Abstract
Background
The disordered metabolism of liver function in liver cancer patients can affect postoperative survival after liver transplantation. We assessed whether the levels of various chemicals in liver metabolism prior to receiving a liver transplant were prognostic factors and metabolism markers in predicting survival rate.
Material/Methods
Seventy-seven patients received a living donor liver transplant between June 2012 and April 2016. The basic level of fasting serum GLU, Crea, TBil, TC, TG, HDL, LDL, ApoA1, ApoB100, INR, and MELD scores of 77 patients were retrospectively analyzed. Each patient’s survival was monitored to evaluate prognosis and long-term survival.
Results
The overall survival rates of all patients post-transplant at 6-, 12-, 24-, and 36-month follow-up were 90.9%, 79.2%, 68.8%, and 64.9% respectively. Fasting serum levels of GLU (P=0.004), HDL (P=0.010), LDL (P=0.008), ApoA1 (P=0.028), and MELD scores (P=0.013) prior to liver transplantation were closely associated with the cumulative survival post-transplant in univariate analyses. Controlled fasting GLU of ≤5.12 mmol/L (P=0.019), LDL of ≤2.62 mmol/L (P=0.031), and MELD scores of ≤9 (P=0.013) before LT were significantly and independently associated with increased cumulative survival in the multivariate analyses.
Conclusions
Decreased fasting serum GLU, LDL, and MELD scores as independent risk factors prior to liver transplantation markedly increase cumulative survival.
MeSH Keywords: Lipid Metabolism Disorders, Liver Transplantation, Survival Rate
Background
Liver cancer is the 5th most common cancer worldwide, [1]. Patients with hepatocellular carcinoma undergoing surgical resection alone have good survival, but actuarial survival at 4 years is low [2]. Liver transplantation (LT) had been shown to be an excellent treatment and it is the only therapeutic scheme that treats the cancer and underlying liver diseases simultaneously in appropriately selected patients with liver cancer. Patients should be considered for LT if they had evidence of fulminant hepatic failure, a liver-based metabolic defect, or cirrhosis with complications such as ascites and bleeding caused by portal hypertension [3]. In recent decades, advances in surgical technology and further development of immunosuppressive regimens had led to improved patient survivals in patients undergoing LT, but basic evaluation of transplant candidacy before the transplant is necessary, including confirming the diagnosis and assessing hepatic synthetic function, electrolytes, and renal function and these may best be primarily determined by MELD score. MELD score is a mathematical score determined from the patient’s laboratory tests and is highly predictive of short-term mortality [4]. However, metabolic disorders are common clinical symptoms after hepatic failure and may lead to increased mortality after LT. Many studies suggest that there are multiple metabolic disorders after LT, such as excessive weight gain, obesity, and dyslipidemia [5,6], but one important aspect that is ignored is that the metabolic disorders existing before LT could be the key point affecting the survival rates after LT, and the identification of risk factors for such metabolic disorders has remained an important issue with regard to long-term survival. Currently, 40% of patients with liver cancer have a chance to extend their lives through LT by assessing their metabolic function preoperatively. In the present study we evaluated the relationship between the patients’ metabolism before LT and long-term survival rates by analyzing metabolic data obtained from fasting patients. The purpose of LT is to offer patients the best chance for long-term survival, and the initial poor function of patients’ metabolism before LT may be a significant risk factors for patient survival after LT.
Material and Methods
Study population
Study data were obtained from primary hepatic carcinoma patients who were admitted for orthotopic living donor liver transplantation at the Third Affiliated Hospital of Sun Yat-Sen University, China, between June 2012 and April 2016. The indication for a living donor liver transplantation in our hospital was evidence of hepatic failure and the clinicopathological features. The preoperative clinicopathological features of patients were analyzed by the tumors’ maximum diameter, the number of tumor lesions, the vascular invasion, and the alpha-fetoprotein (AFP) level. Diagnostic criteria mainly included the following: (1) All of the patients were consistent with hepatocellular carcinoma (HCC) clinical diagnostic criteria; and (2) All of the cases were histologically diagnosed with hepatocellular carcinoma and all of the patients with a living donor liver transplantation met the Milan criteria with early hepatocellular carcinoma (EHCC) in this cohort. Seventy-seven patients, including 72 males and 5 females, who received living donor liver transplantation were recruited. The average age was 51.1±9.81 (28~74) years. One patient with intrahepatic cholangiocarcinoma, 55 patients with hepatocellular carcinoma with viral hepatitis or cirrhosis, 13 patients with hepatocellular carcinoma with B viral hepatitis, and 8 patients with hepatocellular carcinoma were assessed before receiving a liver transplantation.
