | 0.35 | n.s. | n.s. | 0.80 |
| CCpm FA | −0.23 | n.s. | n.s. | −0.58 |
| CCpm MD | 0.27 | n.s. | n.s. | 0.73 |
| CCm FA | −0.54 | 0.60 | n.s. | n.s. |
| CCm MD | 0.48 | n.s. | n.s. | 0.61 |
| CCsp FA | n.s. | n.s. | n.s. | n.s. |
| CCsp MD | 0.40 | n.s. | n.s. | n.s. |
Spearman’s rho; all other columns show Pearson’s r. All correlations shown were signi cant at threshold p<0.05. Correlations with |r|>0.5 are shown in bold.
ALS, amyotrophic lateral sclerosis; ALSFRS-R, revised ALS Functional Rating Scale; CCg, corpus callosum—genu; CCm, corpus callosum—motor; CCpm, corpus callosum—pre-motor; CCsp, corpus callosum—splenium; CST, corticospinal tract; DTI, diffusion tensor imaging; FA, fractional anisotropy; FBI, Frontal Behavioural Inventory; L, left; MD, mean diffusivity; n.s, not signi cant; R, right; UNC, uncinate fasciculus.
The within-subject correlation analysis for the 11 C9+ ALS and ALS-FTD subjects showed that the correlation between changes in clinical measures and changes in whole-tract measures of the CST was significant, indicating that a decline in the ALSFRS-R score was moderately correlated with reduced CST FA and increased CST MD (table 4, figure 3).
Table 4.
Correlation between longitudinal changes in corticospinal tract diffusion measures and changes in ALS Functional Rating Scale (mixed model29)
| Correlation | P value | |
|---|---|---|
| Right Corticospinal Tract FA | 0.4526 | 0.0078 |
| Left Corticospinal Tract FA | 0.6554 | 0.0031 |
| Right Corticospinal Tract MD | −0.5965 | 0.0061 |
| Left Corticospinal Tract MD | −0.6347 | 0.0079 |
FA – fractional anisotropy; MD – mean diffusivity
Correlations Between Disease stage, Imaging
The modified King’s staging system had moderately strong correlations with CST MD and weak correlations with CST FA and FA motor segment of the callosum (table 3). King’s stage was also weakly correlated with FA and MD of anterior segments of the corpus callosum. These callosal segments were strongly correlated with FBI scores, but not with the ALSFRS-R. There was no significant correlation between King’s stage and uncinate diffusion measures.
DISCUSSION
In this cohort of C9orf72 mutation carriers with a mixture of motor and cognitive symptoms, the pattern of clinical deficits corresponded to differing patterns of diffusion changes in white matter tracts. This was best appreciated in the whole-brain analysis which showed that, within the extensive distribution of white matter with diffusion alterations in the C9+ cohort, different white matter regions were correlated with scores on the ALSFRS-R, fluency testing, and the Frontal Behavioural Inventory. These function-specific patterns were detected in comparisons between the C9+ cohort as a whole and healthy controls. The correlation of ALSFRS-R scores with reduced fractional anisotropy of the corticospinal tracts and motor segment of the corpus callosum is consistent with previous studies of sporadic ALS patients.30 These motor tracts were also correlated with the King’s ALS disease stage4 using the tractography method. The similar correlations are not surprising because the King’s stage can be determined by examining the ALSFRS-R. However, the premise of the King’s stages is that it denotes spreading of neurodegeneration from one region of the nervous system to another. It is interesting that the King’s stage was also weakly associated with diffusion measures of white matter regions correlated with cognitive and behavioral function in C9+ patients.31 This correlation may suggest that degeneration of motor and extramotor regions are inseparable components of disease progression caused by C9orf72 mutations. Although previous studies of C9orf72 ALS patients have also reported alterations in frontal white matter and frequent cognitive impairment in C9+ ALS,12,13 this study extends those findings to show that particular tracts are associated with cognitive-behavioural impairment versus motor impairment. Because C9+ cohorts are clinically heterogeneous, a combination of motor and cognitive-behavioural clinical measures is needed to elucidate these specific patterns of white matter disruption.
