Abstract
Background
Meteorin-like (Metrnl) is a novel adipomyokine that may improve glucose tolerance and affect insulin resistance. This study aimed to investigate the association between serum levels of Metrnl with blood glucose status and to its association with insulin resistance.
Material/Methods
The study included 160 subjects with normal glucose tolerance (NGT) (n=40), impaired fasting glucose (IFG) (n=40), impaired glucose tolerance (IGT) (n=40), and newly diagnosed type 2 diabetes mellitus (T2DM) (n=40). An enzyme-linked immunosorbent assay (ELISA) was used to measure the serum levels of Metrnl. Partial correlation analysis was used to analyze the relationship between serum levels of Metrnl and metabolic parameters. Multiple logistic regression analysis was performed to identify the association between serum levels of Metrnl with the risk of diabetes.
Results
Serum levels of Metrnl was highest in patients with T2DM and significantly increased in patients with prediabetes compared with individuals with NGT. After adjusting for age, gender, and body mass index (BMI), serum Metrnl level was significantly correlated with lipid profile, glucose profile, and insulin resistance. Multiple logistic regression analysis showed that Metrnl significantly increased the risk of T2DM (OR=1.727; P=0.008) before adjusting for the homeostatic model assessment of insulin resistance (HOMA-IR). When further adjusted for HOMA-IR, Metrnl was no longer associated with an increased OR for T2DM (OR=1.491; P=0.066), while the HOMA-IR significantly increased the risk of T2DM (OR=1.935; P=0.008).
Conclusions
Serum levels of Metrnl were significantly increased in patients with T2DM and may increase the risk of T2DM independent of insulin resistance.
MeSH Keywords: Cytokines; Diabetes Mellitus, Type 2; Insulin Resistance
Background
Insulin resistance is a risk factor for the development of type 2 diabetes mellitus (T2DM). Improving insulin resistance continues to be an important area of research into the treatment of T2DM and metabolic syndrome, as new insulin-sensitizing treatments are needed [1,2]. Secreted proteins, including adipokines and myokines, have been shown to be closely associated with the development of insulin resistance and have the potential for drug development in the treatment of obesity and T2DM [3–10].
Meteorin-like (Metrnl), a secreted adipomyokine, was recently identified and shown to be induced by exercise or cold exposure [5,11,12]. Metrnl increases energy expenditure and improves insulin sensitivity by inducing the expression of genes associated with brown fat thermogenesis in mice [5]. This finding raises the possibility that Metrnl has a role in the pathophysiological processes of metabolic diseases, including T2DM. The findings of previously published studies have shown that serum levels of Metrnl are increased in patients with T2DM, but the findings are controversial. Lee et al. [13] and Zheng et al. [14] reported lower serum levels of Metrnl in patients with newly diagnosed T2DM, while Chung et al. [15] identified increased serum levels of Metrnl. Also, in preclinical studies, the effects of Metrnl on adipose tissue in mice showed that expression of Metrnl by adipocytes could increase insulin sensitivity by stimulating the peroxisome proliferator-activated receptor gamma (PPARγ) pathway [16]. The overexpression of Metrnl has been shown to reduce lipogenesis and inhibit the expression of PPARγ in human adipocytes, which may lead to hyperinsulinemia and insulin resistance [17].
Given the possible therapeutic role of Metrnl in patients with T2DM, the aim of this study was to investigate the association between serum levels of Metrnl with blood glucose status and to investigate its association with insulin resistance. Serum levels of Metrnl were measured in patients with newly diagnosed T2DM and compared with patients with normal glucose tolerance (NGT), impaired fasting glucose (IFG), and impaired glucose tolerance (IGT).
Material and Methods
Patients and control subjects
This observational study included 160 subjects. Four groups were studied that included subjects with normal glucose tolerance (NGT) or healthy volunteers (n=40), a group with impaired fasting glucose (IFG) (n=40), a group with impaired glucose tolerance (IGT) (n=40), and a group with newly diagnosed type 2 diabetes mellitus (T2DM) (n=40). The study was conducted at the Qilu Hospital of Shandong University from November 2017 to May 2018 and was approved by the Ethics Committee of Shandong University, Qilu Hospital (Approval No. 2017-014).