Laboratory data
Laboratory data and anthropometric measurements were obtained at admission or on the day before surgery for each patient. We obtained data from 77 patients undergoing LT, including fasting serum glucose (GLU), creatinine (Crea), total bilirubin (TBil), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), apolipoproteinsA1 (ApoA1), apolipoproteinsB 100 (ApoB100), and international normalized ratio (INR). Model for end-stage liver disease (MELD) scores were calculated before living donor liver transplantation as a formula 3.8ln [TBil(mg/dl)]+11.2ln(INR)+9.6ln [Cr(mg/dl)]+6.4(Etiology: biliary or alcoholic liver disease =0, other=1.).
Statistical analysis
Data were analyzed using SPSS version 18.0 (SPSS, Inc., Chicago, IL). Data are expressed as the mean±standard error of the mean (SEM). X-tile software was used to determine the optimal cut-off values for each index of cumulative survival for further analysis. Laboratory variables and continuous variables were compared between groups using the t-test and the Mann-Whitney U test for variables with abnormal distribution. The chi-squared test or Fisher’s exact probability test was used to compare the survival rate and gender among groups. Univariate and multivariate analyses were performed with Cox regression. Survival curves between groups were prepared using the Kaplan-Meier method and compared using the log-rank test. A P value <0.05 was considered statistically significant.
Data analysis with X-tile software is a well-established method called the Minimum P value Approach. For continuous variables, patients are usually divided into 2 groups according to the threshold value to evaluate prognostic effects on clinical outcomes, and the difference in survival rate between 2 groups is also analyzed. For the selection of the boundary value, the original data can be divided into 2 groups according to different measured data, and the statistical quantity is selected according to the maximum chi-square value by the logarithmic rank test with X-tile software.
Results
Overall patient survival
The overall survival rates of all patients are shown in Figure 1. The overall follow-up duration and median follow-up duration were 53 months and 22 months, respectively. The 6-, 12-, 24- and 36-month overall survival rates were 90.9%, 79.2%, 68.8%, and 64.9%, respectively.
Figure 1.
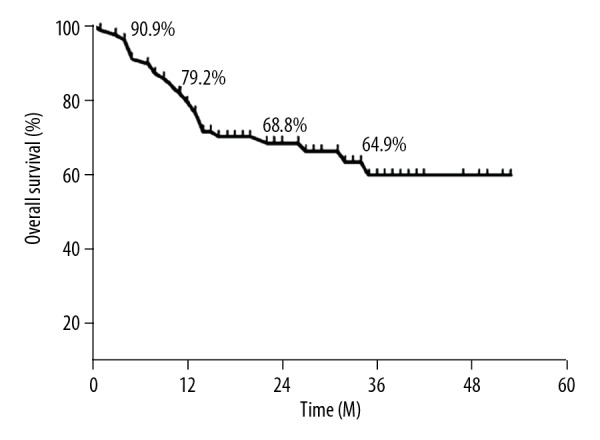
The overall survival curve of all patients with liver transplantation at 6, 12, 24, and 36 months; time is plotted on the x-axis; overall survival rate (%) is plotted on the y-axis.