We used complementary analyses to study white matter integrity. The TBSS whole-brain analysis shows voxel-wise differences in white matter skeletons at the group-level. Fiber tracking provides an average diffusion measure for all voxels within a tract-of-interest for individual subjects. The whole-brain analysis detected extension of regions with affected white matter from baseline to 6 months of follow-up. Regions with DTI changes spread from deep to superficial regions of subcortical white matter, suggestive of Wallerian degeneration, and from anterior to posterior white matter. Fiber tracking was unable to detect changes over time in individual C9+ subjects that differed from controls. The relatively flat slopes of whole-tract measures over time likely occur because the regions of the tract that become affected between scan intervals are a relatively small portion of each tract, with little effect on the average diffusion measurement for the entire tract. In contrast, TBSS can detect differences between small clusters of white matter voxels. Another difference is that tracts-of-interest must be selected for fiber tracking, whereas the whole-brain analysis includes all white matter, including tracts not easily studied by fiber tracking. Fiber tracking showed differences in tracts-of-interest among the C9+ subgroups from the initial scan, which suggests that white matter changes occurred prior to enrolment in the study. In the two C9+ subgroups with ALS, fiber tracking showed changes in corticospinal tract FA and MD occurred with decline in ALSFRS-R over follow-up.
The finding that, over time, regions of affected white matter spread along an anterior- posterior direction agrees with the hypothesis of rostrocaudal spreading of pathology in FTD.32 It is less consistent with the hypothesis of corticofugal spread of disease in ALS, from primary motor cortex to adjacent anterior and posterior cortical areas.33 Although a corticofugal pattern of progression has been supported by cross-sectional imaging studies in sporadic ALS,34 in this cohort of C9orf72 carriers, the pattern of spread in white matter more closely resembles that proposed for FTD. This resemblance may result from the relative enrichment of slower progressing patients, cognitively impaired patients, and unaffected carriers in the longitudinal scans. As shown in Table 1, there was a higher proportion of C9+ ALS patients who were unable to tolerate or return for a follow-up scan due to progression of symptoms. The relatively small sample size, particularly for patient subgroups with different clinical diagnoses, is a limitation of this study. With a sample of only three C9+ patients with pure FTD, no strong conclusions about C9+ FTD can be made. Subgroup diagnoses were based on meeting clinical criteria. There were too few subjects to consider lesser degrees of cognitive and behavioral impairment. Larger cohorts of ALS patients, with and without C9orf72 mutations and characterized for motor and cognitive function, will be needed to establish that patterns of white matter diffusion changes in particular tracts correspond with clinical diagnoses at the level of individual subjects.
Why phenotypic divergence occurs in C9orf72 mutation carriers is not clear. Even within the same family, symptoms of ALS predominate in some individuals and FTD in others.13 One possibility is that distinct disease initiation sites occur in individuals, with spread of degeneration through axonal tracts. Cortical regions where disease frequently begins may express common developmental genes35 that make them similarly vulnerable to the molecular pathways disturbed by the C9orf72 mutation.36 Alternatively, degeneration may occur diffusely in many regions, such that predominant clinical phenotype is determined by the failure of particular critical brain network hubs.37 Understanding the temporal sequence of involvement of different brain areas will require longitudinal multimodal imaging studies and studies of C9+ subjects imaged before and soon after the onset of clinical symptoms.
Familial motor neuron disorders present an opportunity to identify imaging changes associated with early disease activity in presymptomatic gene carriers.38 Determining when disease activity begins is potentially important for deciding the optimal timing for therapy. In Alzheimer disease, for example, CSF proteins and PET imaging findings that can be measured before symptom onset predict progression to cognitive impairment.39 Although pre-clinical imaging changes have been detected by comparing C9orf72 carriers and non-carriers within the same family40 and in large cohorts of asymptomatic carriers of FTD genes,11 we were unable to detect white matter changes at an individual level in this cohort of asymptomatic, unrelated C9orf72 mutation carriers. This group was almost a decade younger than C9+ ALS, and none became symptomatic during the study.