Patients with previously diagnosed T2DM, other types of diabetes, severe cardiovascular or cerebrovascular diseases, severe liver or renal disease, and medication history including the use of antidiabetics, statins, diuretics, corticosteroids, estrogen, and progestin, which influence glucose tolerance and insulin sensitivity, were excluded. T2DM was diagnosed as a fasting blood glucose (FBG) ≥7.0 mmol/L and/or 2-hour postprandial blood glucose (PBG) ≥11.1 mmol/L. IFG was diagnosed as an FBG of 6.1–7.0 mmol/L and a PBG <7.8 mmol/L. IGT was diagnosed as an FBG <6.1 mmol/L and a PBG of 7.8–11.1 mmol/L. These diagnostic criteria were in accordance with the 2006 World Health Organization (WHO) criteria [18]. Overweight and obesity was diagnosed by a body mass index (BMI) ≥25.0 kg/m2 based on the criteria recommended by the WHO [19].
Clinical data
Detailed clinical and demographic data, including past medical history of the study participants in the four study groups, were collected by trained clinical investigators. Height and weight measurements were used to calculate BMI. The waist circumference (WC) was measured from the midpoint between the lower border of the ribcage and the anterior superior iliac spine. After sitting for at least 5 min, blood pressure (BP) was measured consecutively three times on the left arm using an Omron Model HEM-752 sphygmomanometer (Omron Company, Dalian, China), and the average value was recorded.
Laboratory tests
After a 10-hour overnight fast, a sample of fasting venous blood was collected at 7: 00 am and the serum was used for the measurement of fasting blood glucose (FBG), fasting insulin, total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), uric acid (UA), and creatinine by using an Architect ci16200 integrated automatic analyzer (Abbott Labs, Abbott Park, Ill, USA). Glycated hemoglobin (HbA1c) was measured by high-performance liquid chromatography (HPLC) using a VARIANT™ II TURBO (Bio-Rad, Hercules, CA, USA). PBG was measured after all subjects had completed an oral glucose tolerance test (OGTT) of 75 g of glucose.
Measurement of serum Metrnl levels
Serum Metrnl levels were measured using enzyme-linked immunosorbent assay (ELISA) kits (Code: CSB-EL013718HU) (CusaBio Biotech Co., Ltd., Wuhan, China). The homeostatic model assessment of insulin resistance (HOMA-IR) index was calculated as the fasting insulin concentration (mIU/L)×FBG concentration (mmol/L)/22.5. The estimated glomerular filtration rate (eGFR), based on the serum creatinine level was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [20].
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 software (SPSS Inc., Chicago, Ill, USA). The continuous variables with a normal distribution were expressed as the mean ± standard deviation (SD), while those with a non-normal distribution were expressed as the median and interquartile range (IQR). The Kolmogorov-Smirnov test was performed to analyze the normality of the data. Between-group differences were analyzed using one-way analysis of variance (ANOVA) using Tukey’s honestly significant difference (HSD) test or the chi-squared (χ2) test. Partial correlation analysis was used to evaluate the relationship between serum levels of Metrnl and metabolic parameters. Multiple logistic regression analysis was used to determine the association of serum Metrnl levels and the risk of T2DM. A P-value <0.05 was considered to be statistically significant.
Results
Clinical and laboratory characteristics of the study participants
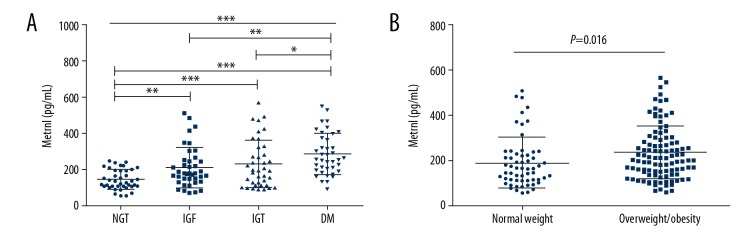
Table 1 shows the comparison of the demographic, clinical, and laboratory data between the fours study groups, subjects with normal glucose tolerance (NGT) or healthy volunteers, the group with impaired fasting glucose (IFG), the group with impaired glucose tolerance (IGT), and the group with newly diagnosed type 2 diabetes mellitus (T2DM). Compared with the other groups, the group with T2DM had significantly higher systolic blood pressure (BP), glycated hemoglobin (HbA1c), fasting blood glucose (FBG), postprandial blood glucose (PBG), homeostatic model assessment of insulin resistance (HOMA-IR) index, alanine aminotransferase (ALT), and aspartate aminotransferase (AST). Serum levels of Metrnl were also highest in patients with T2DM (Figure 1A), and the serum levels of Metrnl level were higher in the prediabetes groups than in the NGT subjects, although there was no significant difference between the IFG and IGT groups. Serum levels of Metrnl level between subjects with normal weight were significantly different when compared with levels in overweight and obese subjects (Figure 1B).