Univariate analysis of preoperative clinical data
Preoperative fasting data were collected on admission before LT. Table 1 lists all the baseline and pre-transplant factors considered. We found the cut-off values for age, GLU, Crea, TC, TG, HDL, LDL, ApoA1, ApoB100, INR, and MELD score of each index were 50, 5.12, 72, 4.09, 0.87, 0.93, 2.62, 1.08, 0.86, 1.15, and 9, respectively, for further analysis by X-tile software. Univariate analysis revealed positive associations for each of these parameters with overall survival rate at 3 years, except for age (≤50 y, P=0.866); Sex (P=0.754); Crea (≤72 umol/L, P=0.054); TC (≤4.09 mmol/L, P=0.517); TG (≤0.87 mmol/L, P=0.813); ApoB100 (≤0.86 g/L, P=0.323) and INR (≤1.15 P=0.772) (GLU, P=0.004; HDL, P=0.010; LDL, P=0.008; ApoA1, P=0.028, and MELD score, P=0.013) (Table 1).
Table 1.
Univariate analysis of the impact of clinical data preoperatively on cumulative survival of 77 patients with liver transplantation.
| Characteristic | Cases | Survival rates (1y, %, X±SD, %) | Survival rates (3y, %, X±SD, %) | χ2 | p Value for difference |
|---|---|---|---|---|---|
| Age (y) | 0.028 | 0.866 | |||
| ≤50 | 39 | 79.5±6.5 | 57.7±9.9 | ||
| >50 | 38 | 78.9±6.6 | 61.9±9.0 | ||
| Sex | 0.098 | 0.754 | |||
| Male | 72 | 79.2±4.8 | 59.7±7.0 | ||
| Female | 5 | 80±17.9 | 60±21.9 | ||
| GLU (mmol/L) | 8.275 | 0.004* | |||
| ≤5.12 | 40 | 85±5.6 | 78.4±6.9 | ||
| >5.12 | 37 | 73±7.3 | 37.1±11.3 | ||
| Crea (umol/L) | 3.708 | 0.054 | |||
| ≤72 | 41 | 68.3±7.3 | 51.2±9.1 | ||
| >72 | 36 | 91.7±4.6 | 68.2±10.3 | ||
| TC (mmol/L) | 0.420 | 0.517 | |||
| ≤4.09 | 39 | 76.9±6.7 | 69.2±7.4 | ||
| >4.09 | 38 | 81.6±6.3 | 48.4±11.2 | ||
| TG (mmol/L) | 0.056 | 0.813 | |||
| ≤0.87 | 40 | 77.5±6.6 | 67.5±7.4 | ||
| >0.87 | 37 | 81.1±6.4 | 54.2±9.8 | ||
| HDL (mmol/L) | 6.550 | 0.010* | |||
| ≤0.93 | 39 | 66.7±7.5 | 42.3±10.0 | ||
| >0.93 | 38 | 92.1±4.4 | 77.7±7.0 | ||
| LDL (mmol/L) | 7.125 | 0.008* | |||
| ≤2.62 | 41 | 85.4±5.5 | 74±8.0 | ||
| >2.62 | 36 | 72.2±7.5 | 42.8±10.6 | ||
| ApoA1 (g/L) | 4.835 | 0.028* | |||
| ≤1.08 | 39 | 66.7±7.5 | 47.3±9.8 | ||
| >1.08 | 38 | 92.1±4.4 | 71±9.2 | ||
| ApoB100 (g/L) | 0.975 | 0.323 | |||
| ≤0.86 | 39 | 76.9±6.7 | 71.8±7.2 | ||
| >0.86 | 38 | 81.2±6.3 | 40.8±10.4 | ||
| INR | 0.084 | 0.772 | |||
| ≤1.15 | 41 | 80.5±6.2 | 61.1±9.0 | ||
| >1.15 | 36 | 69.4±7.7 | 57.3±10.2 | ||
| MELD | 6.155 | 0.013* | |||
| ≤9 | 41 | 85.4±5.5 | 77.8±6.5 | ||
| >9 | 36 | 72.2±7.5 | 40±10.4 |
GLU – glucose; Crea – creatinine; TC – total cholesterol; TG – triglyceride; HDL – high-density lipoprotein; LDL – low density lipoprotein; ApoA1 – apolipoprotein A1; ApoB100 – apolipoprotein B100; INR – international standardized ratio; MELD scores – model for end-stage liver disease; MELD Score=3.8ln[TBil(mg/dl)]+11.2ln(INR)+9.6ln [Cr(mg/dl)]+6.4. Continuous variables are presented as mean ±SD. Categorical variables are presented as number (%).