This study showed the potential for diffusion imaging measures to serve as biomarkers of disease progression in C9orf72 mutation carriers, measuring disease severity for motor, cognitive, and behavioural function. The King’s ALS disease stages4 correlated with corticospinal tract diffusion measures to a similar extent as the ALSFRS-R. However, unlike the ALSFRS-R, where scores can drop because of increased functional severity within the same segment, the King’s stages are based on signs and symptoms indicative that disease has spread to involve additional regions of the central nervous system. In C9orf72 carriers with heterogeneous clinical presentations, King’s stages were also weakly correlated with diffusion measures of non-motor tracts, suggesting that stages may be an indicator of more spatially widespread degeneration. The finding that diffusion changes could be detected over six months is encouraging that DTI measures can contribute information about disease progression that would be relevant on the timescale of a clinical trial. However, clear spread of white matter changes over time was only detected in group-level analyses. Additional work is needed to develop reliable imaging measures that can measure disease progression at the level of individual patients.
Supplementary Material
Supplementary Figure 1. Whole-tract values of FA (Figure 1A) and MD (Figure 1B) for the eight tracts-of-interest for all 28 C9orf72 carriers, all intervals up to 18 months of follow-up. Each line represents measures from an individual C9+ subject. Color code for subgroups: black – C9+ asymptomatic carriers; red – C9+ ALS; green C9+ ALS-FTD; blue – C9+ FTD. The right and left sides are shown for the in corticospinal tracts and the uncinate fasciculus. Four segments of the corpus callosum were defined according to the anatomical scheme of Hofer and Frahm (2006): the genu, the “pre-motor” segment, the “motor” segment and the splenium. Shaded area indicates ± 2 SDs mean of 28 healthy controls’ scan for each tract. Note that the corticospinal tract FA is low and MD is increased in most C9+ ALS patients compared to controls at baseline, but abnormal values remain relatively stable over follow-up. In contrast, C9+ patients with FTD and ALS-FTD, tend to have higher MD in the uncinate and anterior segments of the corpus callosum than controls. Diffusion measures for C9+ asymptomatic subjects tend to lie mostly within the range of controls. Nearly all C9+ subjects’ diffusion measures in the splenium fall within the range of controls.
ACKNOWLEDGMENTS
The study was supported by the intramural program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke Z01 NS003146. We gratefully acknowledge the assistance of Jennifer Farren, R.N. and Carol Hoffman for study coordination. We are grateful to physicians who referred patients, and particularly wish to thank the patients and caregivers who participated in the study. Recruitment was made possible in part by ATSDR’s National ALS Registry Research Notification Mechanism (http://wwwn.cdc.gov/ALS/ALSClinicalResearch.aspx).
Abbreviations
- ALS
Amyotrophic lateral sclerosis
- ALSFRS-R
ALS Functional Rating Scale – Revised
- ANCOVA
Analysis of Covariance
- bvFTD
Behavioural variant frontotemporal dementia
- C9+ Subjects
with C9orf72 hexanucleotide expansion mutations
- CCg
Genu of the corpus callosum
- CCm
Motor segment of the corpus callosum
- CCpm
Premotor segment of the corpus callosum
- CCsp
Splenium of the corpus callosum
- CST
Corticospinal tract
- DTI
Diffusion tensor imaging
- FA
Fractional anisotropy
- FBI
Frontal behavioural inventory
- FTD
Frontotemporal dementia
- FWE
Family-wise error
- MD
Mean diffusivity
- SD
Standard deviation
- TBSS
Tract-Based Spatial Statistics – an FSL program
- UNC
Uncinate fasciculus
Contributor Information
Mary Kay Floeter, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda Maryland.