Table 1.
Characteristics of the subjects in the four study groups with normal glucose tolerance (NGT), impaired fasting glucose (IFG), impaired glucose tolerance (IGT), and type 2 diabetes mellitus (T2DM).
| Characteristic | NGT (n=40) | IFG (n=40) | IGT (n=40) | T2DM (n=40) | P-valuea |
|---|---|---|---|---|---|
| Female (%) | 18 (45.0) | 16 (40.0) | 20 (50.0) | 16 (40.0) | 0.773 |
| Age (years) | 50.68±5.40 | 51.50±5.27 | 50.65±6.27 | 52.13±7.91 | 0.676 |
| BMI (kg/m2) | 24.73±2.57 | 26.02±3.09 | 26.35±3.38 | 27.58±3.10b | 0.001 |
| WC (cm) | 84.28±8.45 | 88.52±8.99 | 88.41±9.37 | 92.11±7.38b | 0.001 |
| Systolic BP (mmHg) | 128.35±14.99 | 132.33±12.00 | 132.28±13.13 | 141.20±14.00bcd | <0.001 |
| Diastolic BP (mmHg) | 78.13±8.63 | 82.55±7.34 | 82.55±8.12 | 85.68±6.44b | <0.001 |
| FBG (mmol/L) | 5.23±0.30 | 6.38±0.20b | 5.53±0.32c | 8.44±2.44bcd | <0.001 |
| PBG (mmol/L) | 5.80±0.91 | 6.90±0.48 | 8.99±0.83bc | 14.85±3.92bcd | <0.001 |
| HbA1c (%) | 4.89±0.35 | 5.35±0.34 | 5.44±0.39b | 7.08±1.55bcd | <0.001 |
| Fasting insulin (μU/mL) | 4.55 (3.32–6.05) | 8.05 (5.98–9.83)b | 8.35 (5.30–10.85)b | 7.75 (4.69–10.10)b | <0.001 |
| HOMA-IR (index) | 1.08 (0.80–1.41) | 2.31 (1.63–2.78)b | 2.13 (1.33–2.65)b | 2.59 (1.66–3.64)bcd | <0.001 |
| TC (mmol/L) | 4.64±0.55 | 4.99±0.72 | 5.02±1.09 | 5.05±0.80 | 0.080 |
| TG (mmol/L) | 0.94 (0.71–1.07) | 1.25 (0.96–2.00)b | 1.35 (0.97–1.98)b | 1.63 (1.10–2.17)bc | <0.001 |
| LDL-C (mmol/L) | 2.90±0.61 | 3.22±0.64 | 3.18±0.77 | 3.33±0.64b | 0.032 |
| HDL-C (mmol/L) | 1.43±0.27 | 1.39±0.43 | 1.40±0.44 | 1.31±0.29 | 0.489 |
| ALT (U/L) | 18.04±4.50 | 17.30±5.24 | 17.89±5.57 | 23.31±8.50bcd | <0.001 |
| AST (U/L) | 19.91±3.83 | 18.80±4.34 | 19.05±4.37 | 22.33±7.45bcd | 0.011 |
| Creatinine (μmol/L) | 61.92±12.81 | 62.56±9.23 | 62.00±10.24 | 63.32±10.26 | 0.943 |
| UA (umol/L) | 269.20±76.06 | 273.12±51.69 | 260.35±63.87 | 273.75±75.65 | 0.801 |
| Metrnl (pg/mL) | 126.86 (103.44–193.94) | 176.76 (135.89–246.20)b | 192.69 (116.84–311.12)b | 256.79 (193.97–384.53)bcd | <0.001 |
Data are presented as the mean ± standard deviation (SD) or the median (interquartile range). NGT – normal glucose tolerance; IFG – impaired fasting glucose; IGT – impaired glucose tolerance; DM – diabetes mellitus; BMI – body mass index; WC – waist circumference; BP – blood pressure; FBG – fasting blood glucose; PBG – postprandial blood glucose; HOMA-IR – homeostatic model assessment of insulin resistance; TC – total cholesterol; TG – triglyceride; LDL-C – low-density lipoprotein cholesterol; HDL-C – high-density lipoprotein cholesterol; ALT – alanine aminotransferase; AST – aspartate aminotransferase; UA – uric acid.