P<0.05.
Preoperative serum GLU ≤5.12 mmol/L was identified as a predictive factor of long post-LT survival in univariate analysis (χ2=8.275, P=0.004). LDL and MELD scores were ≤2.62 mmol/L and ≤9, respectively. Patients with lower LDL (≤2.62 mmol/L) also had significantly longer survival at 12 months (85.4±5.5)% and 36 months (74±8.0)% than patients with higher level (>2.62 mmol/L) at 12 months (72.2±7.5)% and 36 months (42.8±10.6)% (χ2=7.125, P=0.008). Similarly, MELD score was a useful determinant of survival rates in LT patients. Patients with lower MELD scores (≤9) had significantly better survival at 12 months (85.4±5.5)% and 36 months (77.8±6.5)% than patients with higher levels (>9) at 12 months (72.2±7.5)% and 36 months (40±10.4)% in univariate analysis (χ2=6.155, P=0.013). However, patients with higher HDL (>0.93 mmol/L) had a better survival rate at 12 months (92.1±4.4)% and 36 months (77.7±7.0)% than patients with lower levels (≤0.9 3 mmol/L) at 12 months (66.7±7.5)% and 36 months (42.3±10.0)% (χ2=6.550, P=0.010). Patients with higher ApoA1(>1.08 g/L) also had a significantly better survival rate at 12 months (92.1±4.4)% and 36 months (71±9.2)% than patients with lower levels (≤1.08 g/L) at 12 months (66.7±7.5)% and 36 months (47.3±9.8)% (χ2=4.835, P=0.028).
Multivariate analysis of the preoperative clinical data
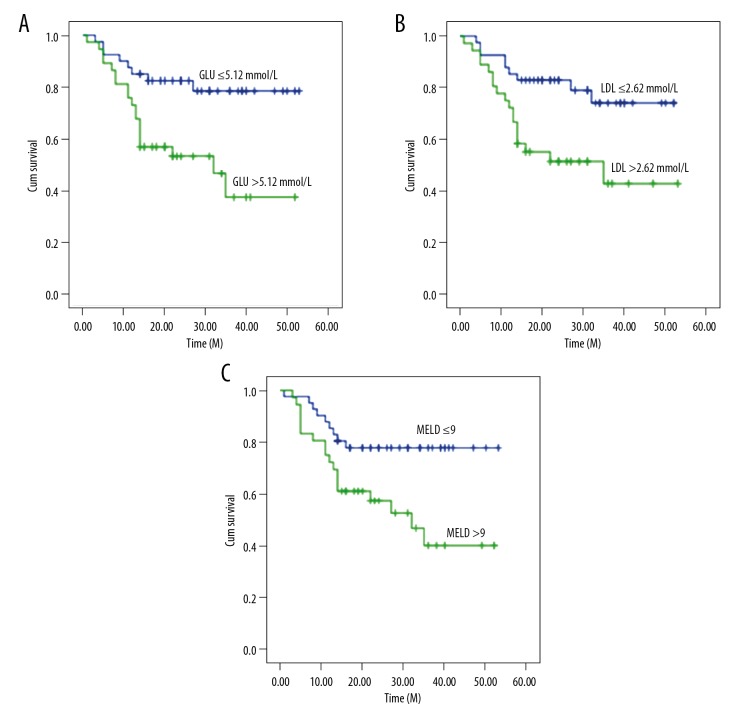
From the results of the univariate analyses identifying factors of significance, multivariate analyses were performed to identify independent variables of significance that could be used to predict patient and graft survival at 36 months. Multivariate analysis indicated that GLU, LDL, and MELD score were the significant parameters (P≤0.05). GLU, LDL, and MELD score were the independent predictors of poor prognosis (GLU, RR=2.926, P=0.019; LDL, RR=2.452, P=0.031; MELD score, RR=2.858, P=0.013; Table 2). We found that the level of serum GLU before LT was 1 unit higher, which would increase the relative long-survival rate by 2.926-fold after LT. Similarly, the 1-unit higher level of LDL before LT increased the relative long-term survival rate by 2.452-fold after LT. If the model for end-stage liver disease score had been elevated by 1 point, the pre-LT survival rate would increase 2.858-fold. Figure 2 shows the overall survival curves according to GLU, LDL, and MELD scores. Independent effects analysis revealed that the cumulative survival rate of patients with fasting serum glucose ≤5.12 mmol/L before LT was about 80% at 36 months, while the cumulative survival rate of patients with preoperative fasting blood glucose higher than 5.12 mmol/L was about 40% at 36 months (Figure 2A). Similarly, the cumulative survival rate of patients with LDL ≤2.62 mmol/L before LT was about 78% at 36 months after surgery, while those with LDL >2.62 mmol/L was about 40% (Figure 2B). Calculating the MELD score of each patient before LT and entering data into a multivariate analysis model to screen MELD scores less than 9, we found that the cumulative survival rate at 36 months reached 80%, but if the patients had pre-LT MELD scores above 9, the postoperative cumulative survival rate was only about 40% (Figure 2C). In addition, we found that each cumulative survival rate of patients with lower fasting blood GLU and blood LDL or lower MELD score before LT were significantly higher than the average level of overall survival rates after LT (Figure 1).