Laura E. Danielian, Email: danielil@ninds.nih.gov, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda Maryland.
Laura E. Braun, Email: laura.braun@nih.gov, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda Maryland.
Tianxia Wu, Email: wuti@mail.nih.gov, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda Maryland.
REFERENCES
- 1.Turner MR, Kiernan MC, Leigh PN, et al. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol 2009;8:94–109. [DOI] [PubMed] [Google Scholar]
- 2.Filippi M, Agosta F, Abrahams S, et al. EFNS guidelines on the use of neuroimaging in the management of motor neuron diseases. Eur J Neurol 2010;17:526–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 1999;169:13–21. [DOI] [PubMed] [Google Scholar]
- 4.Balendra R, Jones A, Jivraj N, et al. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS Functional Rating Scale. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:279–84. [DOI] [PubMed] [Google Scholar]
- 5.Fang T, Al Khleifat A, Stahl DR, et al. Comparison of the King’s and MiToS staging systems for ALS. Amyotroph Lateral Scler Frontotemporal Degener 2017;18:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Chalabi A, Hardiman O, Kiernan MC, et al. Amyotrophic lateral sclerosis: moving towards a new classification system. Lancet Neurol 2016;15:1182–94. [DOI] [PubMed] [Google Scholar]
- 7.Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 2017;18:153–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011;72:257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011;72:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majounie E, Renton AE, Mok K, et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol 2012;11:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohrer JD, Isaacs AM, Mizielinska S, et al. C9orf72 expansions in frontotemporal dementia and amyotrophic lateral sclerosis. Lancet Neurol 2015;14:291–301. [DOI] [PubMed] [Google Scholar]
- 12.Bede P, Bokde AL, Byrne S, et al. Multiparametric MRI study of ALS stratified for the C9orf72 genotype. Neurology 2013;81:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrne S, Elamin M, Bede P, et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurol 2012;11:232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floeter MK, Bageac D, Danielian LE, et al. Longitudinal imaging in C9orf72 mutation carriers: Relationship to phenotype. NeuroImage Clinical 2016;12:1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westeneng HJ, Walhout R, Straathof M, et al. Widespread structural brain involvement in ALS is not limited to the C9orf72 repeat expansion. J Neurol Neurosurg Psychiatry 2016;87:1354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agosta F, Ferraro PM, Riva N, et al. Structural and functional brain signatures of C9orf72 in motor neuron disease. Neurobiol Aging 2017. doi: 10.1016/j.neurobiolaging.2017.05.024 [DOI] [PubMed] [Google Scholar]
- 17.Rohrer JD, Nicholas JM, Cash DM, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol 2015;14:253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agosta F, Galantucci S, Magnani G, et al. MRI signatures of the frontotemporal lobar degeneration continuum. Hum Brain Mapp 2015;36:2602–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Floeter MK, Traynor BJ, Farren J, et al. Disease progression in C9orf72 mutation carriers. Neurology 2017;89:234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludolph A, Drory VE, Hardiman O, et al. A revision of the El Escorial Criteria - 2015. Amyotroph Lateral Scler and Frontotemporal Dementia 2015;16:291–82. [DOI] [PubMed] [Google Scholar]
- 21.Rascovsky K, Salmon DP, Hansen LA, et al. Distinct cognitive profiles and rates of decline on the Mattis Dementia Rating Scale in autopsy-confirmed frontotemporal dementia and Alzheimer’s disease. J Int Neuropsychol Soc 2008;14:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milan G, Lamenza F, Iavarone A, et al. Frontal Behavioural Inventory in the differential diagnosis of dementia. Acta Neurol Scand 2008;117:260–5. [DOI] [PubMed] [Google Scholar]
- 23.Sarlls JE, Pierpaoli C, Talagala SL, et al. Robust fat suppression at 3T in high-resolution diffusion-weighted single-shot echo-planar imaging of human brain. Magn Reson Med 2011;66:1658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–505. [DOI] [PubMed] [Google Scholar]