Difference between the four groups;
P<0.05 compared with the NGT group;
P<0.05 compared with the IFG group;
P<0.05 compared with IGT group.
Figure 1.
Comparison of serum meteorin-like (Metrnl) levels according to blood glucose status and weight in subjects in the four study groups. (A) Serum meteorin-like (Metrnl) levels in subjects with normal glucose tolerance (NGT), impaired fasting glucose (IFG), impaired glucose tolerance (IGT), and newly diagnosed type 2 diabetes mellitus (T2DM). (B) Serum Metrnl levels according to weight status (normal weight vs. overweight and obese). * P<0.05; ** P<0.01; *** P<0.001. Data are shown as the mean ± standard deviation (SD).
Serum levels of Metrnl and metabolic parameters
Partial correlation analysis was used to investigate the relationship between serum levels of Metrnl and metabolic parameters (Table 2). Before adjusting for age, gender, and body mass index (BMI), serum Metrnl levels were positively correlated with BMI, waist circumference (WC), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), FBG, PBG, HbA1c, fasting insulin, and HOMA-IR and was negatively correlated with high-density lipoprotein cholesterol (HDL-C) in all the subjects studied. After adjusting for age, gender, and BMI, these correlations remained statistically significant except for WC, systolic BP, TC, and LDL-C.
Table 2.
Correlation analysis of serum levels of meteorin-like (Metrnl), and metabolic parameters.
| Variable | Metrnl | Metrnl (age, gender, BMI adjusted) | ||
|---|---|---|---|---|
| Coefficient (r) | P-value | Coefficient (r) | P-value | |
| Age (years) | −0.022 | 0.784 | ||
| BMI (kg/m2) | 0.283 | <0.001 | ||
| WC (cm) | 0.194 | 0.014 | 0.014 | 0.862 |
| Systolic BP (mmHg) | 0.086 | 0.278 | 0.090 | 0.263 |
| Diastolic BP (mmHg) | 0.087 | 0.274 | 0.157 | 0.049 |
| TC (mmol/L) | 0.157 | 0.048 | 0.154 | 0.054 |
| TG (mmol/L) | 0.503 | <0.001 | 0.457 | <0.001 |
| LDL-C (mmol/L) | 0.200 | 0.011 | 0.142 | 0.076 |
| HDL-C (mmol/L) | −0.394 | <0.001 | −0.221 | 0.005 |
| FBG (mmol/L) | 0.379 | <0.001 | 0.222 | 0.005 |
| PBG (mmol/L) | 0.420 | <0.001 | 0.313 | <0.001 |
| HbA1c (%) | 0.379 | <0.001 | 0.273 | 0.001 |
| Fasting insulin (μU/mL) | 0.338 | <0.001 | 0.231 | 0.004 |
| HOMA-IR (index) | 0.433 | <0.001 | 0.267 | 0.001 |
BMI – body mass index; WC – waist circumference; BP – blood pressure; TC – total cholesterol; TG – triglyceride; LDL-C – low-density lipoprotein cholesterol; HDL-C – high-density lipoprotein cholesterol; FBG – fasting blood glucose; PBG – postprandial blood glucose; HOMA-IR – homeostatic model assessment of insulin resistance.