Table 2.
Multivariate analysis of the impact of the clinical data preoperatively on the overall survival of 77 patients with liver transplantation.
| Variables | Partial regression coefficient (βi) | SE(βi) | P value | RR | 95% confidence interval |
|---|---|---|---|---|---|
| GLU | 1.074 | 0.458 | 0.019* | 2.926 | 1.192~7.186 |
| LDL | 0.897 | 0.416 | 0.031* | 2.452 | 1.085~5.543 |
| MELD score | 1.050 | 0.422 | 0.013* | 2.858 | 1.251~6.532 |
GLU – glucose; LDL – low density lipoprotein; MELD scores – model for end-stage liver disease; SE(βi) – standard error of partial regression coefficient; RR – risk ratio;
P<0.05.
Figure 2.
(A–C) Cumulative survival curves for patients with liver transplantation in different groups.
Discussion
Metabolic disorders are common complications before liver transplantation (LT) and may lead to increased morbidity and mortality. Unlike previous studies, which focused on determining whether metabolic abnormality of the serum biochemical index had a certain effect on the survival of patients or animal models after LT [7–12] or during the operation [13], our study focused on the effect of basal preoperative metabolic level on postoperative survival in patients with LT.
In our study, the risk of metabolic factors was analyzed for pre-LT survival rate based on data from fasting patients. The relationship between serum indexes and the survival rates among patients were evaluated after LT. Fasting levels of glucose, lipids, and other body metabolism indexes of 77 patients undergoing primary LT were prospectively analyzed and data were evaluated preoperatively. We found that HDL, ApoA1, GLU, LDL, and MELD scores before LT were associated with postoperative survival rate in the univariate analysis (P<0.05). We found that the serum levels of HDL (>0.93 mmol/L), ApoA1 (>1.08 g/L), GLU (≤5.21 mmol/L), and LDL (≤2.62 mmol/L), and MELD score (≤9) among patients before LT were closely associated with postoperative survival, especially at 3 years.
Studies have reported that the increase of fasting plasma glucose before LT is inversely proportional to the survival rate after transplantation [14], and the effects of fasting plasma glucose may be due to underlying microangiopathy. However, we found that the 3 parameters of GLU, LDL, and MELD were independent factors affecting survival after LT in the multivariate analysis. Importantly, patients with serum GLU ≤5.12 mmol/L before LT had significantly better overall survival compared to patients with GLU >5.12 mmol/L during the observation period. Fasting GLU ≤5.12 mmol/L before LT was associated with significantly decreased survival after LT. Our data disagree with recent reports demonstrating that the node of fasting GLU was a value of 100 mg/dL [14]. However, fasting GLU may be a better marker for prognosis than HbA1c [14], because HbA1c and GA are considered useful clinical markers for blood glucose control at all times, but many studies found they were not useful in cirrhotic patients [15]. Our study suggests that the level of fasting GLU should be maintained at no more than 5.12 mmol/L among patients before LT.