- 25.Mori S, Kaufmann WE, Davatzikos C, et al. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med 2002;47:215–23. [DOI] [PubMed] [Google Scholar]
- 26.Danielian LE, Iwata NK, Thomasson DM, et al. Reliability of fiber tracking measurements in diffusion tensor imaging for longitudinal study. Neuroimage 2010;49:1572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwata NK, Kwan JY, Danielian LE, et al. White matter alterations differ in primary lateral sclerosis and amyotrophic lateral sclerosis. Brain 2011;134:2642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 2006;32:989–94. [DOI] [PubMed] [Google Scholar]
- 29.Hamlett A, Ryan L, Wolfinger R. On the use of PROC MIXED to estimate correlation in the presence of repeated measures. Proc Statistics and Data Analysis 2004. www2.sas.com/proceedings/sugi29/198-29.pdf. [Google Scholar]
- 30.Filippini N, Douaud G, Mackay CE, et al. Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology 2010;75:1645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohrer JD, Rosen HJ. Neuroimaging in frontotemporal dementia. International review of psychiatry (Abingdon, England) 2013;25:221–9. [DOI] [PubMed] [Google Scholar]
- 32.Brettschneider J, Del Tredici K, Irwin DJ, et al. Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD). Acta Neuropathol 2014;127:423–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braak H, Brettschneider J, Ludolph AC, et al. Amyotrophic lateral sclerosis-a model of corticofugal axonal spread. Nat Rev Neurol 2013;9:708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kassubek J, Muller HP, Del Tredici K, et al. Diffusion tensor imaging analysis of sequential spreading of disease in amyotrophic lateral sclerosis confirms patterns of TDP-43 pathology. Brain 2014;137:1733–40. [DOI] [PubMed] [Google Scholar]
- 35.Cobos I, Seeley WW. Human von Economo neurons express transcription factors associated with Layer V subcerebral projection neurons. Cereb Cortex 2015;25:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron 2013;79:416–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt R, de Reus MA, Scholtens LH, et al. Simulating disease propagation across white matter connectome reveals anatomical substrate for neuropathology staging in amyotrophic lateral sclerosis. Neuroimage 2016;124:762–9. [DOI] [PubMed] [Google Scholar]
- 38.Benatar M, Wuu J. Presymptomatic studies in ALS: rationale, challenges, and approach. Neurology 2012;79:1732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang JH, Korecka M, Figurski MJ, et al. The Alzheimer’s Disease Neuroimaging Initiative 2 Biomarker Core: A review of progress and plans. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2015;11:772–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walhout R, Schmidt R, Westeneng HJ, et al. Brain morphologic changes in asymptomatic C9orf72 repeat expansion carriers. Neurology 2015;85:1780–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Whole-tract values of FA (Figure 1A) and MD (Figure 1B) for the eight tracts-of-interest for all 28 C9orf72 carriers, all intervals up to 18 months of follow-up. Each line represents measures from an individual C9+ subject. Color code for subgroups: black – C9+ asymptomatic carriers; red – C9+ ALS; green C9+ ALS-FTD; blue – C9+ FTD. The right and left sides are shown for the in corticospinal tracts and the uncinate fasciculus. Four segments of the corpus callosum were defined according to the anatomical scheme of Hofer and Frahm (2006): the genu, the “pre-motor” segment, the “motor” segment and the splenium. Shaded area indicates ± 2 SDs mean of 28 healthy controls’ scan for each tract. Note that the corticospinal tract FA is low and MD is increased in most C9+ ALS patients compared to controls at baseline, but abnormal values remain relatively stable over follow-up. In contrast, C9+ patients with FTD and ALS-FTD, tend to have higher MD in the uncinate and anterior segments of the corpus callosum than controls. Diffusion measures for C9+ asymptomatic subjects tend to lie mostly within the range of controls. Nearly all C9+ subjects’ diffusion measures in the splenium fall within the range of controls.