Serum levels of Metrnl and T2DM
The relationship between Metrnl and T2DM was explored by multiple logistic regression analysis (Table 3). Before adjusting for confounders (model 1), serum Metrnl levels were associated with significantly increased odds ratios (ORs) for T2DM (OR=1.892; P <0.001). After adjusting for age, gender, and BMI (model 2), the association between increased serum Metrnl levels and an increased risk for T2DM was also found (OR=1.780, P=0.001). When further adjusted for systolic BP, TC, TG, ALT (model 3), the estimated glomerular filtration rate (eGFR), and uric acid (UA) and increased serum levels of Metrnl remained significantly correlated with an increased the risk of T2DM (OR=1.727; P=0.008). However, when further statistical adjustment was made for HOMA-IR (model 4), serum levels of Metrnl were no longer significantly correlated with an increased OR for T2DM (OR=1.491; P=0.066), while the HOMA-IR significantly increased the risk of T2DM (OR=1.935; P=0.008).
Table 3.
Multiple logistic regression analysis of the association between serum levels of meteorin-like (Metrnl) with type 2 diabetes mellitus (T2DM).
| Model | Independent variable | beta coefficient | Standard error of beta coefficient | Wald statistic | Odds ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Model 1 | Metrnl, per 100 pg/mL | 0.638 | 0.161 | 15.757 | 1.892 (1.381–2.593) | <0.001 |
| Model 2 | Metrnl, per 100 pg/mL | 0.577 | 0.171 | 11.345 | 1.780 (1.273–2.491) | 0.001 |
| Model 3 | Metrnl, per 100 pg/mL | 0.546 | 0.207 | 6.931 | 1.727 (1.150–2.593) | 0.008 |
| Model 4 | Metrnl, per 100 pg/mL | 0.399 | 0.217 | 3.387 | 1.491 (0.974–2.281) | 0.066 |
| HOMA-IR, per 1 unit | 0.660 | 0.248 | 7.080 | 1.935 (1.190–3.146) | 0.008 |
Model 1: not adjusted; Model 2: adjusted for age, gender, and body mass index (BMI); Model 3: Model 2 plus systolic blood pressure (BP), total cholesterol (TC), triglyceride (TG), alanine aminotransferase (ALT), estimated glomerular filtration rate (eGFR), and uric acid (UA); Model 4: Model 3 plus the homeostatic model assessment of insulin resistance (HOMA-IR).
Discussion
Meteorin-like (Metrnl) is a novel adipomyokine that shows high expression levels in skeletal muscle and white adipose tissue [5,21]. In mice, Metrnl can promote the activation of adipose tissue macrophages and increases the expression of anti-inflammatory and thermogenic genes in white adipose tissue [5]. Therefore, Metrnl might have a potential therapeutic role in inflammatory and metabolic diseases by regulating tissue energy homeostasis [5,22–25].
Although there have been several reported studies on the association between serum levels of Metrnl in type 2 diabetes mellitus (T2DM), the findings have been conflicting. Lee et al. [13] and Zheng et al. [14] reported lower serum levels of Metrnl in patients with newly diagnosed T2DM, whereas Chung et al. [15] reported increased serum levels of Metrnl, which was consistent with the findings of the present study. Because of the limited number of studies on the association between T2DM and serum levels of Metrnl, it is difficult to explain these conflicting findings. However, preclinical studies in mice have shown that Metrnl in adipocytes could increase insulin sensitivity by stimulating the peroxisome proliferator-activated receptor gamma (PPARγ) pathway [16], which has a regulatory role in insulin resistance and adipocyte differentiation [26]. A study conducted in human adipocytes showed that overexpression of Metrnl inhibited the expression of PPARγ, which might lead to hyperinsulinemia and insulin resistance [17]. Therefore, increased serum levels of Metrnl might increase the risk of T2DM by promoting insulin resistance, which is consistent with the findings of the present study.