Cumulative survival of patients with lower LDL (≤2.62 mmol/L) was also better than among patients with higher level of LDL (>2.62 mmol/L). There are no reports on the preoperative level of serum LDL as a survival indicator after LT. The concept of “HCC-MELD-exception” [16] was proposed in 2015. Some research shows that post-transplantation survival of patients with HCC without an “HCC-MELD-exception” is significantly worse than the survival of patients without HCC [17]. The most important predictors of poor post-LT survival were MELD score >20 [17]. But in our study, at aspect of association between cumulative survival and MELD, the cumulative survival of patients over score 9 was significantly less than that of patients scores below 9. This could be due to that patient pre-operation with a physiological MELD scores 7 would develop significant cholestasis one week following liver transplantation [18]. Use of the X-tile software revealed the MELD node preoperatively was 9, but there may be some differences in the characteristics of the population, such as the protopathy with liver cirrhosis or liver cancer or others compared with other research. The basic profiles of our population were 1 patient of intrahepatic cholangiocarcinoma, 55 patients of hepatocellular carcinoma with viral hepatitis or cirrhosis, 13 patients of hepatocellular carcinoma with B viral hepatitis and 8 patients of hepatocellular carcinoma were present before received LT. Thus, large and multicenter case series could be needed to refine the estimates to post-LT outcomes.
In our study, if the level of serum GLU, LDL and MELD score preoperative were elevated every one unit or one point, the relative survival risk would be increased to 2.926, 2.452 and 2.858 times respectively. We also found that the cumulative survival rate of patients with lower fasting GLU, LDL, and lower MELD score preoperative were significantly higher than the average survival rate after LT. Therefore, restraining serum levels of GLU, LDL among patients before LT would significantly affect long-term survival among patients. And the evaluation of MELD score preoperative would also be important to survival.
Survival should be remained the main criteria to assess LT [19] at present. In the cumulative survival curve analysis, the postoperative cumulative survival rate of preoperative patients with higher fasting GLU, higher blood LDL or higher MELD score were significantly lower than the overall survival rates at 36-month after surgery respectively. On contrary, the cumulative survival rate of patients with lower fasting GLU, lower blood LDL and lower MELD score preoperative were significantly higher than the average survival rate after LT. The cumulative survival rate of 36-months was up to 80%. Therefore, pre-operative GLU, LDL, and MELD scores could probably be used as a guide for the introduction of patients undergoing liver transplantation. In addition, the lower level of GLU, LDL and MELD scores were timely evaluated and controlled before LT to improve the survival rate.
Conclusions
Finally, although there are several limitations to our study, we suggest that an examination of factors associated with higher risk factors such as higher GLU, higher LDL, and higher MELD scores pre-LT must be accurately measured and monitored at the right level. Higher GLU and LDL levels and MELD scores preoperatively were found to be independent risk factors for the cumulative survival rate. Assessing the preoperative levels of GLU and LDL and MELD scores could improve liver transplantation outcomes and patient prognosis.
Abbreviations
- GLU
glucose
- Crea
creatinine
- TC
total cholesterol
- TG
triglyceride
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- ApoA1
apolipoprotein A1
- ApoB100
apolipoprotein B100
- INR
international standardized ratio
- MELD scores
model for end-stage liver disease
- SE(βi)
standard error of partial regression coefficient
- RR
risk ratio
Footnotes
Source of support: This work was supported by a grant for Integrated Project of Production, Learning and Research of Guangdong Education Department (2013B090500101) and Natural Science Foundation of Guangdong Province (2014A030313095) and Wu Jieping Medical Fund (320675017353)
Conflict of interests
None.