The findings of this study showed that increased serum levels of Metrnl were significantly associated with T2DM (OR=1.727; P=0.008), in model 3 that used multiple logistic regression analysis after adjusting for confounding factors, except for the homeostatic model assessment of insulin resistance (HOMA-IR). However, when further adjusted for HOMA-IR, in model 4, serum levels of Metrnl were no longer associated with an increased OR for T2DM (OR=1.491; P=0.066). Another explanation for the increased serum levels of Metrnl in patients with T2DM may be attributed to a protective compensatory response against metabolic overload, including insulin resistance [15]. On the basis of these results, the effect of Metrnl on insulin resistance should be confirmed by further studies as at this time, studies on serum Metrnl and its association with T2DM are limited and the findings have been contradictory. To our knowledge, the present study was the first to identify a significant correlation between serum levels of Metrnl with insulin resistance and to report that Metrnl might contribute to the development of T2DM dependent of insulin resistance in human subjects.
Because Metrnl is highly expressed in white adipose tissue, serum levels are likely to be correlated with parameters that include body mass index (BMI) and waist circumference (WC). The findings of this study showed that serum Metrnl levels were increased in overweight and obese subjects when compared with subjects of normal weight (Figure 1B) and was significantly correlated with BMI and WC. Löffler et al. also reported increased Metrnl expression in adipocytes in obese children compared with lean children [17]. However, Chung et al. [15] found that serum Metrnl levels were not associated with body weight, BMI, WC, and mass of adipose tissue. A preclinical study showed that when compared with control mice, neither Metrnl adipose-specific knockout nor transgenic-overexpression mice exhibited a change in body weight, lean to fat body mass, or distribution of adipose tissue [16]. It is clear that the relationship between serum levels of Metrnl and obesity requires further clinical study.
This study also had several limitations. This was an observational study that did not investigate the causal relationship between serum levels pf Metrnl and the development of T2DM. Also, this study included only Chinese subjects, and the findings require validation in subjects of different ethnicity. In this study, it was not possible to exclude other potential confounding factors, especially exercise, as Metrnl expression may be induced in muscle after exercise. Also, as Metrnl is mainly produced by muscle and adipose tissue, the percentage of body fat and skeletal muscle mass should also be assessed in future studies, and statistical analysis of patient data should also be adjusted for these parameters. Finally, the sample size of this study was quite small, and further studies with larger numbers of subjects should be undertaken in the future.
Conclusions
The findings from an observational study conducted at a single center in China showed that serum levels of meteorin-like (Metrnl) were significantly increased in patients with newly diagnosed type 2 diabetes mellitus (T2DM) and were significantly correlated with the lipid profile, glucose profile, and insulin resistance. Further logistic regression analysis showed that serum Metrnl levels were significantly correlated with an increased risk of T2DM, and this association might be due to insulin resistance. Future mechanism research studies are required to explore the mechanism for Metrnl in insulin resistance and T2DM.
Footnotes
Source of support: This study was funded by the National Natural Science Foundation of China (Grant No. 81873650 and No. 81700739), the Fundamental Research Funds of Shandong University (Grant No. 2018JC15), the Shandong Medical and Health Technology Development Project (Grant No. 2014WS0148), and Shandong University Basic Scientific Research Funding (Qilu Hospital Clinical Research Project) (Grant No. 2014QLKY21) awarded to Professor Sun Yu and Professor Kexin Wang
References
- 1.Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152:673–84. doi: 10.1016/j.cell.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 2.Ahima RS, Lazar MA. Physiology. The health risk of obesity – better metrics imperative. Science. 2013;341:856–58. doi: 10.1126/science.1241244. [DOI] [PubMed] [Google Scholar]
- 3.Pfutzner A, Weber MM, Forst T. A biomarker concept for assessment of insulin resistance, beta-cell function and chronic systemic inflammation in type 2 diabetes mellitus. Clin Lab. 2008;54:485–90. [PubMed] [Google Scholar]
- 4.Boström P, Wu J, Jedrychowski MP, et al. A PGC1α-dependent myokine that drives browning of white fat and thermogenesis. Nature. 2012;481:463–68. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao RR, Long JZ, White JP, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–91. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2009;302:179–88. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 7.Tso AW, Xu A, Sham PC, et al. Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: A 10-year prospective study in a Chinese cohort. Diabetes Care. 2007;30:2667–72. doi: 10.2337/dc07-0413. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen BK. IL-6 signalling in exercise and disease. Biochem Soc Trans. 2007;35:1295–97. doi: 10.1042/BST0351295. [DOI] [PubMed] [Google Scholar]
- 9.Lin Z, Tian H, Lam KS, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–89. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Chung HS, Choi KM. Adipokines and myokines: A pivotal role in metabolic and cardiovascular disorders. Curr Med Chem. 2018;25:2401–15. doi: 10.2174/0929867325666171205144627. [DOI] [PubMed] [Google Scholar]
- 11.Eaton M, Granata C, Barry J, et al. Impact of a single bout of high-intensity interval exercise and short-term interval training on interleukin-6, FNDC5, and METRNL mRNA expression in human skeletal muscle. J Sport Health Sci. 2018;7:191–96. doi: 10.1016/j.jshs.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saghebjoo M, Einaloo A, Mogharnasi M, Ahmadabadi F. The response of meteorin-like hormone and interleukin-4 in overweight women during exercise in temperate, warm and cold water. Horm Mol Biol Clin Investig. 2018;36(3) doi: 10.1515/hmbci-2018-0027. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Kang YE, Kim JM, et al. Serum Meteorin-like protein levels decreased in patients newly diagnosed with type 2 diabetes. Diabetes Res Clin Pract. 2018;135:7–10. doi: 10.1016/j.diabres.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Zheng SL, Li ZY, Zhang Z, et al. Evaluation of two commercial enzyme-linked immunosorbent assay kits for the detection of human circulating Metrnl. Chem Pharm Bull (Tokyo) 2018;66:391–98. doi: 10.1248/cpb.c17-00846. [DOI] [PubMed] [Google Scholar]
- 15.Chung HS, Hwang SY, Choi JH, et al. Implications of circulating Meteorin-like (Metrnl) level in human subjects with type 2 diabetes. Diabetes Res Clin Pract. 2018;136:100–7. doi: 10.1016/j.diabres.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 16.Li ZY, Song J, Zheng SL, et al. Adipocyte Metrnl antagonizes insulin resistance through PPARγ signaling. Diabetes. 2015;64:4011–22. doi: 10.2337/db15-0274. [DOI] [PubMed] [Google Scholar]
- 17.Löffler D, Landgraf K, Rockstroh D, et al. METRNL decreases during adipogenesis and inhibits adipocyte differentiation leading to adipocyte hypertrophy in humans. Int J Obes (Lond) 2017;41:112–19. doi: 10.1038/ijo.2016.180. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO) Report of a WHO/International Diabetes Federation (IDF) Consultation. Geneva: WHO; 2006. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia; pp. 1–46. https://apps.who.int/iris/bitstream/handle/10665/43588/9241594934_eng.pdf. [Google Scholar]
- 19.World Health Organization (WHO) Obesity: preventing and managing the global epidemic. Geneva: 2000. pp. 1–268. (WHO Technical Report 894). https://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li ZY, Zheng SL, Wang P, et al. Subfatin is a novel adipokine and unlike Meteorin in adipose and brain expression. CNS Neurosci Ther. 2014;20:344–54. doi: 10.1111/cns.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng SL, Li ZY, Song J, et al. Metrnl: A secreted protein with new emerging functions. Acta Pharmacol Sin. 2016;37:571–79. doi: 10.1038/aps.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo L, Ge S, Ge Y, et al. The adipokine metrnl ameliorates chronic colitis in Il-10−/− mice by attenuating mesenteric adipose tissue lesions during spontaneous colitis. J Crohns Colitis. 2019 doi: 10.1093/ecco-jcc/jjz001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Ushach I, Arrevillaga-Boni G, Heller GN, et al. Meteorin-like/Meteorin-β is a novel immunoregulatory cytokine associated with inflammation. J Immunol. 2018;201:3669–76. doi: 10.4049/jimmunol.1800435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung TW, Lee SH, Kim HC, et al. METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARδ-dependent pathways in skeletal muscle of mice. Exp Mol Med. 2018;50:122. doi: 10.1038/s12276-018-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray SL, Nora ED, Grosse J, et al. Leptin deficiency unmasks the deleterious effects of impaired peroxisome proliferator-activated receptor gamma function (P465L PPARgamma) in mice. Diabetes. 2006;55:2669–77. doi: 10.2337/db06-0389. [DOI] [PubMed] [Google Scholar]