Statement
The study was approved by the institutional ethics committee of the Third Affiliated Hospital of Sun Yat-Sen University. All procedures were performed in accordance with the ethical standards laid down in an appropriate version of the 2000 Declaration of Helsinki as well as the Declaration of Istanbul 2008. Informed consent was obtained from each patient prior to inclusion in the study.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–56. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Chiappa A, Zbar AP, Audisio RA, et al. Factors affecting survival and long-term outcome in the cirrhotic patient undergoing hepatic resection for hepatocellular carcinoma. Eur J Surg Oncol. 2000;26:387–92. doi: 10.1053/ejso.1999.0904. [DOI] [PubMed] [Google Scholar]
- 3.O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008;134:1764–76. doi: 10.1053/j.gastro.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 4.Gyori GP, Silberhumer GR, Zehetmayer S, et al. Dynamic changes in MELD score not only predict survival on the waiting list but also overall survival after liver transplantation. Transpl Int. 2012;25:935–40. doi: 10.1111/j.1432-2277.2012.01519.x. [DOI] [PubMed] [Google Scholar]
- 5.Reuben A. Long-term management of the liver transplant patient: Diabetes, hyperlipidemia, and obesity. Liver Transpl. 2001;7:S13–21. doi: 10.1053/jlts.2001.29167. [DOI] [PubMed] [Google Scholar]
- 6.Akarsu M, Bakir Y, Karademir S, et al. Prevalence and risk factors for obesity after liver transplantation: A single-center experience. Hepat Mon. 2013;13:e7569. doi: 10.5812/hepatmon.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kissler HJ, Hauffen J, Hennig R, et al. Glucose and lipid metabolism after liver transplantation in inbred rats: Consequences of hepatic denervation. Metabolism. 2005;54:881–90. doi: 10.1016/j.metabol.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 8.Park CS. Predictive roles of intraoperative blood glucose for post-transplant outcomes in liver transplantation. World J Gastroenterol. 2015;21:6835–41. doi: 10.3748/wjg.v21.i22.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siirtola A, Antikainen M, Ala-Houhala M, et al. Insulin resistance, LDL particle size, and LDL susceptibility to oxidation in pediatric kidney and liver recipients. Kidney Int. 2005;67:2046–55. doi: 10.1111/j.1523-1755.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 10.Sahin E, Karaman G, Uslu M, et al. Adiponectin levels, insulin resistance and their relationship with serum levels of inflammatory cytokines in patients with Behcet’s disease. J Eur Acad Dermatol Venereol. 2012;26:1498–502. doi: 10.1111/j.1468-3083.2011.04318.x. [DOI] [PubMed] [Google Scholar]
- 11.Zant R, Melter M, Beck D, et al. Glucose metabolism and associated outcome after pediatric liver transplantation. Transplant Proc. 2016;48:2709–13. doi: 10.1016/j.transproceed.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Van Laecke S, Desideri F, Geerts A, et al. Hypomagnesemia and the risk of new-onset diabetes after liver transplantation. Liver Transpl. 2010;16:1278–87. doi: 10.1002/lt.22146. [DOI] [PubMed] [Google Scholar]
- 13.Ammori JB, Sigakis M, Englesbe MJ, et al. Effect of intraoperative hyperglycemia during liver transplantation. J Surg Res. 2007;140:227–33. doi: 10.1016/j.jss.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Katsura E, Ichikawa T, Taura N, et al. Elevated fasting plasma glucose before liver transplantation is associated with lower post-transplant survival. Med Sci Monit. 2016;22:4707–15. doi: 10.12659/MSM.897925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010;57:751–62. doi: 10.1507/endocrj.k10e-138. [DOI] [PubMed] [Google Scholar]
- 16.Northup PG, Intagliata NM, Shah NL, et al. Excess mortality on the liver transplant waiting list: Unintended policy consequences and Model for End-Stage Liver Disease (MELD) inflation. Hepatology. 2015;61:285–91. doi: 10.1002/hep.27283. [DOI] [PubMed] [Google Scholar]
- 17.Ioannou GN, Perkins JD, Carithers RJ. Liver transplantation for hepatocellular carcinoma: Impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342–51. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Agopian VG, Harlander-Locke MP, Markovic D, et al. Evaluation of early allograft function using the liver graft assessment following transplantation risk score model. JAMA Surg. 2018;153:436–44. doi: 10.1001/jamasurg.2017.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seiler CA, Dufour JF. Survival after liver transplantation. Lancet. 2001;357:72. doi: 10.1016/s0140-6736(05)71573-7. [DOI] [PubMed] [Google Scholar]